Abstract
Introduction
Post-traumatic stress disorder (PTSD) is a chronic debilitating psychiatric disorder resulting from exposure to a severe traumatic stressor and an area of great unmet medical need. Advances in pharmacological treatments beyond the currently approved SSRIs are needed.
Areas covered
Background on PTSD, as well as the neurobiology of stress responding and fear conditioning, is provided. Clinical and preclinical data for investigational agents with diverse pharmacological mechanisms are summarized.
Expert opinion
Advances in the understanding of stress biology and mechanisms of fear conditioning plasticity provide a rationale for treatment approaches that may reduce hyperarousal and dysfunctional aversive memories in PTSD. One challenge is to determine if these components are independent or reflect a common underlying neurobiological alteration. Numerous agents reviewed have potential for reducing PTSD core symptoms or targeted symptoms in chronic PTSD. Promising early data support drug approaches that seek to disrupt dysfunctional aversive memories by interfering with consolidation soon after trauma exposure, or in chronic PTSD, by blocking reconsolidation and/or enhancing extinction. Challenges remain for achieving selectivity when attempting to alter aversive memories. Targeting the underlying traumatic memory with a combination of pharmacological therapies applied with appropriate chronicity, and in combination with psychotherapy, is expected to substantially improve PTSD treatment.
Keywords: amygdala, drugs, fear conditioning, prefrontal cortex, PTSD, stress, therapy
1. Introduction
Most people experience traumatic stress at some point in their lives. Evolution has fashioned robust neurobiological mechanisms that allow organisms to respond adaptively to stressors. For most, the intense physiological activation and disrupting impact of aversive memories declines following trauma, allowing life to return to normal.
For some, however, adaptive coping does not occur, as physiological arousal remains and recurring memories of the aversive event persist, strengthen, and disrupt normal functioning. Once post-traumatic stress disorder (PTSD) is diagnosed, treatment may involve psychotherapy, pharmacotherapy, or both. Currently only two drugs are approved in the United States for PTSD, and both are selective serotonin (5-HT) reuptake inhibitor (SSRIs).
Although certain psychosocial interventions and drugs can improve outcomes, there is still considerable unmet need in the treatment of PTSD. We focus primarily on investigational drugs being explored for potential utility in treating PTSD, especially when combined appropriately with therapy. Comprehensive reviews of PTSD psychotherapy are available elsewhere [3,4]. To set the stage for a discussion of investigational drug treatments, Section-2 will provide background on PTSD and explain Diagnostic and Statistical Manual (DSM)-IV criteria. Sections 3 and 4 will then discuss the neurobiology of stress responding and fear conditioning processes, which provide the rationale for psychosocial, pharmacological, and combined approaches.
It is important to acknowledge that many of the principles discussed herein have been derived from data gathered in pre-clinical (animal) studies, and therefore, caution is warranted in extrapolating these results to humans with PTSD. The results from animal studies are intended to help generate hypotheses for human clinical studies which, when tested, could lead to improved treatments.
2. PTSD
In the United States, lifetime prevalence of PTSD is 6.8% [5], and the costs of PTSD to individuals and society are high [6]. Incidence rates and relative costs are even higher in specific populations, such as military, veterans, and first-responders [7,8]. Considerable efforts are underway to improve prevention, diagnosis, and treatment of PTSD in these populations especially, since exposure to severe trauma is common [9].
PTSD (Box 1) is an anxiety disorder that requires exposure to a specific traumatic event. “Exposure” is defined as: experiencing or witnessing an event involving death or serious injury with a resulting feeling of intense fear, helplessness, or horror. Further, a PTSD diagnosis depends on persistent (‡ 1 month) symptoms from three clusters: reexperiencing of the trauma, avoidance/numbing behavior, and hyperarousal. The emphasis on symptom duration is important, as this stems from the recognition that most exposed to severe trauma exhibit PTSD-like symptoms acutely but learn effective coping strategies and recover on their own. Thus, PTSD is at least partly a disorder of recovery [10,11]. Finally, PTSD symptoms must cause severe distress and/or impair normal functioning. Note that criteria for diagnosing and defining PTSD may change for the DSM-V, scheduled for release in 2013.
Currently, both psychotherapy and pharmacotherapy approaches are pursued for the treatment of PTSD. In general, the most effective psychotherapies attempt to treat the pathological memory defining PTSD, whereas drugs are mainly applied to blunt PTSD symptoms. We will argue that PTSD drugs may be particularly useful when they positively interact with therapy-related learning.
The three symptom clusters may have a common neurobio-logical basis, or they may reflect distinct neurobiological mechanisms requiring multiple pharmacological approaches for their treatment [12]. It is notable that the first two core symptom clusters, reexperiencing and avoidance, must be triggered by memories of a specific traumatic event, whereas the third cluster, hyperarousal, is not restricted to associative responding. Thus, PTSD symptoms may result from dissociable, though interacting, associative, and non-associative processes mediated by separate biological systems (i.e., emotional learning vs. stress-responding).
Vulnerability factors, such as prior traumas (e.g., childhood abuse) or genetic variations may prove important for elucidating heterogeneity in PTSD and devising more appropriate treatment approaches. Further, PTSD is often comorbid with other psychiatric disorders (e.g., depression) and/or substance abuse disorders, which may require additional treatments. Although these topics are beyond the scope of the current manuscript, interested readers may refer to other excellent articles for more information [13-22].
3. Stress response pathways
Organisms have innate, automatic mechanisms for responding to threatening stressors. The acute stress response is a highly activated physiological state that prepares the organism in two major ways: i) energy and resources are diverted from non-essential processes (e.g., digestion) and mobilized to prime sensorimotor processes necessary for defensive behavior, and ii) behavioral responding is restricted to defensive behaviors, often simple, innate, species-specific reactions that evolved because they were adaptive [23]. Adaptive defensive behaviors can vary widely and are usually governed by the perceived proximity of the threat and the behavioral options dictated by the environment. For example, rats freeze in a closed space with a predator nearby, but will attempt escape or fight if cornered by an attacking predator [24]. An adaptive stress response system is hardwired to produce reliable activation, yet flexible enough to produce diverse behavioral actions. It should also be transient, so critical bodily resources return to non-defensive processes, like digestion, when the threat wanes.
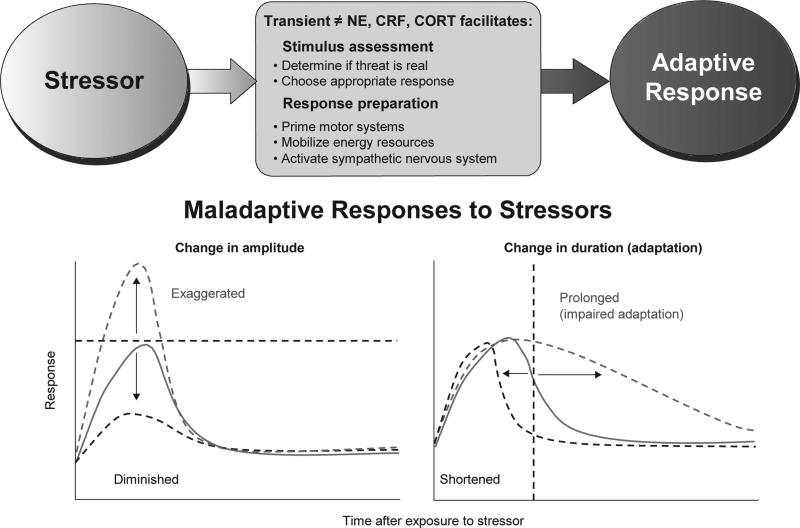
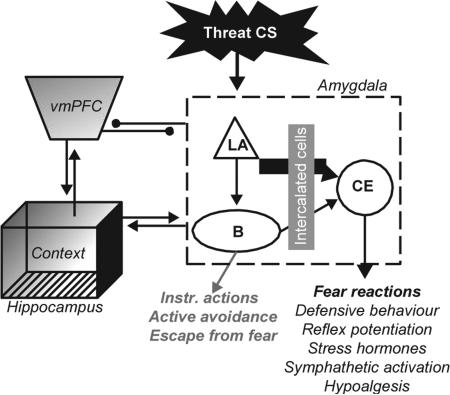
The stress response includes multiple physiological components, including endocrine (hypothalamic-pituitary-adrenal, or HPA, axis) and sympathetic responses [25]. Several key neurochemicals are important for the arousal/activational component of the stress response, and excessive or prolonged activation of these pathways likely contributes to hyperarousal symptoms in PTSD (Figure 1). We will focus here on three powerful modulators of defensive brain systems and sympa-thetic responding: i) norepinephrine (NE), ii) corticotropin-releasing factor (CRF), and iii) cortisol (CORT).
Figure 1.
The Stress Response System (SRS). TOP: The SRS is composed of behavioral, endocrine, and autonomic components which act in concert to generate an appropriate, adaptive response to a stressor. Three key mediators in the SRS are norepinephrine (NE), corticotropin releasing factor (CRF) and cortisol (CORT). Stressor-induced release of NE, CRF and CORT facilitates physiological processes which allow the organism to evaluate the stressor and choose an adaptive response, while in parallel activating effector systems. Execution of a successful response will minimize the impact of the stressor and in parallel, feedback inhibitory systems will ensure that the stress response system will return to normal, pre-stress levels. BOTTOM: Abnormal activation of NE, CRF and CORT pathways can result in a dysfunctional SRS in which normal alarm reactions may be maladaptive. A complex interplay of genetic risk factors, vulnerability factors (prior history), stressor factors (intensity, duration, chronicity), may be expressed neuronally as imbalances in different neurochemical pathways and functionally, as different alterations in the alarm reaction. Thus, the CRF and NE hyperactivation, and perhaps CORT hypoactivation, seen in different disorders may be manifested as alarm reactions with exaggerated or diminished amplitude and/or prolonged or shortened duration. Hypothetical curves represent a normal physiological response to a stressor that, after reaching a maximum, declines to baseline level. Examples are stress hormone responses or levels of physiological arousal. Return to baseline could result from successful removal of the activating threatening stimulus and/or from feedback inhibition processes (e.g. CORT inhibition of pituitary mediated ACTH release). Exposure to traumatic stimuli, possibly in conjunction with vulnerability attributable to genetic and/or environmental (e.g. prior history of stress) factors, has the potential to produce sustained maladaptive responses (dashed lines).
Adapted from [217], printed with permission from Bentham Publishers.
NE is a critical mediator of arousal via direct and indirect effects on both central and peripheral processes. Brainstem NE neurons project to all levels of the neuraxis and NE signaling appears hyperresponsive in PTSD [26]. For instance, PTSD is associated with i) greater plasma NE and stress-induced arousal, especially when stressors are trauma-specific, ii) heightened adrenergic receptor sensitivity, and iii) enhanced yohimbine-facilitated startle (yohimbine increases NE release by blocking autoinhibitory feedback at synapses). NE dysfunction has also been implicated in associative memory, which is discussed in the following section. Norepinephrine's effects are mediated through activation of a1, a2, and b-adrenergic receptors, which are widely distributed in the central nervous system and periphery. As reviewed in Section-5, drugs targeting these various receptors have been evaluated for therapeutic utility in PTSD and may have potential to further improve PTSD treatment if used in novel ways.
The neuropeptide CRF also plays a crucial role in stress/arousal processes and has been implicated in PTSD [16]. Central CRF initiates the HPA-axis response to stress, with hypothalamically released CRF binding to receptors in the anterior pituitary, and causing ACTH release into the circulation. ACTH in turn triggers CORT release from the adrenal cortex, which acts on glucocorticoid receptors in the periphery and brain. CRF is also a powerful modulator of defensive brain systems mediating stress, arousal, and memory. CRF signaling may be dysfunctional in PTSD as cerebrospinal fluid CRF levels are elevated and ACTH responding to exogenous CRF is blunted [27]. Beyond these findings, a crucial role for CRF in PTSD is mainly hypothesized based on CRF's HPA-axis role, preclinical research findings [28], and CRF's known association with related conditions like depression [29]. CRF acts on two receptor subtypes, CRF1 and CRF2. Small molecule antagonists of the CRF1 receptor have been developed and administered to humans [28], but to date there are no published clinical data evaluating the effects of CRF1 receptor antagonists in PTSD.
CORT is a glucocorticoid hormone secreted by the adrenal cortex in response to ACTH or low circulating CORT levels. CORT has a powerful dual role in the coordinated stress response: i) it is a primary mediator of the global stress response, with wide-ranging effects on both peripheral and central processes, and ii) it provides the primary negative feedback signal to halt the stress response by suppressing brain CRF, ACTH and NE release. Thus CORT functions to maintain allostasis and ensure a robust, but transient, response to stressful demands. Not surprisingly, dysfunctions in CORT signaling are associated with PTSD; low circulating CORT and enhanced CORT-mediated negative feedback are usually observed in PTSD patients. This suggests that responses to trauma or distressing reminders may be abnormally prolonged in PTSD [30]. Perhaps most troubling, chronic stress, via a glucocorticoid mechanism, leads to changes in neural and immune functions that weaken the ability to fight off sickness and learn effective coping strategies [31].
Together, it seems clear that dysfunctions throughout the stress-response system contribute to PTSD. NE and CRF systems are hyperresponsive and abnormalities in CORT feedback likely prolong stress-response episodes. This combination could account for hyperarousal symptoms in PTSD, by decreasing thresholds for responding and prolonging the activation of defensive brain and body systems, at the expense of many other bodily functions. This could also indirectly contribute to PTSD by enhancing associative memory symptoms.
4. Fear Conditioning
Dysfunctions in parallel stress-response and defensive-memory systems are evident in PTSD. Many of the defining characteristics of PTSD, such as reliving of trauma, avoidant behavior, and nightmares depend on trauma-related associative learning. Thus, cues present during a traumatic event gain emotional valence and subsequently elicit defensive responses and fear. This learning is normal and even adaptive, however, when dysfunctional, these associative processes disrupt functioning. Although it is useful to consider stress and memory factors in parallel, they are closely related and coordinated systems that surely interact in important ways. For instance, trauma-linked cues can trigger the HPA-axis and circulating stress hormones can have profound effects on associative neural plasticity and thresholds for defensive responding [26,30,31].
Pavlovian fear conditioning (FC) is an important paradigm for studying memory processes and brain circuits that make strong contributions to PTSD. FC occurs when neutral conditioned stimuli (CSs) are temporally paired with naturally aversive unconditioned stimuli (USs). Any sensory stimulus can serve as a CS, and USs can be any unpleasant or painful stimulus. CS and US pairings produce plasticity in the fear system, allowing subsequent CSs to control fearful responding. FC is an extremely powerful form of associative learning; if the CS is sufficiently salient and the US sufficiently aversive, even a single brief episode can lead to strong fear memories that last a lifetime [32]. FC studies have greatly enhanced our understanding of the neurobiology of memory and emotional responding (see Box 2). It is also widely believed that studies of FC processes will identify novel behavioral and pharmacological interventions for PTSD in particular, since this disorder is attributable to a distinct traumatic event and is defined largely by pathologic responses to aversive memories.
