Abstract
Background
Previous studies with adult smokers have shown an association between number of cigarettes smoked per day (CPD) and levels of biomarkers of exposure to the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). This study compared carcinogen and nicotine exposure in adolescent and adult smokers across categories of CPD.
Method
Baseline smoking history and biomarker data were merged from six studies to make two samples: one of adolescent smokers and one of adult smokers. Metabolites of NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides (NNAL-Glucs) and total cotinine were quantified in urine.
Results
CPD was stratified into categories of 5–10, 11–15, and 16–20 CPD. Adolescents tended to have lower mean levels of NNAL plus NNAL-Glucs (total NNAL) compared to adults, although differences were not significant overall. Adolescent mean levels of NNAL/CPD were significantly lower than adult levels only in the 11–15 CPD category (p = 0.045). However, a significant positive relationship was observed for total NNAL/CPD by age. No significant differences between adolescents and adults were found in mean levels of total cotinine or cotinine/CPD. A sub-sample of urines from adolescents and adults were analyzed for NNAL-Glucs and NNAL. Adolescents and adults did not significantly differ in the ratio of NNAL-Glucs to NNAL.
Conclusions
Adolescent uptake of NNK and nicotine tends to be lower, although not statistically different from adults. The lack of significant differences may be due to the wide variation in exposure in adolescents. Some adolescent smokers are exposed to lung carcinogens at levels similar to those of adults.
Keywords: Adolescent, Biomarkers, Toxicant Exposure, Tobacco, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), Cotinine
Introduction
Almost 90% of adult smokers report initiating smoking during childhood and adolescence, before the age of 21 (1). A reported 23% of all high school students are current smokers, (2) and while the number of adolescents who initiate smoking has decreased every year through 2003, no additional reduction has been seen since that time (2). Adolescents who smoke not only experience adverse health effects like reduced maximum lung function, reduced rate of lung growth, reduced overall fitness and increased risk of respiratory illness (3, 4) they are also at risk for becoming highly dependent on nicotine and experiencing difficulty quitting (5). It is predicted that if current smoking patterns persist in the United States, an estimated 6.4 million persons who are currently children or adolescents will eventually die prematurely as adults from a smoking-related illness (6).
Like adults, adolescent smokers are exposed to tobacco related toxicants that have been associated with health outcomes such as heart disease and lung cancer (7, 8). Cigarette smoking is responsible for about 90% of lung cancer deaths in the United States (4). For smoking related cancers, the risk increases with number of cigarettes smoked and duration of smoking (4). Initiating smoking between the ages of 15 and 19 has been shown to be highly correlated with developing lung cancer before the age of 45 (9).
The risk for lung cancer is plausibly associated with the extent of exposure to carcinogens. A urinary biomarker of exposure to a lung carcinogen is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides (NNAL-Glucs). These are metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (8). NNK is a pulmonary carcinogen and is a likely cause of lung cancer in smokers (10). Other biomarkers of exposure include cotinine plus its metabolites (total cotinine) and carbon monoxide (CO). Previous studies have shown an association between number of cigarettes smoked per day (CPD) and levels of total NNAL, total cotinine and expired-air CO in adult smokers (11, 12). Levels of biomarkers increase with increases in CPD, but not linearly, as CO, total NNAL and total cotinine appear to plateau at around 25 to 35 CPD (12).
There are few studies in the literature that have examined the dose-response relationship between CPD and adverse health effects in adolescents (13, 14) or with biomarkers of toxicant exposure in adolescents (15–19). To our knowledge there have been no studies that have investigated carcinogen exposure in adolescents compared to adults.
In the present study, metabolites of NNK in adolescents and adults enrolled in six different studies were assessed. NNAL plus NNAL-Glucs (total NNAL), and the ratio of NNAL-Glucs to NNAL (an indicator of carcinogen detoxification) as well as total cotinine were examined. The aims of this study were threefold: a) to examine carcinogen and nicotine exposure in adolescent smokers across categories of CPD, b) to compare levels between adolescents and adults; and c) to determine whether differences, if they exist, can be attributed to differences in glucuronidation of NNAL.
Materials and Methods
Study Design
Smoking history and biomarker data were merged from six separate study populations to make two samples of smokers: one of adolescent smokers and one of adult smokers. No participants were enrolled in more than one study. Four of the studies were school-based or clinical trials, and the remaining two studies consisted of participants who were recruited specifically to represent stable low-level smokers. From all six studies only baseline samples were used in the analysis.
