SUMMARY
Among the survivors of Ebola virus disease (EVD), complications that include uveitis can develop during convalescence, although the incidence and pathogenesis of EVD-associated uveitis are unknown. We describe a patient who recovered from EVD and was subsequently found to have severe unilateral uveitis during convalescence. Viable Zaire ebolavirus (EBOV) was detected in aqueous humor 14 weeks after the onset of EVD and 9 weeks after the clearance of viremia.
The current outbreak of EVD is believed to have begun in December 2013.1 As of April 26, 2015, a total of 26,312 cases of EVD (including 10,899 deaths) had been reported in six countries in West Africa (i.e., Sierra Leone, Liberia, Guinea, Mali, Nigeria, and Senegal), the United States, the United Kingdom, and Spain.2 The outbreak has also resulted in the largest number of EVD survivors in history.
Among survivors of EVD, late complications that include ocular disease can develop during convalescence.3,4 However, few systematic studies have been conducted on post-EVD sequelae, so the incidence and clinical manifestations of post-EVD ocular complications are unclear. Here, we report the clinical course of a man in whom severe, acute, unilateral uveitis developed during the convalescent phase of EVD. We also report the detection of viable EBOV in aqueous humor obtained from the inflamed eye 14 weeks after the onset of the initial symptoms of EVD and 9 weeks after the clearance of viremia.
CASE REPORT
A previously healthy 43-year-old male physician received a diagnosis of EVD on September 6, 2014, while he was working in an Ebola treatment unit in Kenema, Sierra Leone. He was transferred to Emory University Hospital in Atlanta and arrived 4 days after the onset of symptoms. He was treated with an experimental small interfering RNA antiviral agent (TKM-100802, Tekmira Pharmaceuticals), convalescent plasma, and aggressive supportive care.5
The hospital course was complicated by multiorgan system failure requiring mechanical ventilation for 12 days and hemodialysis for 24 days.6 After extubation, the patient had altered mental status, difficulty walking related to severe proximal weakness and deconditioning, and extreme fatigue. On day 44 of the illness, hemodialysis was no longer required and his mental status had markedly improved, with some residual mild word-finding difficulty. Ambulation was limited to short distances because of exertional fatigue. Blood and urine tested negative for EBOV on quantitative reverse-transcriptase–polymerasechain-reaction (RT-PCR) assay on serial specimens, and he was discharged home. A semen sample obtained on the day of discharge was positive for EBOV RNA on quantitative RT-PCR assay, and EBOV was isolated from semen by means of culture at the Centers for Disease Control and Prevention (CDC).7 The patient was advised to abstain from sex or to use condoms for at least 3 months.8 Longitudinal monitoring of semen specimens for EBOV is ongoing.
After discharge, 10 weeks after the onset of EVD symptoms, the patient’s word-finding difficulty and exercise tolerance were markedly improved, but he had new symptoms, including low back pain involving the right lumbar and sacroiliac region, bilateral enthesitis of the Achilles’ tendon, and paresthesias involving the distal lower limbs. Ophthalmic symptoms, which began shortly after discharge from the hospital, included occasional bilateral ocular burning, foreign-body sensation, and photophobia. He required an adjustment in his prescription for reading glasses, which suggested an accommodative change. His ocular history was clinically significant only for myopia. He was referred to the Emory Eye Center for further evaluation.
