Abstract
Objective:
To evaluate the antimicrobial efficacy and compressive strength of conventional glass ionomer cement (GIC) containing chlorhexidine and antibiotics at varying concentrations.
Materials and Methods:
Chlorhexidine diacetate and antibiotics (ciprofloxacin, metronidazole, and minocycline) were incorporated into GIC Fuji IX at 1.5% and 3% w/w ratio to form the experimental groups. The experimental GIC specimens were placed on brain heart infusion agar plates inoculated with Streptococcus mutans, and the area of inhibition was measured after 48 h. The 24-h compressive strength of the set specimens was evaluated using a Universal Testing Machine.
Results:
The control group demonstrated no zone of inhibition. All experimental groups showed inhibition against S. mutans (P < 0.05), with larger zones of inhibition found in the higher concentration groups. Compressive strength at the end of 24 h decreased in the experimental groups as compared to the control group (P < 0.05), but no difference was found between the experimental groups (P > 0.05).
Conclusion:
The present study demonstrated that experimental GICs containing chlorhexidine diacetate and antibiotics were effective in inhibiting S. mutans, and incorporation of 1.5% ABX was optimal to give the appropriate antibacterial and physical properties.
Keywords: Antibiotics, chlorhexidine diacetate, glass ionomer cement
INTRODUCTION
Dental caries is a disease that dates back to antiquity and still remains a perennial public health problem.[1] Scientific research continues to make progress in identifying the best practices for treating and preventing dental caries.[2] Minimal intervention dentistry is an emerging modern dental practice designed around the principle aim of preservation of as much as natural tooth structure as possible.
Atraumatic restorative treatment is one such paradigm of the Minimal Intervention Dentistry concept. An Alternative Restorative Treatment restoration involves the removal of soft, completely demineralized carious tooth tissue with hand instruments, followed by restoration of the cavity with an adhesive dental material such as glass ionomer cement (GIC) that simultaneously seals any remaining pits and fissures at risk.[3] However, cavities treated by ART have some residual infected dentin, as manual instruments are not as effective as rotary burs in terms of eliminating bacteria.[4] Consequently, cariogenic bacteria can survive incarceration under GIC restoration and remain viable for up to 2 years resulting in secondary caries.[5] Literature has been evidence to the fact that fluoride released from GICs is not sufficiently potent to combat the effects of bacterial destruction over an extensive duration of time.[6]
Different classes of GIC have been used for luting, restorative, core build up, lining purposes, as orthodontic cement, and for sealing pits and fissures. Conventional and metal-reinforced glass ionomers have been superseded by highly viscous GICs like Fuji IX GP, Chem-Flex, and Ketac-Molar. The quest to develop an efficient GIC has led to several advances in the material or modifications of the existing one.[7] The fact that ions can readily travel in and out of the material offers the opportunity to dope the cement with other soluble antimicrobials.[8] Chlorhexidine, a bisbiguanide, is currently the most potent chemotherapeutic agent used to enhance the antimicrobial properties of GICs.[9] This cationic antibacterial agent binds to hydroxyapatite and is gradually released at therapeutic levels, a phenomenon named as substantivity.[10,11,12] Recently, the addition of antibiotics to glass ionomer has been recommended, with an aim to alleviate the total number of viable bacteria.[13]
From dental literature it appears that chlorhexidine (diacetate and digluconate) and antibiotics like doxycycline, metronidazole, ciprofloxacin, cefaclor, cetrimide, and minocycline have frequently been incorporated into GIC and all studies have demonstrated encouraging results regarding the antimicrobial efficacy of the modified cement. However, the incorporation of these antibacterial agents has resulted in jeopardizing the basic physiomechanical characteristics of the material.[4,7,13] Since the requisites of an ideal restorative material remain unaccomplished without the ability of a material to withstand the traumas of occlusion, this highlights the crucial effect of antibacterial additive concentration on GICs’ mechanical performance. Therefore, the present study aims to evaluate the antibacterial activity and physical properties of modified GIC at different concentrations and to determine the optimal concentration of antimicrobials to be incorporated into this emerging biomaterial.