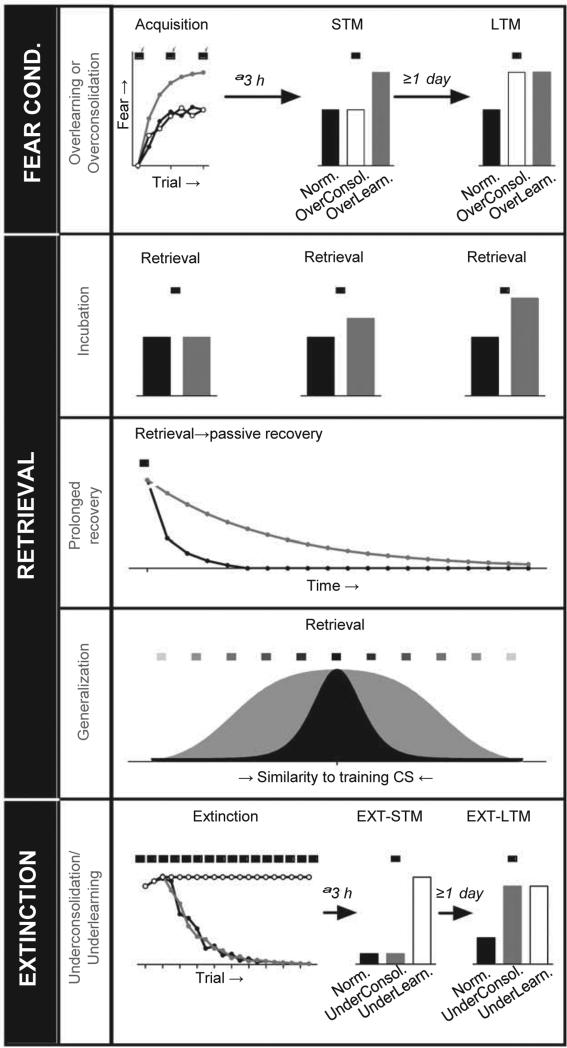
Although FC does not necessarily produce PTSD, psychological and neural processes mediating FC likely contribute to PTSD if dysfunctional. FC has several important features and phases that may make distinct contributions to PTSD or treatment. Further, learning that depends on FC may also be relevant. These are discussed below and depicted in Figure 2.
Figure 2.
FC Processes Could Contribute to PTSD. Prior experience(s) and/or biological factors may interact with stress-responding and associative memory to produce PTSD. Precise analyses of memory processes may continue to refine our understanding of the core memory deficits in PTSD. Identification of specific impairments should help identify brain regions and cellular/molecular mechanisms important for PTSD and its treatment. For instance, recent studies suggest that extinction consolidation/retrieval and generalization/discrimination are impaired in PTSD, highlighting the importance of inhibitory learning and PFC function. Note that this list is not exhaustive and important learning processes like latent inhibition, conditioned inhibition and avoidance learning have been omitted. Black bars/lines represent normal FC and gray or white bars/lines represent potential abnormalities in PTSD.
4.1 Acquisition
This refers to the initial CS-US learning phase that occurs during a traumatic experience. The prevailing view is that acquisition of FC critically depends on synaptic plasticity within the defensive brain circuitry, especially the amygdala [33]. The strongest evidence comes from studies of the lateral nucleus of the amgydala (LA), where strengthening of synapses between sensory afferents and principle neurons is critical for learning [34]. FC plasticity, especially in LA, has been intensely studied and many transmitters, receptors, intracellular messengers, genes, and gene products have been implicated [35,36]. The core mechanism for acquisition in LA is thought to be temporary enhancement of AMPA receptor function, through an LTP-like process [38,39]. Generally speaking, four processes play critical roles: i) glutamate signaling (via AMPA-, NMDA- and metabotropic receptors); ii) feedforward GABAergic inhibition; iii) intracellular kinase activation (e.g., PKA & CAMKII); and iv) modulatory neurotransmitters/neuropeptides. Although theoretically not necessary for FC plasticity, modulators like NE represent important drug targets because they powerfully affect all of the other processes. For instance, NE can enhance PKA and glutamate receptor activation, and suppress GABAergic inhibition in LA—all of which favor FC plasticity [36].
4.2 Consolidation/storage
FC produces learning almost instantly and short-term memory (STM) that lasts at least several hours. However, long-term memory (LTM) requires additional cellular processes to stabilize and maintain learning. This consolidation process is usually complete within 6 – 24 h. Consolidation requires intracellular signaling, gene transcription, translation of new proteins, and remodeling/growth of synaptic connections [36]. Interfering with these key steps can block the formation of LTM and essentially erase the FC memory. Although some forms of memory involve an additional “systems-level” consolidation process, where information is transferred to another brain region, FC memories are likely stored in the same region(s) and neurons where initial learning takes place [42]. Interestingly, recent research suggests that a specific kinase, PKMz, is necessary to store FC memories [43] and interference with this molecule can erase memory even without retrieval (but see [44]). However, it is not clear how this mechanism could be exploited to target PTSD memories specifically.
4.3 Retrieval
Sometimes called expression, recall, or performance, retrieval simply refers to conditioned responding (CR) that depends on the associative CS-US relationship during FC. Retrieval is assessed by presenting the CS alone and measuring newly acquired CRs. In addition to probing the state of the FC memory (STM vs. LTM), retrieval tests can provide important information about the strength and specificity of FC memories. Since defensive responding falls along a fear continuum, the CR type, magnitude, and duration indicate the strength of the FC memory [23]. For instance, a rat that initially freezes at 80% and takes 10 min to stop freezing after a CS presentation has a stronger FC memory than one that initially freezes £80% but stops freezing after 2 min. Retrieval tests that include non-conditioned cues can also assess generalization and discrimination (non-specific fear learning and/or lower thresholds for defensive responding). Note that some drugs may affect memory retrieval without having any effect on learning or memory processes. Such drugs would blunt associative PTSD symptoms, but may not help correct the underlying problem.
4.4 Retrieval-induced learning
Recent exciting research has shown that simple FC retrieval events may induce diametrically opposed plasticity processes that can strengthen or weaken CRs. Retrieval returns the memory to a labile state requiring a second reconsolidation process [45-47]. Reconsolidation and consolidation have over-lapping, but distinct, molecular mechanisms [48]. Reconsolidation likely serves an updating function, allowing for incorporation of new information into the LTM trace [49,50]. Although emotionally neutral updating may be possible, retrieval-related learning usually changes CR strength depending on the nature of the new information. For instance, fear extinction occurs when subjects repeatedly experience the CS without the US [51]. These retrieval episodes contradict the predictive validity of the CS and weaken fear. However, brief or less frequent reminders can strengthen fear (incubation) [52,53]. Little is known about the mechanisms of incubation, although NE makes the clearest contribution [54]. Extinction is better understood. Fear extinction requires prolonged or repeated CS exposure and appears to form a new inhibitory “CS-NoUS” memory that competes for control of emotional behavior [55]. Extinction depends on coordinated activity and plasticity in multiple brain regions including the amygdala [56], periaqueductal gray [57], prefrontal cortex [58], and hippocampus [59]. Extinction also has learning, STM, consolidation, LTM and retrieval phases. Molecularly, extinction depends on some of the same molecules as initial FC (e.g., NMDA receptors and NE) but also distinct molecules (e.g., cannabinoid receptors) [60,61]. And unlike original FC, extinction is highly context-dependent (renewal, reinstatement) and decays with time (spontaneous recovery) [55].
4.5 Other FC-related processes
Additional conditioning processes that depend on FC may have relevance to PTSD, such as conditioned inhibition (CI; safety learning) [62] and instrumental avoidance/escape [63-65]. Dysfunctions in these processes could contribute to the development of PTSD and/or the failure to adequately cope following traumatic experience. However, the exact role for conditioned fear in these processes is unclear, and much less is known about the underlying neurobiology.
4.6 Individual differences and stress-enhanced fear learning (SEFL)
Individual differences in FC behavior may have relevance to understanding and devising better treatments for PTSD. For example, rats that show strong FC, normal retrieval, and extinction, but impaired extinction retrieval [66], may mirror the deficit seen in PTSD. Prior stress can also enhance anxiety and FC [67-69], which could also have relevance to PTSD. Although biomarkers, variability in FC processes and their relation to PTSD vulnerability/resilience are extremely important, this large body of research is beyond the scope of the present discussion and the interested reader is encouraged to consult other excellent reviews [11,14,16-18,70-73].
4.7 Relationship of FC processes to PTSD
Most agree that FC occurs with traumatic experience and contributes to PTSD phenomenology. However, there continue to be debates regarding which FC processes are dysfunctional in PTSD and how to exploit associative learning to enhance treatment. Before proceeding, it is important to note that FC studies, especially those conducted in rodents, relate directly to implicit Pavlovian memories and defensive responses [33]. Thus, one should not assume that findings from FC studies necessarily extend to other types of memory, like explicit/declarative memory, or human emotions and feelings. Strong FC memories likely influence other memory components and emotions, but they are not one in the same. That said, if dysfunctional FC processes are a major contributor to PTSD, addressing this dysfunction should at least dampen PTSD symptoms expressed via other brain systems.
Regarding FC dysfunction as a PTSD cause, two major ideas are prominent: PTSD occurs i) because the FC memory is excessively strong, or ii) because normal fear-coping processes are deficient. Though not mutually exclusive, available data are most consistent with the latter. As mentioned earlier, PTSD may largely be a disorder of recovery, since most traumatized individuals exhibit symptoms acutely but recover without treatment. Further, when subjected to controlled FC procedures measuring implicit fear responses, PTSD cohorts show reliable impairments of fear extinction recall, compared to trauma-exposed, non-PTSD controls [74]. Fear acquisition effects in those with PTSD are less consistent; some studies show facilitated FC, some do not, and still others report normal FC responding to conditioned cues, but overgeneralization to non-conditioned cues or impaired discrimination/CI [74-77]. Thus, the major deficit appears to involve inhibitory fear learning [78], and especially retrieval of the extinction memory, since PTSD patients learn extinction normally but fail to remember it [74]. This specific memory pattern points to dysfunctions in PFC and hippocampal processes that gate amygdala-dependent fear during extinction retrieval [55,79], and those with PTSD show abnormal PFC, hippocampal, and amygdalar activation with fMRI analyses [74].
Another hypothesis suggests that “overconsolidation” and stronger FC memories are the primary problem in PTSD [80]. Although a recent study [74] failed to detect over-consolidation in PTSD patients, this model also hypothesizes a role for reminder-induced strengthening of the FC memory, to account for the progressive worsening of symptoms in PTSD. This is actually predicted by the impaired-extinction model discussed above; retrieval episodes that fail to induce extinction trigger stress-responses like CRF and NE that enhance reconsolidation, leading to incubation of the FC memory [81]. It remains to be determined whether such a secondary “over-reconsolidation” process contributes to PTSD.
Other FC-related processes could also contribute to PTSD, although the data supporting these mechanisms are much weaker. For instance, “overgeneralization” or impaired safety learning could broaden the ability of innocuous stimuli to trigger fear and increase the frequency of fearful episodes [78,82,83]. Finally, therapy tapping into adaptive active avoidance/coping mechanisms, which can powerfully suppress fear and are more durable than extinction [63,64,84], may offer a viable alternative if the extinction deficit in PTSD patients cannot be treated. More research is needed to understand the neurobiology of these processes and their potential contribution to PTSD and treatment.
5. Investigational drugs and PTSD-related memory processes
Pharmacological and psychotherapeutic approaches have been used in PTSD and certain psychotherapy approaches (e.g., prolonged-exposure therapy or PE) are of particular benefit. As the present review is focused on drug treatments, the reader is referred elsewhere for comprehensive reviews regarding therapy [3,4,85-88]. Nevertheless, certain drugs may be particularly useful if they positively interact with psychotherapy, and this will guide our analysis.
Evaluation of relative drug efficacy is challenging in PTSD for several reasons. First, PTSD is often accompanied by other psychiatric afflictions including depression, addiction, chronic pain, and generalized anxiety [3,4]. Second, patients have varied experiences including differences in the nature and/or frequency of the defining trauma, prior traumatic experience, and prior history with different therapeutic approaches. Third, patients are often taking additional medications. Fourth, the lack of reliable, standardized, non-subjective measures to diagnose PTSD and evaluate treatment efficacy severely hinder comparisons between studies, both clinical and preclinical. Finally, FC research strongly suggests that drugs should be evaluated in a more sophisticated way. For instance, although benzodiazepines may blunt anxiety symptoms in patients with PTSD, animal studies demonstrate that these drugs clearly impair fear extinction and could potentially counteract therapy-related learning [89]. This example illustrates that some drugs could be both beneficial and detrimental to PTSD treatment, depending on when they are applied.
Continued research into PTSD biomarkers, PTSD risk/resilience, as well as basic studies of the neurobiology of PTSD-relevant processes may be critical to improving drug, psychosocial, and combination therapies [90]. Nevertheless, recent research suggests that new drugs have great potential to improve PTSD outcomes. This will be discussed below and compared to approved or common drug regimens. Previous reviews have provided overviews of investigational drugs in PTSD with regard to general effects on PTSD symptoms as well as specific symptoms such as anxiety, insomnia, and nightmares [91]. These drugs affect diverse targets, including monoamines (norepinephrine, dopamine, serotonin), amino acids (GABA, glutamate), neurosteroids, neuropeptides (substance P, CRF) and opiates. Although we summarize general results for many of these agents in Table 1 (drugs with monoamine-based mechanisms) and Table 2 (non-monoamine mechanisms), our discussion will emphasize drugs that have potential to significantly alter dysfunctional aversive memories and results from randomized clinical trials (RCTs) [85,92]. Particularly exciting preclinical findings with relevance to PTSD treatment are also included. Finally, Table 3 (monoamine-mechanisms) and Table 4 (non-monoamine mechanisms) summarize ongoing clinical trials with investigational drugs in PTSD.
Table 1.