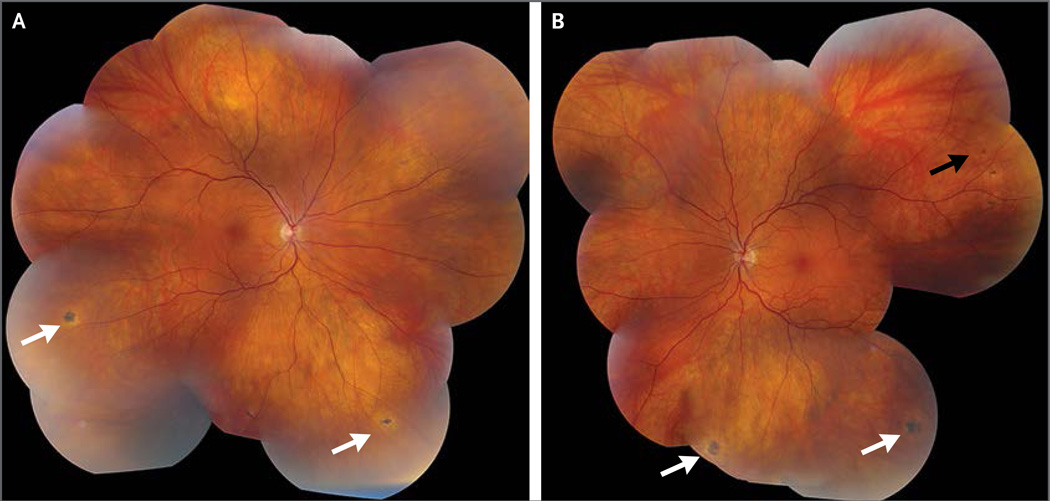
On initial evaluation in November 2014, the patient’s visual acuity was 20/15 bilaterally while wearing eyeglasses. Intraocular pressure, pupils, ocular motility, and confrontational visual fields were normal. The examination of the anterior eye by means of slit lamp was normal. The examination of the dilated posterior eye revealed previously undocumented multiple, peripheral chorioretinal scars with hypopigmented halos in both eyes and a small intraretinal hemorrhage adjacent to one scar in the left eye (Fig. 1). He received the diagnosis of posterior uveitis (i.e., chorioretinitis), a likely sequela of EVD. Close clinical follow-up was planned.
Figure 1. Montage Fundus Photographs 10 Weeks after the Onset of Ebola Virus Disease.
Multiple peripheral chorioretinal scars with hypopigmented haloes are visible in the right eye (Panel A) and left eye (Panel B) (white arrows). A small intraretinal hemorrhage (black arrow) is adjacent to a chorioretinal scar in the left eye.
One month later, 14 weeks after the diagnosis of EVD, he presented with an acute onset of redness, blurred vision with halos, pain, and photophobia in the left eye. Visual acuity was measured at 20/15 in the right eye and 20/20 in the left eye. The left intraocular pressure was highly elevated at 44 mm Hg (normal value, 10 to 21). Slit-lamp examination of the left eye showed conjunctival injection, mild corneal edema, rare nongranulomatous keratic precipitates, and grade 1+ leukocytes and protein (flare) in the anterior chamber (Fig. 2). Examination of the anterior chamber with gonioscopy indicated no signs of angle closure. Dilated funduscopic examination showed stable chorioretinal scars in both eyes with no other signs of ocular inflammation. He received a diagnosis of anterior uveitis and ocular hypertension in the left eye. Treatment was started with eyedrops containing 1% prednisolone acetate four times daily and with ocular hypotensive agents including acetazolamide (at a dose of 500 mg orally twice daily), eyedrops containing 0.2% brimonidine twice daily, and eyedrops containing 2% dorzolamide and 0.5% timolol twice daily. Results of laboratory testing, including measurement of the erythrocyte sedimentation rate and C-reactive protein, were normal; rapid plasma reagin testing and serologic analysis for Toxoplasma gondii were negative.
