Abstract
The filamentous fungus Scedosporium aurantiacum and the bacterium Pseudomonas aeruginosa are opportunistic pathogens isolated from lungs of the cystic fibrosis (CF) patients. P. aeruginosa has been known to suppress the growth of a number of CF related fungi such as Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans. However, the interactions between P. aeruginosa and S. aurantiacum have not been investigated in depth. Hence we assessed the effect of P. aeruginosa reference strain PAO1 and two clinical isolates PASS1 and PASS2 on the growth of two clinical S. aurantiacum isolates WM 06.482 and WM 08.202 using solid plate assays and liquid cultures, in a synthetic medium mimicking the nutrient condition in the CF sputum. Solid plate assays showed a clear inhibition of growth of both S. aurantiacum strains when cultured with P. aeruginosa strains PASS1 and PAO1. The inhibitory effect was confirmed by confocal microscopy. In addition to using chemical fluorescent stains, strains tagged with yfp (P. aeruginosa PASS1) and mCherry (S. aurantiacum WM 06.482) were created to facilitate detailed microscopic observations on strain interaction. To our knowledge, this is the first study describing successful genetic transformation of S. aurantiacum. Inhibition of growth was observed only in co-cultures of P. aeruginosa and S. aurantiacum; the cell fractions obtained from independent bacterial monocultures failed to initiate a response against the fungus. In the liquid co-cultures, biofilm forming P. aeruginosa strains PASS1 and PAO1 displayed higher inhibition of fungal growth when compared to PASS2. No change was observed in the inhibition pattern when direct cell contact between the bacterial and fungal strains was prevented using a separation membrane suggesting the involvement of extracellular metabolites in the fungal inhibition. However, one of the most commonly described bacterial virulence factors, pyocyanin, had no effect against either of the S. aurantiacum strains. This study shows that P. aeruginosa has a substantial inhibitory effect on the growth of the recently described CF fungal pathogen S. aurantiacum. The findings also highlighted that P. aeruginosa biofilm formation is important but not crucial for inhibiting the growth of S. aurantiacum in a lung- mimicking environment.
Keywords: co-culture, S. aurantiacum, P. aeruginosa, interactions, growth inhibition, phenazines, SCFM, biofilms
Introduction
Cystic fibrosis (CF) is one of the most common, potentially lethal, genetically inherited disorders affecting mainly the European Caucasian population (O’Sullivan and Freedman, 2009). Although the disease affects a number of organs and systems in the human body, lungs remain the main site of infection in CF patients (Quinton, 1999). The inherited condition stems from the mutation of the CF transmembrane conductance regulator (CFTR) gene, which regulates the transport of chloride ions across the plasma membrane of the epithelial cells (Boucher, 2007). Impaired ion exchange reduces the mucociliary clearance, which leads to accumulation of hyper-viscous mucus in the airway surfaces, thus providing ideal conditions for the growth of microorganisms (Delhaes et al., 2012). Various molecular and microbiology based approaches have revealed the polymicrobial nature of the infections in CF with the identification of complex microbiota including bacteria, fungi, and viruses (Lynch and Bruce, 2013). Most of these microorganisms are either acquired from the environment or through contact with other infected patients (Lipuma, 2010).
Bacteria constitute the major portion of the microorganisms associated with CF. The most common bacterial inhabitants of the CF airways include Haemophilus influenzae, Staphylococcus aureus, Pseudomonas Aeruginosa, and Burkholderia cepacia complex (BCC) (Harrison, 2007; Lipuma, 2010). Among them, P. aeruginosa is the most dominant bacterial species known to cause chronic respiratory infections in more than 50% of adult CF patients (Coutinho et al., 2008). P. aeruginosa is a ubiquitous Gram-negative bacterium possessing a wide variety of pathogenicity factors to evade the host defense system (Davies, 2002). During the early stages of infection, the bacterium attaches itself to lung epithelial cell surface receptors through specific adhesins and secretes extracellular products to prolong its survival in the CF airways (Tang et al., 1995). The extracellular products secreted by P. aeruginosa include enzymes such as elastase and alkaline protease, exotoxins, siderophores, and phenazines such as pyocyanin with a known role in virulence (Haas et al., 1991). Moreover, P. aeruginosa cells form biofilms in order to proliferate inside the lungs and protect themselves from antibiotic agents (Singh et al., 2000).
In addition to bacteria, some fungal species are also known to colonize the respiratory tracts of CF patients (Cimon et al., 2000; Pihet et al., 2009). Mycological examination of the specimens obtained from CF patients have shown that Aspergillus fumigatus is the most predominant fungal colonizer of the CF lungs as it has been recovered from 6 to 71% of CF patients (Bakare et al., 2003; Horre et al., 2010). However, the presence of non-Aspergillus fungal species often remains unnoticed owing to the lack of sensitive culture techniques to examine the sputum specimens from CF patients (Delhaes et al., 2012). Recently, a more targeted approach has been developed by combining molecular techniques with laboratory culture methods, which can now identify a wide range of fungal pathogens in the expectorated sputa (Middleton et al., 2013). Studies conducted on CF patients in Australia and certain parts of Europe have confirmed the emergence of a new fungal genus Scedosporium (originally called Pseudallescheria) that causes infections in the lungs of immunocompromised hosts (Blyth et al., 2010a; Paugam et al., 2010; Lackner et al., 2014). Scedosporium sp. have been isolated from the sputum specimens of 14.7–17.4% of Australian CF patients which makes it the second most common fungal respiratory pathogen associated with CF (Blyth et al., 2010a,b). Scedosporium aurantiacum is a recently identified, highly virulent member of the Scedosporium sp. complex recovered from one in six CF patients in Sydney (Heath et al., 2009; Blyth et al., 2010b; Harun et al., 2010). The clinical consequences of the S. aurantiacum colonization or infections in the CF patients remain to be explored (Harun et al., 2010).
According to the clinical reports, the prevalence of fungi in the respiratory tracts of CF patients is mainly affected by the bacteria present, and the interactions between the bacteria and fungi potentially impact the disease outcome (Sibley et al., 2006; Chotirmall et al., 2010; Leclair and Hogan, 2010). Several in vitro studies have reported an inhibitory effect of P. aeruginosa against the common lung co-inhabitants such as A. fumigatus or the yeasts Candida albicans, and Cryptococcus neoformans (Hogan and Kolter, 2002; Bandara et al., 2010; Cugini et al., 2010). Similar data for S. aurantiacum are lacking. Reflecting the increasing importance of S. aurantiacum in CF, we examined the effect of clinical P. aeruginosa CF isolates PASS1 and PASS2 and laboratory reference strain PAO1 on the growth of two clinical S. aurantiacum isolates WM 06.482 and WM 08.202 using solid plate assays and liquid co-cultures containing medium that mimics the nutritional content of human CF sputum (Palmer et al., 2007).
Materials and Methods
Growth and Maintenance of Strains
Strains used in the study are listed in Table 1. P. aeruginosa PASS1 and PASS2 were isolated from the sputum samples of CF patients (Penesyan et al., under review). A common laboratory ‘reference’ strain PAO1 (Lewenza et al., 2014) was also included in the study. S. aurantiacum strains WM 06.482 and WM 08.202 were obtained from the culture collection of the Medical Mycology Research Laboratory, Centre for Infectious Diseases and Microbiology, Westmead Hospital, Sydney, NSW, Australia (Kaur et al., 2015). Virulence levels of all P. aeruginosa strains used in this study have been tested previously using Caenorhabditis elegans based infection model (Lewenza et al., 2014; Penesyan et al., under review). Virulence studies of S. aurantiacum have been performed using Galleria mellonella larvae model (Kaur et al., 2015).
Table 1.