The Adolescent Sample consisted of 3 groups of adolescent smokers. Study 1 included participants who were interested in reducing smoking, but were uninterested in quitting in the next 30 days (20). Participants in Study 2 were from a cessation study that included a reduction component. Participants in Study 2 who were unable to quit after 2 weeks were randomly assigned to usual care or to a reduction phase for 4 weeks. Study 3 included participants from a cross-sectional study where measures related to biomarkers of exposure were collected at 2 time points 1 week apart.
Setting and Participants
Participants in the adolescent sample were 13- to 19-years old. Those from Studies 1 and 2 were recruited from 14 traditional and alternative high schools located in suburbs of the Minneapolis-St. Paul area. They attended study visits at their schools. Participants from Study 3 attended visits at the research clinic. The eligibility criteria for Studies 1 and 2 were as follows: (a) smoking at least 5 cigarettes per day (CPD) for at least six months, (b) not using any other tobacco products more than once per week, (c) wanting to reduce smoking, but not having a quit date set within the next 2 months (Study 1) or motivated to quit (Study 2), (d) not using nicotine replacement therapy (NRT) or bupropion, (e) not taking medication contraindicated for use with study medications, (f) not currently abusing alcohol or drugs, (g) not experiencing severe emotional problems within the past year, (h) not taking psychoactive medications that were not stabilized or were likely to change during the course of the study and (i) not pregnant or nursing. The eligibility requirements for Study 3 differed only in that subjects were required to be smoking 15 or fewer cigarettes per day.
Participants from Studies 1 and 2 attended 2 baseline visits 1 week apart. At the 2 baseline visits, expired-air CO samples and urinary samples to assess for carcinogen biomarkers and total cotinine were obtained. The number of CPD was recorded on a daily diary. These measures were also collected from participants in Study 3 at 2 time points 1 week apart. Only the first baseline sample from each participant was analyzed unless it was missing, in which case the second baseline sample was substituted if available.
The Adult Sample was comprised of 3 groups of adult smokers: Study 4 consisted of participants in a clinical trial which recruited cigarette smokers 18–70 years of age who were interested in reducing, but not quitting smoking in the next 30 days; Study 5 included smokers aged 18 to 67 years recruited for a clinical trial study to reduce smoking via scheduled smoking by use of a printed manual or handheld computer; and Study 6 participants were recruited as part of a cross-sectional study of light smokers between the ages of 25 and 70 who smoked 15 or fewer cigarettes per day and were stable at that rate of smoking for at least a year.
Setting and Participants
Subjects from Study 4 were from the Minneapolis-St. Paul area and were screened to ensure that they met the following eligibility requirements: (a) smoking 15 to 45 cigarettes a day for the past year, (b) apparent good health with no unstable medical condition, (c) no contraindications to nicotine replacement use, (d) good mental health, (e) not using other tobacco products, and (f) not pregnant or nursing. Details and results from this study are available in a previous publication (10). Study 5 included adult smokers from the Washington, D.C. – northern Virginia metropolitan area who were considered eligible to participate in the study if they met the following eligibility requirements: (a) self-report of smoking 15 or more cigarettes per day for 1 or more years, (b) an unsuccessful quit attempt in the past year, (c) no specific plan to quit in the next 30 days and willing to attempt smoking reduction as a short-term goal, (d) using no other tobacco products more than 3 times per week in the past week, (e) no current use of nicotine replacement, (f) no use of Zyban in the past 2 weeks, (g) no treatment for drug or alcohol abuse in the past year and (h) not pregnant or nursing. Subjects from Study 6 were from the Minneapolis-St. Paul area and were recruited specifically to represent light smokers smoking 15 or fewer CPD. The other eligibility requirements were the same as for subjects in Study 4.
Participants from Study 4 attended 2 baseline visits 1 week apart. At the 2 baseline visits, expired-air CO samples and urinary samples for assessment of carcinogen biomarkers and total cotinine were obtained. The number of CPD was recorded on a daily diary. These measures were also collected from participants in Study 6 at 2 time points 1 week apart. Subjects in Study 5 had only one baseline visit where biomarker and cigarette diary information was obtained.