MATERIALS AND METHODS
Preparation of molds
A commercially available plastic tubing (linear low-density polyethylene tubing 1/4” outside diameter and 0.165” inside diameter) was obtained. The tube was then cut with the help of a microtome to prepare molds of 4 mm diameter and 10 mm height. Care was taken to have a clean cut surface perpendicular to the long axis of the mold. The molds were grouped as groups I, II, and III, where Group I served as the control group and groups II and III as the experimental groups containing chlorhexidine and antibiotics, respectively. The experimental groups were further sub-categorized into two concentrations: 1.5% and 3%. Seven molds each were assigned to the control group as well as the various concentration subgroups, forming a total of 70 molds.
Preparation of antibacterial cement
Preparation of antibacterial cement is presented in Table 1.
Table 1.
Preparation of antibacterial cement
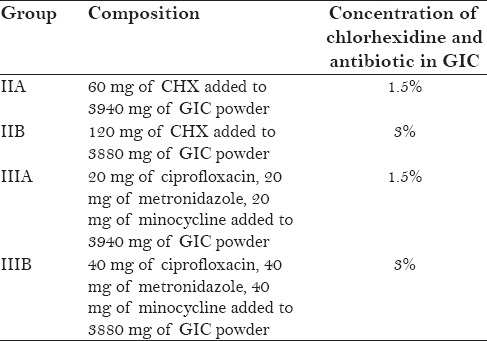
A conventional posterior restorative GIC (Fuji IX; GC Corporation, Tokyo, Japan) was used to fill the molds of the control group (Group I). Chlorhexidine diacetate (CHX), which is commercially available as a solid substance, was weighed using a 10−4 g precision balance (Mettler Toledo Electronic scale) and added to calculated amount of conventional glass ionomer powder, in order to obtain two concentrations of 1.5% and 3% CHX in the GIC formulation.
The antibiotics were obtained in the form of tablets and the surface sugar coating from the tablets was scrapped off. Using a pestle and mortar, the tablets were then ground into fine powder. The antibiotics were proportioned in the ratio 1:1:1 and were added to the GIC powder to form concentrations of 1.5% and 3% w/w antibiotics in the GIC formulation. Following the completion of measurements, all the experimental groups were stored in separate airtight containers containing desiccant (silica gel).
Preparation of samples
The powder and liquid (P/L ratio 3.6:1, as per the manufacturer's instruction) for each experimental group were dispensed on a mixing pad and mixed with a plastic spatula for 30 s. The material was transferred into standardized molds with a plastic instrument from one end of the mold till the material extruded from the other end. Excess material was removed from the molds and both ends of the mold were covered with a glass slide held under pressure. This was done to facilitate the setting of material without any surface defects or voids. The specimens were allowed to set for 30 min at room temperature. Following this, the specimens were carefully teased out of the molds by applying pressure at one end with a condenser. [Figure 1].
Figure 1.
Specimens allowed to set at room temperature and teased out of molds
A 500-grit silicon carbide paper was used to finish any visible irregularities at the ends of the specimen. The height and diameter of each specimen were then measured using digital callipers. The specimens for evaluating antimicrobial efficacy were immediately inoculated, while those for compressive strength evaluation were stored in distilled water at 37°C for 24 h.
Microbial strain and growth media
The antibacterial activity was evaluated against Streptococcus mutans. Stock culture of S. mutans (MTCC No. 497) procured from MTCC, Institute of Microbial Technology (IMTECH), Chandigarh was used in the present study. A loopful of bacterial inoculum from the lypholized culture was transferred to Brain Heart Infusion (BHI) broth and incubated for 24 h at 37°C. The bacterial growth was assessed by the appearance of turbidity in the broth after incubation [Figure 2]. BHI agar was used as a culture medium for agar diffusion test.
Figure 2.

Bacterial growth assessed by appearance of turbidity in the broth
In vitro evaluations
Agar diffusion test
Fresh culture of S. mutans from turbid BHI broth was flood inoculated onto the surface of BHI agar plates. Bacterial lawn was prepared by spread plate method with a volume of 500 μl of bacterial inoculum by using a micropipette (100–1000 μl). The specimens were placed in hot air oven for a period of 1 h to achieve suitable sterilization. The specimens were then placed on BHI agar plates having bacterial strain and incubated at 37°C for 24–48 h. Zones of inhibition around the specimens were measured in millimeters using Hi-Veg Media Antibiotic zone scale (Hi Media Laboratories, Mumbai) [Figures 3 and 4].