Survey of non-SSRIs evaluated for clinical efficacy in treating PTSD: Monoamine mechanisms.
| Drug | Primary mechanism | Pavlovian FC Process* | Population | Trial Type/#Enrolled | Effect on symptoms‡ | Comment |
|---|---|---|---|---|---|---|
| Venlafaxine | SNRI | ” Extinction | Chronic PTSD | DBPC/538 | #[104] | 12 weeks treatment. Similar efficacy vs. sertraline |
| Chronic PTSD | DBPC/329 | #[103] | 24 weeks treatment | |||
| Duloxetine | SNRI | untested | Chronic PTSD, military-related, co-morbid MDD | OL/21 | #[163] | 8 weeks treatment |
| Chronic PTSD, military-related | OL/20 | #[164] | 12 weeks treatment | |||
| Propanolol | NE: b receptor antagonist | # Acquisition | Post-trauma, pediatric | DBPC/29 | $[133] | Trends: #PTSD symptoms in boys, ”PTSD symptoms in girls |
| $ Consolidation | ||||||
| # Retrieval | ||||||
| # Incubation | ||||||
| # Reconsolidation | ||||||
| $ Extinction | ||||||
| Post-trauma | DBPC/48 | $[92] | Gabapentin also failed to provide significant benefit | |||
| Chronic PTSD | DBPC/19 | #[138] | In combination with post-reactivation therapy. Reduced subsequent physiologic responses to recall | |||
| Post-trauma | OL/19 | #[132] | Reduced emergence of PTSD | |||
| Post-trauma | DBPC/41 | #[131] | Pilot study results suggest preventative effect on PTSD | |||
| Chronic PTSD | DBPC/38 | #[130] | Reduced emotional enhancement of memory in PTSD and normal subjects | |||
| Prazosin | NE: a1 receptor antagonist | ” Acquisition | Chronic PTSD, military-related | Historical 237 | $[125] | Prazosin is better tolerated than quetiapine for nighttime PTSD symptoms but both failed to provide significant therapeutic effect (p = 0.54) |
| $ Consolidation | ||||||
| # Extinction | ||||||
| Chronic PTSD | RPCC/13 | #[121] | Improved sleep parameters and decreased nightmares | |||
| Chronic PTSD | Historical/23 | #[127] | Decreased nightmares and overall PTSD symptoms | |||
| Chronic PTSD, military-related | DBPC/34 | #[123] | Decreased nightmares and overall PTSD symptoms; improved sleep parameters | |||
| Chronic PTSD | DBPC/10 | #[126] | Daytime administration reduced PTSD-related psychological distress | |||
| Chronic PTSD, elderly men | OL/9 | #[124] | Reduced nightmares and overall PTSD severity | |||
| Chronic PTSD, military-related | DBPC/10 | #[165] | Decreased nightmares and overall PTSD symptoms; improved sleep parameters | |||
| Chronic PTSD, military-related | Historical/59 | #[122] | Reduced nightmares and overall PTSD severity | |||
| Clonidine | NE: a2 receptor agonist | # Acquisition | Chronic PTSD | Historical/68 | #[168] | Combination with imipramine for PTSD and depression |
| # Retrieval | ||||||
| Guanfacine | NE: a2 receptor agonist | # Retrieval | Chronic PTSD, military-related | DBPC | $[166] | Taking no other psychiatric medication or stable dose of antidepressant |
| Chronic PTSD, military-related | DPBC/63 | $[167] | Taking no other psychiatric medication or stable dose of antidepressant | |||
| MDMA | 5HT: releaser, reuptake inhibitor (DA, NE) | untested | Chronic, treatment-resistant PTSD | DPBC/20 | #[119] | MDMA assisted psychotherapy |
| Bupropion | DA/NE reuptake inhibitor | $ FC | Chronic PTSD | DBPC/30 | $[169] | |
| # Retrieval | ||||||
| Nefazodone | SSRI/5-HT2A Antagonist | # Retrieval | Chronic PTSD, military related | DBPC/41 | #[114] | 12-Week trial |
| Buspirone | 5HT: 5HT1A partial agonist | # FC | Chronic PTSD | OL/8 | #[108] | |
| # Consolidation | ||||||
| # Retrieval | ||||||
| $ Extinction | ||||||
| Risperidone | DA: D2 (and 5HT2A) antagonist | # Retrieval | Chronic PTSD, military-related | DBPC/247 | $[170] | Serotonin reuptake-inhibiting antidepressant resistant PTSD |
| SSRI resistant civilian PTSD | DBPC/45 | $[171] | Post-hoc analysis suggested risperidone augmentation may be helpful in sertraline non-responders | |||
| PTSD in women survivors of domestic abuse and rape trauma | DBPC/20 | #[172] | Risperidone monotherapy | |||
| Psychotic combat-related PTSD | OL /26 | #[173] | Risperidone monotherapy | |||
| Chronic PTSD, military-related | DBPC /73 | #[174] | ||||
| PTSD related to childhood abuse in women | DBPC /21 | #[175] | ||||
| PTSD, military-related | OL /17 | #[176] | ||||
| Chronic PTSD, military-related | DBPC /15 | #[177] | Reduced irritability and intrusive thoughts in combat-related PTSD | |||
| Quetiapine | Mixed DA, 5HT, NE antagonist and H1 antagonist | untested | Chronic PTSD, military-related | Historical /237 | $[125] | Prazosin is better tolerated than quetiapine for nighttime PTSD symptoms but both failed to provide significant therapeutic effect (p = 0.54) |
| Chronic PTSD, military-related | OL /53 | #[178] | Quetiapine monotherapy | |||
| Chronic PTSD | OL /15 | #[179] | Adjunctive therapy to SSRI | |||
| Chronic PTSD, military-related | OL /19 | #[180] | Reduced sleep disturbances | |||
| Chronic PTSD, military related | OL /20 | #[181] | Adjunctive treatment | |||
| Mirtazipine | 5HT, a-adrenergic, H1 antagonist | # Retrieval | Chronic PTSD | DBPC /29 | #[115] | |
| Amitriptyline | TCA with SNRI activity | # Retrieval | Chronic PTSD, military related | DBPC /46 | #[182] | Improved some symptom measures including HAMA-A and HAM-D but not structured interview on PTSD symptoms |
| Imipramine | TCA | #FC #Retrieval | Chronic PTSD, military related | DBPC /34 | #[183] | The MAO inhibitor phenelzine was also effective in this trial |
| Chronic PTSD, military related | 60 | #[184] | The MAO inhibitor phenelzine appeared more effective in this trial | |||
| Olanzapine | Mixed DA, 5HT, NE antagonist and H1 antagonist | # FC | Chronic psychotic PTSD, military related | OL /55 | #[185] | Olanzapine monotherapy was better than fluphenazine in reducing most of the psychotic and PTSD symptoms, and was better tolerated in psychotic PTSD patients |
| Chronic PTSD, military-related | DBPC /19 | #[186] | Adjunctive therapy in SSRI resistant patients. Reduced some PTSD symptom measures and improved sleep. No significant effect on clinician-rated global response | |||
| Chronic PTSD, military-related | OL /48 | #[187] | 30 patients completed the study | |||
| Chronic PTSD | DBPC /15 | $[188] | ||||
| Aripiprazole | DA: D2, partial agonist, 5HT: 5HT1A partial agonist, 5HT2A antagonist | # Retrieval | PTSD, military-related | OL /17 | #[189] | Augmentation of existing pharmacotherapy |
| Chronic PTSD | OL /32 | #[190] | Aripiprazole monotherapy. Nine patients discontinued treatment | |||
| Chronic PTSD | OL /22 | #[191] | Aripiprazole monotherapy. Eight patients discontinued treatment |
Effects of drugs on Pavlovian FC processes: ” = enhanced, # = impaired, $ mixed results. No entry means either the drug has not been tested for that process or available tests show null effects. Both preclinical and clinical studies were considered, but only if drugs were evaluated on explicit FC paradigms. Acquisition: refers to a clear learning effect (either within-session or STM effect, or effect with pre-training but not post- training administration); Consolidation: refers to a clear consolidation effect (drug given post-training but washed out before fear retrieval test). FC is used when data aren't available to distinguish between acquisition and consolidation effects (Note: Pavlovian FC Processes column excludes findings from instrumental avoidance studies).
zEffects of drugs on Symptoms: $ No significant effect; # improved symptoms; ” worsened symptoms.
DBPC: Double Blind Placebo-Controlled; FC: Fear conditioning; Historical: Retrospective chart review; OL: Open Label; RPCC: Randomized Placebo-Controlled Crossover; SNRI: Serotonin–norepinephrine reuptake inhibitor; TCA: Tricyclic antidepressant.
Table 2.
Survey of non-SSRIs evaluated for clinical efficacy in treating PTSD: Non-monoamine mechanisms.
| Drug | Primary mechanism | Pavlovian FC Process* | Population | Trial Type/#Enrolled | Effect on symptoms‡ | Comment |
|---|---|---|---|---|---|---|
| Morphine | Opiate: μ agonist | # FC | Acute trauma, military-related | Historical /696 | #[192] | Reduced risk of developing PTSD following traumatic injury |
| $ Consolidation | ||||||
| # Retrieval | ||||||
| # Extinction | ||||||
| Acute trauma, civilian | Historical /155 | #[193] | Lower morphine dose associated with increased risk of PTSD | |||
| Acute trauma, children | Historical /70 | #[194] | Higher doses of morphine reduced subsequent PTSD symptoms | |||
| Acute trauma, children | Historical /24 | #[195] | Higher doses of morphine reduced subsequent PTSD symptoms | |||
| Nalmefene | Opiate antagonist | Untested | Chronic PTSD | OL /18 | #[196] | Thought to block endogenous opiate effect thereby reducing emotional avoidance |
| Naltrexone | Opiate antagonist | ” FC | Chronic PTSD | OL /8 | #[197] | Reduction in hyperarousal symptoms but at doses producing side-effects |
| $ Consolidation | ||||||
| $ Extinction | ||||||
| D-Cycloserine | NMDA partial agonist (developed as broad spectrum antibiotic) | ” Consolidation | Chronic PTSD | DBPC | $[198,199] | No benefit vs. placebo when combined With CBT. Several other clinical trials in PTSD are ongoing. |
| $ Retrieval | ||||||
| ”Reconsolidation | ||||||
| ” Extinction | ||||||
| Ketamine | GLU: NMDAR antagonist | # Acquisition | Acute stress disorder following trauma | Historical /50 | ”[200] | Ketamine increased symptoms of dissociation, reexperiencing, hyperarousal and avoidance |
| # Retrieval | ||||||
| Acute trauma, military-related | Historical /147 | #[201] | Patients who received ketamine had lower rates of PTSD than those who did not | |||
| Hydrocortisone | Glucocorticoid Receptor: agonist | ” Acquisition | Trauma-exposed, with and without PTSD | OL | #[152] | Decreased fear-potentiated startle in PTSD patients |
| ” Generalization | 100 | |||||
| # Cond. Inhib. | ||||||
| # Retrieval | ||||||
| ” Extinction | ||||||
| Veterans, with and without PTSD | DBPC /63 | #[153] | Decreased fear-potentiated startle in both PTSD and non-PTSD participants | |||
| Chronic PTSD, military-related | DBPC /20 | #[157] | Used in combination with memory reactivation; effect is significant at 1 week but diminished after 1 month | |||
| Cardiac surgery patients | DBPC /28 | #[154] | Hydrocortisone given at time of surgery decreased chronic stress symptoms | |||
| Chronic PTSD | OL /3 | #[151] | Reduced recall of traumatic memories | |||
| Cardiac surgery patients | Randomized 48 | #[155] | Hydrocortisone given at time of surgery decreased chronic stress symptoms | |||
| Septic shock patients | DBPC /20 | #[156] | Decreased development of PTSD | |||
| Topiramate | Anticonvulsant; Multiple effects | untested | Chronic PTSD | DBPC /38 | #[202] | Monotherapy significantly reduced reexperiencing symptoms and Treatment Outcome PTSD scale. |
| Chronic PTSD, military-related | DBPC /40 | $[203] | Augmentation to standard pharmacotherapy and psychotherapy. Higher dropout rate in topiramate group due to CNS adverse events prevented definitive assessment. | |||
| Chronic PTSD | OL /35 | #[204] | Monotherapy and augmentation. Decreased nightmares and other PTSD symptoms. | |||
| Lamotrigine | Anticonvulsant; Na+ channel inhibitor | # FC | Chronic PTSD | DBPC /14 | #[205] | Reduced reexperiencing and avoidance/numbing symptoms |
| Tiagabine | Anticonvulsant; GABA uptake blocker | Untested | Chronic PTSD | DBPC /232 | $[206] | |
| Chronic PTSD | OL/DBPC /18 | #[207] | Responders identified in OL trial had greater incidence of relapse when switched to placebo (p = 0.08) | |||
| Divalproex | Anticonvulsant; GABA enhancer | ” FC | Chronic PTSD, military- related | DBPC /85 | $[208] | |
| ” Generalization | ||||||
| ” Reconsolidation | ||||||
| ” Extinction | ||||||
| Gabapentin | Anticonvulsant: calcium channel a2d subunit | Untested | PTSD | Historical /30 | #[209] | Adjunctive therapy with gabapentin improved sleep and decreased nightmares |
| Levetiracetam | Anticonvulsant; unknown mechanism | Untested | Chronic PTSD | Historical /23 | #[210] | Adjunctive therapy in PTSD patients with partial or no response to antidepressant therapy |
| Eszopiclone | GABA: GABAA PAM (BZD site) | Untested | Chronic PTSD | DBPC /24 | #[211] | 3 weeks treatment improved sleep and overall PTSD symptoms |
| Alprazolam | GABA: GABAA PAM (BZD site) | # Retrieval | PTSD in recent trauma survivors | OL /26 | $[212] | Clonazepam (n = 10) |
| Alprazolam (n = 3) | ||||||
| Chronic PTSD | DBPC /10 | $[213] | Cross-over design. Modest reduction in anxiety with alprazolam. | |||
| Clonazepam | GABA: GABAA PAM (BZD site) | Untested | Chronic PTSD, military-related | Randomized 6 | $[214] | |
| Temazepam | GABA: GABAA PAM (BZD site) | Untested | Acute trauma, civilian | Randomized 21 | $[215] | Treatment for 1 week following trauma; follow-up at 6 weeks |
| GR205171 | NK1 Receptor Antagonist | # Retrieval | Chronic PTSD | DBPC /39 | $[216] |
Effects of drugs on Pavlovian FC processes: ” enhanced, #impaired, $mixed results. No entry means either the drug has not been tested for that process or available tests show null effects. Both preclinical and clinical studies were considered, but only if drugs were evaluated on explicit FC paradigms. Acquisition: refers to a clear learning effect (either within-session or STM effect, or effect with pre-training but not post-training administration); Consolidation: refers to a clear consolidation effect (drug given post-training but washed out before fear retrieval test). FC is used when data aren't available to distinguish between acquisition and consolidation effects (Note: Pavlovian FC Processes column excludes findings from instrumental avoidance studies).
zEffects of drugs on Symptoms: $ No significant effect; # improved symptoms; ” worsened symptoms.
DBPC: Double Blind Placebo-Controlled; FC: Fear conditioning; OL: Open Label; Historical: Retrospective chart review.
Table 3.
Open trials evaluating the effects of investigational drugs on the treatment of PTSD: Monoamine mechanisms.
| Drug | Primary Mechanism | Pavlovian FC Process* | Population/Primary Outcome | Trial Type/# Enroll | Start Date/Estimated Completion | Comment |
|---|---|---|---|---|---|---|
| Propanolol | NE: b receptor antagonist | # Acquisition | Chronic PTSD | DBPC/50 | Feb 2010 | Single dose with memory reactivation each week for six weeks. Evaluation at baseline and after 8 weeks |
| $ Consolidation | CAPS | Jun 2012 | ||||
| # Retrieval | ||||||
| # Incubation | ||||||
| # Reconsolidation | ||||||
| $ Extinction | ||||||
| PTSD and non-PTSD with distress | DBPC/66 | Feb 2010 | Single treatment with memory reactivation. Assessments at baseline and after 4 weeks | |||
| CAPS, IES, TMDM | Sep 2011 | |||||
| Chronic PTSD, military-related | DBPC/60 | Sep 2007 | Single treatment with memory reactivation. Measurements pre and post-treatment | |||
| Psychophysiologic Responses | Oct 2012 | |||||
| Chronic PTSD with Alcohol Depend. | DBPC/50 | Jan 2010 | Propranolol coupled with the elicitation/retrieval of trauma-related memories. Single treatment with 2 week follow-up | |||
| Subjective Distress | Aug 2012 | |||||
| Prazosin | NE: a1 receptor antagonist | ” Acquisition | Chronic PTSD, military-related | DBPC/326 | Jan 2010 | Titrated to stable daily dose to be maintained throughout study. Evaluate changes versus baseline at 6, 10, 14, 18, 22 and 26 weeks |
| $ Consolidation | Nightmares, sleep quality and quantity, and global clinical status | Mar 2013 | ||||
| # Extinction | ||||||
| Chronic PTSD, military-related | DBPC/300 | Sep 2009 | Treatment for 15 weeks to test augmentation of previous psychotropic medications and/or psychotherapy | |||
| CAPS, PSQI, CGIC | Jun 2014 | |||||
| Yohimbine | NE: a2 receptor antagonist | ” Acquisition | Chronic PTSD, military-related | DBPC/60 | Dec 2010 | Yohimbine given one hour before first imaginal exposure in PE. Evaluation at baseline, 15 weeks and 27 weeks |
| $ Retrieval | CAPS | May 2015 | ||||
| ” Extinction | ||||||
| Sertraline | 5-HT: SSRI | # Retrieval | Chronic PTSD, military-related | DBPC/441 | Nov 2011 | Treatment with up to 13 sessions of PE therapy +24 weeks of daily sertraline compared to placebo +PE |
| CAPS | Dec 2015 | |||||
| Venlafaxine | 5-HT, NE: SNRI | ” Extinction | PTSD | OL/150 | Apr 2012 | Six months of daily treatment with venlafaxine combined with CBT compared to sertraline + CBT |
| HTQ | Apr 2014 | |||||
| Mirtazapine | TCA, SSRI | # Retrieval | Chronic PTSD | DBAC | Jun 2010 | Daily doses for 12 weeks. Patients that respond will continue treatment another 12 weeks |
| Sertraline | CAPS and time to discont | 60 | Jun 2013 | |||
| MDMA | 5HT: releaser, reuptake inhibitor (DA, NE) | untested | Chronic PTSD, military-related | DBPC | Sep 2010 | Introductory 90 min psychotherapy followed by two 8 h sessions 3 – 5 weeks apart and combined with MDMA. |
| CAPS | 24 | Dec 2013 | ||||
| Evaluations at baseline; at 1 month following session 2; at 2 months following session 3 and at 12 months | ||||||
| Methylene Blue | MAO: Inhibitor | ” Extinction | Chronic PTSD | DBPC | Sep 2009 | Daily psychotherapy followed by a dose of methylene blue. |
| PTSD symptom severity | 42 | Apr 2013 | Assessment at pre- and post-treatment, 1 and 3 months | |||
| PRX-03140 | 5HT: 5HT4 partial agonist | untested | Chronic PTSD | OL/12 | Apr 2012 | PRX-03140 given daily for 10 weeks with dose escalation |
| Change in adverse events | Dec 2012 |
Source: ClinicalTrials.gov.
