Abstract
Objective
Endogenous cardiotonic steroids (CTS), including marinobufagenin (MBG), stimulate vascular synthesis of collagen. Because mineralocorticoid antagonists competitively antagonize effect of CTS on the Na/K-ATPase, we hypothesized that spironolactone would reverse the pro-fibrotic effects of MBG.
Methods
Experiment 1. Explants of thoracic aortae and aortic vascular smooth muscle cells (VSMC) from Wistar rats were cultured for 24 hours in the presence of vehicle or MBG (100 nmol/L) with or without canrenone (10 µmol/L), an active metabolite of spironolactone. Experiment 2. In 16 patients (56 ± 2 yrs) with resistant hypertension (RH) on a combined (Lisinopril / amlodipine / hydrochlorothiazide) therapy, we determined arterial pressure, pulse wave velocity (PWV), plasma MBG, and erythrocyte Na/K-ATPase before and six months after addition of placebo (n=8) or spironolactone (50 mg/day; n=8) to the therapy.
Results
In rat aortic explants and in VSMC, pretreatment with MBG resulted in a two-fold rise in collagen-1, and a marked reduction in the sensitivity of the aortic rings to the vasorelaxant effect of sodium nitroprusside following endothelin-1-induced constriction (EC50=480±67 nmol/L vs. 23±3 nmol/L in vehicle-treated rings; P<0.01). Canrenone blocked effects of MBG on collagen synthesis and restored sensitivity of vascular rings to sodium nitroprusside (EC50 = 17±1 nmol/L). RH patients exhibited elevated plasma MBG (0.42 ± 0.07 vs. 0.24 ± 0.03 nmol/L; P=0.01) and reduced Na/K-ATPase activity (1.9 ± 0.15 vs 2.8 ± 0.2 µmol Pi/ml/hr, P<0.01) vs. 7 healthy subjects. Six-month administration of spironolactone, unlike placebo treatment, was associated with a decrease in PWV and arterial pressure, and with restoration of Na/K-ATPase activity in the presence of unchanged MBG levels.
Conclusion
MBG-induced vascular fibrosis is a likely target for spironolactone.
INTRODUCTION
Cardiovascular fibrosis is a hallmark of hypertension and chronic kidney disease [1,2]. Mineralocorticoid antagonists exert anti-fibrotic effects [3,4], and, in addition to blocking the effects of aldosterone, are capable to oppose effects of endogenous digitalis-like cardiotonic steroids (CTS) [5–7]. Thus, canrenone, an active metabolite of spironolactone, has reported to reduce arterial pressure in those forms of hypertension in which CTS are elevated [5,6]. CTS, including marinobufagenin (MBG) (Figure 1a), act as physiological ligands of the sodium pump and are implicated in pathogenesis of several diseases including salt-sensitive hypertension, chronic kidney disease and preeclampsia by inducing vasoconstriction [8,9] and causing cardiovascular and renal fibrosis [10,11], all effects antagonized by canrenone in rats with hypertension induced by renal failure [7]. Importantly, mechanisms underlying pro-fibrotic effects of MBG involve inhibition of Fli-1, a nuclear transcription factor which acts as a negative regulator of collagen-1 synthesis [11,12].
Figure 1.
Structure of marinobufagenin (MBG) (a) and canrenone (CAN) (b). Effect of CAN (10 µmol/L) on MBG-induced inhibition of Na/K-ATPase from rat outer medulla (c); by repeated measures ANOVA and Bonferroni test MBG vs. MBG+CAN – P<0.01. D – Effect of CAN (10 µmol/L) on inhibition of Na/K-ATPase from rat erythrocytes by MBG (100 nmol/L) (d); by one-way ANOVA and Bonferroni test: * - P<0.01 vs. control (Ctrl); # - P<0.01 vs. MBG.
The fact that mineralocorticoids antagonists can offset the effects of digitoxin in rat has been first reported by Selye [13]. Subsequently spironolactone and its active metabolite, canrenone (Figure 1b), were reported to reverse digitalis toxicity [14], and to lower blood pressure in rat hypertension models, in which levels of CTS are elevated [5,15,16]. Most recently spironolactone was reported to suppress cardiac fibrosis in rats chronically treated by MBG [7]. Notably, in this study MBG exhibited pro-fibrotic effect in the absence of changes in aldosterone levels [7]. Importantly, high levels of MBG were associated with hypertension [17], stiffening of umbilical vessels and elevated vascular level collagen-1 in preeclamptic patients, and in vitro incubation of the healthy blood vessels in the presence of low MBG concentration produced similar phenotype [18]. We hypothesized that aldosterone antagonists can also reverse MBG-induced vascular fibrosis. To test this hypothesis, in vitro, in the explants of thoracic aorta and in the cultured rat vascular smooth muscle cells (VSMC), we studied effects of canrenone on MBG-induced synthesis of collagen-1. Next, in a pilot study in patients with resistant hypertension we assessed blood pressure, vascular stiffness, plasma levels of MBG and activity of erythrocyte Na/K-ATPase before and after six-month of addition of spironolactone to the conventional antihypertensive therapy.
METHODS
General
The experimental protocol was approved by the Animal Care and Use Committee of the National Institute on Aging. Twenty four 3-month-old (380 ± 7 grams) male Wistar rats (Charles River Laboratories, Wilmington, MA, USA) after one week of adaptation to laboratory environment were anesthetized with a mixture 100 mg/mL ketaject and 20 mg/mL xylazine (1 mL/kg), and exsanguinated from the abdominal aorta. Blood and kidneys were collected for measurement of Na/K-ATPase activity in the erythrocytes and outer medulla. Thoracic aortae from 18 rats were divided into three parts and used for contractility studies, Western blotting and immunohistochemistry. Thoracic aortae from 6 rats were used for isolation and culturing of VSMC.
Erythrocyte Na/K-ATPase
Erythrocytes were washed three times in an isotonic medium (145 mmol/L NaCl in 20 mmol/L Tris buffer; pH = 7.6 at 4°C). Activity of Na/K-ATPase was determined, as reported previously in detail [8]. Erythrocytes were preincubated with Tween-20 (0.5%) in sucrose (250 mmol/L) and Tris buffer (20 mmol/L; pH=7.4, t=37°C) for 30 minutes, and were incubated for 30 minutes in the medium (mmol/L): Na 100, K 10, MgCl2 3, EDTA 0.5, Tris 50, ATP 2 (pH=7.4, t=37°C) in the final dilution 1:40. The reaction was stopped by the addition of trichloracetic acid to final concentration 7%. Total ATPase activity was measured by the production of inorganic phosphate (Pi), and Na/K-ATPase activity was estimated as the difference between ATPase activity in the presence and in the absence of 5 mmol/L ouabain.