Figure 3.
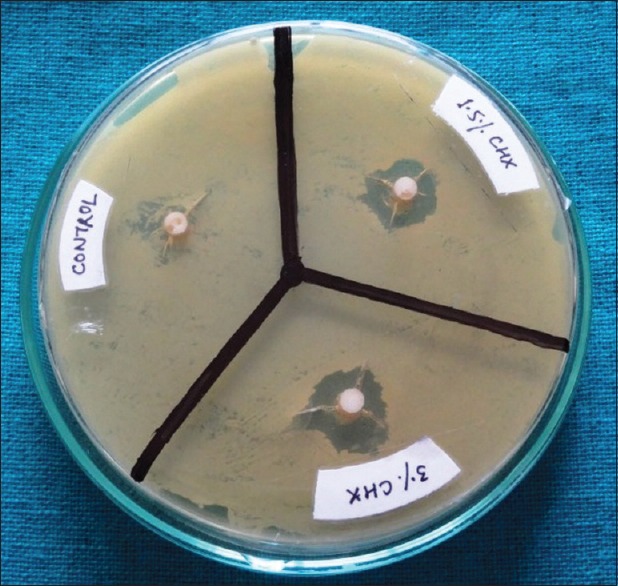
Zones of inhibition around specimens containing chlorhexidine
Figure 4.
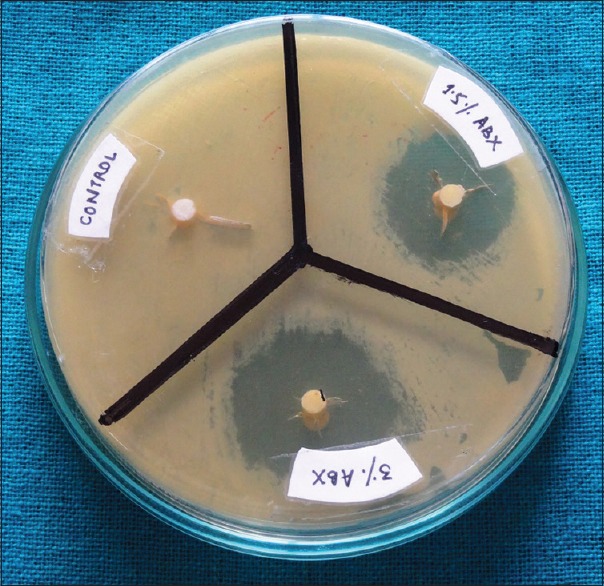
Zones of inhibition around specimens containing antibiotics
Evaluation of compressive strength
After preparation, the specimens were stored in distilled water and 24-h compressive strength was evaluated. Prior to testing, the diameter and height of each specimen were determined using a digital calliper. The specimens were placed with the flat ends up between the plates of the Universal Testing Machine (Hounsfield UTM) [Figure 5]. The strength of the specimen was then recorded in MPa by applying a compressive load along the long axis of the cylindrical pellet at a crosshead speed of 0.5 mm/min.
Figure 5.

Specimen being tested for compressive strength using Universal Testing Machine
Statistical analysis
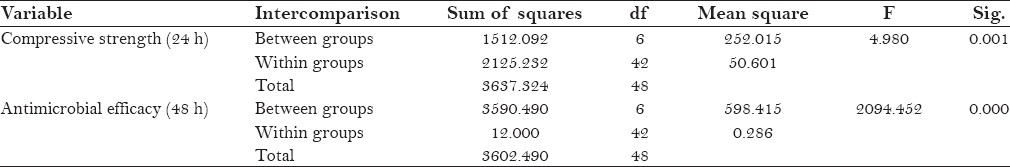
The resultant findings for antimicrobial efficacy (48 h) and compressive strength (24 h) were statistically analyzed using one-way analysis of variance (ANOVA) [Table 2] and Tukey's post hoc test.