Effects of drugs on Pavlovian FC processes: ” = enhanced, # = impaired, $mixed results. No entry means either the drug has not been tested for that process or available tests show null effects. Both preclinical and clinical studies were considered, but only if drugs were evaluated on explicit FC paradigms. Acquisition: refers to a clear learning effect (either within-session or STM effect, or effect with pre-training but not post-training administration); Consolidation: refers to a clear consolidation effect (drug given post-training but washed out before fear retrieval test). FC is used when data aren't available to distinguish between acquisition and consolidation effects (Note: Pavlovian FC Processes column excludes findings from instrumental avoidance studies).
CAPS: Clinician-Administered PTSD Scale; CGIC: Clinical Global Impression of Change Scale; DBAC: Double Blind Active Control; FC: Fear Conditioning; HTQ: Harvard Trauma Questionnaire; IES: Impact of Event; POMS: Profile of Mood States; PSQI: Pittsburgh Sleep Quality Index; TMDM: Traumatic Memory Description Measure.
Table 4.
Open trials evaluating the effects of investigational drugs on the treatment of PTSD: Non-monoamine mechanisms.
| Drug | Primary mechanism | Pavlovian FC Process | Population/Primary Outcome | Trial Type/# Enroll | Start Date/Estimated Completion | Comment |
|---|---|---|---|---|---|---|
| D-Cycloserine | NMDA partial agonist (developed as broad spectrum antibiotic) | ” Consolidation | Chronic PTSD | DBPC/124 | May 2008 | D-Cycloserine prior to cognitive behavioral treatment with exposure therapy (~9 out of 12 – 14 weekly CBT treatments). Assessments at baseline and after sessions 3, 6 and 10 and 6 months after treatment |
| $ Retrieval | CAPS, PCL | May 2013 | ||||
| ” Reconsolidation | ||||||
| ” Extinction | ||||||
| Chronic PTSD | DBPC/40 | Jan 2005 | D-Cycloserine prior to virtual reality exposure therapy (10 – 12 weekly treatments). Assessments at baseline and after sessions 3, 6 and 10 and 6 months after treatment | |||
| CAPS, PCL | Dec 2012 | |||||
| Chronic PTSD | DBPC/56 | Jun 2010 | D-Cycloserine prior to sessions 5 – 12 of the 12-session CBT protocol. Assessments at baseline, after sessions and 3 months after treatment | |||
| Number of PTSD symptoms | Jun 2012 | |||||
| PTSD in youth | DBPC/56 | Jun 2010 | D-Cycloserine prior to sessions 5 – 12 of the 12-session CBT protocol. Assessments at baseline, after sessions and 3 months after treatment | |||
| Number of PTSD symptoms | Jun 2012 | |||||
| Mifepristone | Progesterone receptor antagonist/partial agonist; NMDA partial agonist | Chronic PTSD | DBPC/135 | Mar 2009 | D-Cycloserine prior to script-driven traumatic imagery with Mifepristone also given on treatment days | |
| D-Cycloserine | Psychophys. measures | Jul 2013 | ||||
| Mifepristone | Competitive progesterone receptor antagonist/partial agonist | # Consolidation | Chronic PTSD, military-related | DBPC/80 | May 2008 | Daily doses for 1 week. Evaluations at baseline, 1 week and 4 weeks |
| # Retrieval | CAPS-2, dichotomously defined clinical responder status | Oct 2011 | ||||
| # Reconsolidation | ||||||
| # Extinction | ||||||
| Hydrocortisone | Glucocorticoid Receptor: agonist | ” Acquisition | Chronic PTSD, military-related | DBPC/50 | Feb 2010 | Hydrocortisone given prior to each of 10 weekly PE sessions. Assessments at 0, 10 and 16 weeks |
| ” Generalization | CAPS | Nov 2013 | ||||
| # Cond. Inhib. | ||||||
| # Retrieval | ||||||
| ” Extinction | ||||||
| Chronic PTSD, military-related | DBPC/60 | Apr 2011 | Hydrocortisone given prior to each of 11 weekly PE sessions. Assessments at 0, 12 and 23 weeks | |||
| CAPS | Sep 2015 | |||||
| Acute trauma victims with anxiety | DBPC/120 | Apr 2009 | Single IV injection of hydrocortisone given within 6 h of trauma. Assessments at 0.5, 1, 3, 8 and 13 months | |||
| PTSD diagnosis at the end of the trial | Aug 2014 | |||||
| Females with PTSD | DBPC/25 | Oct 2008 | Comparison of 1 week treatment with hydrocortisone vs. placebo with assessments out to 4 years | |||
| IES-R | Sep 2012 | |||||
| Dexamethasone | Glucocorticoid Receptor: agonist | ” FC | Chronic PTSD vs. non-PTSD | DBPC/150 | Nov 2011 | Baseline and FPS response at 1 h and 10 h following dexamethasone administration |
| ” Generalization | Psychophys. measures | Jun 2013 | ||||
| # Retrieval | ||||||
| ” Extinction | ||||||
| Chronic PTSD, military-related | DBPC/102 | Apr 2010 | Dexamethasone given following traumatic memory reactivation every 7 days for 4 weeks. Evaluation at baseline and at 1, 3 and 6 months after final treatment | |||
| Psychophys. measures | Oct 2012 | |||||
| GSK561679 | CRF: CRF1 antagonist | untested | Women with Chronic PTSD, CAPS | DBPC/150 | Dec 2009 | Daily treatment for 6 weeks. Assessments at baseline and 6 weeks |
| Dec 2013 | ||||||
| Oxytocin | Oxytocin: Receptor agonist | # Retrieval | Chronic PTSD | DBPC/40 | Oct 2011 | Impact of a single intranasal administration on impact on fear renewal and reinstatement at 1 day after treatment |
| # Extinction | Psychophys. measures | Mar 2013 | ||||
| Ketamine | GLU: NMDAR antagonist | # Acquisition | Chronic PTSD | DBAC/40 | Jan 2009 | Single ketamine IV infusion vs. single IV infusion of midazolam |
| # Retrieval | IES-R | Jan 2013 | ||||
| Diazepam | GABA: BZ site PAM | # Acquisition | Acute Trauma | OL/60 | Oct 2011 | Single dose of diazepam given within hours of trauma and before a night of sleep to reduce PTSD development |
| # Retrieval | CAPS | May 2013 | ||||
| # Extinction | ||||||
| Ganaxolone | GABA: steroid site PAM | Untested | Chronic PTSD | DBPC/120 | Apr 2011 | Twelve weeks of daily treatment with assessment of CAPS at week 6 |
| CAPS | Nov 2012 | |||||
| NPY | NPY; NPY receptor agonist | # FC | Chronic PTSD | DBPC/20 | Jan 2012 | Single intranasal administration. Evaluation within 3 h |
| # Retrieval | POMS, BDI-II, BAI, Appetite Scale | Dec 2012 | ||||
| ” Extinction | ||||||
| Omega-3 Fatty Acids | Neurogenesis | # Retrieval | Chronic PTSD, military-related | DBPC | Sep 2008 | Daily dosing with evaluations at baseline and after 10 weeks |
| CAPS, BAC-A | 40 | Dec 2011 | ||||
| Chronic PTSD/CAPS | DBPC/140 | Dec 2008 | Dosing for 12 weeks. Evaluations at baseline, 4 and 12 weeks | |||
| Dec 2013 | ||||||
| NPY | NPY; NPY receptor agonist | # FC | Chronic PTSD | DBPC/20 | Jan 2012 | Single intranasal administration. Evaluation within 3 h |
| # Retrieval | POMS, BDI-II, BAI, Appetite Scale | Dec 2012 | ||||
| ” Extinction |
Source: ClinicalTrials.gov.
*Effects of drugs on Pavlovian FC processes: ” = enhanced, # = impaired, $mixed results. No entry means either the drug has not been tested for that process or available tests show null effects. Both preclinical and clinical studies were considered, but only if drugs were evaluated on explicit FC paradigms. Acquisition: refers to a clear learning effect (either within-session or STM effect, or effect with pre-training but not post-training administration); Consolidation: refers to a clear consolidation effect (drug given post-training but washed out before fear retrieval test). FC is used when data aren't available to distinguish between acquisition and consolidation effects (Note: Pavlovian FC Processes column excludes findings from instrumental avoidance studies).
BAC-A: Brief Assessment of Cognition in Affective Disorders; BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory (Second Edition); CAPS: Clinician-Administered PTSD Scale; DBAC: Double Blind Active Control; IES-R: Impact of Event Scale – Revised; PAM: Positive Allosteric Modulator; PCL: PTSD Checklist; POMS: Profile of Mood States.
5.1 FDA-approved drugs for PTSD: SSRIs
Currently, the SSRIs sertraline and paroxetine are the only FDA-approved drugs for treating PTSD (for reviews of clinical trials, see [85,93,94]). While able to reduce symptoms from all three PTSD clusters, SSRIs alone are not the solution for a large percentage of patients. Effect sizes can be small and anywhere from 70 – 80% of patients fail to achieve complete remission [95-98].
Both preclinical and clinical data indicate that combining SSRIs with psychotherapy may improve outcomes. In mice, extinction + fluoxetine produces “conditioned fear erasure” [99], perhaps by returning the adult fear circuitry to a developmental state where extinction reverses original learning, rather than producing normal context-specific inhibitory learning. Consistent with this, paroxetine combined with PE was more effective than therapy or drug alone in treating PTSD [100]. Chronic post-FC paroxetine also prevents the spontaneous fear incubation seen in rodents subjected to a SEFL procedure [101]. Together these data suggest that, although not always effective in reducing PTSD symptoms, SSRIs may prevent worsening of symptoms and could significantly facilitate the effects of exposure therapy. Further, SSRIs may be superior to other drugs because they are unlikely to exacerbate FC processes [102] or impair natural recovery-related learning occurring outside of therapy.
Venlafaxine, which blocks 5-HT and NE reuptake, demonstrated efficacy in the treatment of PTSD [103,104]. In rodents, acute pre-extinction venlafaxine facilitates extinction retrieval and chronic post-extinction treatment prevents reinstatement, suggesting that combining this drug with extinction may produce FC erasure even when administered post-training in adults [105]. Venlafaxine may selectively improve extinction consolidation, since fear retrieval and extinction learning were unaffected. This profile is consistent with a medial prefrontal cortex (mPFC) mechanism, but this has yet to be tested. Although not FDA-approved for PTSD, venlafaxine is a recommended treatment and is considered to be as efficacious as SSRIs [106]. It is notable that venlafaxine specifically improves extinction consolidation/retrieval in rodents, providing further support for the notion that this FC process is especially relevant to PTSD treatment.
5.2 Other 5-HT drugs
5.2.1 Buspirone
5-HT1A-receptor knockout mice demonstrate excessive generalization (as is suggested to occur in PTSD) [76,107], suggesting that 5-HT1A receptor agonism may be of therapeutic utility in PTSD. Buspirone, an anxiolytic 5-HT1A receptor partial agonist, reduced PTSD symptoms in case studies or small trials [108-110]. Buspirone also potentiates SSRI responses in PTSD [111] though larger trials are needed to confirm these findings. Vilazodone, which has both 5-HT reuptake inhibition and 5-HT1A receptor partial agonism, was active prophylactically in a rat model of hypervigilance following severe stress [112] and would be of potential interest to explore clinically in PTSD.
5.2.2 5-HT2 receptor antagonists
5-HT2A receptor antagonists may have utility in PTSD, based on animal studies [113], though clinical studies are lacking. Nefazodone, a 5-HT reuptake inhibitor/5-HT2A antagonist, showed promising results in a small PTSD pilot study [114], although this drug has fallen out of favor because of liver toxicity issues. Positive findings were also reported for mirtazapine, an antidepressant with antagonist actions at multiple 5-HT2 receptor subtypes as well as at a2-adrenergic receptors [115]. In preclinical FC tests, mirtazapine suppressed FC retrieval [116,117].
5.2.3 3,4-methylenedioxymethamphetamine (MDMA)
MDMA (“Ecstacy”) is a substituted amphetamine that increases 5-HT release from presynaptic terminals and a drug that has been explored previously as an adjunct to psychotherapy [118]. MDMA decreased PTSD symptoms in subjects who were non-responsive to pharmacological or psychotherapeutic interventions [119], with 83% responding to drug versus 25% for placebo. The authors reported that there were no apparent long-term side effects noted in this study but the ongoing discussion of the potential for neurotoxicity in humans following chronic MDMA use (e.g., [120]) necessitates that caution be exercised in evaluating this drug.
5.3 Norepinephrine (NE)
NE contributes to hyperarousal and FC processes, providing the rationale for evaluating compounds affecting a1-, a2-, and b-adrenergic receptors in PTSD.
5.3.1 Prazosin
Hyperarousal is linked to sleep disturbances which are difficult to treat with SSRIs. Prazosin, an a1-adrenergic receptor antagonist, decreased sleep-related disturbances in PTSD, as measured by latency to sleep and trauma-related nightmares [121-127]. These effects may result from decreased arousal, though it is possible that prazosin is affecting FC memory processes (e.g., disrupting reconsolidation or incubation of trauma memories causing nightmares), a hypothesis requiring formal testing. Notably, a1 blockers increase fear learning and impair fear extinction in animals [128,129] suggesting that further clinical research is needed to evaluate whether a1 blockers given before exposure therapy sessions are detrimental.