Measures
Subjects in all 6 groups (3 adolescent and 3 adult) provided urine samples for biomarker assessments. They also completed tobacco use questionnaires and provided demographic information. Expired-air CO was measured using a Bedfont Micro Smokerlyzer. Total NNAL, NNAL, NNAL-Glucs and total cotinine were quantified as previously described (21, 22). From all six studies, only the smoking and biomarker data from baseline visits were analyzed for the current study. All studies except for Study 5 had 2 baseline urine samples. Study 5 had only one baseline urine sample. For studies with 2 baseline visits, the first non-missing biomarker level was used in the analysis.
Statistical Analysis
Analysis was restricted to Caucasian or Hispanic Caucasian subjects smoking 5 to 20 cigarettes per day. Descriptive analysis stratified by study is presented for demographic characteristics, current amount of smoking (in CPD), duration of daily smoking, and brand type of cigarette based on nicotine and tar yields. Between-study heterogeneity was assessed by testing the differences across the three adolescent studies and across the three adult studies, respectively. These same variables were also compared between adolescent and adult smokers. The chi-square test was used for gender and brand type, the t test and F test were used for age and current amount and duration of smoking.
The number of CPD was stratified by intervals of 5–10, 11–15, and 16–20. Typically NNAL and cotinine measures are corrected by taking into account creatinine. Because of the differences between adolescents and adults in body composition and because creatinine is related to muscle mass, a comparison of creatinine levels between age groups was conducted using a mixed effect model to control for between study variability. The results of this comparison showed that adolescents had significantly higher levels of creatinine than did adults (p = 0.008). Thus creatinine, although widely used in the adult literature as the denominator of choice when examining biomarkers, is an inappropriate denominator when comparing biomarker levels between adolescents and adults (23). Therefore, biomarkers for exposure to NNK included total NNAL (NNAL plus NNAL-Glucs) per ml urine and total NNAL per ml urine per CPD. Biomarkers for exposure to nicotine included total cotinine (cotinine plus its glucuronide) per ml urine and total cotinine per ml urine per CPD. Levels of NNAL and cotinine observed in this study were similar to levels found in other studies (24). In addition, the ratio of NNAL-Glucs to NNAL per ml urine was examined as a measure of NNK metabolism.
An aggregate data analysis for biomarkers was undertaken by using the mixed effect model with unequal random effect variances across groups (adolescents vs. adults), with the random effect of study taking care of the between-study heterogeneity. Factors other than group being investigated in the model included CPD and the interaction of group and CPD. Group effects were represented by fixed effects. To ensure validity of tests and regression analyses, all biomarkers with skewed distributions are transformed using the natural logarithm to approximate normality and were summarized as geometric mean. All statistical tests were two-sided. P-values less than 0.05 were considered statistically significant.
Human Subjects Approval
Studies 1–4 and Study 6 were reviewed and approved by the Institutional Review Board of the University of Minnesota. Study 5 was reviewed and approved by The Personal Improvement Computer Systems (PICS) Institutional Review Board. Written consent was obtained from all subjects at orientation visits where the studies were explained in detail.
Results
Study Samples
There were 403 participants whose urine was analyzed for total NNAL: 273 from the 3 adolescent populations (Study 1, n=94; Study 2, n=165; and Study 3, n=14) and 130 from the 3 adult populations (Study 4, n=49; Study 5, n=43; and Study 6, n=38). For the cotinine analysis there was a total of 341 participants; 255 from the 3 adolescent populations (Study 1, n=89; Study 2, n=152; and Study 3, n=14) and 86 from adult populations (Study 4, n=48; and Study 6, n=38.) There were no urine cotinine samples from Study 5. The above participants included in these analyses represent subsets of the original studies which had much larger numbers of participants. Because preliminary analyses showed that there were differences between African Americans and Caucasians in number of cigarettes smoked per day (Caucasians had a higher CPD than African Americans), subsets of each study consisted of all Caucasian and Hispanic Caucasian subjects smoking between 5 and 20 CPD who provided baseline samples. Very few adolescents smoked 21 or more CPD, so to enable comparisons between adolescents and adults, no subjects smoking 21 or more CPD were included in the analysis.
Comparison of study samples
The demographic data and smoking history by categories of brand type (regular/. light/ultra-light) are summarized in Table 1a and b.
Table 1.