Renal Na/K-ATPase
The in vitro ability of canrenone to reverse MBG-induced Na/K-ATPase inhibition has been studied in membranes purified from rat renal outer medulla, as described recently in detail [17]. Medullary slices were homogenized in a solution containing (in mmol/L) sucrose 250, histidine 30, imidazole 5, EDTA 1 (4°C; pH 7.4), and then centrifuged (6,000 g, 15 min, 4°C). The supernatant was respun at 15,000 g for 30 min at 4°C, and the resultant supernatant centrifuged at 148,000 g for 90 min at 4°C. The pellet (membranes) was suspended in a homogenizing medium, applied to discontinuous sucrose gradients, consisting of 0.32 – 1.2 M layers of sucrose buffered with 30 mM histidine and 5 mM imidazole (pH 7.4), and centrifuged at 148,000 g for 90 minutes. The band appearing at the 0.8 M layer was aspirated, re-sedimented at 148,000 g for 90 minutes. Final pellet was re-suspended in a homogenizing medium to a protein concentration 3–4 mg/ml, and stored in liquid nitrogen. Na/K-ATPase activity was determined as reported recently in detail [17].
Isolated rat aorta contractile studies
Explants of rat thoracic aortae were placed in Dulbecco's Modified Eagle Medium (DMEM; Life Technologies, Karlsbad, CA) supplemented with gentamicin (25 mg/L). Explants were incubated for 24 h in a 5% CO2 atmosphere at 37°C in the presence of vehicle (control), MBG (100 nmol/L), canrenone (10 µmol/L), and combination of MBG and canrenone. Explants were washed 3 times in the fresh incubation media. Endothelium-denuded rings of rat thoracic aortae (2 mm) were suspended at a resting tension of 1.0 g in a 15-mL organ bath (Ugo Basile, Italy) and superfused at 37°C with a solution containing in mmol/L: NaCl 130, KCl 4.0, CaCl2 1.8, MgCl2 1.0, NaH2PO4 0.4, NaHCO3 19, and glucose 5.4, and gassed with a mixture of 95% O2 and 5% CO2 (pH 7.45), and isometric contractions were recorded as reported previously [17]. The aortic rings were constricted twice with 80 mmol/L of KCl. Next we studied the vasorelaxation of aortic rings by sodium nitroprusside (1 nmol/L – 10 µmol/L) following constriction of vascular rings with 100 nmol/L endothelin-1, as described previously [17]. The force of contractions was expressed as the percent of vasoconstrictor response to 80 mmol/L KCl. The percent of relaxation was calculated relative to the plateau of contractile force that was achieved in response to 100 nmol/L endothelin-1.
Western blot analyses of Fli-1 and collagen-1
Following the 24-hr preincubation of aortic explants with MBG, canrenone and combination of both compounds, segments of thoracic aortae were homogenized in lysis RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Western Blot analysis was performed as previously reported [18]. Solubilized proteins from aortic sarcolemma was separated by 10% Tris-Glycine polyacrylamide gel (Life Technologies) electrophoresis and transferred to a nitrocellulose membrane. Bands were visualized by 1–5 minute exposure of nitrocellulose membrane on Kodak SAR5 film, and optical density was quantified. Goat anti-type1 collagen antibody (Southern Biotech, Birmingham, AL, USA) was used to probe for collagen-1, with secondary anti-goat antibody from Santa Cruz Biotechnology. To probe for Fli-1, we used rabbit polyclonal anti-Fli1 (C19) antibody (Santa-Cruz Biotechnology; 1:500) and peroxidase-conjugated anti-rabbit antiserum (Life Technologies, 1:1000). For protein detection, we used ECL and ECL plus (Life Technologies). To normalize levels of proteins against levels of glyceraldehydes-3-phosphate dehydrogenase (GAPDH), membranes were stripped and reprobed with a rabbit monoclonal anti-GAPDH antibody (Cell Signaling Technology, Danvers, MA, USA).
Histochemistry
Aortic rings were fixed in 4% formalin-buffered solution (pH 7.2) for 20 hours, and dehydrated in 70% ethanol. Tissues were embedded in paraffin and 6 µm sections were cut using a microtome. Sirius red staining (American MasterTech Scientific, Inc., Lodi, CA) was carried out to determine the extent of fibrosis by staining collagen type I and III fibers in red color.
VSMC preparation
VSMC from six rat aortae were prepared according to the previous publication [19] with some modifications. Briefly, aortae were incubated with collagenase type I (Worthington Biochemical Corporation, Lakewood, NJ) in HBSS buffer (Hank’s balanced salt solution; Life Technologies, Grand Island, NY) with antibiotic mixture (penicillin and streptomycin with addition of antibiotic-antimycotic mixture; Life Technologies) at 37°C for 30 min. Then adventitia and endothelial cells were removed, and the resulting tissues were incubated in DMEM (Life Technologies) with 10% FBS (fetal bovine serum; Life Technologies), and antibiotic mixture at 37°C overnight. Next the tissues were minced with scissors and incubated for 1.5 hours with elastase (0.5 mg/ml; Sigma, St. Louis, MO) and collagenase type II (Worthington Biochemical Corporation) in HBSS buffer with antibiotics. Then the digestion was stopped by adding DMEM with 10% FBS and antibiotics. The cells were filtered through a 70 um cells strainer, collected by centrifugation, and plated on collagen covered tissue culture plate in DMEM with 10% FBS with antibiotics.
VSMC prior to fifth passage (~ 90% confluence) were used for 24 hours incubation with MBG (1, 10, or 100 nmol/L), canrenone (10 µmol/L; Spectrum MFG Corp, New Brunswick, NJ), or mixture of both compounds. After 24 hours VSMC were washed twice in PBS (phosphate buffered saline; Life Technologies) and fixed with 4% paraformaldehyde (Sigma) in PBS for 20 min, then permeabilized with 0.2% TritonX-100 (Sigma) for 15 min. Cells were washed 3 times with PBS then blocked with 1% BSA (bovine serum albumin, Life Technologies) in PBS for 1 hour. VSMC were incubated with primary rabbit polyclonal anti-collagen-1 antibody (1:250; Novus Biological, Littleton, CO) at 4°C overnight. Next VSMC were incubated with secondary fluorescent antibody (1:1000; Alexa fluor 488 donkey anti-rabbit IgG (H+L); Life Technologies) for 1 hour a room temperature following by 15 min incubation with 1µg/ml DAPI (4',6-diamidino-2-phenylindole; Thermo Scientific, Waltham, MA). Immunofluorescent images were obtained by EVOS® FL Cell Imaging System (Life Technologies). Collagen-1 abundance as a green fluorescence intensity was measured by MetaMorph 7.8.0.0 (Molecular Devices, Sunnyvale, CA) in each image (seven-eight images for each condition), and presented as a fluorescence intensity per cell.