Pseudomonas aeruginosa and Scedosporium aurantiacum strains used in the study.
| Strain | Strain name | Source | Virulence level | Reference |
|---|---|---|---|---|
| PASS1 | P. aeruginosa | Sputum sample of a cystic fibrosis (CF) patient Sydney, NSW, Australia | High | Penesyan et al. (under review) |
| PASS2 | P. aeruginosa | Sputum sample of a CF patient Sydney, NSW, Australia | Low | Penesyan et al. (under review) |
| PAO1 (ATCC 15692) | P. aeruginosa | Wound exudate Melbourne, VIC, Australia | High | Holloway (1955) |
| WM 06.482 | S. aurantiacum | Invasive clinical isolate from CF patient Sydney, NSW, Australia | High | Kaur et al. (2015) |
| WM 08.202 | S. aurantiacum | Type strain from a wound exudate Santiago de Compostela (Spain) | Low | Kaur et al. (2015) |
Pseudomonas aeruginosa strains were revived from frozen stocks stored at -80°C by streaking on LB (Luria Bertani, Sigma) plates and incubation overnight at 37°C. Bacterial colonies were inoculated into LB broth and incubated at 37°C on an orbital shaker (200 rpm) overnight. Following fractions were prepared from overnight cultures of the P. aeruginosa strains: (1) Heat killed cells were obtained by incubating 1 ml of an overnight cell culture at 80°C for 60 min. Absence of any viable cells was confirmed by plating on LB agar medium; (2) Cell lysates were obtained after sonicating the cells (50 ml) on ice for 10 min in an ultrasonic processor followed by collection of the supernatant after centrifugation at 10,000 × g for 30 min; (3) Cell culture supernatants were collected by centrifuging 50 ml of overnight cultures of P. aeruginosa strains at 10,000 × g for 30 min. Supernatants were then freeze dried and resuspended in 100 μl of 1x PBS and stored at 4°C until use.
Fungal strains were maintained on PDA (potato dextrose agar, BD, DifcoTM) plates at 37°C. After 5 days of growth, the conidia were scraped into sterile saline solution (0.9% w/v NaCl and 0.01% v/v Tween 80) and the suspension was filtered through a sterile cotton wool to separate the conidia from the hyphal debris. Conidia were washed with 1x PBS to remove traces of saline and the inoculum was adjusted to a McFarland standard concentration of 2.5 × 105 conidia/ml. Concentration of conidia was confirmed using Neubauer counting chamber and additional plate counting.
Construction of Strains Tagged with Fluorescent Proteins
Pseudomonas aeruginosa Strain Expressing Yellow Fluorescent Protein (YFP)
Plasmid pUCPyfp (Gloag et al., 2013) encoding yellow fluorescent protein (YFP) was used to transform the P. aeruginosa PASS1 strain. In order to make electrocompetent cells, PASS1 was cultured in 5 ml of LB broth overnight at 42°C and 200 rpm. Cells were harvested by centrifugation (14,000 g for 15 min at 4°C) and the ionic strength of the suspension was reduced by rigorous washing with 1x M9 minimal salts medium (Sigma) followed by two washes with ice-cold sterile milliQ water. Bacterial cells were transformed by electroporation as described by Dower et al. (1988) by adding 1 μg of the plasmid DNA to 20 μl of the washed cell aliquots. At the end of the procedure, cells were streaked on LB plates containing 8 mg/ml ampicillin and incubated for up to 48 h at 37°C to select for the transformants.
Construction of the S. aurantiacum Strain Expressing mCherry
The mCherry gene was PCR amplified from the pmcherry-c1 vector (Clontech Laboratories, USA) using mCherry.fwd and mCherry.rev primers (Table 2) and was expressed under the Trichoderma reesei pyruvate kinase (pki) promoter, which was amplified from the pCBH1corlin vector (Te’o et al., 2000) using pki.fwd and pki.rev primers. In addition, a DNA fragment featuring the pki promoter together with the hygromycin B resistance gene (pki-hph) was PCR amplified using primer pkihph.fwd and pki-hph.rev to allow selection of transformants. The fragments were engineered to contain restriction sites as needed (Table 2).
Table 2.
Sequence of primers used for the construction of transformation cassettes.
| Primer name | Sequence (5′–3′) |
|---|---|
| mCherry. fwd | GAA GAACCT CTT AAC CTC TAG (pki sequence) ATG GTG AGC AAG GGC GAG G |
| mCherry. rev | CAT GCG GGT ACC (KpnI) CTA TTA CTT GTA CAG CTC GTC CAT GC |
| pki. fwd | TGC TGC GAT ATC (EcoRV) CTT AAG TTA G TA ACT AGT GGA TC |
| pki.rev | CTC GCC CTT GCT CAC CAT (mCherry sequence) CTA GAG GTT AAG AGG TTC TTC |
| pki-hph. fwd | TAC GCG GCG CGC CCT TAA G (AflII) TT AG T AAC TAG TGG ATC |
| pki-hph.rev | CAT GCT AAG CTT (HindIII) CTA TTC CTT TGC CCG CGG AC |
The primers contain engineered restriction sites shown in shading and the overlapping sequences are shown in bold.
The primers pki.fwd and mCherry.rev were used to fuse the separately amplified pki and mCherry fragments in an overlap extension PCR as described by Thornton (2015). The fragment pki-hph was digested with restriction enzymes HindIII and AflII (Fermentas, Thermo Scientific, USA) and fragment pki-mcherry was digested with EcoRV and KpnI. The digested products pki-hph and pkimcherry were gel purified using QIAquick gel extraction kit (Qiagen, USA) and inserted into MCS-1 (multiple cloning site) and MCS-2 of the pETDuet-1 plasmid, respectively (Supplementary Figure S1). Finally, the purified vectors and inserts were ligated using T4 ligase (Fermentas, USA) at a 1:3 molar ratio for 2 h at room temperature. The final ligated vector (pETDuet-phpm) was introduced into Escherichia coli DH5α competent cells as described by Inoue et al. (1990). Selection of transformants was performed on LB agar plates containing ampicillin (100 μg/ml) and incubating at 37°C. Selected transformants were grown in 3 ml of LB and plasmid DNA was isolated using QIAprep Spin Miniprep kit (Qiagen, USA). The plasmid pETDuet-phpm was sequenced by AGRF, Sydney, NSW, USA to check sequence alignment of the inserted gene cassettes.
The pETDuet-phpm DNA was introduced into highly virulent S. aurantiacum WM 06.482 using protoplast-mediated transformation based on the method adopted from Penttilä et al. (1987) with modifications. The young hyphae obtained from an overnight culture of WM 06.482 on PDA plates with cellophane at 28°C were digested with 10 mg/ml of lysing enzyme from T. harzianum (Sigma–Aldrich, Australia) to obtain protoplasts which were then filtered through a sterile sintered glass filter (porosity 1). Osmotically stabilized protoplasts were transformed with 5 μg of plasmid DNA as described by Penttilä et al. (1987). Transformed protoplasts were mixed with 10 ml of molten agar (1.5% w/v KH2PO4, 0.5% w/v NH4SO4, 2% w/v glucose, 1 M sorbitol, pH 5.5) containing hygromycin B (410 U/ml) and overlayed onto PDA plates which were incubated at 28°C for 3–5 days. Hygromycin resistant colonies were restreaked onto fresh PDA plates containing hygromycin B (410 U/ml) for a second round of selection. Transformation efficiency was calculated as number of transformants per μg of plasmid DNA. Expression of the mCherry protein in selected transformants was confirmed using Fluoview FV1000 inverted confocal microscope (Olympus) with an excitation and emission wavelength 488/633 nm (HeNe).
Growth Inhibition Assays
The effect of P. aeruginosa on the growth of S. aurantiacum was tested in different combinations on both solid and liquid growth media. Combinations of bacterial and fungal strains for the testing are presented in Table 3.
Table 3.
Types of cultures used to investigate the effect of different P. aeruginosa strains on S. aurantiacum.
| Type of co-culture | P. aeruginosa strains | S. aurantiacum strains |
|---|---|---|
| Solid plate (cross streak, disk inhibition assay) | PASS1, PASS2, PAO1 | WM 06.482, WM 08.202 |
| Liquid cultures (chemical fluorescent dyes) | PASS1, PASS2, PAO1 (stained with Syto9) | WM 06.482, WM 08.202 (stained with Mito tracker FR) |
| Liquid culture (genetically tagged strains) | PASS1 (yfp-labeled) | WM 06.482 (mCherry- labeled) |
| Liquid culture (addition of an antibiotic) | PASS1 (yfp-labeled) | WM 06.482 (mCherry- labeled) |
Cross Streak Assay using Live Cells
The effect of bacteria on fungal growth was assessed using an agar plate method described by Kerr (1999), with slight modifications adopted from Chen et al. (2013). P. aeruginosa strains PASS1, PASS2, and PAO1; and S. aurantiacum strains WM 06.482 and WM 08.202, were cultured together on a synthetic cystic fibrosis medium (SCFM) that mimics the nutritional content of human CF sputum. SCFM contains average concentrations of ions, free amino acids, glucose, and lactate present in the CF sputum samples (Palmer et al., 2007). Solid SCFM agar plates were made with an addition of 2% w/v agar to liquid SCFM medium. A sterile cotton swab was used to draw a straight vertical line of P. aeruginosa cells (1 × 108 CFU/ml=0.5 McFarland standard concentration) across the plate. At the same time, S. aurantiacum conidia (2.5 × 105 conidia/ml = 0.5 McFarland standard concentration) were inoculated with a cotton swab horizontally across the upper part of the plate preventing any direct contact between fungi and bacteria. The plates were dried at room temperature for 15 min and incubated at 37°C. Digital photography was performed after 24 h to visualize the growth of both bacterial and the fungal strains tested on the plate.