5.3.2 Propranolol
Blocking b-adrenergic receptors with propranolol may improve PTSD, though RCT findings are mixed. Propranolol attenuated retrieval of an emotionally arousing narrative in both normal volunteers and those with PTSD [130], suggesting utility when administered after exposure to trauma. In a small emergency room study, propranolol administered within 6 h of trauma, and daily thereafter for 10 days [131], showed a non-significant trend for PTSD reduction at 1 month. A similar study where propranolol was given for 7 days (3x/day, beginning 2 – 20 h post-trauma) reduced PTSD development at 1 month [132]. In contrast, a third study reported no effect of propranolol administered up to 48 h post-trauma on the development of PTSD symptoms [92]. In this latter study, the small sample size, dosing, and long delay between trauma and treatment may account for the different outcome. Another study in juveniles found that a moderate/low dose of propranolol given within 12 h of admission increased PTSD symptoms in girls but non-significantly decreased the same measures in boys [133]. A recent study found that propranolol given within 12 h of trauma and continued for 19 days failed to improve clinical outcomes but did have a modest effect on script-driven physiological arousal [134]. Thus, propranolol does not appear to strongly block the consolidation of traumatic memories to prevent PTSD, although it is important to note that subjects in these studies received the drug as the consolidation window was closing, or in many cases, after FC consolidation was likely complete.
Recent preclinical (rodent) work may help explain the mixed results for propranolol in PTSD. Although it clearly disrupts consolidation of hippocampal-dependent memories when given immediately post-training [135], consolidation of Pavlovian FC is not hippocampal-dependent and propranolol fails to disrupt fear when given post-conditioning [136]. However, propranolol does impair the acquisition, retrieval and reconsolidation of FC [137], and thus may be useful for PTSD if given i) before a trauma (limited potential except for military and first responders, perhaps), ii) between therapy sessions (to block reminder-induced reconsolidation/incubation) or, iii) during therapy designed to reactivate, but not extinguish, FC (to intentionally block reconsolidation). Two recent studies in humans support the notion that propranolol given after traumatic-memory retrieval (PTSD patients; [138]), or before FC reactivation (normals; [139]), weakens reconsolidation and blunts subsequent memory-induced arousal and fear behavior. However, null results have also been reported and methodological issues temper enthusiasm for these early results [140], so further work is needed to determine whether propranolol can blunt fear by blocking reconsolidation. Finally, propranolol administered to the PFC in rats impairs fear extinction [141], thus, mixed effects could relate to propranolol having both positive and negative effects on PTSD-relevant FC processes.
5.3.3 Yohimbine
a2-Adrenergic antagonists like yohimbine can exacerbate PTSD symptoms, presumably by blocking autoinhibitory feedback and increasing synaptic NE. Indeed, pre-training yohimbine induces stronger LTM and more generalization [142], and pre-test yohimbine enhances FC retrieval. However, yohimbine facilitates fear extinction in both rodents [143,144] and humans [145] and thus may be a useful adjunct to exposure-based psychotherapies. This idea is supported by a recent study using a mouse strain with a poor-extinction phenotype: pre-training yohimbine treatment led to significant LTM for extinction whereas vehicle- and d-cycloserine-treatment did not [146]. Yohimbine is currently being evaluated in an open clinical trial as an adjunct to exposure therapy (Table 3).
5.4 GABA
Benzodiazepines (which facilitate GABAA receptors and are used as anxiolytics) have well-documented amnestic effects on anterograde memory. Benzodiazepines administered in the post-trauma period did not prevent, and possibly even enhanced, the subsequent development of PTSD (Table 2). Time after trauma may be an important factor here, as drugs were generally administered 2 or more days post-trauma, well beyond the FC consolidation window. An ongoing clinical study (Table 4) is administering diazepam in the emergency room within hours after trauma (and before the first night's sleep) in an effort to prevent fear memory consolidation and development of PTSD.
5.5 Opiates
Clinical studies (Table 2) have indicated that administration of opiates for pain relief at the time of acute physical trauma may reduce the subsequent incidence of PTSD, possibly from impairment of memory consolidation, though controlled studies are needed to confirm this finding. One preclinical study of FC suggests that morphine can block consolidation if given soon after conditioning [147].
5.6 Glutamate
Drugs clinically tested for efficacy in treating PTSD symptoms interact with the NMDA receptor complex as ion channel blockers (ketamine), partial (d-cycloserine) or full (d-serine) agonists.
5.6.1 D-cycloserine
D-cycloserine, first shown to facilitate fear extinction in rodents [148], has demonstrated utility for facilitating the beneficial effects of psychotherapy in treating phobia as well as other anxiety disorders. Though two pilot studies suggested efficacy as a monotherapy in PTSD [149] and for decreasing negative symptoms as an adjunctive therapy to neuroleptics [150], recent trials presented in abstract form suggest no benefit when combined with cognitive behavior therapy (see Table 2 references). A recent preclinical study found that d-cycloserine can enhance reconsolidation and strengthen fear, though the clinical implications of these findings for combining this drug with exposure therapy need to be assessed [49]. Numerous clinical trials evaluating d-cycloserine are underway (Table 4).
5.7 Glucocorticoids
There are a number of agents which can work at various levels of the HPA axis to affect its functioning, but of these, only hydrocortisone (CORT), an agonist at glucocorticoid receptors, has been evaluated in PTSD. In chronic PTSD, low-dose hydrocortisone reduced traumatic memories and reexperiencing symptoms [151]. Hydrocortisone decreased fear responses as measured with fear-potentiated startle (a FC paradigm) in civilian and combat-related PTSD patients as well as non-PTSD controls [152,153].
Other studies assessed hydrocortisone given near the time of trauma for its ability to prevent the development of PTSD. Hydrocortisone administered during cardiac surgery decreased chronic stress symptoms [154,155] and decreased the development PTSD when administered to patients with septic shock [156].
Hydrocortisone was evaluated for activity in augmenting memory extinction and reducing clinical symptoms in veterans with combat-related PTSD [157]. Subjects dosed with hydrocortisone after a memory reactivation task showed a reduction of PTSD symptoms 1 week later, though this effect was attenuated at 1 month. The authors explained this as glucocorticoid-mediated enhancement of extinction, though a reduction in memory reconsolidation is possible.
5.8 Neuropeptides
5.8.1 GSK561679
CRF, a key stress-response mediator, may be hyperactive in PTSD. CRF1 receptor antagonists are proposed to be useful in treating PTSD, and, while there are no clinical data available, a trial is currently underway evaluating GSK561679 in women with chronic PTSD (Table 4). As reviewed elsewhere [158], animal FC studies indicate that CRF1 receptor blockade may impact both consolidation and expression of conditioned fear. Furthermore, recent analyses indicate that CRF1 receptor antagonism may facilitate extinction (Figure 3; [159]), suggesting that clinically, these agents should be evaluated in combination with psychotherapy for their effects on moderating fear memories in PTSD.
Figure 3.
The CRF1 receptor antagonist antalarmin weakens FC and accelerates fear extinction in rats. CRF powerfully modulates memory and stress-responding through activation of CRF1 receptors. However, no data exists on the potential utility of these drugs for treating PTSD. We reanalyzed data from a 1999 study by Deak et al published in Endocrinology (experiment 1) examining the effect of antalarmin on context FC and retrieval [159]. Since this experiment used 20-min LTM tests that produced significant extinction, we hypothesized that antalarmin reduced retrieval primarily by facilitating fear extinction. Original data were generously provided by Terrence Deak for the new analysis. Since antalarmin did slightly weaken initial fear retrieval as assessed by freezing behavior, we normalized responding to freezing in the first 2-min of the test to evaluate extinction. Rats were injected with Drug or Vehicle prior to context FC on Day 1. On Day 2, half of each group received the same treatment and half received the opposite treatment. (Left) Antalarmin accelerated extinction learning in rats that were conditioned drug-free. (Middle) Rats conditioned after antalarmin injections showed faster extinction when tested drug-free on Day 2. (Right) These effects were additive, as rats injected with antalarmin pre-conditioning and pre-extinction extinguished faster than any other group. These data support the notion that CRF1 receptors antagonists may be particularly useful drugs for PTSD since they can blunt fear learning and facilitate fear suppression when combined with CS exposure. However, since the experiment wasn't designed to assess extinction processes it remains unclear whether the facilitation by antalarmin acutely translates to LTM for extinction in the drug-free state.
5.9 Drug combinations
Although there are no clinical data available, drug combination studies may be worth pursuing. For example, preclinical work has shown that combination of dexamethasone and d-cycloserine facilitate extinction better than either treatment alone [160].
6. Conclusions
Mechanistically diverse agents have been evaluated in a large number of studies for their potential efficacy in treating PTSD. However, a relatively small number of studies have specifically evaluated the ability of drugs, either alone or in combination with psychotherapy, to affect specific memory processes and thereby attempt to “correct” the core underlying problem in PTSD. These agents have primarily targeted adrenergic and glutamatergic pathways. Ongoing trials demonstrate a greater focus on evaluating drug effects on learning processes.
7. Expert opinion
PTSD is a large problem, especially given the upswing of terrorism in the last decade and the nature of modern warfare, where soldiers operate under chronically stressful conditions with uncertain enemies using novel tactics like improvised explosive devices (IEDs). Advances in modern medicine and protective equipment also raise the PTSD incidence rate as more survive traumatic experiences and are subjected to multiple deployments. PTSD is also a major concern for civilians, and especially those with dangerous careers (e.g., first-responders).
PTSD treatment usually involves psychotherapy, pharmacotherapy, or, more likely, some combination of the two, even if not by design. However, even the most successful monotherapies (e.g., PE or SSRIs) leave many with significant symptoms. In our opinion, the cure for PTSD has remained elusive because pharmacotherapies and psychotherapies have largely developed in parallel without a full understanding of how they may interact. Even combined therapies guided by knowledge of the stress response and FC neurobiology often underutilize basic findings that could improve efficacy. Below we highlight recommendations based on clinical, preclinical and neurobiological findings that could speed the development of a PTSD cure. Since many of these ideas are inspired by studies of memory and neurobiology in rodents, these points should not be taken as recommendations for immediate changes in clinical practice, but rather, recommendations for new research to evaluate novel PTSD treatment strategies at the preclinical and clinical levels.
7.1 Blunt original consolidation
Weakening consolidation in the hours post-trauma may be the most efficacious strategy for preventing PTSD altogether, regardless of whether PTSD is caused by excessive conditioning or impaired recovery learning. This is not novel, however, enthusiasm for this strategy has waned since the disappointing results of propranolol treatment post-trauma. Propranolol was predicted to block consolidation based on studies of emotional learning, however, recent work shows that consolidation of Pavlovian FC is not sensitive to propranolol. Thus, agents with potential to block FC consolidation, such as morphine and others (Tables 1, 2, 3, 4; [48]), should be explored and applied immediately (£6 h) post-trauma to prevent PTSD. Encouraging results from victims with physical injuries support this strategy.
7.2 Target the dysfunctional memory
At its core, PTSD is an associative memory disorder. It is defined by specific experience and the major symptoms relate to specific traumatic memories. Normal living is disrupted by intense, recurring memories and avoidance of trauma-related cues. Although hyperarousal is also a defining feature, this may be largely secondary to the memory problem as traumatic memories activate the stress response. Even if hyperarousal is independent of memory dysfunction, treating hyperarousal alone will likely not correct the memory dysfunction and significant symptoms will therefore remain. However, if the pathological PTSD memory is corrected, the core problem is removed and even hyperarousal symptoms should wane.
7.3 Combined therapies may be necessary to cure PTSD
If one accepts that specific traumatic memories must be targeted for correction to cure PTSD, then monotherapies may never be adequate. First, although drugs can alleviate PTSD symptoms temporarily, it is difficult to envision a way that drugs alone could dampen a traumatic memory. Available neurobiology findings suggest that memory retrieval is necessary for targeted disruption. Second, although psychotherapy can target a specific memory for correction, PTSD patients show clear impairments in learning processes known to effectively suppress conditioned fear (e.g., extinction). Combining drug treatment with memory-specific psychotherapy may offer the best route to permanent recovery—by returning the pathological memory to a state amenable to change, inducing new learning to correct the dysfunction, and facilitating the underlying neuroplasticity processes with drugs targeting key molecules.
7.4 Target neurobiological systems known to be dysfunctional
A great deal is known about the psychological and neurobiological systems that are awry in PTSD. For instance, the stress-response system depends critically on NE, CRF, ACTH and CORT and FC depends on glutamate receptors, intracellular kinases, transcription and translation, growth factors, and neuromodulators. Yet the currently approved/recommended drugs are antidepressants that largely affect 5-HT signaling. 5-HT clearly modulates stress responding and conditioning, however, the core stress-response and FC mechanisms do not require 5-HT. It appears that these drugs were evaluated based on their demonstrated safety and efficacy in treating depression and anxiety [86] rather than their ability to alter learning processes which might improve memory-related core PTSD symptoms. Recent findings indicate that SSRIs have a positive interaction with extinction-based therapy, though this extinction facilitation was only discovered after they were approved for treating PTSD. Advances in drug treatment strategies are likely to come quicker if limited clinical-trial resources are first devoted to mechanisms that are awry in PTSD and/or known to contribute to therapy-related learning processes. We predict that dual-role drugs, those that counteract stress-response abnormalities and make a positive contribution to therapy-related learning, will be especially effective for PTSD. One promising but untested drug type is CRF1 receptor antagonists. These drugs may weaken HPA-axis activation and facilitate fear extinction learning (see Figure 3).
7.5 Apply drugs more selectively
If deciding which drugs to use is important, deciding when to use them is equally important. For instance, a recent survey found that approximately 30 – 40% of U.S. veterans with PTSD are taking benzodiazepines for comorbid conditions like anxiety [161]. FC research suggests that benzodiazepines may have beneficial and detrimental effects, depending on when they are taken. Benzodiazepines are anxiolytic and amnestic, and if taken post-trauma, could blunt consolidation, reconsolidation and/or retrieval of FC to prevent or ameliorate PTSD symptoms. However, if taken before therapy they could impair therapy-related learning. Indeed, it has been known for >20 years that benzodiazepine treatment impairs fear extinction. Propranolol is another example. Although propranolol has little effect on FC consolidation, it does interfere with acquisition, incubation and reconsolidation of FC. Thus, propranolol is unlikely to have a profound effect when given post-trauma, but could be useful if given pre-trauma or post-retrieval, to blunt or erase the FC memory. At minimum, drugs given chronically should be evaluated to ensure that they do not impair learning processes necessary for normal or therapy-assisted recovery.
7.6 PFC processes may be particularly important
Although many components of the stress-response and fear circuitry show abnormalities in PTSD, it can be a challenge to determine which component(s) represent the primary dysfunction and which are secondary. Targeting the primary problem is the clearest path to developing a real cure. Data suggest that PFC dysfunction may be particularly important. PTSD is at least partly a disorder of recovery and the PFC is particularly important for extinction and other forms of emotion regulation (e.g., active coping) [162]. PTSD patients can acquire, consolidate, retrieve and extinguish fear normally, but have significantly impaired extinction retrieval. Ventral PFC is critical for extinction retrieval and PTSD patients show PFC hypoactivity. Finally, the best currently available drugs for PTSD treatment are SSRIs and the mixed 5-HT/NE reuptake blocker venlafaxine and these agents have been shown to enhance fear extinction. Although the SSRIs may enhance extinction learning, venlafaxine has no effect on extinction learning but strongly facilitates extinction retrieval when given only after extinction training. This pattern is most consistent with an extinction consolidation enhancement, most likely PFC-mediated. Thus, it is likely that drug/therapy combinations that effectively normalize PFC function will contribute to the PTSD cure. Most of the other PTSD symptoms could also result from PFC impairments since PFC suppresses amygdala-dependent fear responding including HPA-axis activation.
Article highlights.
- Although robust neurobiological mechanisms mediate adaptive responding to traumatic stressors, some individuals fail to adequately adapt, leading to the severe disruption of normal functioning characteristic of PTSD.
