Abstract
Objective
To assess whether the pattern of diffusion changes among a cohort of individuals showing BMI-related increases in white matter volume reflects healthy expansion of myelin or damaged white matter.
Design and Methods
Diffusion MRI measures (axial, radial, and fractional anisotropy) were obtained from 94 females, ages 52–92. Relationships between BMI and diffusion measures were assessed controlling for age, hypertension, and diabetes status using general linear modelling. Associations between diffusion measures and cognitive status (memory, executive functions, and visuomotor speed) were assessed using multiple regressions, controlling for age, education, hypertension, and diabetes status.
Results
Higher levels of BMI were associated with lower axial diffusion in frontal, temporal, parietal, internal capsule, and cerebellar white matter. Lower fractional anisotropy was observed in bilateral temporal white matter and the right corticospinal tract, with high radial diffusion in temporal and temporoparietal white matter. Importantly, diffusion measures predicted reductions in executive functioning, memory, and visuomotor speed.
Conclusions
The pattern of diffusion changes in regions of white matter showing BMI-related volume increases are not due to expansion of normal myelin, but instead suggest damage to white matter that has important consequences for cognitive functioning.
Keywords: Aging, Cognition, Diffusion tensor imaging, Obesity
Introduction
There is growing evidence suggesting that increased body fat during mid-life and late-life is associated with functional and structural changes in the brains of older adults. Overweight and obese individuals show lower levels of cognitive performance in the areas of executive function, sustained attention, and memory (1–4), although other studies have failed to find similar relationships (5,6). The rate of cognitive decline in older adults increases as body weight increases (1,7), and risk for developing neurodegenerative diseases, including Alzheimer’s dementia, also increases (8–10).
These cognitive effects may be mediated by structural brain changes associated with increased body weight. In studies using morphometric analysis of MRI and CT, greater body weight has been associated with smaller whole brain volumes in middle-aged individuals (5,11) and smaller hippocampal volumes (12). Voxel-based morphometry (VBM) studies (13) have provided evidence of a complex pattern of volumetric differences, identifying both larger and smaller cortical volumes in obese individuals compared to normal-weight controls in the frontal, temporal and parietal lobe, cerebellum, and subcortical regions (11,14,15).
In a recent VBM study (4) we examined the association between BMI, regional volume differences in gray and white matter, and cognitive functioning in 95 community-dwelling older females ages 52 to 92. Higher BMI was associated with smaller volumes in multiple gray matter regions including the inferior and orbital frontal cortex, a large region of right posterior cortex extending from the parahippocampal gyrus to the occipital lobe, and the right cerebellum. The most intriguing finding, however, was extensive larger regions of anterior and posterior white matter volumes associated with higher BMI. This finding was consistent with other studies reporting larger white matter volumes in the temporal lobe, cerebellum, and brainstem (16) and striatal and orbito-frontal white matter (14) in obese compared with normal weight individuals. Remarkably, the effect in one study (16) was partially reversed when participants dieted for six weeks. The authors speculated that deposition of visceral body fat may be associated with the accumulation of fat in the central myelin resulting in denser myelinisation in obese individuals. This hypothesis, however, is difficult to reconcile with studies suggesting that increased body fat results in white matter damage including increased matter hyperintensities (12) and lower concentrations of N-acetylaspartate and choline-containing compounds in white matter, suggestive of axonal and/or myelin abnormalities (17). Thus, whether increases in white matter volume associated with higher BMI are signaling increased myelin density or damage to white matter remains unclear.
Diffusion MRI may be helpful in shedding light on this issue, as it provides measures that are sensitive to the microstructural integrity of white matter. Several parameters can be obtained from the diffusion tensor that relate to different aspects of white matter structure and pathology. The most commonly used index is fractional anisotropy (FA) which describes the directionality of the molecular diffusion, likely reflecting a complex combination of parallelism of fibers within a voxel, fiber density, axonal diameter, and myelination. Consequently, FA is a sensitive but relatively nonspecific marker of white matter integrity that decreases in many neurological diseases (18–20) as well as normal aging (21–22). Component parameters of the diffusion tensor may provide more specific information regarding axonal properties and myelinisation. Axial diffusion represents motion of water parallel to the axon tracts (λ1), while radial diffusion is defined as the magnitude of water movement perpendicular to the axon tracts (average of λ2 and λ3). Recent animal models suggest that changes to axial diffusion reflect axonal degradation while radial diffusion is related to the integrity of the myelin sheath (23–24).