Disk Inhibition Method using Live Cells and Cell Fractions
Sterile filter paper disks (Whatman no. 1; Sigma–Aldrich), 7 mm in diameter, were impregnated with 20 μl of the P. aeruginosa PASS1, PASS2, and PAO1 cell fractions, i.e., cell lysates, cell culture supernatant and heat inactivated cells (see preparation in section 1.1) and placed on an SCFM plate that was freshly surface seeded with 100 μl (2.5 × 105 conidia/ml) of S. aurantiacum conidia (WM 06.482 or WM 08.202). A suspension of live P. aeruginosa cells was included for comparison. The plates were incubated at 37°C for up to 3 days and observed at regular intervals for the appearance of any clear inhibition zones around the disks. Assays were repeated in three biological replicates. A relative inhibition index was calculated for each P. aeruginosa isolate by dividing the area of activity (difference between the area of the inhibition zone and area of the colony) by the area of the colony.
Effect of Bacteria on the Fungal Growth in Liquid Co-cultures
Interactions between P. aeruginosa and S. aurantiacum were observed in liquid medium using both chemical fluorescent stains and genetically labeled strains of bacteria and fungi in a direct contact with each other. In case of fluorescently labeled co-cultures, 1 × 108 CFU/ml of P. aeruginosa PASS1, PASS2, and PAO1 and 2.5 × 105 conidia/ml of S. aurantiacum WM 06.482 and WM 08.202 were inoculated in 20 ml SCFM medium in 100 ml shake flasks and incubated for 24 h at 37°C on an orbital shaker at 150 rpm. Aliquots were taken on a sterile glass slide from the co-cultures after every 4 h, washed with 1x PBS and fixed using 2% v/v paraformaldehyde (Sigma–Aldrich). The co-cultures were stained with DNA specific Syto9 (0.6 μM) and mitochondria specific Mito-TrackerR Red FM (25 nM) for 15 min in the dark as per the manufacturer’s protocol (Molecular Probes, Life Technologies). Bacterial cells were expected to stain with Syto9 whereas fungal cells would stain with Mito-TrackerR Red FM. Fixed specimens were imaged using Fluoview FV1000 inverted confocal microscope (Olympus) with an excitation and emission wavelength of 488 nm (Ar) and 633 nm (HeNe).
The genetically tagged P. aeruginosa PASS1yfp strain and S. aurantiacum WM 06.482mCherry strain were also cultured together in 20 ml of SCFM for 24 h at 37°C, shaking at 150 rpm. At the end of the incubation period, cells were washed and fixed on sterile glass slides as above. Imaging was performed with a confocal microscope using an excitation and emission wavelength of 488 nm (blue laser diode for yfp) and 561 nm (yellow–green laser for mCherry) respectively. Liquid co-cultures with genetically labeled PASS1 and WM 06.482 strains were also repeated by adding different concentrations of gentamicin (2.5–10 mg/ml), which is a commonly used antibiotic against bacteria (Doring et al., 2000; Lin et al., 2011). Image analysis for both types of co-cultures was performed using IMARIS imaging software.
Transwell Assay with Polycarbonate Membranes
In order to explore the role of secreted bacterial metabolites on the fungi, P. aeruginosa strains PASS1, PASS2, and PAO1 and S. aurantiacum strains WM 06.482 and WM 08.202 were co-cultured in SCFM in sterile six-well Transwell plates (Corning) with polycarbonate cell culture inserts (0.4 μm, Sigma–Aldrich) in order to prevent direct contact between the fungal and bacterial strains. P. aeruginosa (1 × 108 CFU/ml) and S. aurantiacum (2.5 × 105 conidia/ml) were inoculated in the bottom and top of the membrane insert, respectively. The plates were incubated at 37°C for 24 h and any inhibition of the growth of S. aurantiacum was measured as a difference in the dry weight of the S. aurantiacum cultured with or without P. aeruginosa. The method of calculating dry weight was adopted from Kaur et al. (2015).
Effect of Phenazines
Phenazines were extracted from all P. aeruginosa strains (PASS1, PASS2, and PAO1) cultured in 5 ml of LB (in three biological replicates) for 2 days at 37°C using chloroform according to a method described by Mavrodi et al. (2001). Crude phenazine extracts were dried under reduced pressure to remove the solvent, resuspended in 80% acetonitrile (ACN) and applied to the filter paper disks (Whatman paper no. 1). The presence of pyocyanin in the crude phenazine extracts was confirmed using Ultra High Performance Liquid Chromatography (UHPLC) as described by Penesyan et al. (under review). While the absolute concentration of pyocyanin in the crude phenazine extracts from the three P. aeruginosa strains was not known, major experimental discrepancy was minimized by using same amount (20 μl) of crude extracts for the testing. The effect of these crude extracts on the fungal growth was determined using a disk inhibition assay (as described in section Disk Inhibition Method using Live Cells and Cell Fractions) where disks containing 20 μl of phenazine extracts from different P. aeruginosa isolates were air-dried and placed on SCFM agar plates freshly spread with 2.5 × 105 conidia/ml of S. aurantiacum strains (WM 06.482 and WM 08.202). The activity of blank 80% ACN, blank LB medium extract and the solution of commercial pyocyanin (10 mM, Sigma–Aldrich) were also tested against S. aurantiacum for comparison. Plates were incubated for 48 h at 37°C and observed for the presence of clearing zones around the filter paper disks as an indication of inhibitory activity of the extracts on fungal growth.
Statistical Analysis
Statistical significance between the means of different experimental datasets was analyzed using two-tailed Student’s t-test. SD with p-value less than 0.05 was considered significant. All experiments were performed in biological triplicates.
Results
Inhibition of S. aurantiacum Growth by Live P. aeruginosa Cells
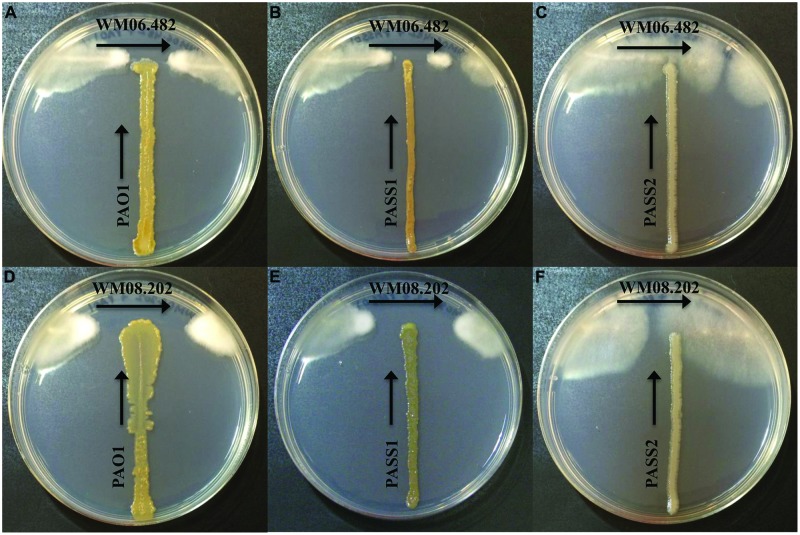
When S. aurantiacum strains WM 06.482 (high virulence) and WM 08.202 (low virulence) were cross streaked against three different P. aeruginosa isolates PAO1, PASS1, and PASS2 on the SCFM agar medium, an area of inhibition was observed in the growth of both S. aurantiacum strains after 24 h (Figures 1A–F). It was evident from the size of the inhibition area that the bacterial strains had lesser impact on the highly virulent S. aurantiacum strain WM 06.482 (Figures 1A,B) compared to the less virulent WM 08.202 strain (Figures 1D,E). Out of the three bacterial strains studied, P. aeruginosa PASS2 had the weakest inhibitory effect on the S. aurantiacum strains in the plate test as seen in Figures 1C,F.
FIGURE 1.