- PTSD is a unique anxiety disorder that is induced by specific traumatic experience(s) and characterized by dysfunctional fear memories, although hyperarousal also makes a strong contribution.
- Studies of fear conditioning provide insights into the mechanisms underlying the formation and maintenance of aversive memories. Potential therapeutic approaches for modulating such memories include altering processes of fear memory consolidation, reconsolidation and/or extinction.
- Selective serotonin reuptake inhibitors (SSRIs) are currently the only FDA-approved pharmacological treatment for PTSD, however, there is a considerable amount of clinical data for investigational drugs that target diverse neurotransmitter systems, including monoamines (norepinephrine, dopamine, serotonin), amino acids (GABA, glutamate), and peptides. Tables of investigational drugs are provided to summarize findings from completed clinical trials, highlight ongoing clinical trials, and facilitate comparisons between preclinical and clinical studies.
- Approaches that combine pharmacological agents with psychotherapy to modulate the core aversive fear memories in PTSD have considerable potential for improving treatment outcomes.
- Conversely, preclinical findings suggest that some drugs commonly prescribed for comorbid conditions may actually impair psychotherapy-related learning processes. More clinical research is needed to determine whether drugs can facilitate or impair psychotherapies for PTSD.
This box summarizes key points contained in the article.
Box 1. DSM-IV-TR Diagnostic Criteria for PTSD.
| A1. The person experience, witnessed, or was confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others | ||
| A2. The person's response involved intense fear, helplessness or horror | ||
| B. Re-experiencing Symptoms (1 or more) B1. Intrusive recollections B2. Distressing nightmares B3. Acting/feeling as though event were recurring (flashbacks) B4. Psychological distress when expose to traumatic reminders B5. Physiological reactivity when exposed to traumatic reminders |
C. Avoidant/Numbing Symptoms (3 or more) C1. Avoidance of thoughts, feelings or conversations associated with the stressor C2. Avoidance of activities, places or people associated with the stressor C3. Inability to recall important aspects of traumatic aspects of traumatic event C4. Diminished interest in significant activities C5. Detachment from others C6. Restricted range of affect C7. Sense of foreshortened future |
D. Hyperarousal Symptoms (2 or more) D1. Sleep problems D2. Irritability D3. Concentration problems D4. Hypervigilance D5. Exaggerated startle response |
| E. Duration of the disturbance is at least 1 month | ||
| “Acute”: duration of symptoms is less than 3 months; “Chronic”: symptoms last 3 months or longer; “With Delayed Onset”: at least 6 months have elapsed between the traumatic event and onset of symptoms | ||
| F. Requires significant distress or functional impairment | ||
| Key Changes Recommended for DSM-5 (see [1,2]) | ||
| - Define a “Trauma and Stressor-Related Disorders” section (move PTSD out of Anxiety Disorder into this new section which recognizes trauma as etiological factor) | ||
| - Modify PTSD Criteria, including switch from a 3-factor model to a 4-factor model (Intrusion Symptoms; Persistent Avoidance; Alterations in Cognitions and Mood; Hyperarousal and Reactivity Symptoms) | ||
Box 2. Neurobiology of Pavlovian FC and Extinction.
LA is necessary for all aspects of FC1. LA neurons (below) receive CS (e.g.tones) and US (e.g. shocks) information. Before conditioning, CS afferents excite LA neurons weakly and fail to elicit fear. During conditioning, US inputs strongly depolarize LA neurons, trigger action potentials and drive defensive responses. Coincident CS-US activity opens calcium channels at CS synapses triggering plasticity mechanisms necessary for STM and LTM. After conditioning, CS-synapses are potentiated and CS-alone presentations now excite LA neurons enough to spike and drive fear responding. Although plasticity occurs throughout the network with FC, LA plasticity is essential for learning and most extra-amygdala plasticity. Unlike some other memory systems, FC memories are believed to be stored in the same neurons responsible for acquisition. Thus, FC permanently links CSs with the defensive brain system. In this view, retrieval is simply the consequence of CS-processing changes in the fear circuit, an automatic readout of FC plasticity, not a process requiring conscious thought or decision making.
LA lies at the center of a broader defensive network that can control fear responding. CS information reaches LA via thalamus and cortex. LA then projects to CE, both directly and indirectly through the basal nucleus (B) and intercalated (IC) cell masses. CE coordinates fear responding via its projections to downstream effector regions that mediate specific responses. For instance, projections to hypothalamus and LC allow the CS to activate NE and HPA-axis hormones. CE plasticity is also necessary for consolidation and linking the CS-US memory to appropriate responses. B has a complex role in FC, extinction and avoidance, via connections to CE, hippocampus, striatum and PFC.
Several regions and processes are critical for extinction2. In LA, strengthening of GABAergic inhibition is associated with extinction learning. PAG may provide the error signal that drives learning. B neurons allow for switching from high to low fear states. PFC plays a crucial role in extinction consolidation/retrieval, which may be a core deficiency in PTSD. PFC may suppress amygdala fear outputs by exciting inhibitory IC cells that lie between LA and CE. Finally, hippocampus provides key contextual information that gates extinction memory retrieval.
Acknowledgments
CK Cain has received funding from New York University, Nathan Kline Institute for Psychiatric Research and the National Institutes of Health (U.S.).
Footnotes
Declaration of interest
JH Kehne is a former consultant for PGx Health. GD Maynard has no competing interests to declare.
This explanation is based on prominent ‘serial processing’ (e.g., [37]) and ‘synaptic plasticity’ (e.g., [38]) models of amygdala-based Pavlovian fear conditioning. For an alternative ‘parallel processing’ view, see [40].
See [41] for a detailed review of fear extinction neural mechanisms.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Friedman MJ, Resick PA, Bryant RA, Brewin CR. Considering ptsd for dsm-5. Depress Anxiety. 2011;28(9):750–69. doi: 10.1002/da.20767. [DOI] [PubMed] [Google Scholar]
- 2.Friedman MJ, Resick PA, Bryant RA, et al. Classification of trauma and stressor-related disorders in dsm-5. Depress Anxiety. 2011;28(9):737–49. doi: 10.1002/da.20845. [DOI] [PubMed] [Google Scholar]
- 3.Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–5. [PubMed] [Google Scholar]
- 4.Kowalik J, Weller J, Venter J, Drachman D. Cognitive behavioral therapy for the treatment of pediatric posttraumatic stress disorder: a review and meta-analysis. J Behav Ther Exp Psychiatry. 2011;42(3):405–13. doi: 10.1016/j.jbtep.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of- onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Brunello N, Davidson JR, Deahl M, et al. Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001;43(3):150–62. doi: 10.1159/000054884. [DOI] [PubMed] [Google Scholar]
- 7.Hoge CW, Castro CA, Messer SC, et al. Combat duty in iraq and afghanistan, mental health problems and barriers to care. US Army Medical Department journal. 2008:7–17. [PubMed] [Google Scholar]
- 8.McFarlane AC, Williamson P, Barton CA. The impact of traumatic stressors in civilian occupational settings. J Public Health Policy. 2009;30(3):311–27. doi: 10.1057/jphp.2009.21. [DOI] [PubMed] [Google Scholar]
- 9.Ledford H. Neuroscientists unite for. ‘moon shot’. Nature. 2011 doi:10.1038/news.2011.324. [Google Scholar]
- 10.Shalev AY. PTSD: A disorder of recovery? In: Kirmayer L, Lemelson R, Barad M, editors. Understanding trauma: integrating biological, clinical, and cultural perspectives. Cambridge University Press; New York, NY: 2007. pp. 207–23. [Google Scholar]
- 11.Yehuda R. Risk and resilience in posttraumatic stress disorder. J Clin Psychiatry. 2004;65(Suppl 1):29–36. [PubMed] [Google Scholar]
- 12.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003;417:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 13•.Ressler KJ, Mercer KB, Bradley B, et al. Post-traumatic stress disorder is associated with pacap and the pac1 receptor. Nature. 2011;470(7335):492–7. doi: 10.1038/nature09856. [A fascinating study implicating the PACAP-PAC1 pathway with abnormal stress responses in PTSD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmar CR, McCaslin SE, Metzler TJ, et al. Predictors of posttraumatic stress in police and other first responders. Ann NY Acad Sci. 2006;(1071):1–18. doi: 10.1196/annals.1364.001. [DOI] [PubMed] [Google Scholar]
- 15.Heim C, Nemeroff CB. Neurobiology of early life stress: clinical studies. Semin Clin Neuropsychiatry. 2002;7(2):147–59. doi: 10.1053/scnp.2002.33127. [DOI] [PubMed] [Google Scholar]
- 16.Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40(1):1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 17••.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding ptsd. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [An excellent review detailing the need for more research on the neurobiology of individual differences in stress and fear responding to improve PTSD treatments.] [DOI] [PubMed] [Google Scholar]
- 18.Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26(11):984–92. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 20.Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress. 2006;19(5):571–81. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- 21.Kozaric-Kovacic D. Pharmacotherapy treatment of ptsd and comorbid disorders. Psychiatr Danub. 2009;21(3):411–14. [PubMed] [Google Scholar]
- 22.Delahanty DL, Nugent NR. Predicting ptsd prospectively based on prior trauma history and immediate biological responses. Ann NY Acad Sci. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- 23••.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and learning. Erlbaum; Hillsdale, NJ: 1988. pp. 185–211. [Excellent piece about the organization of fear/defensive behavior systems.] [Google Scholar]
- 24.Blanchard DC, Yang M, Herbert M, Blanchard RJ. Defensive behaviors. In: Fink G, editor. Encyclopedia of stress. Academic Press; Oxford: 2007. pp. 722–26. [Google Scholar]
- 25.Selye H. The stress of life. McGraw-Hill; New York: 1978. [Google Scholar]
- 26.Southwick SM, Bremner JD, Rasmusson A, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46(9):1192–204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 27.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 28•.Kehne JH, Cain CK. Therapeutic utility of non-peptidic crf1 receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol Ther. 2010;128(3):460–87. doi: 10.1016/j.pharmthera.2010.08.011. [Comprehensive review of CRF in fear, anxiety and depression with a focus on CRF1 receptor antagonists and circumstances/conditions where these drugs may have utility.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasckow JW, Baker D, Geracioti TD., Jr Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides. 2001;22(5):845–51. doi: 10.1016/s0196-9781(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346(2):108–14. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 31•.Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15(12):576–84. doi: 10.1016/j.tics.2011.10.005. [Reviews influential research and hypotheses about the detrimental effects of chronic stress and its relationship to anxiety disorders.] [DOI] [PubMed] [Google Scholar]
- 32•.Gale GD, Anagnostaras SG, Godsil BP, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24(15):3810–15. doi: 10.1523/JNEUROSCI.4100-03.2004. [Nice, simple demonstration in rats that a single traumatic (FC) experience can produce a robust memory that lasts a lifetime and is stored in the same brain region (amygdala).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–76. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maren S. Neurobiology of pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44(1):75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–24. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cain CK, LeDoux JE. In: Handbook of anxiety and fear 17. Nutt DJ, Blanchard RJ, Blanchard DC, Griebel G, editors. Elsevier Academic Press; Amsterdam: 2008. pp. 103–25. [Google Scholar]
- 38.Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52(1):215–27. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 39•.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–8. doi: 10.1126/science.1103944. [Shows how fear learning depends on enhancement of glutamate signaling in LA.] [DOI] [PubMed] [Google Scholar]
- 40.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29(5):272–9. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annual review of psychology. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Josselyn SA. Continuing the search for the engram: examining the mechanism of fear memories. J Psychiatry Neurosci. 2010;35(4):221–8. doi: 10.1503/jpn.100015. [Describes a beautiful set of data showing that FC memories are stored in a subset of LA neurons – and can be deleted by targeting these neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacktor TC. How does pkmzeta maintain long-term memory? Nat Rev Neurosci. 2011;12(1):9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- 44.Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following pkmzeta inhibition in the amygdala. Nat Neurosci. 2011;14(3):295–6. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7(2):73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 46•.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–6. doi: 10.1038/35021052. [Seminal paper reviving the field of ‘reconsolidation’, where FC and other specific memories can be erased by reactivating the memory and disrupting subsequent cellular storage processes.] [DOI] [PubMed] [Google Scholar]
- 47•.Suzuki A, Josselyn SA, Frankland PW, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24(20):4787–95. doi: 10.1523/JNEUROSCI.5491-03.2004. [One of the first papers to demonstrate that fear extinction and reconsolidation, which both are triggered by CS-alone memory retrieval episodes, have distinct molecular profiles.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cain CK, Debiec J, LeDoux JE. Consolidation and reconsolidation of pavlovian fear conditioning: roles for intracellular signaling and extracellular modulation in memory storage. In: Fields R, editor. Beyond the synapse: cell-cell signaling in synaptic plasticit. Cambridge University Press; New York, NY: 2008. pp. 72–88. [Google Scholar]
- 49•.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26(39):10051–6. doi: 10.1523/JNEUROSCI.2466-06.2006. [Very interesting paper showing how drugs like DCS that improve memory could strengthen or weaken fear depending on the amount of CS exposure and the degree that reconsolidation versus extinction processes are activated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiller D, Monfils MH, Raio CM, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36(4):567–84. doi: 10.1016/s0896-6273(02)01064-4. [Excellent review of fear extinction including psychological, anatomical, physiological and molecular mechanisms – although slightly outdated in 2012.] [DOI] [PubMed] [Google Scholar]
- 52.Pickens CL, Golden SA, Adams-Deutsch T, et al. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009;65(10):881–6. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Eysenck HJ. A theory of the incubation of anxiety-fear responses. Behav Res Ther. 1968;6(3):309–21. doi: 10.1016/0005-7967(68)90064-8. [Introduction of the fear ‘incubation’ hypothesis, where fear memory retrieval can strengthen responding, perhaps by a combination of stress-response systems and memory reconsolidation.] [DOI] [PubMed] [Google Scholar]
- 54.Debiec J, Bush DE, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats-a possible mechanism for the persistence of traumatic memories in ptsd. Depress Anxiety. 2011;28(3):186–93. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60(4):352–60. doi: 10.1016/j.biopsych.2005.12.015. [Summarizes data and ideas about the context-dependency of fear extinction – a major impediment to extinction-based cognitivebehavioral therapies.] [DOI] [PubMed] [Google Scholar]
- 56.Likhtik E, Popa D, Apergis-Schoute J, et al. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–5. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci. 2004;24(31):6912–19. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–4. doi: 10.1038/nature01138. [Seminal paper implicating ventromedial prefrontal cortex neurobiology in fear extinction retrieval.] [DOI] [PubMed] [Google Scholar]
- 59.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17(9):749–58. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 60.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 61.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90(2):419–63. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostroff LE, Cain CK, Bedont J, et al. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci USA. 2010;107(20):9418–23. doi: 10.1073/pnas.0913384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cain CK, LeDoux JE. Escape from fear: a detailed behavioral analysis of two atypical responses reinforced by cs termination. J Exp Psychol Anim Behav Process. 2007;33(4):451–63. doi: 10.1037/0097-7403.33.4.451. [DOI] [PubMed] [Google Scholar]
- 64.Lazaro-Munoz G, LeDoux JE, Cain CK. Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated pavlovian processes. Biol Psychiatry. 2010;67(12):1120–7. doi: 10.1016/j.biopsych.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biol Psychiatry. 1999;46(9):1140–52. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 66•.Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20(4):413–22. doi: 10.1002/jts.20261. [Simple analysis of FC behaviors in rats demonstrating distinct patterns of variability in fear learning and fear extinction, with a discussion of the relationship to anxiety disorders.] [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto S, Morinobu S, Takei S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26(12):1110–17. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 68.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29(8):1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 70.Yehuda R, Koenen KC, Galea S, Flory JD. The role of genes in defining a molecular biology of ptsd. Dis Markers. 2011;30(2-3):67–76. doi: 10.3233/DMA-2011-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker DG, Nievergelt CM, O'Connor DT. Biomarkers of ptsd: neuropeptides and immune signaling. Neuropharmacology. 2012;62(2):663–73. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 72.Skelton K, Ressler KJ, Norrholm SD, et al. Ptsd and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62(2):628–37. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jovanovic T, Norrholm SD, Blanding NQ, et al. Impaired fear inhibition is a biomarker of ptsd but not depression. Depress Anxiety. 2010;27(3):244–51. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–82. doi: 10.1016/j.biopsych.2009.06.026. [Important paper showing a specific deficit in fear extinction consolidation/ retrieval in PTSD with associated reductions in PFC/Hippocampal activity (and amygdala hyperactivity).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Jovanovic T, Norrholm SD, Fennell JE, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Res. 2009;167(1-2):151–60. doi: 10.1016/j.psychres.2007.12.014. [Nicely designed study demonstrating that safety learning (conditioned inhibition) is impaired in PTSD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grillon C, Morgan CA III. Fear-potentiated startle conditioning to explicit and contextual cues in gulf war veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108(1):134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 77.Orr SP, Metzger LJ, Lasko NB, et al. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109(2):290–8. [PubMed] [Google Scholar]
- 78.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of ptsd. Am J Psychiatry. 2010;167(6):648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–31. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80•.Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectr. 2005;10(2):99–106. doi: 10.1017/s109285290001943x. [Excellent review outlining how understanding psychological and neurobiological stress and memory processes will inform the use of drugs to treat PTSD.] [DOI] [PubMed] [Google Scholar]
- 81.Quirk GJ. Learning not to fear, faster. Learn Mem. 2004;11(2):125–6. doi: 10.1101/lm.75404. [DOI] [PubMed] [Google Scholar]
- 82.Golub Y, Mauch CP, Dahlhoff M, Wotjak CT. Consequences of extinction training on associative and non-associative fear in a mouse model of posttraumatic stress disorder (ptsd). Behav Brain Res. 2009;205(2):544–9. doi: 10.1016/j.bbr.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 83.Casada JH, Amdur R, Larsen R, Liberzon I. Psychophysiologic responsivity in posttraumatic stress disorder: generalized hyperresponsiveness versus trauma specificity. Biol Psychiatry. 1998;44(10):1037–44. doi: 10.1016/s0006-3223(98)00182-6. [DOI] [PubMed] [Google Scholar]
- 84.van der Kolk BA. Clinical implications of neuroscience research in ptsd. Ann N Y Acad Sci. 2006;1071:277–93. doi: 10.1196/annals.1364.022. [DOI] [PubMed] [Google Scholar]
- 85.Institute of Medicine Treatment of posttraumatic stress disorder: An assessment of the evidence. 2008. Press Release.