Relatively few studies have determined how diffusion properties of white matter relate to body fat. Most consistently these studies report decreases in FA associated with higher BMI in the frontal white matter (25–26) and prominent tracts including the corpus callosum (27) and cingulum bundle (28). Decreased frontal white matter FA, relative to normal-weight controls, has been observed in patients with metabolic syndrome (25) as well as morbidly obese individuals (26–27). In a sample of 103 otherwise healthy adults, ages 21–86, age-related decreased in FA were exacerbated by higher BMI in the corpus callosum and fornix bundle (28). Verstynen et al. (29) suggested that BMI-related changes in white matter FA are distributed globally throughout the cortex and brainstem. BMI-related differences in other measures of diffusion have been reported less frequently, including axial diffusivity in the corpus callosum in young males and females consistent with axonal damage, and differences in radial diffusivity in the corpus callosum only among female participants, suggesting that some components of myelin damage may be gender-specific (30).
The present cross-sectional study investigated the association between BMI and white matter diffusion obtained from 94 of the 95 older females (BMI 19–45) who participated in Walther et al. (4). Axial, radial, and FA parameters were calculated to determine whether axonal or myelin integrity (or both) are affected by increased BMI. Voxel-based analysis was used to examine these measures as a function of increasing BMI across the whole brain. Because this method requires a conservative correction for multiple comparisons, we performed additional region of interest (ROI) analyses in order to examine specific regions showing larger white matter volumes reported previously (4). We were interested in whether increased white matter volumes reflect enhanced density of the myelin sheath (16) or damaged white matter (17). Diffusion measures extracted from the ROI’s were related to three domains of cognitive functioning – memory, executive function, and visuomotor speed – to determine whether diffusion changes associated with higher BMI have implications for cognitive functions.
Results
Demographics and cognitive measures
BMI was entered as a continuous variable in all analyses. For descriptive purposes only, demographic data is presented using the classification system of the World Health Organization (WHO; http://www.who.int/topics/obesity/en/). Normal weight, overweight, and obese participants were well matched on age (Table 1). Not unexpectedly, the obese group included a higher percentage of individuals with diagnosed hypertension. One participant in the obese group had a BMI above 40 (WHO Class III obesity). One overweight and two obese participants were diagnosed with type 2 diabetes, and one obese participant met criteria for metabolic syndrome. Although the obese group had significantly fewer years of education compared to the normal weight controls, the groups were well matched on vocabulary scores, which provides a good premorbid indication of intellectual attainment. On average, memory and visuomotor speed scores did not differ across the groups. However, executive function scores for the obese group were significantly lower than the normal weight group, p<.05, but did not differ from the overweight group.
Table 1.
Demographic information and neuropsychological test results for three groups based on body-mass index (BMI).
| Normal-weight (n = 52) | Overweight (n = 22) | Obese (n = 20) | |
|---|---|---|---|
| Age (years) | 71.2 ± 9.8 | 69.9 ± 8.1 | 66.9 ± 9.9 |
| Education (years) | 16.2 ± 2.3 | 15.9 ± 2.9 | 14.2 ± 2.0a |
| BMI (kg/m2) | 22.3 ± 1.6 | 27.6 ± 1.4 | 34.9 ± 3.3 |
| Hypertension (n) | 8 (15%) | 5 (23%) | 10 (50%)a |
| Diabetes (n) | 0 | 1 (5%) | 2 (10%) |
| MMSE (raw score) | 28.9 ± 1.5 | 29.2 ± 0.9 | 29.1 ± 0.9 |
| Vocabulary (raw score) | 69.5 ± 5.6 | 70.0 ± 5.4 | 67.8 ± 6.9 |
| Memory Function (z-score) | 0.31 ± 0.71 | 0.44 ± 0.46 | 0.43 ± 0.69 |
| Executive Function (z-score) | 0.11 ± 0.59 | 0.04 ± 0.60 | −0.28 ± 0.62a |
| Trails A (seconds) | 34.5 ± 10.6 | 34.6 ± 11.7 | 34.9 ± 13.9 |
Except for hypertension (number and percentage of participants), values represent means and standard deviations;
significantly different from normal weight females.