Summary statistics for smoking variables across treatment groups
| a. Demographics and smoking history among adolescent and adult smokers | |||||
|---|---|---|---|---|---|
|
| |||||
| N=403 | Gender (% male) |
Mean age SD) |
Mean CPD (SD) |
Mean duration (SD) |
|
| Adolescent smokers | |||||
| Study 1 | 94 | 40.4 | 16.6±1.2 | 12.7±5.1 | 3.4±2.0 |
| Study 2 | 165 | 52.7 | 17.1±1.1 | 10.3±4.5 | 2.6±1.5 |
| Study 3 | 14 | 14.3 | 16.8±1.2 | 11.4±4.2 | 3.3±1.7 |
| Pooled | 273 | 46.5 | 16.9±1.2 | 11.2±4.8 | 2.9±1.7 |
| P-value (difference across studies 1–3) | – | 0.01 | <0.01 | <0.01 | <0.01 |
|
| |||||
| Adult smokers | |||||
| Study 4 | 49 | 44.9 | 44.3±11.4 | 19.9±0.7 | 25.5±11.4 |
| Study 5 | 43 | 60.5 | 42.2±16.2 | 18.1±1.8 | 24.6±15.5 |
| Study 6 | 38 | 44.7 | 46.8±13.0 | 9.7±3.2 | NA |
| Pooled | 130 | 50.0 | 44.3±13.6 | 16.3±4.8 | 25.1±13.4 |
| P-value (difference across studies 4–6) | – | 0.24 | 0.31 | <0.01 | 0.76 |
|
| |||||
| P-value (adolescent vs. adult) | – | 0.51 | <0.01 | <0.01 | <0.01 |
| b. Brand type among adolescent and adult smokers | ||||
|---|---|---|---|---|
|
| ||||
| Regular N (%) |
Light N (%) |
Ultra light N (%) |
Uncoded N (%) |
|
| Adolescent smokers | ||||
| Study 1 | 48(51.1) | 38 (40.4) | 2(2.1) | 6 (6.4) |
| Study 2 | 91 (55.2) | 68 (41.2) | 2 (1.2) | 4 (2.4) |
| Study 3 | 0 | 9 (64.3) | 5 (35.7) | 0 |
| Pooled | 139 (50.9) | 115(42.1) | 9 (3.3) | 10 (3.7) |
| P-value (difference across studies 1–3) | <0.01 | |||
|
| ||||
| Adult smokers | ||||
| Study 4 | 9 (18.4) | 20 (40.8) | 12 (24.5) | 8(16.3) |
| Study 5 | 4 (9.3) | 28(65.1) | 9 (20.9) | 2 (4.7) |
| Study 6 | 17 (44.7) | 13 (34.2) | 3 (7.9) | 5 (13.2) |
| Pooled | 30(23.1) | 61 (46.9) | 24 (18.5) | 15(11.5) |
| P-value (difference across studies 4–6) | <0.01 | |||
|
| ||||
| P-value (adolescent vs. adult) | <0.01 | |||
Expected significant differences were observed between adolescents and adults on variables of age (p <0.01), mean number of CPD (p <0.01) and duration of smoking (p <0.01).
When cigarette brand type was examined, pooled adolescent and adult smokers differed significantly (p < 0.01) in the nicotine/tar yield (regular, light or ultra-light) of cigarettes smoked. More adolescents smoked regular cigarettes than did adults (p <0.01).
Correlations between biomarkers and CPD
Levels of total NNAL per ml urine were significantly associated with number of CPD for both the pooled adolescents (r=0.35, p<0.0001) and the pooled adults (r=0.30, p<0.0005). Levels of total cotinine per ml urine were also significantly associated with number of CPD for pooled adolescents (r=0.34, p<0.0001) and pooled adults (r=0.51, p<0.0001).
Total NNAL
Figure 1a shows the mean levels of total NNAL per ml of urine for adolescents and adults for each of the CPD strata (5–10, 11–15 and 16–20 CPD). While adults had higher mean values across all strata of cigarette smoking, differences between levels of total NNAL for adolescents and adults were not significant overall and only approached significance in the 11–15 CPD category (p = 0.074). Figure 1b shows the mean levels of total NNAL divided by CPD for adolescents and adults across the CPD strata. The difference between adolescents and adults was significant only for the 11–15 CPD smoking category (p = 0.045). Adults had non-significantly higher mean values in two smoking categories.
Figure 1.