MBG immunoassay
Plasma samples were extracted on Sep-Pak C-18 cartridges (Waters, Milford, MA, USA), and MBG competitive fluoroimmunoassay based on a murine monoclonal anti-MBG 4G4 antibody was performed as described recently [17]. The cross-reactivity of 4G4 anti-MBG antibody is: MBG - 100%, ouabain – 0.005%, digoxin – 0.03%, digitoxin < 0.001%, bufalin – 0.08%, cinobufagin – 0.07%, cinobufatalin – 40%, prednisone, spironolactone, aldosterone, proscillaridin, and progesterone < 0.001%.
Clinical data and hemodynamic measurements
The study was approved by Almazov Federal Centre Ethic Committee (St. Petersburg, Russia) and by Medstar Institute IRB (Washington, DC), and the informed consent was obtained from all participants. Sixteen consecutive patients with resistant hypertension (7 males and 9 females, mean age 56±2 years) from the Outpatient Resistant Hypertension Centre of the Almazov Federal Heart, Blood and Endocrinology Centre were enrolled. The inclusion criteria were: essential hypertension with duration over 5 years, stable three drug full dose antihypertensive therapy, including diuretic, with office blood pressure greater than 140/90 mmHg. Patients were examined to exclude secondary hypertension. Exclusion criteria were: concomitant coronary artery disease, previous stroke or transient ischemic attacks, known poor treatment adherence, drug abuse, and body mass index over 35 kg/m2. Patients were administered a standard three-drug antihypertensive therapy (ACE inhibitor lisinopril 20 mg/day, calcium channel blocker amlodipine 10 mg/day, and diuretic hydrochlorothiazide 25 mg/day). The diagnosis of resistant hypertension was reconfirmed after two weeks. Patients were examined before and six months after the addition of placebo (4 males and 4 females, n=8) or spironolactone (50 mg/day; 3 males and 5 females, n=8) to the conventional therapy. The control group included 7 age-matched normotensive subjects (4 males and 3 females, mean age 50±3 years).
Blood pressure was measured by finger plethysmography (Finometer; TNO, Amsterdam, Netherlands) of the right index or middle finger intermittently recalibrated against oscillometry. Pulse wave velocity (PWV) was calculated using the SphygmoCor System (Vx Pulse Wave Velocity), (AtCor Medical, West Ryde, Australia) with a subject in a supine decubitus position. Carotid and femoral pulse wave was analyzed, estimating the delay in the electrocardiographic wave and calculating the PWV. The distance from the suprasternal notch to the carotid and femoral arteries at the sensor location was estimated with measuring tape. Ten ml of venous blood was collected before and after addition of placebo or spironolactone to conventional therapy. Blood was used for standard clinical analyses.
Statistical analyses
Data are presented as means ± SEM (Standard Error of the Mean). Statistical analyses utilized two-tailed t-test or one-way ANOVA followed by a post-hoc t-test utilizing Newman-Keuls correction for multiple comparisons. A two-sided P value of less than 0.05 was considered to be statistically significant (GraphPad Instat and GraphPad Prism, GraphPad Software Inc., San Diego, CA).
RESULTS
Data illustrating effect of an active metabolite of spironolactone, canrenone, on inhibition of Na/K-ATPase by MBG are summarized in Figure 1c,d. MBG inhibited Na/K-ATPase from rat renal medulla in a concentration-dependent fashion, and addition of 10 µmol/L canrenone to the incubation medium markedly reduced sensitivity of the Na/K-ATPase to MBG (IC50 = 1.9±0.5 µmol/L and 113±11 µmol/L, respectively; Figure 1c). Because activity of erythrocyte Na/K-ATPase could be a marker for circulating MBG activity [8,17], we next tested Na/K-ATPase inhibitory effect of MBG in the absence and in the presence of canrenone in rat erythrocytes in vitro. As presented in Figure 1d, canrenone alone did not alter Na/K-ATPase activity, but reversed the inhibitory effects of 100 nmol/L MBG.