Table 2.
Results of analysis of variance (ANOVA) comparing different groups for compressive strength and antimicrobial efficacy
RESULTS
Antimicrobial activity screening test
At the end of 48 h, Group I (control) exhibited no zone of inhibition against the test organism. However, large zones of inhibition, as determined by the Hi-Veg Media Antibiotic zone scale, were observed around specimens of groups II and III [Figure 6]. Also, the size of inhibition zones was dependant on the amount of antimicrobial agent added.
Figure 6.
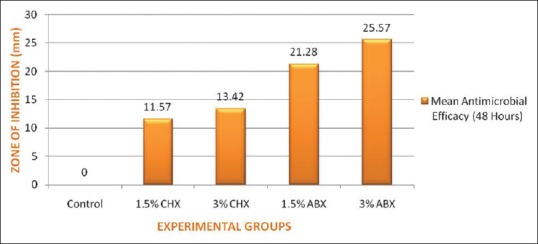
Mean values of zone of inhibition for control and experimental groups in millimeters
Significant differences existed in the size of the inhibition zones produced among the control and experimental groups (P < 0.05). Zones of inhibition recorded for Group III were significantly higher (P < 0.05) as compared to Group II.
Evaluation of compressive strength
At the end of 24 h, the experimental groups showed lower compressive strength when compared to the control group, with a statistically significant difference (P < 0.05). However, there was no significant difference (P > 0.05) in the mean compressive strength values of groups II and III for both concentrations. The compressive strength decreased in a concentration-dependant manner [Figure 7].
Figure 7.
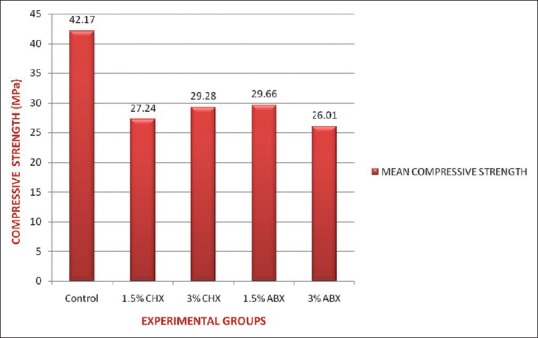
Mean values of compressive strength for control and experimental groups in megapascals
DISCUSSION
The quest to search an ideal restorative material has been a challenge for the researchers and academicians in the fraternity of restorative dentistry. Glass ionomers are a class of biomaterials in widespread use in modern dentistry.[14] GICs are capable of releasing fluoride, which contributes to some reduction in the number of residual bacteria in cavities as well as remineralization of the softened dentin.[15,16,17,18] However, even after the removal of infected dentin and adequate sealing, viable bacteria have been found in the remaining affected dentine after different periods of evaluation.[19] Literature is a testimony to the fact that therapeutic benefits have been gained when antimicrobial substances like chlorhexidine and antibiotics are used in association with GIC; however, a compromise of the strength characteristics has always emerged unconcealed.[4,7,13,16] Therefore, a comparison of the influence of incorporating CHX and triple antibiotic mixture (ABX) consisting of ciprofloxacin, metronidazole, and minocycline on the antibacterial efficacy and compressive strength of GIC was the prime objective of our study.
Fuji IX GIC was used as the control in the present study, since this is the most frequently reported material in in vivo and in vitro studies in the past.[4,13,20] Considering the material as a gold standard in high-strength posterior restoratives, it could be efficiently used for the assessment of compressive strength of the modified cement. CHX and triple antibiotic mixture were the preferred choice of antimicrobials. The efficacy of chlorhexidine has been proven against oral pathogens, primarily S. mutans.[21] Different salts of chlorhexidine, mainly digluconate and diacetate, are commercially available as pre-weighed packages. CHX was preferred to other CHX derivates (chlorhexidine gluconate) in the present study, as it is a more stable material, not prone to decomposition, and can be easily blended with GIC powder.[22]
The antibacterial efficacy of the modified cements was illustrated against S. mutans. S. mutans bacteria are the most cariogenic pathogens as they induce an acid tolerance response that enables this pathogen to survive and grow in low-pH environments. Considering the impact of S. mutans as an initiator of the pathological process of dental caries, it was selected as the test organism.