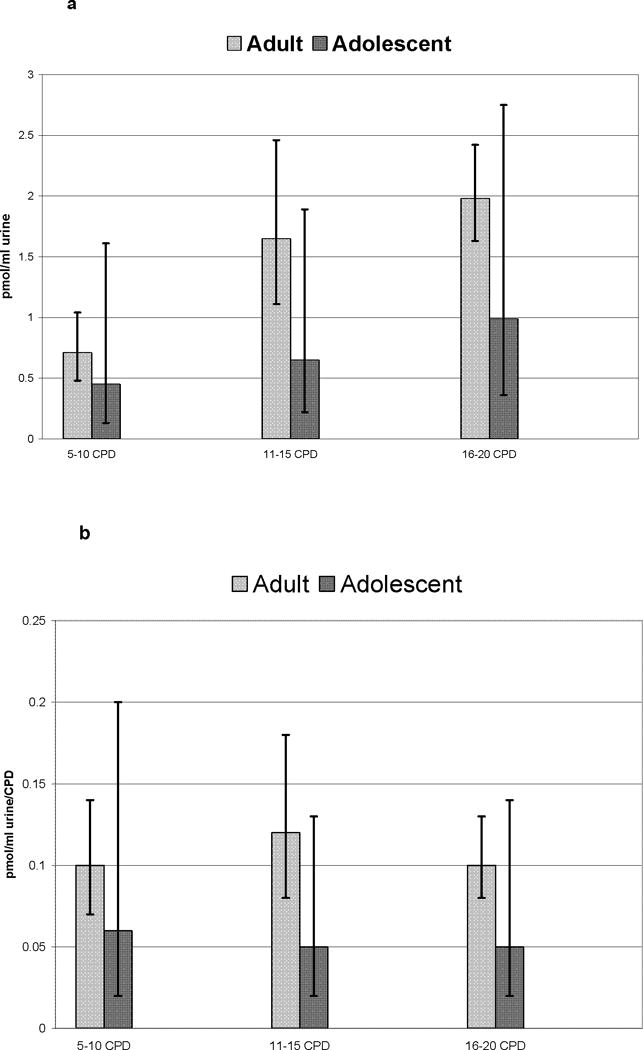
a and b. Mean levels of total NNAL per ml of urine (1a) and mean levels of total NNAL divided by CPD (1b) for adolescents and adults for each of the CPD strata (5–10 CPD, Adolescent N=167, Adult N=23; 11–15 CPD, Adolescent N=58, Adult N=21; and 16–20 CPD, Adolescent N=48, Adult N=86).
Glucuronidation of NNAL
The urines of a subset of adolescents (50 from Study 2) and adults (114 from Study 4) were analyzed for levels of NNAL and NNAL-Glucs per ml urine. Because few subjects in the adult sample smoked less than 15 CPD, only subjects who smoked 15–20 CPD were included in this data analysis. As the carcinogen NNK is metabolized, NNAL is formed and then glucuronidated. NNAL is a lung carcinogen, but NNAL-Glucs is non-carcinogenic (25). The ratio of NNAL-Glucs to NNAL has been suggested as an indicator of risk with higher ratios indicating lower risk (26). Adolescent ratios (geometric mean: 3.43, 95% CI: 2.77–4.25) were not significantly different from those of adults (geometric mean: 3.12, 95% CI: 1.46–5.52), p = 0.65.
Cotinine
For total cotinine per ml urine and total cotinine per ml urine per CPD, no significant differences were found between the adolescents and adults at any CPD category (p> .13).
Biomarkers by Age
Figure 3a shows total NNAL/CPD by age. It indicates that total NNAL/CPD is lower in younger smokers than older smokers. The Spearman correlation between total NNAL/CPD and age was 0.34 (p<0.0001). Younger smokers had larger variation in log total NNAL/CPD than older smokers (SD of log total NNAL/CPD: teen 0.99, adults 0.82, p-value of equal variance test = 0.02). Figure 3b shows total cotinine/CPD by age. The Spearman correlation between total cotinine/CPD and age was 0.07 (p=0.23). Younger smokers had larger variation in log cotinine/CPD than older smokers (SD of log cotinine/CPD: adolescent 1.19, adults 0.66, p-value of equal variance test < 0.0001). The significant p values indicate that equal variances cannot be assumed. For both NNAL/CPD and cotinine/CPD, younger smokers had larger variation than older smokers.
Figure 3.