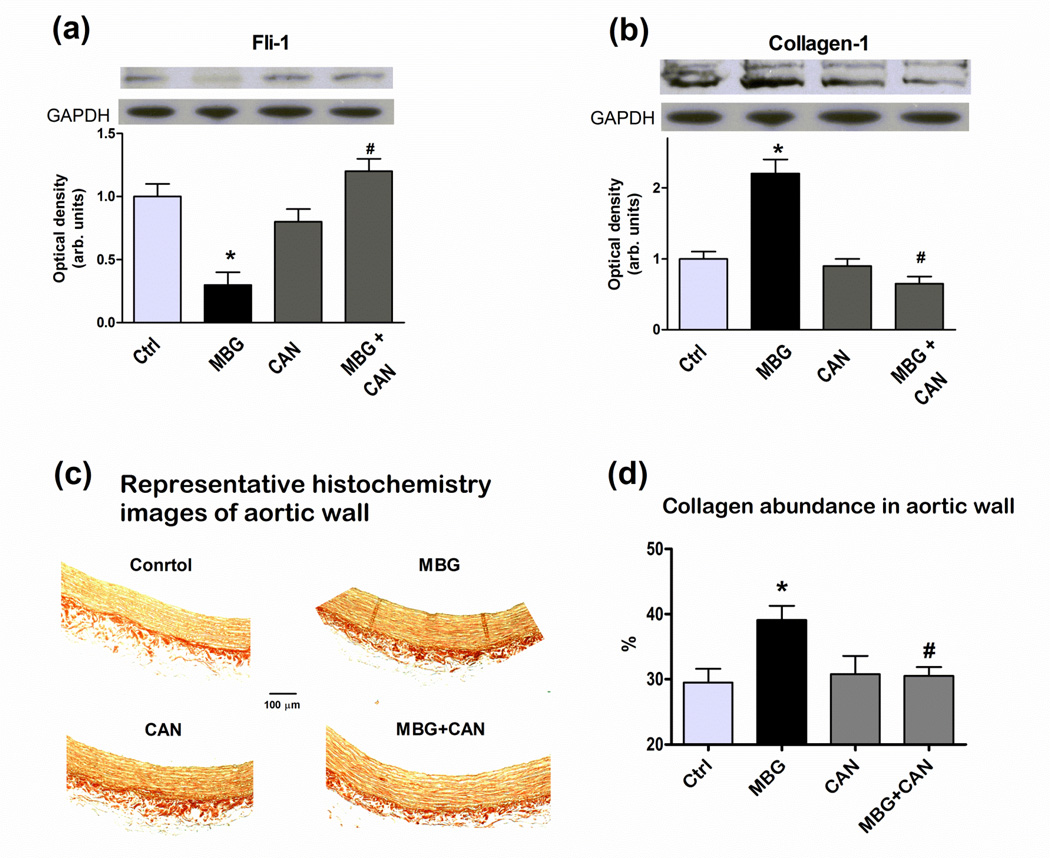
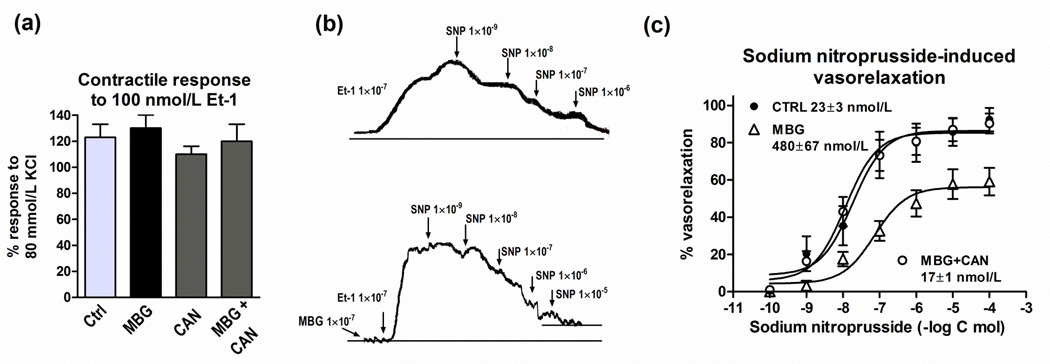
Data on the effect of MBG on Fli-1 and collagen-1 levels in rat aortic explants, in the presence and in the absence of canrenone, are presented in Figure 2. Incubation of aortic rings for 24 hours in the presence of 100 nmol/L MBG resulted in a reduction in the levels of Fli-1, a concomitant increase in the levels of collagen-1, and an increase in vascular levels of fibrosis assessed by Sirius red staining (collagen is stained red; Figure 2c). While canrenone alone did not significantly alter aortic levels of Fli-1, collagen-1, and fibrosis, in the presence of canrenone MBG failed to inhibit Fli-1 and to increase aortic levels of collagen-1 and fibrosis (Figure 2). Data on the vasorelaxation of rat aortic explants, preconstricted with 100 nmol/L endothelin-1, are summarized in Figure 3. As shown in Figure 3a, aortic rings, preincubated with vehicle, MBG, canrenone, or combination of MBG and canrenone, exhibited similar sensitivity to submaximal (100 nmol/L) concentration of endothelin-1. At the same time, in MBG-pretreated aortic rings with increased collagen abundance (Figure 2), which were precontracted with 100 nmol/L endothelin-1, responsiveness to the relaxant effect of sodium nitroprusside was markedly reduced (EC50 = 480±67 nmol/L) as compared to rings pretreated with vehicle (EC50 = 23±3 nmol/L, p<0.01) (Figure 3b,c). After pretreatment of blood vessels with MBG in the presence of canrenone, sensitivity of aortic rings to sodium nitroprusside was the same as in vehicle pretreated tissue (EC50 = 17±1 nmol/L) (Figure 3c). Pretreatment of intact aortic rings with canrenone did not affect their ability to vasorelax in response to sodium nitroprusside (EC50 = 28±2 nmol/L).
Figure 2.
Effect of 24-hour incubation of rat aortic explants with canrenone (CAN), MBG and their combination on levels Fli-1 (a), collagen-1 (b), and fibrosis assessed by Sirius red staining (c,d) where collagen types I and III fibers are stained red. Upper panels are representative immunoblots; lower panels are mean ± SEM from six immunoblots (a,b). Representative histochemistry images (c), mean ± SEM from six-seven images of aortic explants for each condition (d). By one-way ANOVA and Bonferroni test: * - P<0.01 vs. control (Ctrl); # - P<0.01 vs. MBG.
Figure 3.
Effect of MBG (100 nmol/L), canrenone (CAN)(10 umol/L) and their combination on responsiveness of rat aortic rings to 100 nmol/L endothelin-1 (Et-1) (a), and on sodium nitroprusside-induced vasorelaxation following contraction induced by 100 nmol/L Et-1, representative recordings (b). Concentration-response curves determined 5–8 times and averaged to give means ± SEM for EC50 (c). By repeated measures ANOVA and Bonferroni test: control (CTRL) vs. MBG – P<0.01; MBG+CAN vs. MBG – P<0.01.
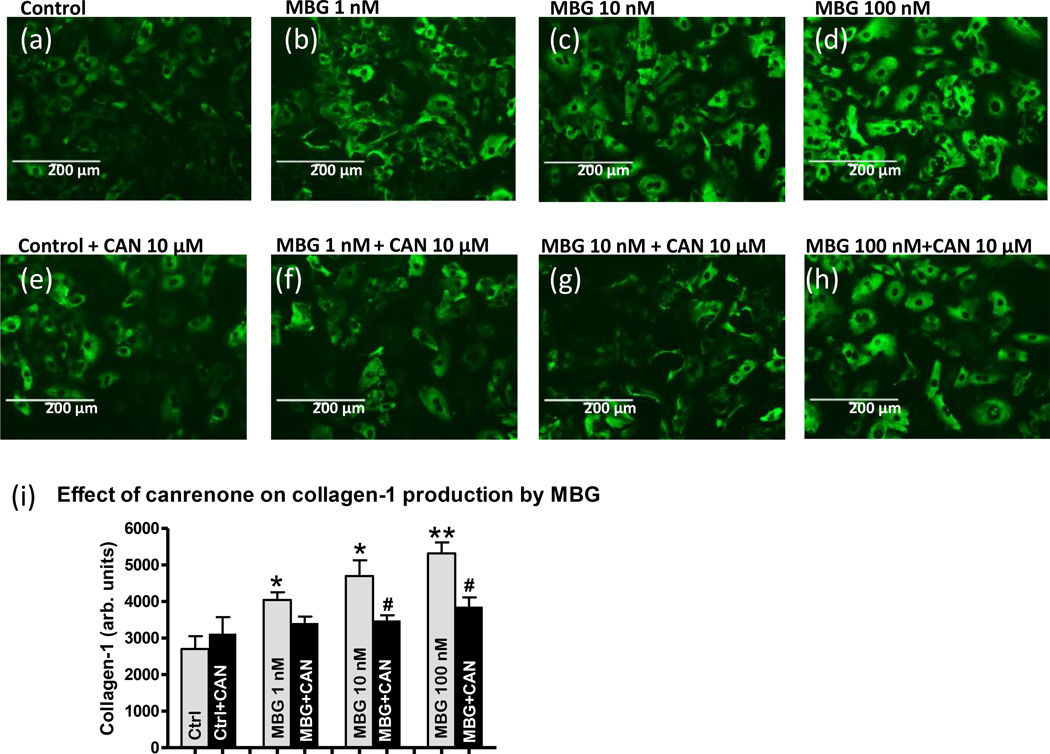
Data on the effect of MBG on collagen production by rat aortic VSMC in primary cell culture are presented in Figure 4. The representative histochemistry images of VSMC are shown in Figure 4a–h. Collagen-1, which is shown by antibody staining (green fluorescence), dose-dependently increased after 24 hours of incubation of VSMC with various MBG concentrations (Figure 4a–d and i). Incubation of VSMC with MBG in the presence of canrenone (10 µmol/L) significantly decreased collagen-1 abundance (Figure 4e–f and i) compared to MBG-treated VSMC, i.e. canrenone reversed collagen production initiated by MBG (Figure 4a–d and i). Canrenone alone did not change collagen abundance (Figure 4e and i).