VBM whole brain analysis
The relationships between white matter diffusion measures and BMI were examined using a GLM analysis in SPM2 that included BMI as a continuous predictor with three control variables: age (mean-centered continuous variable), hypertension and diabetes status (categorical variables). Voxel-wise alpha was set at p < 0.05 employing a false discovery rate (FDR) correction for multiple comparisons. Clusters of 10 or more contiguous voxels are reported.
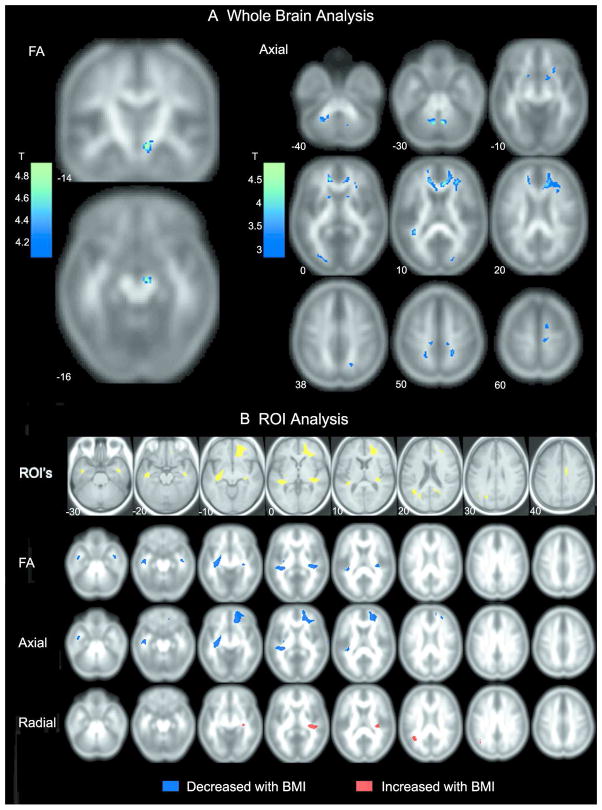
The results (summarized in Table 2 and Figure 1) indicated that higher BMI was associated with a pattern of diffusion change consistent with damage to white matter. BMI-related decreases in FA were evident in the right cerebral peduncle likely including both the corticospinal and corticobulbar tracts. Importantly, no voxels showed a positive association between BMI and FA. Axial diffusion decreased in the genu, the arcuate fasciculus bilaterally, the right uncinate fasciculus, the anterior limb of the internal capsule bilaterally, bilateral regions of the superior corona radiata in frontal and parietal white matter, the left superior longitudinal fasciculus, left and right occipital lobe, and the superior and inferior cerebellar peduncles bilaterally. No voxels showed a positive correlation between BMI and axial diffusion. Additionally, no voxels were positively or negatively correlated with radial diffusivity.
Table 2.
Brain regions where BMI predicts diffusion measures in a VBM whole brain analysis, controlling for age, hypertension, and diabetes status.