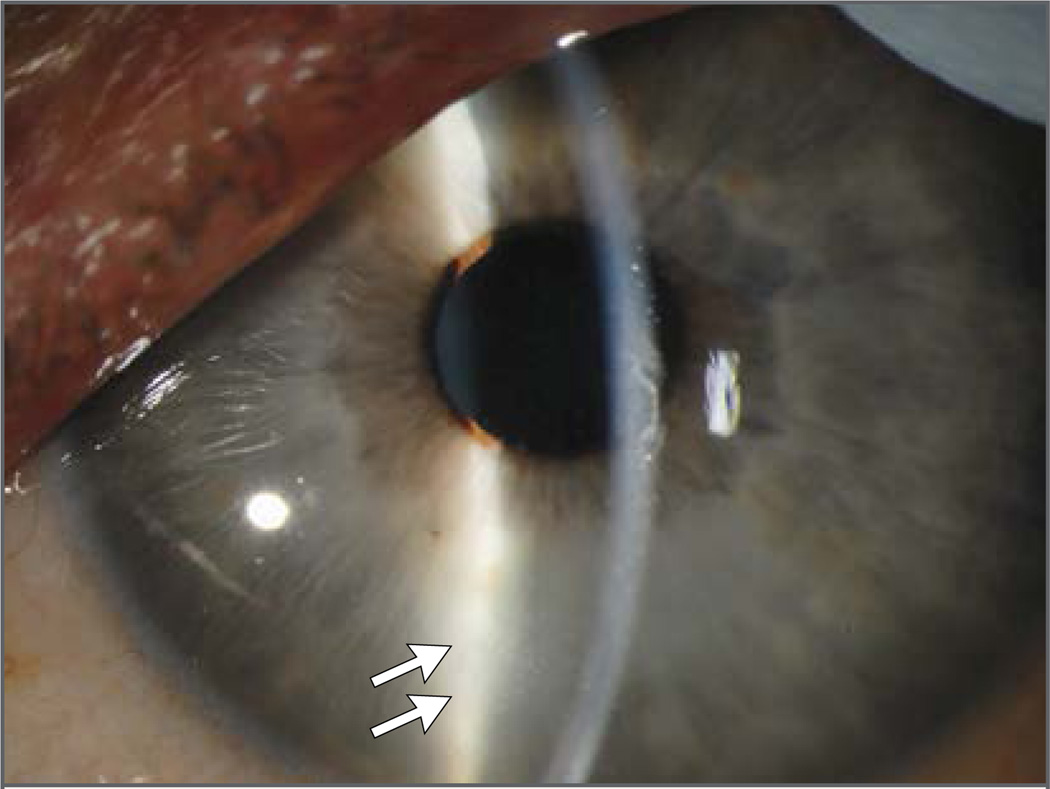
Figure 2. Slit-Lamp Photograph of the Left Eye 14 Weeks after the Onset of Ebola Virus Disease.
Mild corneal edema, rare keratic precipitates (arrows), and inflammatory cells and protein in the anterior chamber are consistent with acute anterior uveitis.
Because of escalating anterior-chamber inflammation and worsening symptoms during the subsequent 48 hours, the frequency of administration of prednisolone acetate was increased to every 2 hours, and eyedrops containing 1% atropine twice daily were added. After another 24 hours of increasing inflammation and concern about an infectious cause, paracentesis of the anterior chamber was performed, with aspiration of 170 µl of aqueous humor through a sterile 30-gauge needle while the practitioner was wearing gloves and a surgical mask. The specimen was double-bagged and delivered to a dedicated laboratory at Emory University Hospital for testing samples from patients with EVD. Testing was performed by clinical laboratory technologists who were trained in the safe handling of infectious pathogens and with the use of the standard institutional operating protocols.9 The aqueous humor tested positive for EBOV RNA on quantitative RT-PCR assay, with a cycle threshold value of 18.7. EBOV was isolated from this specimen by means of a viral culture performed at the CDC.7 A conjunctival swab obtained before the procedure and tear-film specimens collected before the procedure and 24 hours after the procedure tested negative for EBOV RNA on quantitative RT-PCR assay. In addition, a specimen of peripheral blood tested negative for EBOV RNA on quantitative RT-PCR assay. Preliminary analyses of EBOV sequenced from blood during the patient’s hospitalization for symptomatic EVD, as compared with EBOV sequenced from ocular fluid, identified a single nonsynonymous mutation, as well as two silent mutations and two mutations in noncoding regions. The significance of these mutations is unknown. However, these findings are in contrast to results that showed no changes in viral consensus sequences acquired over several days from a single patient.10 All personal protective equipment and materials that were used during paracentesis and laboratory testing were sterilized by means of autoclaving before disposal.11
The uveitis continued to progress; by 5 days after the onset of symptoms, visual acuity in the left eye was decreased to 20/60. Anterior-segment examination revealed scleritis and persistent anterior uveitis. Intermediate uveitis (i.e., vitritis, with grade 0.5+ haze) was noted on examination of the dilated posterior segment. Oral prednisone (at a dose of 1 mg per kilogram of body weight per day) was started. Ophthalmic drops of 1% prednisolone acetate every 2 hours, 0.5% timolol twice daily, and 1% atropine twice daily were continued in the left eye.