Figure 4.
Collagen-1 abundance in the primary culture of rat aortic vascular smooth muscle cells, pretreated with various (0, 1, 10, 100 nmol/L) concentrations of MBG for 24 hours in the absence of canrenone (a–d), or in the presence of 10 µmol/L canrenone (CANR) (e–h). Collagen-1 is shown by anti-collagen-1 antibody staining (green fluorescence) on the representative histochemistry images (a–h). Grey bars represents collagen-1 amount in the presence of MBG vs. control; black bars represents combined treatment of MBG and canrenone; each bar represents mean ± SEM of green fluorescence intensity (collagen-1) per cell from seven-eight histochemistry images for each condition (i). By one-way ANOVA and Newman-Keuls test: *- P<0.05 vs. control; **- P<0.01 vs. control; # - P<0.05 MBG+CANR vs. MBG.
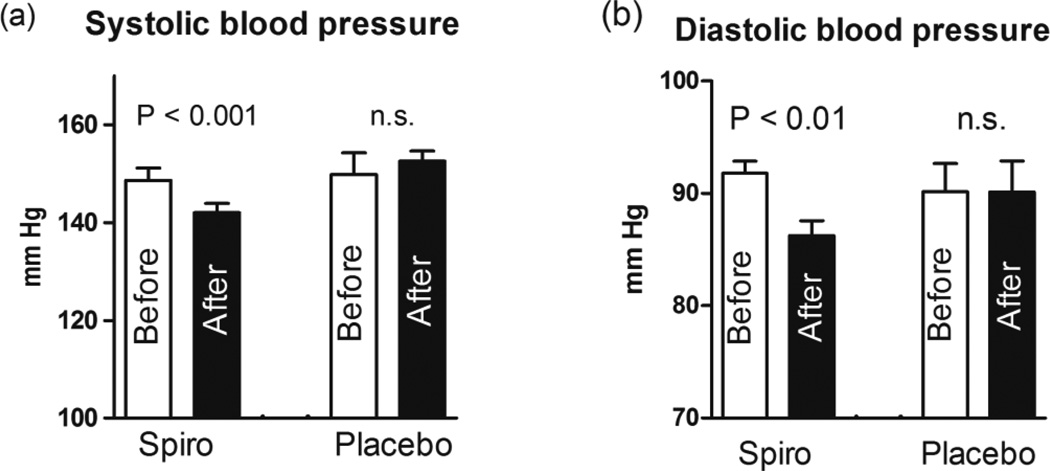
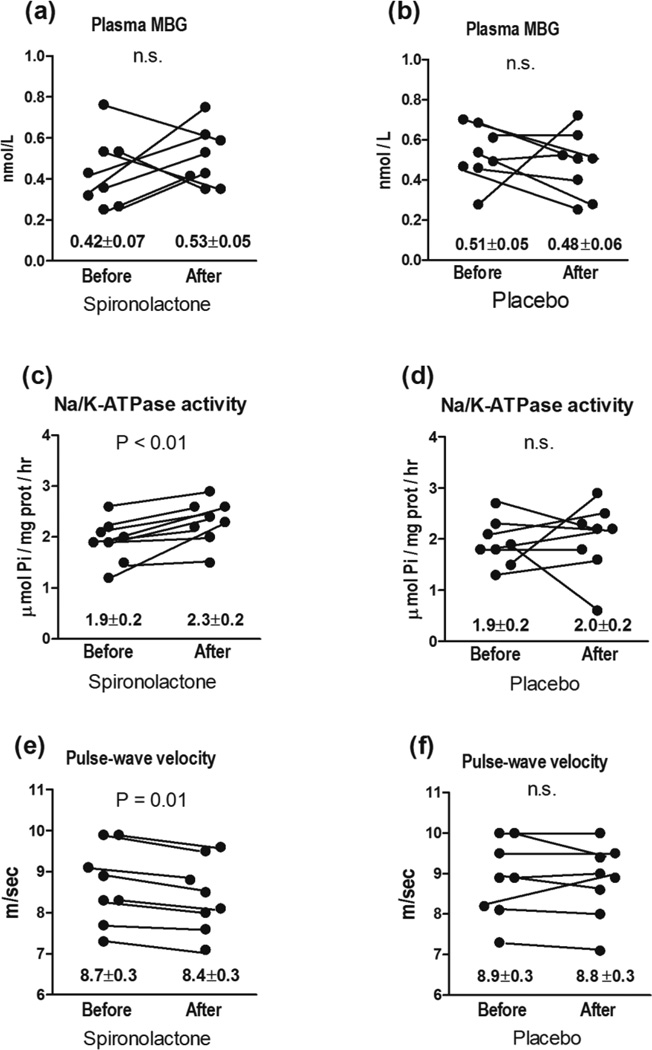
Pilot clinical data on the levels of arterial pressure, PWV, and erythrocyte Na/K-ATPase activity before and six month after administration of spironolactone or placebo to patients with resistant hypertension are summarized in Table 1 and in Figures 5 and 6. Patients with resistant hypertension exhibited greater systolic and diastolic blood pressure and PWV, higher serum creatinine, reduced glomerular filtration rate, elevated plasma MBG levels, and decreased erythrocyte Na/K-ATPase activity, as compared to control subjects (Table 1). As presented in Figure 6, administration of spironolactone (50 mg/day) to hypertensive patients for six month was associated with restoration of erythrocyte Na/K-ATPase, a target enzyme for MBG, occurring in the presence of unchanged plasma levels of MBG. Spironolactone treatment also produced reduction in systolic and diastolic blood pressure (Figure 5), and modest but significant reduction in PWV (Figure 6). At the same time, placebo treatment of RH patients during six months did not have effects of blood pressure (Figure 5), and on plasma MBG, erythrocyte Na/K-ATPase and PWV (Figure 6).