Considering that the development of dental caries is attributed to a diverse, abundant, and complex microbial community, the use of a mixture of antibiotics is a better alternative than the use of a single antibiotic.[13,23] Pinherio et al.[24] suggested that a GIC containing triple antibiotic mixture may be used for the treatment of carious lesions, as it reduces total viable bacteria. These studies were contemplated and ciprofloxacin, metronidazole, and minocycline were selected for the mixture of antibiotics that was tested in the present research.
The concentration of antibiotics and material preparation were determined based on the study conducted by Yesilyurt et al.[13] CHX was proportioned into GIC in similar ratios to ensure adequate intercomparison between the two antimicrobial agents. The antibacterial efficacy of the modified cements was illustrated against S. mutans. Agar plate diffusion was the method of choice to evaluate the antimicrobial efficacy as the process is relatively inexpensive and can be performed rapidly and easily with a large number of specimens.[16] The diameter of the inhibition zone is related to the susceptibility of the isolate and to the diffusion rate of the drug through the agar medium.[25,26,27]
The agar diffusion test in our study demonstrated that Fuji IX GIC showed no antibacterial effect against S. mutans. These results were consistent with the findings of Botelho et al.,[28] Yap et al.,[29] and Yesilyurt et al.[13] On the contrary, Shashibhushan et al.[30] demonstrated that some degree of growth inhibition of mutans streptococci is exhibited by GICs due to the release of fluoride and zinc ions into an aqueous medium, which may inhibit the growth of mutans streptococci. However, the release of these ions from GICs is governed by intrinsic and extrinsic factors such as preparation of the material, its P/L ratio, manipulation time, temperature, specimen geometry, surface protection, storage/dissolution of the medium, and the analytic method used. A combination of these factors could have possibly contributed to the absence of inhibition zones around the control specimens.
The addition of triple antibiotic mixture and CHX to GIC enhanced its antimicrobial efficacy in a concentration-dependant manner. The results of our study were complementary to the findings of Ribeiro et al.[31] and Prabhakar et al.,[7] who demonstrated a similar dose response effect. The antimicrobial efficacy of both concentrations of the antibiotic group was significantly higher as compared to the chlorhexidine group. This could possibly be accredited to the difference in mechanism of action of the two antimicrobial agents. At low concentrations, the bacteriostatic effect of CHX is based on disturbance of bacterial cell functions, enzymes, and cell receptors and at high concentrations, CHX causes cytoplasmic precipitation or coagulation.[32,33] In contrast, the triple antibiotic mixture has a broader spectrum of antibacterial activity and a versatile antibacterial action. Though metronidazole is a narrow-spectrum antibiotic effective only against obligate anaerobes, ciprofloxacin and minocycline have proven efficacy against S. mutans.[34] Moreover, the agar diffusion assay is highly affected by material diffusibility through the agar. The powdered antibiotic particles easily absorb water and disseminate through the agar medium, as compared to CHX.
The clinical utility of a material is defined by its ability to endure the stresses and strains induced during mastication and function. The most commonly used strength value to characterize dental cements is compressive strength.[4] In the present study, compressive strength of the experimental groups was considerably lower as compared to the control group. The recorded values of compressive strength (MPa) for both control and experimental groups were less than that reported in literature.[4,16,35] This could be due to the difference in the method of casting the specimens and the mechanical testing procedure. Since the detection of internal defects was beyond the confines of our study, their effect on the compressive strength of the material cannot be ignored.
The compressive strength of experimental groups containing CHX and antibiotics decreased in a concentration-dependant manner. The cross-linking in GIC is because of the coordination of Al3+ and Ca2+ with the COOH groups on the acidic polymers. Due to vitrification of GIC with antimicrobials, many of these COOH groups are prevented from participating in these coordination complexes.[36] In addition, variation in the P/L ratio by addition of antimicrobials may also have attributed to the decrease observed in compressive strength.[37,38,39,40,41] Moreover, the powdered antibiotic particles which are added to GIC easily absorb water.[13] The absorption of water can decrease the compressive strength of the GIC.