Cross-streak plate assay between different strains of Pseudomonas aeruginosa and Scedosporium aurantiacum on synthetic cystic fibrosis medium (SCFM) agar plates. All bacterial strains were inoculated vertically whereas the fungal strains were streaked horizontally across the upper part of the SCFM agar plate. The plates were incubated at 37°C for 24–48 h. (A–C) Inhibition of S. aurantiacum strain WM 06.482 by PAO1, PASS1, and PASS2 strains of P. aeruginosa. (D–F) Inhibition of S. aurantiacum strain WM 08.202 by PAO1, PASS1, and PASS2 strains of P. aeruginosa.
Effect of P. aeruginosa Cell Extracts on S. aurantiacum
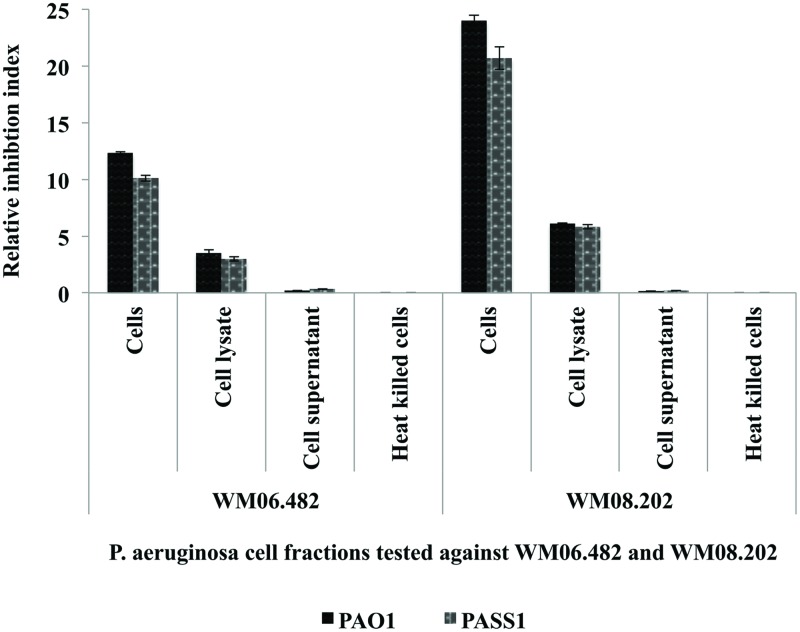
The effect of different cell fractions, i.e., the culture supernatant and cell lysate, and heat inactivated cells of P. aeruginosa strains PASS1, PASS2, and PAO1 was further tested on the growth of S. aurantiacum WM 06.482 and WM 08.202 using the disk inhibition method. Following 48 h incubation, clear inhibition zones were observed on plates inoculated with living cells of P. aeruginosa PASS1 and the reference strain PAO1 and their respective cell lysates. The inhibitory effect of P. aeruginosa was expressed as a relative inhibition index (Figure 2).
FIGURE 2.
Susceptibility of S. aurantiacum (WM 06.482 and WM 08.202) to P. aeruginosa (PAO1, PASS1, and PASS2) and their cell lysate fractions. Relative inhibition index was calculated as the average value of three replicates (n = 3) with a p-value <0.05 considered as significant.
Living cells of both PAO1 and PASS1 and their corresponding cell lysates displayed a higher inhibitory activity against the less virulent S. aurantiacum strain WM 08.202 compared to the high virulence strain WM 06.482. Cell supernatants and heat killed P. aeruginosa cells failed to elicit a response against either of the fungal strains. In a separate experiment, the effect of S. aurantiacum was also tested against P. aeruginosa by incubating filter disks impregnated with S. aurantiacum conidia and cell fractions on the plates freshly seeded with P. aeruginosa cells. As S. aurantiacum failed to display any inhibition against P. aeruginosa, these interactions were not studied further (data not shown).
Effect of P. aeruginosa on Fungal Physiology
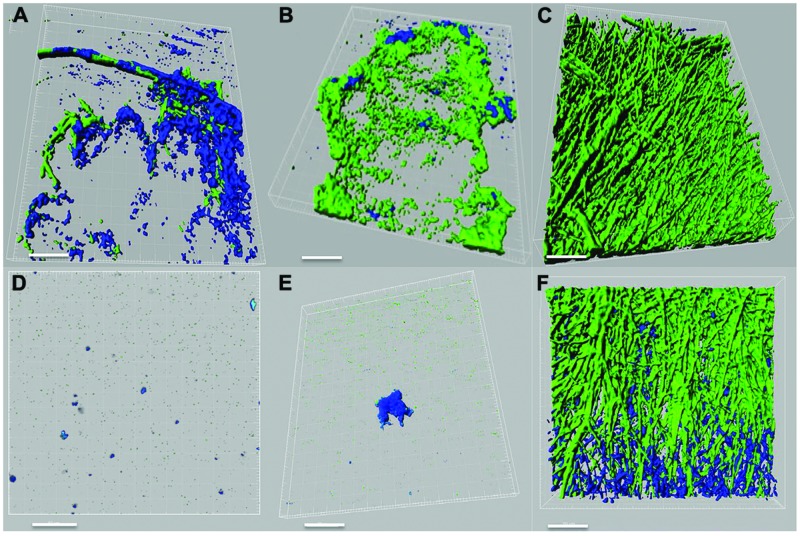
Pseudomonas–Scedosporium interactions were also studied using confocal microscopy by imaging cellular aggregates from liquid co-cultures labeled with fluorescent stains. Confocal images demonstrated an inhibitory effect of the P. aeruginosa PASS1 (isolated from sputum of a CF patient) and the reference strain PAO1 on the growth and development of both S. aurantiacum strains tested (Figures 3A–F). In the course of 24 h, the bacteria had attached to the surface of fungal hyphae and formed biofilm-like structures containing a high density of bacterial cells but very few fungal hyphae. The tested bacterial strains had a weaker impact on the more virulent WM 06.482 compared to the less virulent WM 08.202, showing the resistant nature of the more virulent strain also highlighted in the plate tests. Although different fluorescent stains were used to distinguish between P. aeruginosa and S. aurantiacum in liquid cultures, it was difficult to visualize the detailed effect of bacteria on the fungal hyphae due to permeabilisation of the Syto9 dye by both P. aeruginosa and S. aurantiacum. No growth inhibiting effect was observed when the fungal strains were co-cultured with PASS2 as indicated by dense growth of fungi in Figures 3C,F. These observations were also consistent with the results seen in the assays carried on plates.
FIGURE 3.
Confocal laser scanning microscope (CLSM) images of interactions between P. aeruginosa (PAO1, PASS1, and PASS2) and S. aurantiacum (WM 06.482 and WM 08.202) as observed after co-incubating both the organisms in SCFM liquid medium at 37°C for 24 h. P. aeruginosa cells are stained with Syto9 (shown in green) and S. aurantiacum with Mito-tracker deep red FM (shown in blue). 3D re-construction of CLSM datasets was performed using IMARIS software package (Bitplane). Scale bar = 50 μm. (A–C) CLSM images of co-culture of WM 06.482 with PAO1, PASS1, and PASS2, respectively. (D–F) CLSM images of co-culture of WM 08.202 with PAO1, PASS1, and PASS2, respectively.
Interactions between Genetically Tagged P. aeruginosa and S. aurantiacum Strains
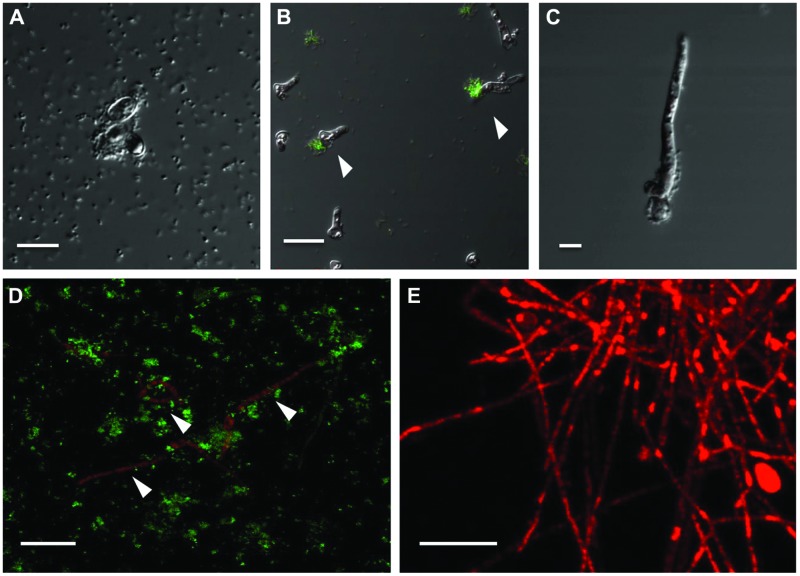
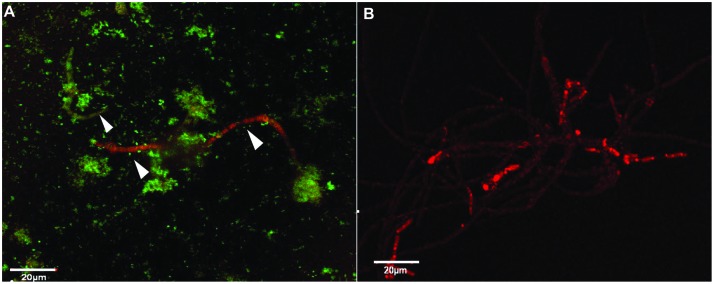
To circumvent the difficulty in differentiating between bacteria and fungi in liquid co-cultures, genetically tagged P. aeruginosa strain PASS1 expressing yfp and S. aurantiacum strain WM 06.482 expressing mCherry were developed. With this arrangement, it was observed that the bacteria started colonizing the fungal conidia soon after incubating them together in the SCFM (Figure 4A). Thus, within 8 h, bacteria began aligning themselves along the length of fungal hyphae as seen in Figures 4B,C. After 24 h, large clumps of P. aeruginosa cells were observed on S. aurantiacum hyphal filaments (Figure 4D), and the amount of hyphae was also reduced in number compared to the S. aurantiacum control without the bacteria (Figure 4E).