| MNI | Cluster size | |||||
|---|---|---|---|---|---|---|
| Lobe | x | y | z | tvalue | ||
| FA decrease with BMI increase | ||||||
| R cst/cbt | BS | 8 | −14 | −16 | 4.96 | 45 |
| AD decrease with BMI increase | ||||||
| R genu, arc, unc, alic | F | 8 | 32 | 8 | 4.79 | 823 |
| L genu, arc | F | −14 | 40 | 0 | 4.39 | 273 |
| R frontal wm | F | 30 | 18 | 32 | 3.25 | 12 |
| R alic | SC | 12 | 12 | −8 | 3.63 | 46 |
| L alic | SC | 16 | 10 | 6 | 3.63 | 24 |
| L alic | SC | −16 | 10 | 4 | 3.61 | 33 |
| R scr | F | 16 | 0 | 60 | 3.89 | 16 |
| R scr | F | 10 | −28 | 56 | 3.51 | 65 |
| L scr | F | −10 | −30 | 52 | 3.14 | 13 |
| R scr | P | 20 | −66 | 38 | 3.38 | 17 |
| R scr | P | 22 | −44 | 52 | 4.17 | 59 |
| L scr | P | −20 | −50 | 48 | 3.35 | 36 |
| L slf | T | −38 | −44 | 10 | 4.12 | 70 |
| R occipital wm | O | 20 | −84 | 18 | 3.66 | 19 |
| L occipital wm | O | −24 | −88 | −2 | 3.56 | 55 |
| R scp/icp | CB | 6 | −62 | −32 | 4.97 | 69 |
| L scp/icp/mcp | CB | −8 | −62 | −32 | 4.65 | 191 |
The coordinates of the location of maximal significance (from Montreal Neurological Institute template [MNI]), the t value, and cluster size (voxel number) of each cluster are provided. arc: arcuate fasciculus, alic: anterior limb of the internal capsule, AD: axial diffusion, BS: brain stem, CB: cerebellum, cbt: cortico bulbar tract, cst: corticospinal tract, F: frontal, FA: fractional anisotropy, icp: inferior cerebellar peduncle, L: left hemisphere, mcp: middle cerebellar peduncle, O: occipital, R: right, P: parietal, scp: superior cerebellar peduncle, scr: superior corona radiata, slf: superior longitudinal fasciculus, T: temporal, unc: uncinate fasciculus.
Figure 1.
A) Regions identified from the whole brain analysis showing significant negative correlations between BMI and FA (left side) and BMI and axial diffusion (right side). B) Results of the ROI analysis. Axial sections in the first row depict areas of increased white matter volume with increasing BMI identified previously in VBM analysis (4). Diffusion measures were extracted from these regions of interest. ROIs of significant correlations between BMI and FA, axial, and radial diffusion are shown in the following rows. Blue represents negative correlations with BMI and red represents a positive correlation with BMI.
ROI analyses
For the eleven ROIs, the relationship between BMI and diffusion measures was determined using GLM analyses. For each region and each diffusion measure, BMI was entered as a continuous predictor variable controlling for age, hypertension and diabetes status. Because diabetes did not enter as a significant predictor into any model (all p’s>.25), this variable was dropped from the models. Table 3 shows the variance accounted for (adjusted R2) and F statistic for all models where BMI significantly predicted diffusion measures after controlling for age and hypertension status. Consistent with previous literature (21–22), increasing age was consistently associated with changes in FA, axial, and radial diffusion in all significant models, and hypertension significantly predicted diffusion in six of the eleven ROIs.
Table 3.
GLM models significantly predicting diffusion measures (FA, axial, radial) from BMI controlling for age and hypertension status in white matter regions previous shown to increase in volume with higher BMI (4).
| FA | Axial | Radial | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| R2Adj | F | R2Adj | F | R2Adj | F | |
| R orbital frontal (12, 51, −14) | .17 | 8.35 *** | ||||
| R inferior frontal | .16 | 6.23 ** | ||||
| R superior frontal | .25 | 14.01 *** | ||||
| L temporal stem (−32, −12, −13) | .27 | 12.54 *** | .25 | 11.13 *** | ||
| R temporal stem (39, −1, −31) | .11 | 5.59 ** | .25 | 11.22 *** | ||
| R superior temporal (35, −27, 6) | .27 | 12.28 *** | .19 | 8.14 ** | ||
| R cingulate (9, −6, 41) | ||||||
| R posterior cingulate (22, −57, 20) | ||||||
| L parietal (−16, −35, 59) | ||||||
| L temporoparietal (medial; −40, −57, 23) | .19 | 11.54 *** | ||||
| L temporoparietal (lateral) | ||||||
p <.001;
p <.01,
p <.05
Additional significant correlations between BMI and diffusion measures were observed in regions that were not observed in the VBM whole brain analysis (Table 3, Figure 1B). Higher BMI was associated with lower FA values bilaterally in the temporal stem and the right superior temporal white matter, regions not observed in the whole brain analysis. Axial diffusion decreased in the right orbital, inferior, and superior frontal white matter and the temporal stem bilaterally. While the whole brain analysis did not reveal any significant correlations between radial diffusion and BMI, the ROI analysis demonstrated that radial diffusion was positively correlated with BMI in two regions; the right superior temporal and left medial temporoparietal white matter.