Table 1.
Clinical data on the enrollment
| Control subjects (n=7) |
Patients with resistant hypertension (before treatment) |
||
|---|---|---|---|
| Spironolactone group (n=8) |
Placebo group (n=8) |
||
| Age (years) | 50 ± 3 | 55 ± 2 | 57 ± 3 |
| Gender (M/F) | 4/3 | 3/5 | 4/4 |
| BMI (kg/m2) | 27 ± 1 | 33 ± 2 | 31 ± 3 |
| 24-hour SBP (mmHg) | 125 ± 2 | 149 ± 3* | 150 ± 4* |
| 24-hour DBP (mmHg) | 75 ± 2 | 95 ± 2* | 90 ± 4* |
| PWV (m/sec) | 6.3 ± 0.6 | 8.7 ± 0.3* | 8.8 ± 0.4* |
| Heart rate (beats/ min) | 69 ± 2 | 67 ± 3 | 69 ± 2 |
| Hemoglobin (g/dL) | 148 ± 4 | 150 ± 4 | 143 ± 3 |
| Hematocrit (%) | 44 ± 1 | 44 ± 1 | 44 ± 2 |
| Serum cholesterol (mmol/L) | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.6 ± 0.2 |
| Serum creatinine (mmol/L) | 76 ± 4 | 86 ± 2* | 88 ± 2* |
| GFR (mL/min) | 94 ± 8 | 72 ± 3* | 73 ± 4* |
| Blood glucose (mmol/L) | 5.5 ±0.2 | 5.7 ± 0.2 | 5.5 ± 0.3 |
| Plasma Na (mmol/L) | 141 ± 2 | 143 ± 2 | 141 ± 2 |
| Plasma MBG (nmol/L) | 0.24 ± 0.03 | 0.42 ± 0.07* | 0.43 ± 0.09* |
| Erythrocyte Na/K-ATPase (µmol Pi/mL/hr) | 2.8 ± 0.2 | 1.9 ± 0.2* | 2.0 ± 0.1* |
BMI – body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, PWV – pulse wave velocity, GFR – glomerular filtration rate, MBG - marinobufagenin.
- P<0.05 vs. controls by ANOVA followed by Newman-Keuls test.
Figure 5.
Systolic blood pressure (a) and diastolic blood pressure (b) in patients with resistant hypertension before (Bef) and after (Aft) six months of spironolactone (Spiro) or placebo treatment. Numbers on the graphs are means ± SEM. Two-tailed paired t-test.
Figure 6.
Plasma MBG (a), erythrocyte Na/K-ATPase activity (c), and PWV (e) in patients with resistant hypertension prior to and following six-month administration of spironolactone (50 mg/day). Plasma MBG (b), erythrocyte Na/K-ATPase activity (d), and PWV (f) in patients with resistant hypertension prior to and following six-month administration of placebo. Numbers on the graphs are means ± SEM. Two-tailed paired t-test.
DISCUSSION
The main finding of our study is that ex vivo, in explants of rat aorta, canrenone, an active metabolite of spironolactone, antagonizes MBG-induced synthesis of collagen and impairment of endothelium-independent vasorelaxation. These experimental results are consistent with the present pilot clinical data demonstrating that in patients with resistant hypertension, effect of spironolactone administration on arterial blood pressure and on PWV is associated with restoration of erythrocyte activity of Na/K-ATPase, a target enzyme for circulating MBG. Notably, in spironolactone-treated patients with resistant hypertension, Na/K-ATPase was not inhibited even in the presence of elevated plasma MBG, suggesting that spironolactone desensitized sodium pumps to MBG-induced inhibition (Figure 6).
Previous studies indicated that mineralocorticoid antagonists are capable of binding to the receptor site on the Na/K-ATPase and acting as antagonists of digitalis glycosides and of endogenous CTS including MBG [6,7,20]. Accordingly, canrenone was reported to reduce blood pressure in rat hypertensive models in which plasma levels of CTS were elevated [5,15]. Subsequently, in hypertensive human subjects canrenone was shown to prevent ouabain-induced vasoconstriction [21], and to block inhibition of erythrocyte Na/K-ATPase induced by intravenous saline loading [22]. During last two decades it became obvious that contribution of CTS to pathogenesis of hypertension, chronic kidney disease and preeclampsia is not limited to induction of vasoconstriction, and that CTS induce cell signaling events resulting in hypertrophic growth, apoptosis and fibrosis [9,23–25]. Thus, elevated levels of MBG were shown to accompany development of cardiac fibrosis in uremic rats, while active immunization of partially nephrectomized rats against MBG prevented cardiac fibrosis [10]. In agreement with the in vivo results, MBG stimulated collagen synthesis in cardiac and renal fibroblasts [11]. Recently the capacity of spironolactone to reverse cardiac fibrosis has been studied in uremic and MBG-treated rats [7]. Notably, in that experiment spironolactone reversed cardiac fibrosis not only in partially nephrectomized rats, but also in MBG-treated rats, in which hypertension and cardiac hypertrophy developed in the absence of elevations in the levels of aldosterone [7]. This finding indicated that interaction between Na/K-ATPase and digitalis-like CTS could be a target for aldosterone antagonists [5,7,16] which is in agreement with our present observations.
Decrease of Fli-1, a nuclear transcription factor and a member of ETS family is implicated in MBG-induced fibrosis [11,12,18]. Fli-1 acts as a negative regulator of collagen-1 synthesis and it competes with another transcription factor, ETS-1, to maintain a balance between stimulation and repression of Col1a2 gene [26,27]. Previously, in partially nephrectomized rats we demonstrated that development of cardiac fibrosis in the presence of elevated MBG levels was associated with decrease of Fli-1 [12]. In preeclampsia, which is also associated with elevated levels of MBG, fibrosis of umbilical arteries was accompanied by a dramatic reduction in the levels of Fli-1 [18]. In uremic rats a single administration of monoclonal anti-MBG antibody markedly reduced cardiac levels of collagen-1 in parallel with an increase in the levels of Fli-1, suggesting a causative relationship between MBG and Fli-1 [12]. Accordingly, MBG downregulated Fli-1, and stimulated synthesis of collagen in rat renal and cardiac fibroblasts [11], and in the explants of human umbilical arteries [18]. Our present results support these previous observations, and for first time demonstrate that MBG-induced decrease of Fli-1 followed by initiation of the pro-fibrotic signaling in vascular tissues is reversed by mineralocorticoid antagonists. Signaling pathways underlying effect of MBG on Fli-1-dependent pro-fibrotic signaling have to be determined.