FIGURE 4.
Adhesion and colonization of mCherry-tagged S. aurantiacum strain WM 06.482 (shown in red) by P. aeruginosa strain PASS1 tagged with yfp (shown in green) during coculturing in SCFM for 24 h at 37°C. Scale bar = 20 μm. (A) P. aeruginosa cells adhered to germinating S. aurantiacum conidia after 2 h of incubation as viewed by DIC. (B,C) after 8 h, some young hyphae were surrounded by bacterial cells. (D) Bacteria can be seen attached to the hyphal filaments after incubation for 24 h. (E) Healthy growing culture of WM 06.482 expressing mCherry in the absence of bacteria. ∗White arrows indicate fungal filaments that are being colonized by the bacteria.
Effect of Antibiotics used in Clinical Practice on Co-cultures
Analysis of the plate cultures and confocal images confirmed that P. aeruginosa had an inhibitory effect on the growth of S. aurantiacum. Therefore, in order to further validate this finding and to reveal the possible effect of antibiotic therapy on S. aurantiacum and P. aeruginosa mixed populations present in CF lungs, co-culturing was repeated with an addition of varying amounts of gentamicin (2.5–10 mg/ml) to selectively inhibit the growth of P. aeruginosa S. aurantiacum-P. aeruginosa co-cultures were also maintained without the addition of gentamicin for comparison (Figure 5A). All bacteria were killed at a concentration of 8 mg/ml of gentamicin. As seen from Figure 5B, S. aurantiacum strain WM 06.482 was growing actively in the absence of P. aeruginosa strain PASS1 indicating the reversal of the inhibitory effect caused by live bacteria against the fungus.
FIGURE 5.
The effect of gentamicin on P. aeruginosa (PASS01) and S. aurantiacum (WM 06.482) co-cultures growing in SCFM at 37°C for 24 h. Scale = 20 μm. (A) Co-culture of WM 06.482 and PASS01 without the antibiotic. (B) Active growth of S. aurantiacum in a co-culture treated with 8 mg/ml of gentamicin to eradicate the bacterial growth.
Indirect (non-physical) Interactions between P. aeruginosa and S. aurantiacum
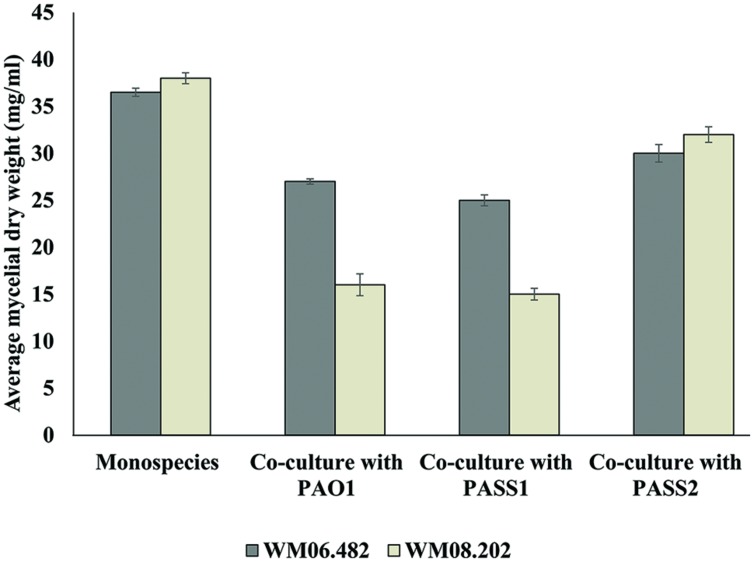
To investigate whether physical contact between P. aeruginosa and S. aurantiacum was important to trigger growth inhibition, co-cultures were performed in six-well plates fitted with polycarbonate membranes to prevent direct contact between P. aeruginosa and S. aurantiacum cells while allowing free exchange of nutrients and extracellular molecules between the organisms. Growth of the less virulent S. aurantiacum strain WM 08.202 was inhibited when co-cultured with PAO1 and PASS1, evident from the substantial decrease in the fungal biomass (Figure 6) when compared to the culture of WM 08.202 maintained for the same amount of time.
FIGURE 6.
The inhibitory effect of P. aeruginosa on S. aurantiacum during co-culture in SCFM, determined by the change in dry weight of S. aurantiacum hyphae. A sterile polycarbonate membrane was used to separate P. aeruginosa from S. aurantiacum in six well culture plates. Error bars on each data point represent SEM of three independent experiments. P-values were calculated by student’s t-test, where p < 0.05 was considered significant.
Pseudomonas aeruginosa isolate PASS1 and the reference strain PAO1 showed a milder inhibitory effect against the high virulence S. aurantiacum strain WM 06.482. The PASS2 strain had little or almost no effect on growth of either of the S. aurantiacum strains. The results suggested that cell–cell contact was in fact not necessary to bring about inhibition of the growth of S. aurantiacum by P. aeruginosa and that the inhibition might involve bacterial metabolites and/or extracellular signaling molecules. In addition, S. aurantiacum strains WM 06.482 and WM 08.202 produced a red colored pigment when co-cultured with clinical P. aeruginosa strain PASS1 and reference strain PAO1. No such pigment was observed in the co-cultures involving S. aurantiacum and PASS2 strain (Supplementary Figure S2).
Effect of Phenazines on the Growth of S. aurantiacum
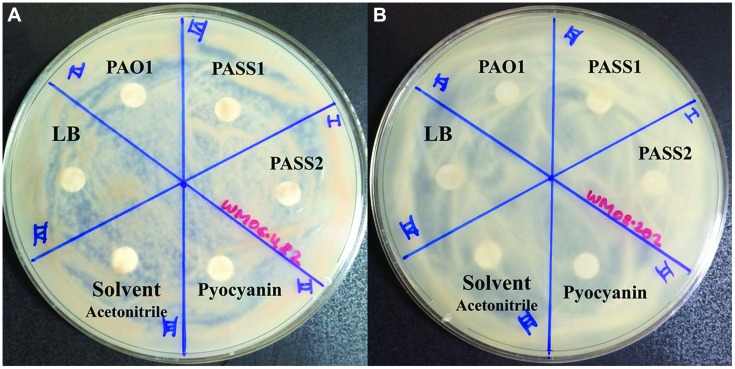
To test whether known virulence factors such as phenazines secreted by P. aeruginosa were involved in the inhibition of S. aurantiacum growth, the effect of crude phenazine extracts from P. aeruginosa strains PAO1, PASS1, and PASS2 were tested on the two S. aurantiacum strains using a disk inhibition assay. No inhibition was observed with disks saturated with the crude extracts as seen in Figure 7. All S. aurantiacum strains also showed resistance to a high concentration (10 mM) of commercial phenazine pyocyanin. Phenazines are known to have an inhibitory effect against a wide range of fungal species (Kerr et al., 1999).
FIGURE 7.
Effect of P. aeruginosa phenazines on two S. aurantiacum isolates (A) WM 06.482 and (B) WM 08.202. Phenazines were extracted from different P. aeruginosa strains (PAO1, PASS1, and PASS2) and redissolved in acetonitrile (ACN). LB medium extract, ACN solvent and commercial phenazine (pyocyanin) were also tested against S. aurantiacum.