Diffusion measures and cognition
Multiple regression analyses were carried out predicting memory, executive functioning, and visuomotor speed from diffusion measures, controlling for age, years of education, and hypertension status. Table 4 includes the standardized beta coefficients (all p’s <.05) for diffusion measures that were obtained from models that met or exceeded p<.0001. Executive functioning was positively predicted by axial diffusion in three regions of right frontal white matter – superior, inferior, and orbital frontal, as well as left parietal white matter. Memory functioning was predicted by increased radial diffusion in three temporal lobe regions, the left and right temporal stem and the left temporoparietal white matter, as well as the right posterior cingulate. Memory scores were also predicted by decreased FA in two of these same regions, the right posterior cingulate and the left temporoparietal white matter region. Visuomotor speed was predicted by differences in both radial diffusion and FA in the right orbital and superior frontal white matter.
Table 4.
Regression models showing diffusion measures (Axial, Radial, FA) predicting cognitive measures after controlling for age, hypertension status, and years of education. Listed are the standardized beta coefficients (all p’s<.05) for diffusion measures from regression models that were significant at p<.0001.
| Standardized Beta Coefficient | |||
|---|---|---|---|
|
| |||
| Axial | Radial | FA | |
| Memory function | |||
| Left temporal stem (−32, −12, −13) | −.21 | ||
| Right temporal stem (39, −1, −31) | −.21 | ||
| Right posterior cingulate (22, −57, 20) | −.32 | .27 | |
| Left temporoparietal (medial; −40, −57, 23) | −.23 | .22 | |
| Executive function | |||
| Right inferior frontal | .24 | ||
| Right orbital frontal | .27 | ||
| Right superior frontal | .20 | ||
| Left parietal (−16, −35, 59) | .23 | ||
| Visuomotor speed | |||
| Right orbital frontal (12, 51, −14) | .22 | −.27 | |
| Right superior frontal | .22 | −.24 | |
Discussion
The present study expands upon our previous study demonstrating larger white matter volumes associated with higher BMI in a sample of older females. The results indicated diffusion changes that exceed expected age-related changes in diffusion (21,22), consistent with previous reports of white matter damage in obese individuals (12,17). In this relatively healthy sample, BMI-related differences in diffusion were observed throughout frontal and temporal white matter extending into the parietal and occipital lobes, as well as cerebellar white matter. In regions of white matter where larger volumes were previously observed, higher BMI was associated with lower axial diffusion in both frontal and temporal lobe white matter, and lower FA in temporal lobe white matter. Higher radial diffusion was also present in the temporal and temporoparietal white matter.
The VBM diffusion analysis demonstrated that BMI-related alterations in white matter integrity go beyond previously observed regions of increased white matter volumes. Axial diffusion, in particular, was affected in multiple tracts including the genu, arcuate fasciculus, internal capsule, and the cerebellar peduncles. Diffusion may be a more sensitive marker of white matter pathology than white matter volume, suggesting that white matter is compromised throughout the brain and brainstem, consistent with a recent whole brain diffusion study by Verstynen et al. (29) reporting that the distributions of FA, radial, and axial diffusivity shift globally with higher BMI.