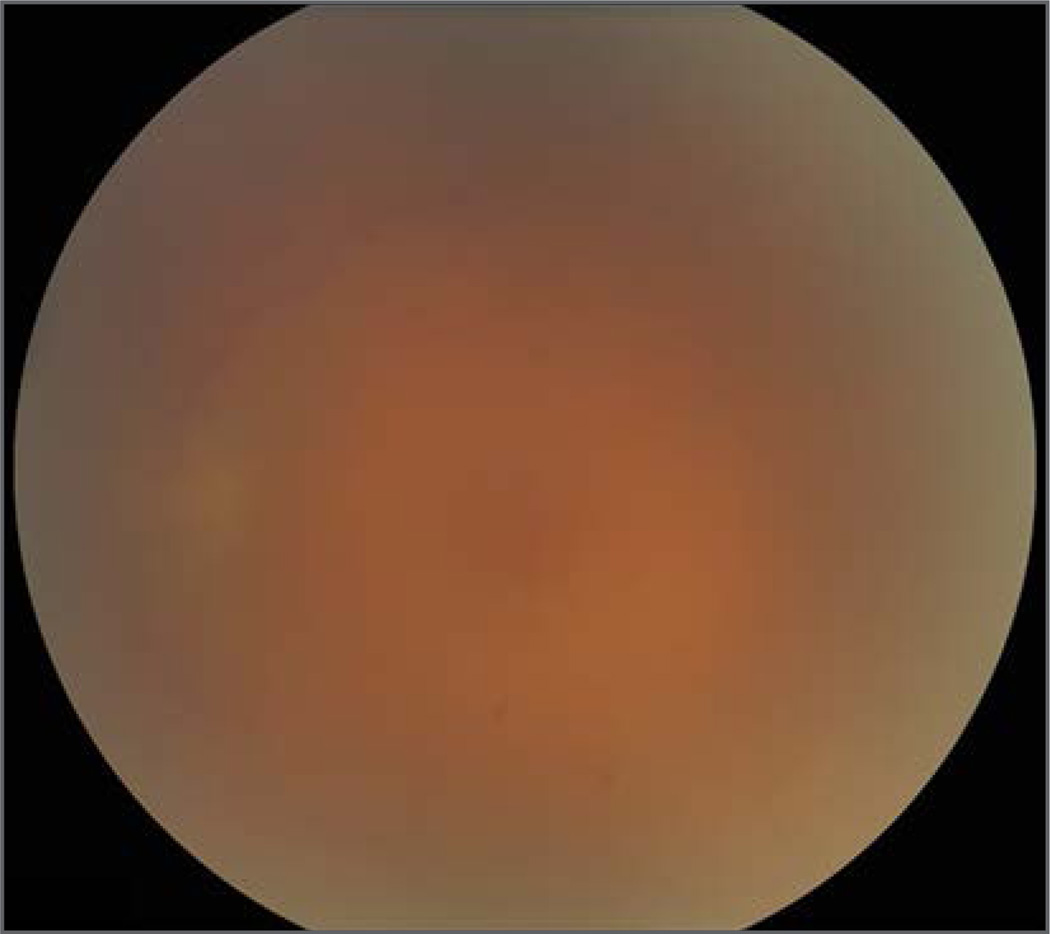
During the subsequent 72 hours, the patient’s condition improved, with resolution of the scleritis and a decrease in the anterior uveitis. Despite this improvement in the anterior-segment inflammation, the vitritis worsened, resulting in a decrease in visual acuity to 20/400 in the left eye at 1 week (Fig. 3). Continued clinical deterioration of the patient’s left eye prompted the initiation of treatment with topical difluprednate (Alcon Laboratories), a 21-day course of oral favipiravir (MediVector), a periocular injection of oral prednisone. On examination 3 months after presentation with uveitis, the vitritis was resolving and left visual acuity had recovered to 20/15. Follow-up ophthalmic evaluations are ongoing.