Since the addition of antimicrobial agents to GIC can affect the mechanical properties of the cement,[42] the particular antimicrobial agent and its quantity are important aspects to be determined. The antimicrobial efficacy of the 1.5% ABX group was significantly higher than that in the 1.5% CHX and 3% CHX groups. The mean compressive strength of 1.5% ABX was also better as compared to both 1.5% CHX and 3% CHX. Although the compressive strength of the 1.5% ABX group was significantly compromised as compared to the control group, therapeutic properties obtained from these materials might outdo the disadvantages of altered mechanical properties. Nevertheless, the amount of antimicrobial agent must be kept as low as possible, as high amounts of additives would weaken the scaffold and the glass ionomer network, thereby compromising the physical properties.
CONCLUSION
The present in vitro study demonstrated that the addition of chlorhexidine and antibiotics to GIC decreased the compressive strength in all experimental groups. However, within the limitations of the present study, incorporation of 1.5% ABX into glass ionomer appears to provide an acceptable combination of properties. Further in vivo studies are required to test the clinical efficacy of this concentration before advocating the use of antibiotic-modified GIC in ART procedures. Endeavors to compare lower concentrations of chlorhexidine with antibiotics for other properties like fluoride release, bond strength, diametral tensile strength, and biaxial flexure strength must be attempted in future studies. Therefore, until the long-term effects of the antibiotic-modified GIC are investigated, this modified cement can be used as a base material under the conventional glass ionomer restorations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ahluwalia P, Chopra S, Thomas AM. Strength characteristics and marginal sealing ability of chlorhexidine-modified glass ionomer cement: An in vitro study. J Indian Soc Pedod Prev Dent. 2012;30:41–6. doi: 10.4103/0970-4388.95580. [DOI] [PubMed] [Google Scholar]
- 2.Moses J, Rangeeth BN, Gurunathan D. Prevalence of dental caries, socio-economic status and treatment needs among 5 to 15 year old school going children of Chidambaram. J Clin Diagn Res. 2011;5:146–51. [Google Scholar]
- 3.Frencken JE, van Amerongen WE. The atraumatic restorative treatment approach. In: Fejerskov O, Kidd E, Bente N, editors. Dental Caries: The Disease and its Clinical Management. 2nd ed. Oxford, UK: Blackwell Munksgaard; 2008. pp. 427–42. [Google Scholar]
- 4.Deepalakshmi M, Poorni S, Miglani R, Rajamani I, Ramachandran S. Evaluation of the antibacterial and physical properties of glass ionomer cements containing Chlorhexidine and Cetrimide: An in-vitro study. Indian J Dent Res. 2010;21:552–6. doi: 10.4103/0970-9290.74217. [DOI] [PubMed] [Google Scholar]
- 5.Weerheijm KL, Kreulen CM, de Soet JJ, Groen HJ, van Amerongen WE. Bacterial counts in carious dentine under restorations: 2-year in vivo effects. Caries Res. 1999;33:130–4. doi: 10.1159/000016506. [DOI] [PubMed] [Google Scholar]
- 6.Weng Y, Guo X, Gregory R, Xie D. A novel antibacterial dental glass-ionomer cement. Eur J Oral Sci. 2010;118:531–4. doi: 10.1111/j.1600-0722.2010.00770.x. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar AR, Prahlad D, Kumar SR. Antibacterial activity, fluoride release, and physical properties of an antibiotic-modified glass ionomer cement. Pediatr Dent. 2013;35:411–5. [PubMed] [Google Scholar]
- 8.Hook ER, Owen OJ, Bellis CA, Holder JA, O’sullivan DJ, Barbour ME. Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J Nanobiotechnology. 2014;12:3. doi: 10.1186/1477-3155-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emilson CG. Potential efficacy of chlorhexidine against mutans streptococci and human dental caries. J Dent Res. 1994;73:682–91. doi: 10.1177/00220345940730031401. [DOI] [PubMed] [Google Scholar]