Some differences between the present study and previous reports may relate to the impact of conditions that commonly co-occur with obesity. For example, Segura and colleagues (26) reported increased ADC in frontal and temporal regions associated with metabolic syndrome (reporting health conditions including hypertension, high cholesterol, hyperlipidemia, and type 2 diabetes in addition to central obesity) compared to controls with no vascular risk factors. ADC changes could be driven by increases in axial diffusion, radial diffusion, or both. However, in the present study, axial diffusion decreased as a function of BMI. In the present study, while half the obese participants reported a diagnosis of hypertension, type 2 diabetes was rare, and only one individual met criteria for metabolic syndrome, suggesting that diffusion differences were likely related directly to BMI. Consistent with our findings, Karlsson et al. (26) reported decreases in mean diffusivity of frontal white matter in morbidly obese middle-aged adults compared to normal-weight controls. Verstynen et al. (29) reported both lower axial and higher radial diffusivity in a healthy sample of individuals with a range of BMI scores (19.5–45.7) similar to this study. The health conditions included in metabolic syndrome may have differential effects on diffusion measures compared to the isolated effect of BMI, and more work needs to be done to separate the influence of these various conditions on white matter integrity.
There is a growing literature emphasizing the involvement of the frontal lobes in obesity. Recent studies have linked obesity to reductions in frontal gray matter volumes (11,14), white matter volumes (26), and changes in white matter diffusion (26–28). Functional changes, including decreased glucose metabolism in prefrontal areas (3), and increased regional cerebral blood flow in responses to satiety (32), have also been reported. Symptoms of hyperphagia, cravings for sweet food, and compulsive overeating have been associated with more prominent grey matter atrophy in the orbitofrontal cortex of frontotemporal dementia patients (33,34). The frontal lobes have been implicated in many processes related to food intake, including multimodal integration of sensory information and the representation of flavor (32), inhibitory responses to satiety (34), and response to the rewarding properties of food (35). Interestingly, diffusion changes as well as white matter volume increases associated with BMI in our female sample were more prominent in the right frontal hemisphere whereas other regions showed a more bilateral pattern, consistent with recent research emphasizing a crucial role of the right prefrontal cortex in the regulation of food intake (36).
A relevant question is whether the pattern of diffusion change observed here can provide clues to the pathology associated with increased body fat. Recent evidence suggests that increased radial diffusion may relate to the integrity of the myelin sheath whereas decreases in axial diffusion reflect axonal degradation. Song et al. (23) observed increased radial diffusion with no change in axial diffusion in Shiverer mice that carry a mutation resulting in a lack of myelin without axonal injury. In contrast, decreased axial diffusion with preserved radial diffusion was associated with damage to axons in a mouse model of acute retinal ischemia resulting in degeneration of the axons of the optic nerve with intact myelin sheath (24). Animal studies may have limited generalizability to the present study since these may not apply to a chronic condition such as obesity. Further, the use of axial and radial diffusion to infer alterations in underlying tissue structure can be problematic, particularly in regions of white matter with crossing fibers (37). Nevertheless, the present finding of widespread decreases in axial diffusion is suggestive of axonal degeneration. Increased radial diffusion was also observed in temporal and parietal lobe white matter, suggesting that myelin damage may also occur in some brain regions. Alternatively, changes to diffusion parameters may be due to the presence of inflammatory cells or cytotoxic edema, suggested by recent work in HIV positive individuals (38). Consistent with this notion, Cazettes and colleagues (39) found that both frontal white matter volumes and DTI measures in overweight/obese individuals were predicted by plasma fibrinogen levels, a marker of inflammation. Ultimately, the specific white matter microstructural changes associated with increasing body fat will only be addressed fully with appropriate animal models and human post-mortem studies.
Finally, an important finding of this study was that BMI-related white matter damage in frontal, temporal, and parietal white matter regions have significant relevance to cognitive functioning. Diffusion measures in frontal white matter regions predicted executive function scores and visuomotor speed, while temporal and parietal white matter measures predicted memory scores. The results are consistent with a recent PET study (3) demonstrating that higher BMI was associated with lower baseline glucose metabolism in prefrontal regions which, in turn, predicted poorer performance on executive and memory tasks. In our previous study (4), although gray matter volume decreases were related to lower performance in both executive function and memory, white matter volumes did not predict cognitive performance in either domain. This suggests that diffusion provides a more sensitive and functionally-relevant measure of the integrity of normal-appearing white matter than volume. Taken together, these findings suggest that BMI-related structural and metabolic brain changes result in impairments in executive and memory functions, two areas of cognitive functions that probably have important implications for the control and monitoring of food intake, as well as processing speed.