In the present experiment canrenone improved the MBG-induced impairment of endothelium-independent vasorelaxation by sodium nitroprusside and reversed MBG-dependent increase of collagen in rat aortic explants. It is likely, that the mechanism which is involved in profibrotic effect of MBG is based on the inhibition Na/K-ATPase by MBG, which initiated the pro-fibrotic Fli-1-dependent signaling [11] resulted in the tissue remodeling. Canrenone desensitizes Na/K-ATPase to the inhibitory and pro-fibrotic effects of MBG resulting in the restoration of Na/K-ATPase activity in the presence of MBG, as it was observed in rat renal medulla and erythrocytes (Figure 1) and in the clinical study (Figure 6) when heightened MBG did not inhibit Na/K-ATPase in the presence of spironolactone. Importantly, in the present study we used denuded aortic rings for the preincubation with MBG and canrenone. In the present experiment, data on the tissue remodeling induced by MBG are in accordance with the previous studies. In these studies it was demonstrated that MBG induced pro-collagen synthesis in experimental cardiomyopathy [7,10,11,12]. In the present experiment, MBG-dependent fibrotic changes in the vascular wall and VSMC were reversed by canrenone, which supports the findings of our previous experiments [7]. We have demonstrated for the first time that MBG-induced compromised vasorelaxation of rat aorta was reversed by the mineralocorticoid receptor antagonist canrenone. In a previous study [28], endothelium-independent (but not endothelium-dependent) vasorelaxation by sodium nitroprusside was impaired in the aortic rings of angiotensin-II treated mice which showed increased aortic collagen abundance and PWV. This observation is in agreement with our present results. The authors of this previous study had suggested [28] that the impaired redox signaling might be responsible for endothelium-independent dysfunction in response to angiotensin-II. We hypothesized that in addition to the signaling responsible for fibrosis, several other intracellular signaling pathways (i.e. cGMP, oxidative stress) might be altered and add to the impairment of SNP relaxation. Therefore, we assume that in our study the impaired endothelium-independent responses, induced by MBG, may not only be due to MBG-induced fibrosis, but also due to activation of other signaling pathways. Although previously endothelium-free vessels from pregnant animals demonstrated unchanged sensitivity to vasoconstrictors [29,30], it would be interesting to study the impact of endothelium on vasorelaxation in the presence of activated pro-fibrotic and other pathways.
Our present preliminary clinical data agree with experimental results and demonstrate that in patients with resistant hypertension with heightened MBG levels effect of spironolactone on blood pressure and PWV is paralleled by restoration of erythrocyte Na/K-ATPase in the presence of an unchanged MBG, indicating on the protective properties of mineralocorticoid antagonists. Interestingly, that in previous publications it was demonstrated that arterial stiffness and augmentation index were independently associated with blood pressure in uremic patients [31,32], a condition, associated with an increased MBG levels [33]. In this study [31], both endothelial-dependent flow-mediated dilatation and endothelial-independent maximal vasodilatory capacity were impaired in uremic patients compared to healthy controls. Causative relations of PWV and MBG were previously observed in a group of hypertensives under the conditions of dietary sodium restriction [34]. It was demonstrated that in these patients, dietary sodium restriction was associated with reduction of MBG excretion, and that MBG levels positively correlated to aortic PWV [34]. Thus, our experimental and clinical findings warrant further large scale clinical studies of the impact of aldosterone antagonists/MBG interactions on vascular fibrosis.
In conclusion, in the present study we have demonstrated for the first time that mineralocorticoid receptor antagonists reversed MBG-induced vascular collagen deposition which may affect relaxation of rat aorta. These data support our finding that the ability of spironolactone to reduce arterial stiffness in patients with resistant hypertension and restore their Na/K-ATPase in the presence of increased plasma MBG may be linked to a reduction in arterial collagen. Thus, MBG and MBG-induced arterial remodeling and arterial stiffness are novel targets for mineralocorticoid receptor antagonists.
ACKNOWLEDGEMENTS
Supported by Intramural Research Program, National Institute on Aging, National Institutes of Health (OVF, OJ, WW, CAM, EGL, AYB), and by a grant from Ministry of Science and Education of Russian Federation Nr. 14.740.11.0928 (IVE, YNG, AOK). The authors are grateful to Ruth Sadler for editorial assistance.
Footnotes
Conflicts of interest
Authors declare no conflicts of interest.