Figure 3. Fundus Photograph of the Left Eye 14 Weeks after the Onset of Ebola Virus Disease.
Severe vitritis is indicated by the obscuration of the optic nerve and blood vessels.
DISCUSSION
We describe the detection of viable EBOV in the aqueous humor of the eye in a patient in recovery from EVD with acute panuveitis (a combination of anterior, intermediate, and posterior uveitis). In a previous EVD outbreak, EBOV RNA was detected on RT-PCR assay in a conjunctival sample obtained from a 25-year-old patient 22 days after the onset of symptoms and 10 days after viremia had cleared. In that patient, 45 days after the onset of EVD symptoms, posterior uveitis developed; the patient did not undergo any additional testing of ocular tissue.4 Marburg virus, a filovirus like EBOV, has also been associated with uveitis during convalescence. In 1975, Marburg virus was cultured from the aqueous humor of a patient with acute anterior uveitis that developed nearly 3 months after the onset of acute illness.12 Viral culture of the patient’s aqueous humor that was sampled 2 weeks later was negative.13
Although the pathogenesis of EVD-associated uveitis is unknown, we believe that the severe, acute panuveitis that developed in our patient was a direct cytopathic effect of active replication of EBOV persisting in an immune-privileged organ. The acute onset of symptoms, unilateral location, and extreme elevation of intraocular pressure that were seen in our patient are clinical findings similar to infectious uveitis syndromes caused by herpesviruses, in which the pathogenesis is known to be a direct consequence of active viral replication.14,15 Although the relative contribution of lytic viral infection, as compared with immunologic reaction to EBOV, in the pathogenesis of our patient’s aggressive panuveitis is unclear, the low cycle threshold on quantitative RT-PCR assay shows that a high burden of viable EBOV was present at the time that the patient’s ocular symptoms were worsening. Further studies to investigate the mechanisms responsible for the ocular persistence of EBOV and the possible presence of the virus in other immune-privileged sites (e.g., in the central nervous system, gonads, and articular cartilage) are warranted.
Few systematic studies have examined post-EVD sequelae, so the incidence and clinical manifestations of post-EVD ocular complications are uncertain. Of 71 EVD survivors from the 1995 Ebola outbreak in the Democratic Republic of Congo, 20 were enrolled in a small, retrospective study.4 Three of the 20 survivors in this limited sample were found to have evidence of uveitis (anterior, posterior, or panuveitis) that occurred 42 to 72 days after the onset of EVD. Data on the incidence of ocular complications among survivors of the current West African EVD outbreak are also limited. Although 40% of participants in a recent survey of 85 EVD survivors in Sierra Leone reported having “eye problems,” the incidence of uveitis in this cohort is unknown.16
In conclusion, our patient’s recovery from EVD was complicated by acute anterior uveitis, which rapidly evolved into a sight-threatening panuveitis with detection of persistent EBOV within the eye. This case highlights an important complication of EVD with major implications for both individual and public health that are immediately relevant to the ongoing West African outbreak. It is reassuring that samples of conjunctivae and tears tested negative for EBOV, a finding that supports previous studies suggesting that patients who recover from EVD pose no risk of spreading the infection through casual contact.3,17 Further studies are needed to assess the persistence of EBOV during convalescence, to elucidate the mechanisms underlying this persistence in ocular and other immune-privileged tissue sites, and to identify effective treatment strategies for the clinical management of EVD complications.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Supported by a grant from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000454, to the Atlanta Clinical and Translational Science Institute), an unrestricted grant from Research to Prevent Blindness and a grant from the National Eye Institute (P30-EY06360, to the Department of Ophthalmology, Emory University School of Medicine), and a fellowship grant from the Australian Research Council (FT130101648, to Dr. Smith). Favipiravir was provided by the Department of Defense Joint Project Manager Medical Countermeasure Systems.