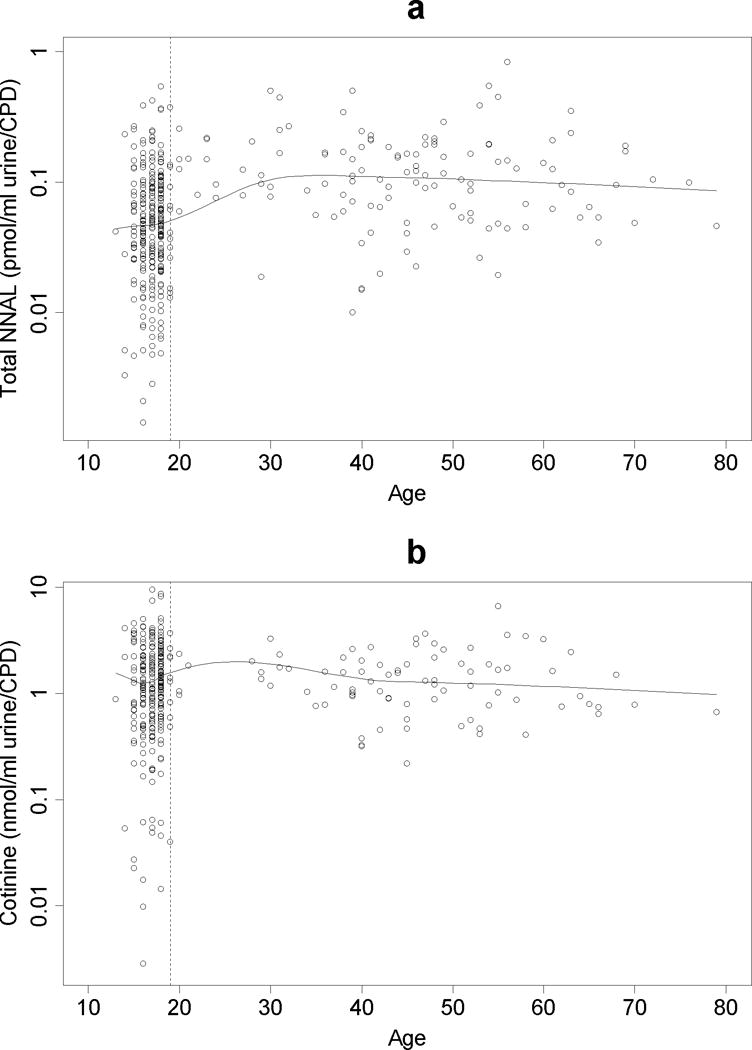
a and b. Mean total NNAL per CPD (3a) and cotinine levels per CPD (3b) by age.
Discussion
In general, the differences in levels of the biomarkers of exposure measured here in adolescent and adult smokers were not statistically significant when smokers were stratified by amount of cigarettes smoked, however adolescents tended to have lower levels of total NNAL and total NNAL per CPD with significance found for the latter variable in those who smoked 11–15 CPD. Furthermore, correlational analyses showed a positive and significant relationship between age and total NNAL/CPD although not for total cotinine per CPD. In one other study that directly compared biomarkers in adults and adolescents, adults smoking 20 to 35 CPD did have significantly higher mean baseline serum cotinine levels compared with adolescents matched for the same self-reported baseline smoking rate (19). There are several reasons for the lack of differences observed between adolescents and adults in our study.
The most likely reason for the lack of significant differences in levels of total NNAL between adolescent and adult smokers observed in this study was the large amount of variability in the biomarker levels of the younger smokers. One potential reason why adolescents might show higher variability in their levels of biomarkers is the lack of stabilization of smoking. Although the pooled adolescent sample showed a mean duration of smoking of 2.9 years (±1.7), about half the sample (49.8%) had been smoking daily for 2 years or less. Inexperienced smokers may smoke differently from smokers who have been smoking longer. Thus, it could be that once a mature pattern of smoking intake is established, less variability in patterns of biomarker uptake would follow.
The lack of differences may also be a function of more “regular” cigarettes smoked among adolescents than adults (higher tar and nicotine). Although recent studies have found no significant differences in total NNAL and total cotinine uptake in adult smokers of regular, light and ultra-light cigarettes (8), it is not known if the same holds true for adolescents. Post hoc analysis showed no differences in total NNAL between regular versus light and ultra-light adolescent smokers. This finding would indicate that differences in types of cigarettes smoked would not account for the lack of significant differences in adult versus adolescent smokers.
In this study, we observed generally lower levels of total NNAL in adolescent than in adult smokers, although the differences were not significant. It was possible that the lower total NNAL levels in adolescents resulted from lower activity of glucuronidation enzymes in adolescents. Therefore, we analyzed free NNAL and NNAL-Glucs in a subset of adolescents, but found no difference between NNAL-Glucs: NNAL ratio in these subjects vs. adults.