REFERENCES
- 1.Susic D, Frohlich ED. The aging hypertensive heart: a brief update. Nat Clin Pract Cardiovasc Med. 2008;5:104–110. doi: 10.1038/ncpcardio1091. [DOI] [PubMed] [Google Scholar]
- 2.Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2013;50:89–99. doi: 10.1159/000345243. [DOI] [PubMed] [Google Scholar]
- 3.Duprez DA. Is vascular stiffness a target for therapy? Cardiovasc Drugs Ther. 2010;24:305–310. doi: 10.1007/s10557-010-6250-z. [DOI] [PubMed] [Google Scholar]
- 4.Safar ME, Blacher J, Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease-is it possible to break the vicious circle? Atherosclerosis. 2011;218:263–271. doi: 10.1016/j.atherosclerosis.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 5.de Mendonça M, Grichois ML, Pernollet MG, Wauquier I, Trouillet-Thormann B, Meyer P, Devynck MA, Garay R. Antihypertensive effect of canrenone in a model where endogenous ouabain-like factors are present. J Cardiovasc Pharmacol. 1988;11:75–83. doi: 10.1097/00005344-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Semplicini A, Serena L, Valle R, Ceolotto G, Felice M, Fontebasso A, Pessina AC. Ouabain-inhibiting activity of aldosterone antagonists. Steroids. 1995;60:110–133. doi: 10.1016/0039-128x(94)00005-w. [DOI] [PubMed] [Google Scholar]
- 7.Tian J, Shidyak A, Periyasamy SM, Haller S, Taleb M, El-Okdi N, Elkareh J, Gupta S, Gohara S, Fedorova OV, Cooper CJ, Xie Z, Malhotra D, Bagrov AY, Shapiro JI. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009;54:1313–1320. doi: 10.1161/HYPERTENSIONAHA.109.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. An endogenous ligand of alpha-1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 9.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh B, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid, marinobufagenin, in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 11.Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroeder J, Raju V, Hariri IM, El-Okdi N, Gupta S, Fedorova L, Liu J, Fedorova OV, Kahaleh MB, Xie Z, Malhotra D, Watson DK, Bagrov AY, Shapiro JI. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: Implications for uremic cardiomyopathy. Am J Physiol Renal Electrolyte. 2009;296:F922–F934. doi: 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haller ST, Kennedy DJ, Shidyak A, Budny GV, Malhotra D, Fedorova OV, Shapiro JI, Bagrov AY. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. Am J Hypertens. 2012;25:690–696. doi: 10.1038/ajh.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selye H, Krajny M, Savoie L. Digitoxin poisoning: prevention by spironolactone. Science. 1969;164:842–843. doi: 10.1126/science.164.3881.842. [DOI] [PubMed] [Google Scholar]
- 14.Yeh BK, Chiang BN, Sung PK. Antiarrhythmic activity of potassium canrenoate in man. Am Heart J. 1976;92:308–314. doi: 10.1016/s0002-8703(76)80112-3. [DOI] [PubMed] [Google Scholar]
- 15.Pamnani MB, Whitehorn WV, Clough DL, Haddy FJ. Effects of canrenone on blood pressure in rats with reduced renal mass. Am J Hypertens. 1990;3:188–195. doi: 10.1093/ajh/3.3.188. [DOI] [PubMed] [Google Scholar]
- 16.Balzan S, Nicolini G, Bellitto L, Ghione S, Biver P, Montali U. Effect of canrenone on the digitalis site of Na+/K(+)-ATPase in human placental membranes and in erythrocytes. J Cardiovasc Pharmacol. 2003;42:32–36. doi: 10.1097/00005344-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Fedorova OV, Simbirtsev AS, Kolodkin NI, Kotov AY, Agalakova NI, Kashkin VA, Tapilskaya NI, Bzhelyansky AM, Reznik VA, Nikitina ER, Frolova EV, Budny GV, Longo DL, Lakatta EG, Bagrov AY. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition in lowers blood pressure in NaCl-sensitive hypertension. J Hypertens. 2008;26:2414–2425. doi: 10.1097/HJH.0b013e328312c86a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikitina ER, Mikhailov AV, Nikandrova ES, Frolova EV, Fadeev AV, Shman VV, Shilova VY, Tapilskaya NI, Shapiro JI, Fedorova OV, Bagrov AY. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J Hypertens. 2011;29:769–776. doi: 10.1097/HJH.0b013e32834436a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauly RR, Bilato C, Cheng L, Monticone R, Crow MT. Vascular smooth muscle cell cultures. Methods Cell Biol. 1997;52:133–154. doi: 10.1016/s0091-679x(08)60377-5. [DOI] [PubMed] [Google Scholar]
- 20.Finotti P, Palatini P. Canrenone as a partial agonist at the digitalis receptor site of sodium-potassium-activated adenosine triphosphatase. J Pharmacol Exp Ther. 1981;217:784–790. [PubMed] [Google Scholar]
- 21.Semplicini A, Buzzaccarini F, Ceolotto G, Marzola M, Mozzato MG, Giusto M, Campagnolo M, Simonella C, Pessina AC. Effects of canrenoate on red cell sodium transport and calf flow in essential hypertension. Am J Hypertens. 1993;6:295–301. doi: 10.1093/ajh/6.4.295. [DOI] [PubMed] [Google Scholar]
- 22.Boero R, Guarena C, Deabate MC, Rolando B, Rosati C, Quarello F, Piccoli G. Erythrocyte Na+, K+ pump inhibition after saline infusion in essentially hypertensive subjects: effects of canrenone administration. Int J Cardiol. 1989;25(Suppl 1):S47–S52. doi: 10.1016/0167-5273(89)90092-2. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 24.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293:C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 25.Dvela M, Rosen H, Ben-Ami HC, Lichtstein D. Endogenous ouabain regulates cell viability. Am J Physiol Cell Physiol. 2012;302:C442–C452. doi: 10.1152/ajpcell.00336.2011. [DOI] [PubMed] [Google Scholar]
- 26.Czuwara-Ladykowska J, Shirasaki F, Jackers P, Watson DK, Trojanowska M. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J Biol Chem. 2001;276:20839–20848. doi: 10.1074/jbc.M010133200. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Fan PS, Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the Fli1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–2279. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]
- 28.Tham DM, Martin-McNulty B, Wang Y-X, da Cunha V, Wilson DW, Athanassious CN, Powers AF, Sullivan ME, Rutledge JC. Angiotensin II injuries the arterial wall causing increased aortic stiffening in apolipoprotein E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1442–R1449. doi: 10.1152/ajpregu.00295.2002. [DOI] [PubMed] [Google Scholar]
- 29.Xie H, Triggle CR. Endothelium-independent relaxations to acetylcholine and A23187 in the human umbilical artery. J Vasc Res. 1994;31(2):92–105. doi: 10.1159/000159035. [DOI] [PubMed] [Google Scholar]
- 30.Sexton AJ, Loesch A, Turmaine M, Miah S, Burnstock G. Nitric oxide and human umbilical vessels: pharmacological and immunohistochemical studies. Placenta. 1995;16:277–288. doi: 10.1016/0143-4004(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 31.Hornum M, Clausen P, Kjaergaard J, Hansen JM, Mathiesen ER, Feldt-Rasmussen B. Pre-diabetes and arterial stiffness in uraemic patients. Nephrol Dial Transplant. 2010;25:1218–1225. doi: 10.1093/ndt/gfp558. [DOI] [PubMed] [Google Scholar]
- 32.Hornum M, Clausen P, Idorn T, Hansen JM, Mathiesen ER, Feldt-Rasmussen B. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol Dial Transplant. 2011;26:2370–2377. doi: 10.1093/ndt/gfq704. [DOI] [PubMed] [Google Scholar]
- 33.Kolmakova EV, Haller ST, Kennedy DJ, Isachkina AN, Budny GV, Frolova EV, Piecha G, Nikitina ER, Malhotra D, Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant. 2011;26:2912–2919. doi: 10.1093/ndt/gfq772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, Fleenor BS, Lakatta EG, Bagrov AY, Seals DG. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol. 2013;8:1952–1959. doi: 10.2215/CJN.00900113. [DOI] [PMC free article] [PubMed] [Google Scholar]