- 86.Sharpless BA, Barber JP. A clinician's guide to ptsd treatments for returning veterans. Prof Psychol Res Pr. 2011;42(1):8–15. doi: 10.1037/a0022351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Cukor J, Olden M, Lee F, Difede J. Evidence-based treatments for ptsd, new directions, and special challenges. Ann N Y Acad Sci. 2010;1208:82–9. doi: 10.1111/j.1749-6632.2010.05793.x. Excellent review. [DOI] [PubMed] [Google Scholar]
- 88.Choi DC, Rothbaum BO, Gerardi M, Ressler KJ. Pharmacological enhancement of behavioral therapy: Focus on posttraumatic stress disorder. Curr Top Behav Neurosci. 2010;2:279–99. doi: 10.1007/7854_2009_10. [DOI] [PubMed] [Google Scholar]
- 89•.Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behavioral Neuroscience. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [Nice study showing that benzodiazepines impair fear extinction, at least partly because extinction is context-dependent and extinction learned on benzodiazepines cannot be recalled well without drug.] [DOI] [PubMed] [Google Scholar]
- 90•.Pratchett LC, Daly K, Bierer LM, Yehuda R. New approaches to combining pharmacotherapy and psychotherapy for posttraumatic stress disorder. Expert Opin Pharmacother. 2011;12(15):2339–54. doi: 10.1517/14656566.2011.604030. Excellent review. [DOI] [PubMed] [Google Scholar]
- 91•.Ravindran LN, Stein MB. Pharmacotherapy of post-traumatic stress disorder. Curr Top Behav Neurosci. 2010;2:505–25. doi: 10.1007/7854_2009_15. Excellent review. [DOI] [PubMed] [Google Scholar]
- 92.Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent ptsd: results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20(6):923–32. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 93.Stein DJ, Zungu-Dirwayi N, van Der Linden GJ, Seedat S. Pharmacotherapy for posttraumatic stress disorder. Cochrane Database Syst Rev. 2000;4:CD002795. doi: 10.1002/14651858.CD002795. [DOI] [PubMed] [Google Scholar]
- 94.Post-traumatic stress disorder: the management of ptsd in adults and children in primary and secondary care. British Psychological Society; London: 2005. [PubMed] [Google Scholar]
- 95.Stein DJ, Ipser J, McAnda N. Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines. CNS Spectr. 2009;14(1 Suppl 1):25–31. [PubMed] [Google Scholar]
- 96.Ipser J, Seedat S, Stein DJ. Pharmacotherapy for post-traumatic stress disorder – a systematic review and meta-analysis. S Afr Med J. 2006;96(10):1088–96. [PubMed] [Google Scholar]
- 97.Ursano RJ, Bell C, Eth S, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry. 2004;161(11 Suppl):3–31. [PubMed] [Google Scholar]
- 98.Londborg PD, Hegel MT, Goldstein S, et al. Sertraline treatment of posttraumatic stress disorder: results of 24 weeks of open-label continuation treatment. J Clin Psychiatry. 2001;62(5):325–31. doi: 10.4088/jcp.v62n0503. [DOI] [PubMed] [Google Scholar]
- 99•.Karpova NN, Pickenhagen A, Lindholm J, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334(6063):1731–4. doi: 10.1126/science.1214592. [Exciting recent finding that SSRIs combined with extinction can lead to permanent blunting (erasure) of FC memories.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schneier FR, Neria Y, Pavlicova M, et al. Combined prolonged exposure therapy and paroxetine for ptsd related to the world trade center attack: a randomized controlled trial. Am J Psychiatry. 2012;169(1):80–8. doi: 10.1176/appi.ajp.2011.11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang W, Liu Y, Zheng H, et al. A modified single-prolonged stress model for post-traumatic stress disorder. Neurosci Lett. 2008;441(2):237–41. doi: 10.1016/j.neulet.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 102.Inoue T, Kitaichi Y, Koyama T. Ssris and conditioned fear. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(8):1810–19. doi: 10.1016/j.pnpbp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158–65. doi: 10.1001/archpsyc.63.10.1158. [DOI] [PubMed] [Google Scholar]
- 104.Davidson J, Rothbaum BO, Tucker P, et al. Venlafaxine extended release in posttraumatic stress disorder: a sertraline- and placebo-controlled study. J Clin Psychopharmacol. 2006;26(3):259–67. doi: 10.1097/01.jcp.0000222514.71390.c1. [DOI] [PubMed] [Google Scholar]
- 105•.Yang CH, Shi HS, Zhu WL, et al. Venlafaxine facilitates between-session extinction and prevents reinstatement of auditory-cue conditioned fear. Behav Brain Res. 2012;230(1):268–73. doi: 10.1016/j.bbr.2012.02.023. [Beautiful behavioral data showing that post-extinction venlafaxine profoundly improves LTM for fear extinction – clearly demonstrates that post-extinction processes are an important target for improving PTSD especially since PTSD patients show a specific impairment in this process (see Milad reference above).] [DOI] [PubMed] [Google Scholar]
- 106.U.S. Departments of Veterans Affairs and Defense Clinical Practice Guideline for the Management of Post-Traumatic Stress. 2010.
- 107.Klemenhagen KC, Gordon JA, David DJ, et al. Increased fear response to contextual cues in mice lacking the 5-ht1a receptor. Neuropsychopharmacology. 2006;31(1):101–11. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- 108.Duffy JD, Malloy PF. Efficacy of buspirone in the treatment of posttraumatic stress disorder: an open trial. Ann Clin Psychiatry. 1994;6(1):33–7. doi: 10.3109/10401239409148837. [DOI] [PubMed] [Google Scholar]
- 109.Wells BG, Chu CC, Johnson R, et al. Buspirone in the treatment of posttraumatic stress disorder. Pharmacotherapy. 1991;11(4):340–3. [PubMed] [Google Scholar]
- 110.LaPorta LD, Ware MR. Buspirone in the treatment of posttraumatic stress disorder. J Clin Psychopharmacol. 1992;12(2):133–4. doi: 10.1097/00004714-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 111.Hamner M, Ulmer H, Horne D. Buspirone potentiation of antidepressants in the treatment of ptsd. Depress Anxiety. 1997;5(3):137–9. [PubMed] [Google Scholar]
- 112.Adamec R, Bartoszyk GD, Burton P. Effects of systemic injections of vilazodone, a selective serotonin reuptake inhibitor and serotonin 1a receptor agonist, on anxiety induced by predator stress in rats. Eur J Pharmacol. 2004;504(1-2):65–77. doi: 10.1016/j.ejphar.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 113.Jiang X, Zhang ZJ, Zhang S, et al. 5-ht2a receptor antagonism by mdl 11,939 during inescapable stress prevents subsequent exaggeration of acoustic startle response and reduced body weight in rats. J Psychopharmacol. 2011;25(2):289–97. doi: 10.1177/0269881109106911. [DOI] [PubMed] [Google Scholar]
- 114.Davis LL, Jewell ME, Ambrose S, et al. A placebo-controlled study of nefazodone for the treatment of chronic posttraumatic stress disorder: a preliminary study. J Clin Psychopharmacol. 2004;24(3):291–7. doi: 10.1097/01.jcp.0000125685.82219.1a. [DOI] [PubMed] [Google Scholar]
- 115.Davidson JR, Weisler RH, Butterfield MI, et al. Mirtazapine vs. Placebo in posttraumatic stress disorder: a pilot trial. Biol Psychiatry. 2003;53(2):188–91. doi: 10.1016/s0006-3223(02)01411-7. [DOI] [PubMed] [Google Scholar]
- 116.Kakui N, Yokoyama F, Yamauchi M, et al. Anxiolytic-like profile of mirtazapine in rat conditioned fear stress model: functional significance of 5-hydroxytryptamine 1a receptor and alpha1-adrenergic receptor. Pharmacol Biochem Behav. 2009;92(3):393–8. doi: 10.1016/j.pbb.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 117.Villard V, Meunier J, Chevallier N, Maurice T. Pharmacological interaction with the sigma1 (sigma1)-receptor in the acute behavioral effects of antidepressants. J Pharmacol Sci. 2011;115(3):279–92. doi: 10.1254/jphs.10191fp. [DOI] [PubMed] [Google Scholar]
- 118.Sessa B. Is there a case for mdma-assisted psychotherapy in the uk? J Psychopharmacol. 2007;21(2):220–4. doi: 10.1177/0269881107069029. [DOI] [PubMed] [Google Scholar]
- 119.Mithoefer MC, Wagner MT, Mithoefer AT, et al. The safety and efficacy of {+/−} 3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25(4):439–52. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.den Hollander B, Schouw M, Groot P, et al. Preliminary evidence of hippocampal damage in chronic users of ecstasy. J Neurol Neurosurg Psychiatry. 2012;83(1):83–5. doi: 10.1136/jnnp.2010.228387. [DOI] [PubMed] [Google Scholar]
- 121.Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629–32. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565–8. doi: 10.4088/jcp.v63n0705. [DOI] [PubMed] [Google Scholar]
- 123.Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928–34. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 124.Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165–71. doi: 10.1177/0891988703256050. [DOI] [PubMed] [Google Scholar]
- 125.Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225–9. doi: 10.1097/JCP.0b013e3181dac52f. [DOI] [PubMed] [Google Scholar]
- 126.Taylor FB, Lowe K, Thompson C, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59(7):577–81. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 127.Boynton L, Bentley J, Strachan E, et al. Preliminary findings concerning the use of prazosin for the treatment of posttraumatic nightmares in a refugee population. J Psychiatr Pract. 2009;15(6):454–9. doi: 10.1097/01.pra.0000364287.63210.92. [DOI] [PubMed] [Google Scholar]
- 128•.Bernardi RE, Lattal KM. A role for alpha-adrenergic receptors in extinction of conditioned fear and cocaine conditioned place preference. Behav Neurosci. 2010;124(2):204–10. doi: 10.1037/a0018909. [Shows that a1-adrenergic blockers like prazosin, often used to combat nightmares in PTSD, impair fear extinction. Illustrates how certain drug-therapy combinations could be problematic.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lazzaro SC, Cain CK, Cunha C, LeDoux JE. Terazosin, an alpha1 adrenergic receptor antagonist, enhances acquisition and impairs extinction, but does not affect consolidation of pavlovian cue fear. Soc Neurosci Abstr. 2009;479:22. [Google Scholar]
- 130.Reist C, Duffy JG, Fujimoto K, Cahill L. Beta-adrenergic blockade and emotional memory in ptsd. Int J Neuropsychopharmacol. 2001;4(4):377–83. doi: 10.1017/S1461145701002607. [DOI] [PubMed] [Google Scholar]
- 131.Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51(2):189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 132.Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54(9):947–9. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 133.Nugent NR, Christopher NC, Crow JP, et al. The efficacy of early propranolol administration at reducing ptsd symptoms in pediatric injury patients: a pilot study. J Trauma Stress. 2010;23(2):282–7. doi: 10.1002/jts.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoge EA, Worthington JJ, Nagurney JT, et al. Effect of acute posttrauma propranolol on ptsd outcome and physiological responses during script-driven imagery. CNS Neurosci Ther. 2012;18(1):21–7. doi: 10.1111/j.1755-5949.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem. 2002;78(3):539–52. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- 136•.Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129(2):267–72. doi: 10.1016/j.neuroscience.2004.08.018. [Demonstrates that b-adrenergic blockers could be useful for reconsolidation-based therapies but may not be useful for blocking original consolidation following trauma.] [DOI] [PubMed] [Google Scholar]
- 137.Bush DE, Caparosa EM, Gekker A, Ledoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but. not the consolidation of auditory fear conditioning. Front Behav Neurosci. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brunet A, Orr SP, Tremblay J, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42(6):503–6. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 139.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256–8. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 140.Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28(2):369–75. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soeter M, Kindt M. Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology. 2012;37(5):1204–15. doi: 10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11(2):179–87. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121(3):501–14. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- 145.Powers MB, Smits JA, Otto MW, et al. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. J Anxiety Disord. 2009;23(3):350–6. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 146.Hefner K, Whittle N, Juhasz J, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28(32):8074–85. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rudy JW, Kuwagama K, Pugh CR. Isolation reduces contextual but not auditory-cue fear conditioning: a role for endogenous opioids. Behav Neurosci. 1999;113(2):316–23. doi: 10.1037//0735-7044.113.2.316. [Only preclinical study showing that morphine can block post-trauma consolidation of Pavlovian FC.] [DOI] [PubMed] [Google Scholar]
- 148.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of d-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22(6):2343–51. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [Original paper showing how NMDAR augmentation with DCS can facilitate fear extinction. Also implicates amygdala processes in fear extinction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heresco-Levy U, Kremer I, Javitt DC, et al. Pilot-controlled trial of d-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2002;5(4):301–7. doi: 10.1017/S1461145702003061. [DOI] [PubMed] [Google Scholar]
- 150.Heresco-Levy U, Ermilov M, Shimoni J, et al. Placebo-controlled trial of d-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry. 2002;159(3):480–2. doi: 10.1176/appi.ajp.159.3.480. [DOI] [PubMed] [Google Scholar]