One of the limitations of the study is the reliance on self-report. Studies of both adults (8) and adolescents (19) have observed that inaccuracies in self-reported numbers of cigarettes smoked per day can have many causes, from faulty recall to rounding errors to over- or under-reporting of the number of cigarettes smoked. Smith and colleagues (1996) suggest that over-reporting among teenagers could be considered consistent with “the rebellious nature of some adolescents”. Another limitation of this study was that only baseline cross-sectional data was available for analysis such that it was not possible to distinguish whether the difference or lack of difference between age groups was due to cohort effect or within-subject effect. A longitudinal population-based investigation might be a more appropriate design. This study is also limited by the fact that across six different study cohorts, only Caucasian and Hispanic Caucasian smokers smoking between 5 and 20 CPD were included in the analyses. Thus, our results cannot be generalized to other populations nor to smokers at lower or upper ranges of smoking intake (CPD <5 or >20).
In spite of these caveats, the results of this study show that while there is wide variability in their levels of exposure, some adolescent smokers are exposed to lung carcinogens and nicotine at levels scarcely distinguishable from those of adults. Early experience with smoking and duration of smoking exposure are associated with increased risk of smoking related cancers. Adolescents who smoke daily from an early age are in the highest category of potential risk.
Figure 2.
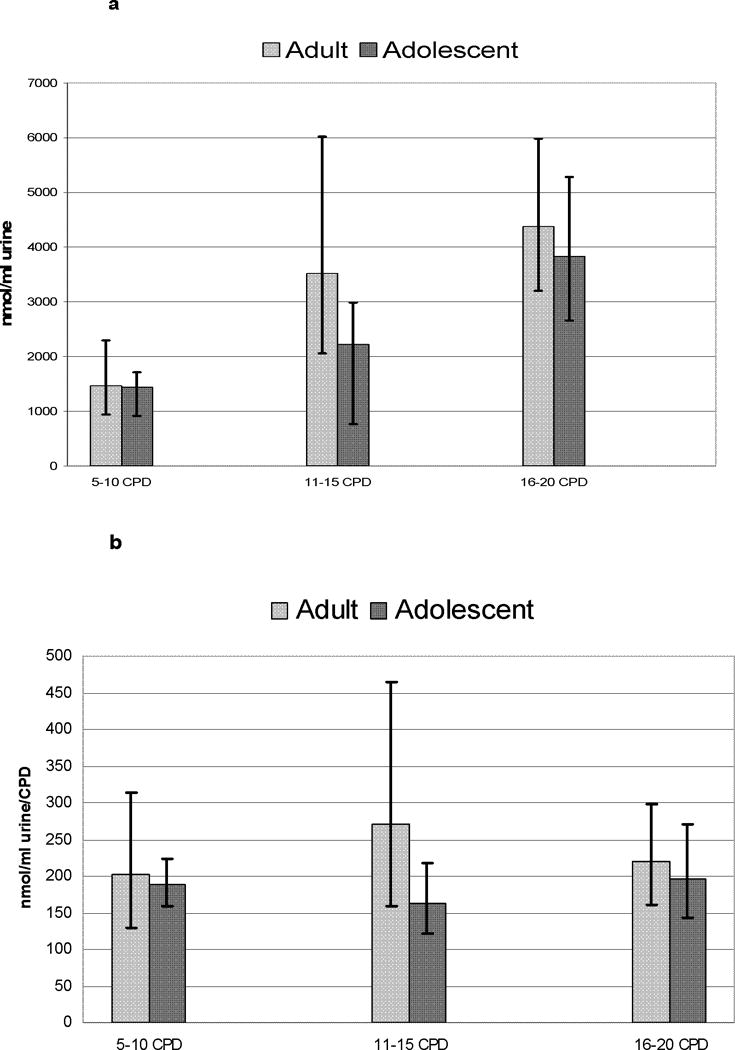
a and b. Mean levels of total cotinine per ml of urine (2a) and mean levels of total cotinine divided by CPD (2b) for adolescents and adults for each of the CPD strata (5–10 CPD, Adolescent N=157, Adult N=23; 11–15 CPD, Adolescent N=53, Adult N=16; and 16–20 CPD, Adolescent N=45, Adult N=47).
Acknowledgments
Grant Support: This research was supported by NIH grants P50-DA013333, R01-DA014538 and National Cancer Institute CA083451.