In summary, diffusion MRI revealed a pattern of changes that suggests pathology to the white matter, although the specific nature of that pathology warrants further examination. The study further supports the notion of a negative impact of higher body fat on cognitive functioning in the areas of executive functioning, memory, and processing speed.
Methods
Image acquisition
Images were acquired on a GE 3.0T Signa VH/I whole body echospeed scanner equipped with an 8-channel phased array coil (HD Signa Excite, General Electric, Milwaukee, WI). High resolution T1-weighted structural images were acquired using a 3D SPGR pulse sequence, section thickness 0.7mm, no skip (TE/TR/TI=2ms/5.1ms/500ms; Flip Angle=15°; Matrix=256×256; FOV=260×260mm2). T2 fluid-attenuated inversion recovery (T2-FLAIR) images were collected with fifty-eight axial images covering the whole brain (2.6 mm slice thickness, no gap, TE/TR/TI=120 ms/11,000 ms/2250 ms, matrix=256×192, FOV=260×260 mm2). In the same session, echo planar diffusion images were collected and corrected for spatial distortion (GE ASSET). Diffusion was measured in 25 directions with 2 averages (b0=1000s/mm2, 2 NEX) in fifty-eight axial sections, 2.6mm, no gap, covering the whole brain (TE/TR=71ms/13000ms, Matrix 96×96, FOV=250×250mm2), and resampled at 256×256 using sinc interpolation.
Image processing
Images were realigned to remove eddy current distortions using FSL software (www.fmrib.ox.ac.uk/fsl). DTI Studio Version 2.4 (https://www.dtistudio.org/) was used to compute a diffusion tensor for each voxel that included three eigenvalues and eigenvectors. Based on these values, FA, axial diffusion (λ1), and radial diffusion (mean of λ2 and λ3) maps were computed for each participant. SPM2 (http://www.fil.ion.ucl.ac.uk/spm/) was used for all post-processing and statistical analyses.
Whole-brain white matter analysis
Diffusion data were normalize to a custom template created from T2-weighted b0 images of all participants. Images were normalized to the echo-planar MNI template using an affine transformation followed by non-linear normalization, then averaged and smoothed using an 8-mm FWHM isotropic Gaussian kernel. Each participant’s original b0 volume was then normalized to the customized template using the same algorithm, and the resulting transformation parameters were applied to the FA, axial, and radial diffusion maps. Diffusion maps were smoothed with an 8mm FWHM kernel. FA maps were thresholded at an absolute value of 0.2 in order to restrict the analyses to white matter. The mask created from the thresholded FA map was applied to the axial and radial diffusion maps to ensure that the same white matter voxels were included in all regression analyses. The thresholded FA maps were overlaid on the T2 Flair image to ensure that hyperintense voxels were not included in the mask.
Regions of interest (ROI) analysis
Eleven ROIs with a cluster size of 200 voxels or more were identified from regions of white matter volume that increased with BMI in the previously reported VBM study (4). The ROI masks (Figure 1B, first row) were transformed back to native MRI space for each participant by applying the inverse of the spatial normalization parameter matrix. The inverse of the transformation matrix was also applied to the normalized, unmodulated white matter segmented image for each individual. The white matter segment was thresholded conservatively to include only voxels with a 90% probability of being white matter. Diffusion maps were co-registered to the participant’s T1-weighted image. The eleven ROI masks were then applied and multiplied by the individualized white matter segmented image using MarsBaR (http://marsbar.sourceforge.net/), thus ensuring that only fully-volumed white matter voxels were included in the ROI and removing regions of white matter hyperintensity. Diffusion measures were obtained for each ROI for a given individual by averaging the diffusion values across all extracted voxels.
What is already known about this subject?
Higher BMI is associated with smaller gray matter volumes, particularly in brain regions associated with control of food intake.
Surprisingly, higher BMI has also been associated with larger white matter volumes, but it is unclear as to whether these changes are related to healthy expansion of white matter or structural damage.