- 151.Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161(8):1488–90. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 152.Jovanovic T, Phifer JE, Sicking K, et al. Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(10):1540–52. doi: 10.1016/j.psyneuen.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miller MW, McKinney AE, Kanter FS, et al. Hydrocortisone suppression of the fear-potentiated startle response and posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(7):970–80. doi: 10.1016/j.psyneuen.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weis F, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg. 2006;131(2):277–82. doi: 10.1016/j.jtcvs.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 155.Schelling G, Kilger E, Roozendaal B, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55(6):627–33. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 156.Schelling G, Briegel J, Roozendaal B, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50(12):978–85. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 157.Suris A, North C, Adinoff B, et al. Effects of exogenous glucocorticoid on combat-related ptsd symptoms. Ann Clin Psychiatry. 2010;22(4):274–9. [PMC free article] [PubMed] [Google Scholar]
- 158.Kehne JH, Cain CK. Therapeutic utility of non-peptidic crf(1) receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol Ther. 2010 doi: 10.1016/j.pharmthera.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159•.Deak T, Nguyen KT, Ehrlich AL, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140(1):79–86. doi: 10.1210/endo.140.1.6415. [Source of the data in Figure 3 – reanalyzed data support the notion that CRF1 receptor antagonists can blunt fear learning and facilitate fear extinction.] [DOI] [PubMed] [Google Scholar]
- 160.Yang YL, Chao PK, Ro LS, et al. Glutamate nmda receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharmacology. 2007;32(5):1042–51. doi: 10.1038/sj.npp.1301215. [DOI] [PubMed] [Google Scholar]
- 161.Bernardy NC, Lund BC, Alexander B, Friedman MJ. Prescribing trends in veterans with posttraumatic stress disorder. J Clin Psychiatry. 2012;73(3):297–303. doi: 10.4088/JCP.11m07311. [DOI] [PubMed] [Google Scholar]
- 162.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–46. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Walderhaug E, Kasserman S, Aikins D, et al. Effects of duloxetine in treatment-refractory men with posttraumatic stress disorder. Pharmacopsychiatry. 2010;43(2):45–9. doi: 10.1055/s-0029-1237694. [DOI] [PubMed] [Google Scholar]
- 164.Villarreal G, Canive JM, Calais LA, et al. Duloxetine in military posttraumatic stress disorder. Psychopharmacol Bull. 2010;43(3):26–34. [PubMed] [Google Scholar]
- 165.Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other ptsd symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371–3. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 166.Davis LL, Ward C, Rasmusson A, et al. A placebo-controlled trial of guanfacine for the treatment of posttraumatic stress disorder in veterans. Psychopharmacol Bull. 2008;41(1):8–18. [PubMed] [Google Scholar]
- 167.Neylan TC, Lenoci M, Samuelson KW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006;163(12):2186–8. doi: 10.1176/appi.ajp.163.12.2186. [DOI] [PubMed] [Google Scholar]
- 168.Kinzie JD, Leung P. Clonidine in cambodian patients with posttraumatic stress disorder [see comments]. J Nerv Ment Dis. 1989;177(9):546–50. doi: 10.1097/00005053-198909000-00005. [DOI] [PubMed] [Google Scholar]
- 169.Becker ME, Hertzberg MA, Moore SD, et al. A placebo-controlled trial of bupropion sr in the treatment of chronic posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27(2):193–7. doi: 10.1097/JCP.0b013e318032eaed. [DOI] [PubMed] [Google Scholar]
- 170.Krystal JH, Rosenheck RA, Cramer JA, et al. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related ptsd: a randomized trial. JAMA. 2011;306(5):493–502. doi: 10.1001/jama.2011.1080. [DOI] [PubMed] [Google Scholar]
- 171.Rothbaum BO, Killeen TK, Davidson JR, et al. Placebo-controlled trial of risperidone augmentation for selective serotonin reuptake inhibitor-resistant civilian posttraumatic stress disorder. J Clin Psychiatry. 2008;69(4):520–5. doi: 10.4088/jcp.v69n0402. [DOI] [PubMed] [Google Scholar]
- 172.Padala PR, Madison J, Monnahan M, et al. Risperidone monotherapy for post-traumatic stress disorder related to sexual assault and domestic abuse in women. Int Clin Psychopharmacol. 2006;21(5):275–80. doi: 10.1097/00004850-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 173.Kozaric-Kovacic D, Pivac N, Muck-Seler D, Rothbaum BO. Risperidone in psychotic combat-related posttraumatic stress disorder: an open trial. J Clin Psychiatry. 2005;66(7):922–7. doi: 10.4088/jcp.v66n0716. [DOI] [PubMed] [Google Scholar]
- 174.Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 2005;57(5):474–9. doi: 10.1016/j.biopsych.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 175.Reich DB, Winternitz S, Hennen J, et al. A preliminary study of risperidone in the treatment of posttraumatic stress disorder related to childhood abuse in women. J Clin Psychiatry. 2004;65(12):1601–6. doi: 10.4088/jcp.v65n1204. [DOI] [PubMed] [Google Scholar]
- 176.David D, De Faria L, Lapeyra O, Mellman TA. Adjunctive risperidone treatment in combat veterans with chronic ptsd. J Clin Psychopharmacol. 2004;24(5):556–9. doi: 10.1097/01.jcp.0000138771.46353.59. [DOI] [PubMed] [Google Scholar]
- 177.Monnelly EP, Ciraulo DA, Knapp C, Keane T. Low-dose risperidone as adjunctive therapy for irritable aggression in posttraumatic stress disorder. J Clin Psychopharmacol. 2003;23(2):193–6. doi: 10.1097/00004714-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 178.Kozaric-Kovacic D, Pivac N. Quetiapine treatment in an open trial in combat-related post-traumatic stress disorder with psychotic features. Int J Neuropsychopharmacol. 2007;10(2):253–61. doi: 10.1017/S1461145706006596. [DOI] [PubMed] [Google Scholar]
- 179.Ahearn EP, Mussey M, Johnson C, et al. Quetiapine as an adjunctive treatment for post-traumatic stress disorder: an 8-week open-label study. Int Clin Psychopharmacol. 2006;21(1):29–33. doi: 10.1097/01.yic.0000182116.49887.ae. [DOI] [PubMed] [Google Scholar]
- 180.Robert S, Hamner MB, Kose S, et al. Quetiapine improves sleep disturbances in combat veterans with ptsd: sleep data from a prospective, open-label study. J Clin Psychopharmacol. 2005;25(4):387–8. doi: 10.1097/01.jcp.0000169624.37819.60. [DOI] [PubMed] [Google Scholar]
- 181.Hamner MB, Deitsch SE, Brodrick PS, et al. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J Clin Psychopharmacol. 2003;23(1):15–20. doi: 10.1097/00004714-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 182.Davidson J, Kudler H, Smith R, et al. Treatment of posttraumatic stress disorder with amitriptyline and placebo. Arch Gen Psychiatry. 1990;47(3):259–66. doi: 10.1001/archpsyc.1990.01810150059010. [DOI] [PubMed] [Google Scholar]
- 183.Frank JB, Kosten TR, Giller EL, Jr, Dan E. A randomized clinical trial of phenelzine and imipramine for posttraumatic stress disorder. Am J Psychiatry. 1988;145(10):1289–91. doi: 10.1176/ajp.145.10.1289. [DOI] [PubMed] [Google Scholar]
- 184.Kosten TR, Frank JB, Dan E, et al. Pharmacotherapy for posttraumatic stress disorder using phenelzine or imipramine. J Nerv Ment Dis. 1991;179(6):366–70. doi: 10.1097/00005053-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 185.Pivac N, Kozaric-Kovacic D, Muck-Seler D. Olanzapine versus fluphenazine in an open trial in patients with psychotic combat-related post-traumatic stress disorder. Psychopharmacology (Berl) 2004;175(4):451–6. doi: 10.1007/s00213-004-1849-z. [DOI] [PubMed] [Google Scholar]
- 186.Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for ssri-resistant combat-related ptsd: a double-blind, placebo-controlled study. Am J Psychiatry. 2002;159(10):1777–9. doi: 10.1176/appi.ajp.159.10.1777. [DOI] [PubMed] [Google Scholar]
- 187.Petty F, Brannan S, Casada J, et al. Olanzapine treatment for post-traumatic stress disorder: an open-label study. Int Clin Psychopharmacol. 2001;16(6):331–7. doi: 10.1097/00004850-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 188.Butterfield MI, Becker ME, Connor KM, et al. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Int Clin Psychopharmacol. 2001;16(4):197–203. doi: 10.1097/00004850-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 189.Robert S, Hamner MB, Durkalski VL, et al. An open-label assessment of aripiprazole in the treatment of ptsd. Psychopharmacol Bull. 2009;42(1):69–80. [PubMed] [Google Scholar]
- 190.Mello MF, Costa MC, Schoedl AF, Fiks JP. Aripiprazole in the treatment of posttraumatic stress disorder: an open-label trial. Rev Bras Psiquiatr. 2008;30(4):358–61. doi: 10.1590/s1516-44462008000400011. [DOI] [PubMed] [Google Scholar]
- 191.Villarreal G, Calais LA, Canive JM, et al. Prospective study to evaluate the efficacy of aripiprazole as a monotherapy in patients with severe chronic posttraumatic stress disorder: an open trial. Psychopharmacol Bull. 2007;40(2):6–18. [PubMed] [Google Scholar]
- 192.Holbrook TL, Galarneau MR, Dye JL, et al. Morphine use after combat injury in iraq and post-traumatic stress disorder. N Engl J Med. 2010;362(2):110–17. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 193.Bryant RA, Creamer M, O'Donnell M, et al. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry. 2009;65(5):438–40. doi: 10.1016/j.biopsych.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 194.Stoddard FJ, Jr, Sorrentino EA, Ceranoglu TA, et al. Preliminary evidence for the effects of morphine on posttraumatic stress disorder symptoms in one- to four-year-olds with burns. J Burn Care Res. 2009;30(5):836–43. doi: 10.1097/BCR.0b013e3181b48102. [DOI] [PubMed] [Google Scholar]
- 195.Saxe G, Stoddard F, Courtney D, et al. Relationship between acute morphine and the course of ptsd in children with burns. J Am Acad Child Adolesc Psychiatry. 2001;40(8):915–21. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 196.Glover H. A preliminary trial of nalmefene for the treatment of emotional numbing in combat veterans with post-traumatic stress disorder. Isr J Psychiatry Relat Sci. 1993;30(4):255–63. [PubMed] [Google Scholar]
- 197.Lubin G, Weizman A, Shmushkevitz M, Valevski A. Short-term treatment of post-traumatic stress disorder with naltrexone: an open-label preliminary study. Hum Psychopharmacol. 2002;17(4):181–5. doi: 10.1002/hup.395. [DOI] [PubMed] [Google Scholar]
- 198.Henn-Haase HC, Best BS, Metzler MT, et al. Exposure based cbt therapy and d-cycloserine treatment for ptsd in veterans and civilians. 2010.
- 199.Guay GS, Marchand MA, Landry LP. Results from a six-month follow-up of a randomized controlled trial assessing the efficacy of cognitive-behavior therapy combined with d-cycloserine for treating ptsd. 2010.
- 200.Schonenberg M, Reichwald U, Domes G, et al. Ketamine aggravates symptoms of acute stress disorder in a naturalistic sample of accident victims. J Psychopharmacol. 2008;22(5):493–7. doi: 10.1177/0269881107082481. [DOI] [PubMed] [Google Scholar]
- 201.McGhee LL, Maani CV, Garza TH, et al. The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma. 2008;64(2 Suppl):S195–8. doi: 10.1097/TA.0b013e318160ba1d. Discussion S197-198. [DOI] [PubMed] [Google Scholar]
- 202.Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(2):201–6. doi: 10.4088/jcp.v68n0204. [DOI] [PubMed] [Google Scholar]
- 203.Lindley SE, Carlson EB, Hill K. A randomized, double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27(6):677–81. doi: 10.1097/jcp.0b013e31815a43ee. [DOI] [PubMed] [Google Scholar]
- 204.Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15–20. doi: 10.4088/jcp.v63n0104. [DOI] [PubMed] [Google Scholar]
- 205.Hertzberg MA, Butterfield MI, Feldman ME, et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;45(9):1226–9. doi: 10.1016/s0006-3223(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 206.Davidson JR, Brady K, Mellman TA, et al. The efficacy and tolerability of tiagabine in adult patients with post-traumatic stress disorder. J Clin Psychopharmacol. 2007;27(1):85–8. doi: 10.1097/JCP.0b013e31802e5115. [DOI] [PubMed] [Google Scholar]
- 207.Connor KM, Davidson JR, Weisler RH, et al. Tiagabine for posttraumatic stress disorder: effects of open-label and double-blind discontinuation treatment. Psychopharmacology (Berl) 2006;184(1):21–5. doi: 10.1007/s00213-005-0265-3. [DOI] [PubMed] [Google Scholar]
- 208.Davis LL, Davidson JR, Ward LC, et al. Divalproex in the treatment of posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial in a veteran population. J Clin Psychopharmacol. 2008;28(1):84–8. doi: 10.1097/JCP.0b013e318160f83b. [DOI] [PubMed] [Google Scholar]
- 209.Hamner MB, Brodrick PS, Labbate LA. Gabapentin in ptsd: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry. 2001;13(3):141–6. doi: 10.1023/a:1012281424057. [DOI] [PubMed] [Google Scholar]
- 210.Kinrys G, Wygant LE, Pardo TB, Melo M. Levetiracetam for treatment-refractory posttraumatic stress disorder. J Clin Psychiatry. 2006;67(2):211–14. doi: 10.4088/jcp.v67n0206. [DOI] [PubMed] [Google Scholar]
- 211.Pollack MH, Hoge EA, Worthington JJ, et al. Eszopiclone for the treatment of posttraumatic stress disorder and associated insomnia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(7):892–7. doi: 10.4088/JCP.09m05607gry. [DOI] [PubMed] [Google Scholar]
- 212.Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390–4. [PubMed] [Google Scholar]
- 213.Braun P, Greenberg D, Dasberg H, Lerer B. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990;51(6):236–8. [PubMed] [Google Scholar]
- 214.Cates ME, Bishop MH, Davis LL, et al. Clonazepam for treatment of sleep disturbances associated with combat-related posttraumatic stress disorder. Ann Pharmacother. 2004;38(9):1395–9. doi: 10.1345/aph.1E043. [DOI] [PubMed] [Google Scholar]
- 215.Mellman TA, Bustamante V, David D, Fins AI. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183–4. doi: 10.4088/jcp.v63n1214h. [DOI] [PubMed] [Google Scholar]
- 216.Mathew SJ, Vythilingam M, Murrough JW, et al. A selective neurokinin-1 receptor antagonist in chronic ptsd: A randomized, double-blind, placebo-controlled, proof-of-concept trial. Eur Neuropsychopharmacol. 2011;21(3):221–9. doi: 10.1016/j.euroneuro.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kehne JH. The crf1 receptor, a novel target for the treatment of depression, anxiety, and stress-related disorders. CNS Neurol Disord Drug Targets. 2007;6(3):163–82. doi: 10.2174/187152707780619344. [DOI] [PubMed] [Google Scholar]