Discussion
Most of the studies targeting bacterial-fungal interactions in vitro have been performed with bacterial laboratory reference strains using either fungus-specific culture media (PDA, SABD) and/or minimal salts medium (Kerr et al., 1999; Hogan and Kolter, 2002; McAlester et al., 2008; Bandara et al., 2010; Manavathu et al., 2014). Differently to previous studies and to provide a better focus, we used a CF sputum-mimicking medium, i.e., SCFM to explore the possible effect of P. aeruginosa on S. aurantiacum in the CF lung environment. We also used two recently isolated clinical CF strains of P. aeruginosa, PASS1 and PASS2, together with PAO1, a commonly used reference strain, and a clinical S. aurantiacum isolate (WM 06.482) with a high established virulence and a less-virulent type strain WM 08.202 to add to the clinical relevance of the findings.
Our results demonstrated that P. aeruginosa strains exhibit an inhibitory effect against S. aurantiacum. Consistent with the co-culture studies involving P. aeruginosa and other fungi, initial screening using plate assays suggested that presence of metabolically active (live) bacteria was necessary to inhibit the growth of the fungus as heat killed cells had no effect on S. aurantiacum growth (Mowat et al., 2010). Further on, extracts obtained from the bacterial monocultures failed to show any inhibitory effect. Thus it is possible that inhibition pathways might involve genes that are expressed only in bacterial-fungal co-cultures. In this respect our findings are similar to those of Rella et al. (2012) who showed that the growth of C. neoformans was not affected by the cell extracts obtained from P. aeruginosa strains PAO1 and PA14 cultured separately. The inhibition of S. aurantiacum by cell lysates of P. aeruginosa may be explained by the presence of bacterial exotoxins that are released during the cell lysis.
Confocal microscopy has been used to study interactions between chemically stained P. aeruginosa and major fungal lung pathogens such as C. albicans and A. fumigatus in liquid co-cultures (Bandara et al., 2010; Manavathu et al., 2014). However, the use of chemical stains was limited by the cross staining of bacteria and fungi thereby making it impossible to differentiate between them under a confocal microscope (Bandara et al., 2010). One of the key features of the current study is the use of P. aeruginosa and S. aurantiacum strains that were genetically tagged with fluorescent proteins in order to characterize the interactions in detail. To the best of our knowledge, this is the first report on successful genetic transformation of the newly described S. aurantiacum species. As no homologous promoters are available for this fungal species as yet, the fluorescent marker mCherry and the E. coli hph gene encoding hygromycin B phosphotransferase were expressed under a heterologous pki (pyruvate kinase) promoter derived from another ascomycetous fungus, T. reesei (Te’o et al., 2002; Boon et al., 2008; Klix et al., 2010). In previous studies, heterologous promoters such as pki and gpdA have been successfully used for gene expression across various phylogenetically close species (Punt et al., 1990; Jieh-Juen Yu, 1998; Ruiz-Diez and Martinez-Suarez, 1999; Almeida et al., 2007). The amount of hygromycin B required to inhibit the growth of S. aurantiacum was relatively high (410 U/ml) compared to some other fungi, which shows the highly resistant nature of S. aurantiacum also observed in antifungal susceptibility tests described in other studies (Lackner et al., 2012). Although the transformation efficiency was low (2.2 μg of plasmid DNA), transformant strains expressing the mCherry protein were obtained.
Confocal microscopy of the bacterial-fungal co-cultures revealed that bacteria elicit a specific inhibitory response by establishing a physical contact with the fungal hyphae. Similar types of interactions have also been observed in yeasts such as C. albicans and ascomycetous fungi such as A. nidulans and Alternaria alternata (Hogan and Kolter, 2002; Jarosz et al., 2011). This association might be directed toward utilization of the fungus by bacteria as an additional source of nutrients, or as an additional matrix support to form biofilms (Hibbing et al., 2010), or it may be a strategy to promote their own survival by inhibiting the fungal growth owing to nutrient limiting conditions in the medium (Brand et al., 2008).
Under nutrient limiting conditions, biofilm formation has been described as an important characteristic for P. aeruginosa mediated killing of other fungi such as C. albicans and A. fumigatus (Hogan and Kolter, 2002; Manavathu et al., 2014). Similarly, an inhibitory effect was also displayed by the biofilm forming strains of P. aeruginosa (PASS1 and PAO1) against the two S. aurantiacum strains in this study. PASS1 and PAO1 are high virulence strains, which share many similarities in their respective genomes. In contrast, the least virulent bacterial strain PASS2 (Penesyan et al., under review) that failed to show an effect against the fungi lacks several virulence related genes such as those encoding phenazines and the psl (polysaccharide synthesis locus) gene cluster which is required for biofilm formation (Ma et al., 2009; Penesyan et al., under review). The effect of bacteria on the growth of the less virulent S. aurantiacum strain WM 08.202 was much higher compared to the more virulent WM 06.482 both in the plate assays and in liquid co-cultures. This difference probably results from their different physiology as shown by Kaur et al. (2015) and possibly higher resistance to antifungals of the more virulent S. aurantiacum strain WM 06.482. These factors will be studied further when annotated S. aurantiacum genomes are available.
While biofilm formation and colonization of fungal hyphae in the nutrient limited SCFM liquid medium clearly contributed to the inhibition of S. aurantiacum by P. aeruginosa, it was not absolutely essential for the inhibitory effect as the cross streak assay with cultures not touching each other and disk inhibition experiments using cell lysates also resulted in inhibition of fungal growth. These indicated the possible involvement of secreted diffusible bacterial exoproducts/metabolites in fungal growth inhibition. One of these metabolites pyocyanin, a phenazine, is an extracellular redox-active virulence factor which is widely known to affect the growth of a large number of fungal species such as A. fumigatus, C. albicans, and C. neoformans (Kerr et al., 1999; Laursen and Nielsen, 2004; Gibson et al., 2009). Corroborating the highly resistant nature of S. aurantiacum, the amount of commercial pyocyanin (i.e., 10 mM) included in the test for comparison, was much higher than the MIC (minimum inhibitory concentration) of pyocyanin used for C. albicans and A. fumigatus (>0.3 mM; Kerr et al., 1999). These amounts are significantly higher than the amount of pyocyanin normally detected in the lungs of CF patients (100 μM; Wilson et al., 1988). However, neither crude phenazines nor pyocyanin showed an inhibitory effect against S. aurantiacum in our assays. A similar phenomenon has been observed in some ascomycetous fungi such as A. sclerotiorum (Hill and Johnson, 1969). Although it is not yet known if the phenazines are modified or sequestered by S. aurantiacum, the production of a red colored pigment in co-cultures could be due to a detoxification mechanism used by the fungus against bacterial phenazines. However, further studies into the chemical structure and UV and visible absorption spectra are required in order to ascertain if the red pigment indicates a modified phenazine.
In addition to phenazines, P. aeruginosa has also been reported to produce a wide variety of other exoproducts/metabolites such as proteases, elastases, haemolysin, and rhamnolipids that contribute to bacterial virulence (McAlester et al., 2008; Ben Haj Khalifa et al., 2011; Heeb et al., 2011; Rella et al., 2012; Mear et al., 2013). Their possible activity against S. aurantiacum will be worthy of a further study.
Most of the CF associated filamentous fungal species have been isolated from the lungs of patients with prolonged antibiotic therapies (Bakare et al., 2003). Previous clinical reports by Blyth et al. (2010b) have also showed an increased prevalence of S. aurantiacum in CF patients administered with antibacterial drugs indicating that the presence of bacteria has an effect on the susceptibility of the lungs to fungal infection. In support of this view, an increase in the growth of the fungus was observed upon a decline in the bacterial growth through addition of gentamicin to the co-culture medium in the present study. Therefore, it seems that the P. aeruginosa strains prevalent in CF patients during early stages of CF hinder fungal infection of lungs by inhibiting their growth.
Conclusion
We have assessed the effect of clinically relevant strains of P. aeruginosa on a newly discovered fungal lung pathogen S. aurantiacum in a synthetic lung-mimicking medium (SCFM) that closely resembles the chemistry of CF sputum. An inhibitory effect of P. aeruginosa was observed on the growth of S. aurantiacum, which can be mediated by the production of biologically active metabolites. Biofilm formation and colonization of fungal hyphae by bacteria were also important for S. aurantiacum growth inhibition. Surprisingly, the toxic P. aeruginosa phenazine pigments, such as pyocyanin, known to have an inhibitory effect against other fungal species including A. fumigatus and C. albicans, proved to be ineffective against S. aurantiacum. This suggests involvement of other virulence determinants and emphasizes the resilient nature of S. aurantiacum compared to other fungi present in lung infections. Further research may include transcriptomic studies of P. aeruginosa – S. aurantiacum co-cultures in order to reveal detailed molecular mechanisms underlying these interactions; these studies will be facilitated by the upcoming annotated S. aurantiacum genome.