- 10.Rölla G, Löe H, Schiott CR. The affinity of chlorhexidine for hydroxyapatite and salivary mucins. J Periodontal Res. 1970;5:90–5. doi: 10.1111/j.1600-0765.1970.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal S, Spångberg L, Safavi K. Chlorhexidine substantivity in root canal dentine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:488–92. doi: 10.1016/j.tripleo.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Khadeimi AA, Mohammadi Z, Havaee A. Evaluation of the antibacterial substantivity of several intra-canal agents. Aust Endod J. 2006;32:112–5. doi: 10.1111/j.1747-4477.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 13.Yesilyurt C, Er K, Tasdemir T, Buruk K, Celik D. Antibacterial activity and physical properties of glass-ionomer containing antibiotics. Oper Dent. 2009;34:18–23. doi: 10.2341/08-30. [DOI] [PubMed] [Google Scholar]
- 14.Sidhu SK. Glass ionomer cement restorative materials: A sticky subject? Aust Dent J. 2011;56(Suppl 1):23–30. doi: 10.1111/j.1834-7819.2010.01293.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrera M, Castillo A, Baca P, Carrión P. Antibacterial activity of glass-ionomer restorative cements exposed to cavity-producing microorganisms. Oper Dent. 1999;24:286–91. [PubMed] [Google Scholar]
- 16.Türkün LS, Türkün M, Ertuğrul F, Ateş M, Brugger S. Long-term antibacterial effects and physical properties of a chlorhexidine-containing glass ionomer cement. J Esthet Restor Dent. 2008;20:29–45. doi: 10.1111/j.1708-8240.2008.00146.x. [DOI] [PubMed] [Google Scholar]
- 17.Herrera M, Castillo A, Bravo M, Liébana J, Carrión P. Antibacterial activity of resin adhesives, glass ionomer and resin-modified glass ionomer cements and a compomer in contact with dentin caries samples. Oper Dent. 2000;25:265–9. [PubMed] [Google Scholar]
- 18.Massara ML, Alves JB, Brandão PR. Atraumatic restorative treatment: Clinical, ultrastructural and chemical analysis. Caries Res. 2002;36:430–6. doi: 10.1159/000066534. [DOI] [PubMed] [Google Scholar]
- 19.Bjørndal L, Larsen T, Thylstrup A. A clinical and microbiological study of deep carious lesion during stepwise excavation using long treatment intervals. Caries Res. 1997;31:411–7. doi: 10.1159/000262431. [DOI] [PubMed] [Google Scholar]
- 20.Tuzuner T, Kuşgöz A, Kursat ER, Taşdemir T, Buruk K, Kemer B. Antibacterial activity and physical properties of conventional glass-ionomer cements containing chlorhexidine diacetate/cetrimide mixtures. J Esthet Restor Dent. 2011;23:46–55. doi: 10.1111/j.1708-8240.2010.00385.x. [DOI] [PubMed] [Google Scholar]
- 21.Emilson CG. Susceptibility of various microorganisms to chlorhexidine. Scand J Dent Res. 1977;85:255–65. doi: 10.1111/j.1600-0722.1977.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 22.Block SS. 4th ed. Malvern: Lea and Febiger; 1991. Disinfection, Sterilization and Preservation; pp. 274–5. [Google Scholar]
- 23.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 24.Pinheiro SL, Simionato MR, Imparato JC, Oda M. Antibacterial activity of glass-ionomer cement containing antibiotics on caries lesion microorganisms. Am J Dent. 2005;18:261–6. [PubMed] [Google Scholar]
- 25.Al-Khatib ZZ, Baum RH, Morse DR, Yesilsoy C, Bhambhani S, Furst ML. The antimicrobial effect of various endodontic sealers. Oral Surg Oral Med Oral Pathol. 1990;70:784–90. doi: 10.1016/0030-4220(90)90022-k. [DOI] [PubMed] [Google Scholar]