We thank Alison Boess and the family of Dr. Ian Crozier; staff members at the Biotechnology Core Facility Branch of the CDC for their assistance in generating viral sequences; Dr. William Bornstein, Dr. Bryce Gartland, Crystal Evans, Maureen Lindsey, Brian Frislie, Emily Beck, Connie Wilbanks, Paula DesRoches, and all the investigators at the Emory Serious Communicable Diseases Unit; Kimberly Lovitt, Rhonda Waldron, Debora Jordan, Jannah Dobbs, Matthew Raeber, and the staff at the Department of Ophthalmology at Emory University; Dr. Monica Farley and the staff at the Division of Infectious Diseases at Emory University; and MediVector.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Global alert and response: Ebola virus disease. Geneva: World Health Organization; 2014. ( http://www.who.int/csr/don/archive/disease/ebola/en). [Google Scholar]
- 2.Ebola situation report - 29 April 2015. Geneva: World Health Organization; 2015. ( http://www.who.int/csr/disease/ebola/situation-reports/en/?m=20141231). [Google Scholar]
- 3.Kibadi K, Mupapa K, Kuvula K, et al. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J Infect Dis. 1999;179(Suppl 1):S13–S14. doi: 10.1086/514288. [DOI] [PubMed] [Google Scholar]
- 4.Sierra Leone: helping the Ebola survivors turn the page. Geneva: World Health Organization; 2014. ( http://www.who.int/features/2014/post-ebola-syndrome/en). [Google Scholar]
- 5.Kraft CS, Hewlett AL, Koepsell S, et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015 Apr 22; doi: 10.1093/cid/civ334. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor MJ, Jr, Kraft C, Mehta AK, et al. Successful delivery of RRT in Ebola virus disease. J Am Soc Nephrol. 2015;26:31–37. doi: 10.1681/ASN.2014111057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S170–S176. doi: 10.1086/514291. [DOI] [PubMed] [Google Scholar]
- 8.Ebola (Ebola virus disease): Q&As on transmission. Atlanta: Centers for Disease Control and Prevention; 2015. ( http://www.cdc.gov/vhf/ebola/transmission/qas.html). [Google Scholar]
- 9.Hill CE, Burd EM, Kraft CS, et al. Laboratory test support for Ebola patients within a high-containment facility. Lab Med. 2014;45(3):e109–e111. doi: 10.1309/LMTMW3VVN20HIFS. [DOI] [PubMed] [Google Scholar]
- 10.Gire SK, Goba A, Anderson KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345:1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebola (Ebola virus disease): Ebola-associated waste management. Atlanta: Centers for Disease Control and Prevention; 2015. ( http://www.cdc.gov/vhf/ebola/hcp/medical-waste-management.html). [Google Scholar]
- 12.Gear JS, Cassel GA, Gear AJ, et al. Outbreake of Marburg virus disease in Johannesburg. Br Med J. 1975;4:489–493. doi: 10.1136/bmj.4.5995.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuming BS, Kokoris N. Uveal involvement in Marburg virus disease. Br J Ophthalmol. 1977;61:265–266. doi: 10.1136/bjo.61.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amano S, Oshika T, Kaji Y, Numaga J, Matsubara M, Araie M. Herpes simplex virus in the trabeculum of an eye with corneal endotheliitis. Am J Ophthalmol. 1999;127:721–722. doi: 10.1016/s0002-9394(99)00018-5. [DOI] [PubMed] [Google Scholar]
- 15.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;145:834–840. doi: 10.1016/j.ajo.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Trenchard T. Survivors cope with new Ebola after-effects. Al-Jazeera. 2014 ( http://www.aljazeera.com/news/africa/2014/12/survivors-cope-with-new-ebola-after-effects-2014121573521561384.html). [Google Scholar]
- 17.Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow- up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo: Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(Suppl 1):S28–S35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]