References
- 1.Centers for Disease Control. Cigarette Smoking Among Adults – United States, 2003. MMWR. 2005;54:509–513. [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Cigarette use among high school students – United States, 1991–2005. MMWR. 2006;55:724–726. [PubMed] [Google Scholar]
- 3.Prokhorov AV, Emmons KM, Pallonen UE, Tsoh JY. Respiratory response to cigarette smoking among adolescent smokers: a pilot study. Prev Med. 1996;25:633–40. doi: 10.1006/pmed.1996.0099. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health & Human Services. The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 5.DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescents. Tob Control. 2000;9:313–9. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Sustaining State Programs for Tobacco Control Data Highlights 2006. Department of Health and Human Services: Center for Disease Control and Prevention; 2006. [Google Scholar]
- 7.Hecht SS. Carcinogen biomarkers for lung or oral cancer chemoprevention trials. [comment] IARC Scientific Publications. 2001;154:245–55. [PubMed] [Google Scholar]
- 8.Hecht SS, Murphy SE, Carmella SG, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:693–8. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- 9.Strand TE, Malayeri C, Eskonsipo PKJ, Grimsrud TK, Norstein J, Grotmol T. Adolescent smoking and trends in lung cancer incidence among young adults in Norway 1954–1998. Cancer Causes Control. 2004;15:27–33. doi: 10.1023/B:CACO.0000016575.31651.b0. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS, Murphy SE, Carmella SG, et al. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–15. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- 11.Etter JF, Vu Duc T, Perneger TV. Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol. 2000;151:251–8. doi: 10.1093/oxfordjournals.aje.a010200. [DOI] [PubMed] [Google Scholar]
- 12.Joseph AM, Hecht SS, Murphy SE, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14:2963–8. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 13.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of Cigarette Smoking on Lung Function in Adolescent Boys and Girls. N Engl J Med. 1996;335:931–937. doi: 10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- 14.Stice E, Martinez EE. Cigarette smoking prospectively predicts retarded physical growth among female adolescents. J Adol Health. 2005;37:363–370. doi: 10.1016/j.jadohealth.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Corrigall WA, Zack M, Eissenberg T, Belsito L, Scher R. Acute subjective and physiological responses to smoking in adolescents. Addiction. 2001;96:1409–17. doi: 10.1046/j.1360-0443.2001.961014095.x. [DOI] [PubMed] [Google Scholar]
- 16.Kandel DB, Schaffran C, Griesler PC, Hu MC, Davies M, Benowitz N. Salivary cotinine concentration versus self-reported cigarette smoking: Three patterns of inconsistency in adolescence. Nicotine Tob Res. 2006;8:525–37. doi: 10.1080/14622200600672732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeill AD, Jarvis MJ, Stapleton JA, West RJ, Bryant A. Nicotine intake in young smokers: longitudinal study of saliva cotinine concentrations. Am J Public Health. 1989;79:172–5. doi: 10.2105/ajph.79.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinstein ML, Thompson PJ, Benowitz NL, Shiffman S, Moscicki AB. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine Tob Res. 2007;9:129–135. doi: 10.1080/14622200601078517. [DOI] [PubMed] [Google Scholar]
- 19.Smith TA, House RF, Jr, Croghan IT, et al. Nicotine patch therapy in adolescent smokers. Pediatrics. 1996;98:659–67. [PubMed] [Google Scholar]
- 20.Hanson K, Zylla E, Allen S, Li Z, Hatsukami DK. Cigarette Reduction: An intervention for adolescent smokers. Drug Alchol Depend. 2008;95:164–168. doi: 10.1016/j.drugalcdep.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- 22.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12:1257–61. [PubMed] [Google Scholar]
- 23.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hecht SS, Carmella SG, Murphy SE, et al. Similar Exposure to a Tobacco-Specific Carcinogen in Smokeless Tobacco Users and Cigarette Smokers. Cancer Epidemiol Biomarkers Prev. 2007;16:1567–1572. doi: 10.1158/1055-9965.EPI-07-0227. [DOI] [PubMed] [Google Scholar]
- 25.Upadhyaya P, Kenney PM, Hochalter JB, Wang M, Hecht SS. Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers and metabolites in the A/J mouse. Carcinogenesis. 1999;20:1577–82. doi: 10.1093/carcin/20.8.1577. [DOI] [PubMed] [Google Scholar]
- 26.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]