Author Contributions
Conceived and designed the experiments: JK, LK, AP, AS, IP, HN. Performed the experiments: JK, SK, BP, MK. Analysed the data: JK, AP, HN. Wrote the paper: JK, HN.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Microscopy unit at Macquarie University for providing access to the confocal microscopy. We also acknowledge the help provided by Debra Birch during the confocal microscopy based examination of liquid cultures. Super Science Fellowship (SSF) awarded to N. H. Packer, M. P. Molloy, HN, IP, and P. A. Haynes by Australian Research Council (ARC) supported the project.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2015.00866
References
- Almeida A. J., Carmona J. A., Cunha C., Carvalho A., Rappleye C. A., Goldman W. E., et al. (2007). Towards a molecular genetic system for the pathogenic fungus Paracoccidioides brasiliensis. Fungal Genet. Biol. 44 1387–1398. 10.1016/j.fgb.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Bakare N., Rickerts V., Bargon J., Just-Nubling G. (2003). Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses 46 19–23. 10.1046/j.1439-0507.2003.00830.x [DOI] [PubMed] [Google Scholar]
- Bandara H. M., Yau J. Y., Watt R. M., Jin L. J., Samaranayake L. P. (2010). Pseudomonas aeruginosa inhibits in-vitro Candida biofilm development. BMC Microbiol. 10:125 10.1186/1471-2180-10-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haj Khalifa A., Moissenet D., Vu Thien H., Khedher M. (2011). [Virulence factors in Pseudomonas aeruginosa: mechanisms and modes of regulation]. Ann. Biol. Clin. (Paris) 69 393–403. 10.1684/abc.2011.0589 [DOI] [PubMed] [Google Scholar]
- Blyth C. C., Harun A., Middleton P. G., Sleiman S., Lee O., Sorrell T. C., et al. (2010a). Detection of occult Scedosporium species in respiratory tract specimens from patients with cystic fibrosis by use of selective media. J. Clin. Microbiol. 48 314–316. 10.1128/JCM.01470-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth C. C., Middleton P. G., Harun A., Sorrell T. C., Meyer W., Chen S. C. (2010b). Clinical associations and prevalence of Scedosporium spp. in Australian cystic fibrosis patients: identification of novel risk factors? Med. Mycol. 48(Suppl. 1) S37–S44. 10.3109/13693786.2010.500627 [DOI] [PubMed] [Google Scholar]
- Boon C., Deng Y., Wang L. H., He Y., Xu J. L., Fan Y., et al. (2008). A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2 27–36. 10.1038/ismej.2007.76 [DOI] [PubMed] [Google Scholar]
- Boucher R. C. (2007). Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58 157–170. 10.1146/annurev.med.58.071905.105316 [DOI] [PubMed] [Google Scholar]
- Brand A., Barnes J. D., Mackenzie K. S., Odds F. C., Gow N. A. (2008). Cell wall glycans and soluble factors determine the interactions between the hyphae of Candida albicans and Pseudomonas aeruginosa. FEMS Microbiol. Lett. 287 48–55. 10.1111/j.15746968.2008.01301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. W., Chin S., Tee K. K., Yin W. F., Choo Y. M., Chan K. G. (2013). N-acyl homoserine lactone-producing Pseudomonas putida strain T2-2 from human tongue surface. Sensors (Basel) 13 13192–13203. 10.3390/s131013192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotirmall S. H., Greene C. M., McElvaney N. G. (2010). Candida species in cystic fibrosis: a road less travelled. Med. Mycol. 48(Suppl. 1) S114–S124. 10.3109/13693786.2010.503320 [DOI] [PubMed] [Google Scholar]
- Cimon B., Carrere J., Vinatier J. F., Chazalette J. P., Chabasse D., Bouchara J. P. (2000). Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 19 53–56. 10.1007/s100960050011 [DOI] [PubMed] [Google Scholar]
- Coutinho H. D., Falcao-Silva V. S., Goncalves G. F. (2008). Pulmonary bacterial pathogens in cystic fibrosis patients and antibiotic therapy: a tool for the health workers. Int. Arch. Med. 1 24 10.1186/1755-7682-1-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugini C., Morales D. K., Hogan D. A. (2010). Candida albicans-produced farnesol stimulates Pseudomonas quinolone signal production in LasR-defective Pseudomonas aeruginosa strains. Microbiology 156 3096–3107. 10.1099/mic.0.037911-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. C. (2002). Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr. Respir. Rev. 3 128–134. 10.1016/S1526-0550(02)00003-3 [DOI] [PubMed] [Google Scholar]
- Delhaes L., Monchy S., Frealle E., Hubans C., Salleron J., Leroy S., et al. (2012). The airway microbiota in cystic fibrosis: a complex fungal and bacterial community–implications for therapeutic management. PLoS ONE 7:e36313 10.1371/journal.pone.0036313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring G., Conway S. P., Heijerman H. G., Hodson M. E., Hoiby N., Smyth A., et al. (2000). Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur. Respir. J. 16 749–767. 10.1034/j.1399-3003.2000.16d30.x [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. (1988). High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16 6127–6145. 10.1093/nar/16.13.6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J., Sood A., Hogan D. A. (2009). Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 75 504–513. 10.1128/AEM.01037-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloag E. S., Turnbull L., Huang A., Vallotton P., Wang H., Nolan L. M., et al. (2013). Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc. Natl. Acad. Sci. U.S.A. 110 11541–11546. 10.1073/pnas.1218898110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B., Kraut J., Marks J., Zanker S. C., Castignetti D. (1991). Siderophore presence in sputa of cystic fibrosis patients. Infect. Immun. 59 3997–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison F. (2007). Microbial ecology of the cystic fibrosis lung. Microbiology 153 917–923. 10.1099/mic.0.2006/004077-0 [DOI] [PubMed] [Google Scholar]
- Harun A., Gilgado F., Chen S. C., Meyer W. (2010). Abundance of Pseudallescheria/Scedosporium species in the Australian urban environment suggests a possible source for scedosporiosis including the colonisation of airways in cystic fibrosis. Med. Mycol. 48(Suppl. 1) S70–S76. 10.3109/13693786.2010.515254 [DOI] [PubMed] [Google Scholar]
- Heath C. H., Slavin M. A., Sorrell T. C., Handke R., Harun A., Phillips M., et al. (2009). Population based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clin. Microbiol. Infect. 15 689–693. 10.1111/j.1469-0691.2009.02802.x [DOI] [PubMed] [Google Scholar]
- Heeb S., Fletcher M. P., Chhabra S. R., Diggle S. P., Williams P., Camara M. (2011). Quinolones: from antibiotics to autoinducers. FEMS Microbiol. Rev. 35 247–274. 10.1111/j.1574-6976.2010.00247.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbing M. E., Fuqua C., Parsek M. R., Peterson S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8 15–25. 10.1038/nrmicro2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. C., Johnson G. T. (1969). Microbial transformation of phenazines by Aspergillus sclerotiorum. Mycologia 61 452–467. 10.2307/3757234 [DOI] [PubMed] [Google Scholar]
- Hogan D. A., Kolter R. (2002). Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296 2229–2232. 10.1126/science.1070784 [DOI] [PubMed] [Google Scholar]
- Holloway B. W. (1955). Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13 572–581. 10.1099/00221287-13-3-572 [DOI] [PubMed] [Google Scholar]
- Horre R., Symoens F., Delhaes L., Bouchara J. P. (2010). Fungal respiratory infections in cystic fibrosis: a growing problem. Med. Mycol. 48(Suppl. 1) S1–S3. 10.3109/13693786.2010.529304 [DOI] [PubMed] [Google Scholar]
- Inoue H., Nojima H., Okayama H. (1990). High efficiency transformation of Escherichia coli with plasmids. Gene 96 23–28. 10.1016/0378-1119(90)90336-P [DOI] [PubMed] [Google Scholar]
- Jarosz L. M., Ovchinnikova E. S., Meijler M. M., Krom B. P. (2011). Microbial spy games and host response: roles of a Pseudomonas aeruginosa small molecule in communication with other species. PLoS Pathog. 7:e1002312 10.1371/journal.ppat.1002312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jieh-Juen Yu G. T. C. (1998). Biolistic transformation of the human pathogenic fungus Coccidioides immitis. J. Microbiol. Methods 33 129–141. 10.1016/S0167-7012(98)00046-3 [DOI] [Google Scholar]