MRI diffusion changes have been reported in white matter related to higher BMI, but no studies to date have considered how the pattern of diffusion relates to white matter volumes in the same participants, or their association with cognitive functions.
What does this study add?
Diffusion measures (fractional anisotropy, axial, and radial) suggest a pattern of white matter damage related to high BMI that likely reflects damage to axons and myelin.
These changes were observed in regions co-occurring with larger white matter volumes, but also extending beyond these regions, suggesting that diffusion is a more sensitive measure of BMI-related white matter abnormality than white matter volume.
Diffusion measures predicted cognitive functioning in memory, executive functioning, and visuomotor speed. These findings suggest that diffusion is a functionally relevant measure of white matter integrity.
Acknowledgments
The work was supported by the Arizona Biomedical Research Commission, the Arizona Alzheimer’s Research Consortium (HB 2354, Dept. of Health Services, Arizona), the Evelyn F. McKnight Brain Institute, the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), and the National Institute on Neurologic Disorder and Stroke (RO1 NS044107). We thank Ms. Kristina Irwin for coordinating and administering neuropsychological testing, and Dr. Thomas Beach at Banner Sun Health Research Institute for his generous support.
Footnotes
Disclosure statement
The authors have no competing interests.
References
- 1.Cournot M, Marquié JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 2.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang GJ, Telang F, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–64. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweat V, Starr V, Bruehl H, et al. C-reactive protein is linked to lower cognitive performance in overweight and obese women. Inflammation. 2008;31:198–207. doi: 10.1007/s10753-008-9065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: The Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of body mass index and risk for Alzheimer’s disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 9.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Whitmer RA, Gunderson EP, Barrett-Connor E. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity. 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 12.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 13.Ashburner J, Friston KJ. Voxel-based morphometry - the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 14.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–64. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haltia LT, Viljanen A, Parkkola R, et al. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- 17.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–19. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 19.Roosendaal SD, Geurts JJ, Vrenken H, et al. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44:1397–403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Salat DH, Tuch DS, van der Kouwe AJ, et al. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiol Aging. 2010;31:244–56. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan L, Walther K, Bendlin BB, Liu L-F, Walker DG, Glisky EL. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 24.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Segura B, Jurado MA, Freixenet N, Falcón C, Junqué C, Arboix A. Microstructural white matter changes in metabolic syndrome: a diffusion tensor imaging study. Neurology. 2009;73:438–44. doi: 10.1212/WNL.0b013e3181b163cd. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson HK, Tuulari JJ, Hirvonen J, Lepomaki V, Parkkola R, Hiltunen J, et al. Obesity is associated with white matter atrophy: A combined diffusion tensor imaging and voxel-based morphometric study. Obesity. 2013;21:2530–2537. doi: 10.1002/oby.20386. [DOI] [PubMed] [Google Scholar]
- 27.Shimoji K, Abe O, Uka T, Yasmin H, Kamagata K, Asahi K, et al. White matter alteration in metabolic syndrome. Diabetes Care. 2013;36:696–700. doi: 10.2337/dc12-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen R, Gunstad JJ. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity. 2011;19:500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- 29.Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Eirckson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med. 2012;74:682–690. doi: 10.1097/PSY.0b013e318261909c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller K, Anwander A, Moller HE, Horstmann A, Lepsien J, Busse F, et al. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion –tensor imaging. PLOS ONE. 2011;6:1–9. doi: 10.1371/journal.pone.0018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. J Exp Psychol Learn Mem Cogn. 2008;34:809–822. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gautier JF, Chen K, Salbe AD, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- 33.Whitwell JL, Sampson EL, Loy CT, et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage. 2007;35:207–213. doi: 10.1016/j.neuroimage.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 34.de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- 35.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 36.Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–22. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 38.Wright P, Heaps J, Shimony JS, Thomas JB, Ances BM. The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS. 2012;26:1501–1508. doi: 10.1097/QAD.0b013e3283550bec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cazettes F, Cohen JL, Yau PL, Talbot H, Covit A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res. 2011;1373:101–109. doi: 10.1016/j.brainres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]