- 26.Abdulkader A, Deguid R, Saunders EM. The antimicrobial activity of endodontic sealers to anaerobic bacteria. Int Endod J. 1996;29:280–3. doi: 10.1111/j.1365-2591.1996.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 27.Siqueira JF, Jr, Favieri A, Gahyva SM, Moraes SR, Lima KC, Lopes HP. Antimicrobial activity and flow rate of newer and established root canal sealers. J Endod. 2000;26:274–7. doi: 10.1097/00004770-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Botelho MG. Inhibitory effects on selected oral bacteria of antibacterial agents incorporated in a glass ionomer cement. Caries Res. 2003;37:108–14. doi: 10.1159/000069019. [DOI] [PubMed] [Google Scholar]
- 29.Yap AU, Khor E, Foo SH. Fluoride release and antibacterial properties of new generation tooth-colored restoratives. Oper Dent. 1999;24:297–305. [PubMed] [Google Scholar]
- 30.Shashibhushan KK, Basappa N, Subba Reddy VV. Comparison of antibacterial active ity of three fluorides- and zinc-releasing commercial glass ionomer cements on strains of mutans streptococci: An in vitro study. J Indian Soc Pedod Prev Dent. 2008;26(Suppl 2):S56–61. [PubMed] [Google Scholar]
- 31.Ribeiro J, Ericson D. In vitro antibacterial effect of chlorhexidine added to glass-ionomer cements. Scand J Dent Res. 1991;99:533–40. doi: 10.1111/j.1600-0722.1991.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 32.Marsh PD, Keevil CW, McDermid AS, Williamson MI, Ellwood DC. Inhibition by the antimicrobial agent chlorhexidine of acid production and sugar transport in oral streptococcal bacteria. Arch Oral Biol. 1983;28:233–40. doi: 10.1016/0003-9969(83)90152-8. [DOI] [PubMed] [Google Scholar]
- 33.Hennessey TD. Antibacterial properties of Hibitane. J Clin Periodontol. 1977;4:36–48. doi: 10.1111/j.1600-051x.1977.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 34.Choudhary S, Singh V, Chauhan PK, Tyagi A, Kumar M. In vitro antibacterial activity of Eugenia Jambolana against Streptococcus mutans causing dental plaque formation. Int J Inst Pharm Life Sci. 2011;1:91–9. [Google Scholar]
- 35.Chandana PS, Munaga S, Reddy MN, Devabhaktuni D, Swathi CL. Evaluation of compressive strength for a combination of glass ionomer cement and antibiotics. J Orofac Res. 2013;3:245–8. [Google Scholar]
- 36.Moshaverinia A, Brantley WA, Chee WW, Rohpour N, Ansari S, Zheng F, et al. Measure of microhardness, fracture toughness and flexure strength of N-vinylcaprolactam (NVC)- containing glass-ionomer cement. Dent Mater. 2010;26:1137–43. doi: 10.1016/j.dental.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Billington RW, Williams JA, Pearson GJ. Variation in powder/liquid ratio of restorative glass-ionomer cement used in dental practice. Br Dent J. 1990;169:164–7. doi: 10.1038/sj.bdj.4807311. [DOI] [PubMed] [Google Scholar]
- 38.Crisp S, Lewis BG, Wilson AD. Characterization of glass-ionomer cements. 2. Effect of the powder: Liquid ratio on the physical properties. J Dent. 1976;4:287–90. doi: 10.1016/s0300-5712(76)80008-5. [DOI] [PubMed] [Google Scholar]
- 39.Ewoldsen N, Covey D, Lavin M. The physical and adhesive properties of dental cements used for atraumatic restorative treatment. Spec Care Dentist. 1997;17:19–24. doi: 10.1111/j.1754-4505.1997.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 40.Kerby RE, Knobloch L. Strength characteristics of glass-ionomer cements. Oper Dent. 1992;17:170–4. [PubMed] [Google Scholar]
- 41.Wilder AD, Boghsian AA, Bayne SC, Heymann HO, Sturdevant JR, Roberson TM. Effect of powder/liquid ratio on the clinical and laboratory performance of resin-modified glass-ionomers. J Dent. 1998;26:369–77. doi: 10.1016/s0300-5712(97)00018-3. [DOI] [PubMed] [Google Scholar]
- 42.Sanders BJ, Gregory RL, Moore K, Avery DR. Antibacterial and physical properties of resin modified glass-ionomers combined with chlorhexidine. J Oral Rehabil. 2002;29:553–8. doi: 10.1046/j.1365-2842.2002.00876.x. [DOI] [PubMed] [Google Scholar]