- Kaur J., Duan S. Y., Vaas L. A., Penesyan A., Meyer W., Paulsen I. T., et al. (2015). Phenotypic profiling of Scedosporium aurantiacum, an opportunistic pathogen colonizing human lungs. PLoS ONE 10:e0122354 10.1371/journal.pone.0122354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. R. (1999). Bacterial inhibition of fungal growth and pathogenicity. Microb. Ecol. Health Dis. 11 129–142. 10.1080/089106099435709 [DOI] [Google Scholar]
- Kerr J. R., Taylor G. W., Rutman A., Hoiby N., Cole P. J., Wilson R. (1999). Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 52 385–387. 10.1136/jcp.52.5.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klix V., Nowrousian M., Ringelberg C., Loros J. J., Dunlap J. C., Poggeler S. (2010). Functional characterization of MAT1-1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and nonessential sexual regulators. Eukaryot. Cell 9 894–905. 10.1128/EC.00019-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner M., de Hoog G. S., Verweij P. E., Najafzadeh M. J., Curfs-Breuker I., Klaassen C. H., et al. (2012). Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob. Agents Chemother. 56 2635–2642. 10.1128/AAC.0591011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner M., de Hoog G. S., Yang L., Moreno L. F., Ahmed S. A., Andreas F., et al. (2014). Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Divers. 67 1–10. 10.1007/s13225-014-0295-4 [DOI] [Google Scholar]
- Laursen J. B., Nielsen J. (2004). Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 104 1663–1686. 10.1021/cr020473j [DOI] [PubMed] [Google Scholar]
- Leclair L. W., Hogan D. A. (2010). Mixed bacterial-fungal infections in the CF respiratory tract. Med. Mycol. 48(Suppl. 1)S125–S132. 10.3109/13693786.2010.521522 [DOI] [PubMed] [Google Scholar]
- Lewenza S., Charron-Mazenod L., Giroux L., Zamponi A. D. (2014). Feeding behaviour of Caenorhabditis elegans is an indicator of Pseudomonas aeruginosa PAO1 virulence. PeerJ 2 e521. 10.7717/peerj.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Wagner M. C., Cocklin R., Kuzma A., Harrington M., Molitoris B. A., et al. (2011). The antibiotic gentamicin inhibits specific protein trafficking functions of the Arf1/2 family of GTPases. Antimicrob. Agents Chemother. 55 246–254. 10.1128/AAC.00450-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipuma J. J. (2010). The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 23 299–323. 10.1128/CMR.00068-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. V., Bruce K. D. (2013). The cystic fibrosis airway microbiome. Cold Spring Harb. Perspect. Med. 3:a009738 10.1101/cshperspect.a009738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Conover M., Lu H., Parsek M. R., Bayles K., Wozniak D. J. (2009). Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 5:e1000354 10.1371/journal.ppat.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavathu E. K., Vager D. L., Vazquez J. A. (2014). Development and antimicrobial susceptibility studies of in vitro monomicrobial and polymicrobial biofilm models with Aspergillus fumigatus and Pseudomonas aeruginosa. BMC Microbiol. 14:53 10.1186/1471-2180-14-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrodi D. V., Bonsall R. F., Delaney S. M., Soule M. J., Phillips G., Thomashow L. S. (2001). Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 183 6454–6465. 10.1128/JB.183.21.64546465.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlester G., O’Gara F., Morrissey J. P. (2008). Signal-mediated interactions between Pseudomonas aeruginosa and Candida albicans. J. Med. Microbiol. 57 563–569. 10.1099/jmm.0.47705-0 [DOI] [PubMed] [Google Scholar]
- Mear J. B., Kipnis E., Faure E., Dessein R., Schurtz G., Faure K., et al. (2013). Candida albicans and Pseudomonas aeruginosa interactions: more than an opportunistic criminal association? Med. Mal. Infect. 43 146–151. 10.1016/j.medmal.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Middleton P. G., Chen S. C., Meyer W. (2013). Fungal infections and treatment in cystic fibrosis. Curr. Opin. Pulm. Med. 19 670–675. 10.1097/MCP.0b013e328365ab74 [DOI] [PubMed] [Google Scholar]
- Mowat E., Rajendran R., Williams C., McCulloch E., Jones B., Lang S., et al. (2010). Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol. Lett. 313 96–102. 10.1111/j.1574-6968.2010.02130.x [DOI] [PubMed] [Google Scholar]
- O’Sullivan B. P., Freedman S. D. (2009). Cystic fibrosis. Lancet 373 1891–1904. 10.1016/S0140-6736(09)60327-5 [DOI] [PubMed] [Google Scholar]
- Palmer K. L., Aye L. M., Whiteley M. (2007). Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189 8079–8087. 10.1128/JB.01138-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugam A., Baixench M. T., Demazes-Dufeu N., Burgel P. R., Sauter E., Kanaan R., et al. (2010). Characteristics and consequences of airway colonisation by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med. Mycol. 48(Suppl. 1)S32–S36. 10.3109/13693786.2010.503665 [DOI] [PubMed] [Google Scholar]
- Penttilä M., Nevalainen H., Rättö M., Salminen E., Knowles J. (1987). A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61 155–164. 10.1016/0378-1119(87)90110-7 [DOI] [PubMed] [Google Scholar]
- Pihet M., Carrere J., Cimon B., Chabasse D., Delhaes L., Symoens F., et al. (2009). Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis–a review. Med. Mycol. 47 387–397. 10.1080/13693780802609604 [DOI] [PubMed] [Google Scholar]
- Punt P. J., Dingemanse M. A., Kuyvenhoven A., Soede R. D., Pouwels P. H., van den Hondel C. A. (1990). Functional elements in the promoter region of the Aspergillus nidulans gpdA gene encoding glyceraldehyde-3-phosphate dehydrogenase. Gene 93 101–109. 10.1016/0378-1119(90)90142-E [DOI] [PubMed] [Google Scholar]
- Quinton P. M. (1999). Physiological basis of cystic fibrosis: a historical perspective. Physiol. Rev. 79 S3–S22. [DOI] [PubMed] [Google Scholar]
- Rella A., Yang M. W., Gruber J., Montagna M. T., Luberto C., Zhang Y. M., et al. (2012). Pseudomonas aeruginosa inhibits the growth of Cryptococcus species. Mycopathologia 173 451–461. 10.1007/s11046-011-9494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Diez B., Martinez-Suarez J. V. (1999). Electrotransformation of the human pathogenic fungus Scedosporium prolificans mediated by repetitive rDNA sequences. FEMS Immunol. Med. Microbiol. 25 275–282. 10.1111/j.1574-695X.1999.tb01352.x [DOI] [PubMed] [Google Scholar]
- Sibley C. D., Rabin H., Surette M. G. (2006). Cystic fibrosis: a polymicrobial infectious disease. Future Microbiol. 1 53–61. 10.2217/17460913.1.1.53 [DOI] [PubMed] [Google Scholar]
- Singh P. K., Schaefer A. L., Parsek M. R., Moninger T. O., Welsh M. J., Greenberg E. P. (2000). Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407 762–764. 10.1038/35037627 [DOI] [PubMed] [Google Scholar]
- Tang H., Kays M., Prince A. (1995). Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect. Immun. 63 1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te’o V. S., Bergquist P. L., Nevalainen K. M. (2002). Biolistic transformation of Trichoderma reesei using the Bio-Rad seven barrels Hepta Adaptor system. J. Microbiol. Methods 51 393–399. 10.1016/S0167-7012(02)00126-4 [DOI] [PubMed] [Google Scholar]
- Te’o V. S., Cziferszky A. E., Bergquist P. L., Nevalainen K. M. (2000). Codon optimization of xylanase gene xynB from the thermophilic bacterium Dictyoglomus thermophilum for expression in the filamentous fungus Trichoderma reesei. FEMS Microbiol. Lett. 190 13–19. 10.1111/j.1574-6968.2000.tb09255.x [DOI] [PubMed] [Google Scholar]
- Thornton J. A. (2015). Splicing by overlap extension PCR to obtain hybrid DNA products. Methods Mol. Biol. 10.1007/7651_2014_182 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Watson D., Rutman A., Taylor G. W., Cole P. J. (1988). Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 56 2515–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.