Abstract
The goal of this review is to introduce the biomaterials community to the emerging field of self-healing materials, and also to suggest how one could utilize and modify self-healing approaches to develop new classes of biomaterials. A brief discussion of the in vivo mechanical loading and resultant failures experienced by biomedical implants is followed by presentation of the self-healing methods for combating mechanical failure. If conventional composite materials that retard failure may be considered zeroth generation self-healing materials, then taxonomically-speaking, first generation self-healing materials describe approaches that “halt” and “fill” damage, whereas second generation self-healing materials strive to “fully restore” the pre-failed material structure. In spite of limited commercial use to date, primarily because the technical details have not been suitably optimized, it is likely from a practical standpoint that first generation approaches will be the first to be employed commercially, whereas second generation approaches may take longer to implement. For self-healing biomaterials the optimization of technical considerations is further compounded by the additional constraints of toxicity and biocompatibility, necessitating inclusion of separate discussions of design criteria for self-healing biomaterials.
Keywords: self-healing, biomaterials, material failure, bone cement, mechanical failure
1. Introduction
Biological materials such as bone, skin, and muscle, when healthy, undergo in situ self-healing through a cycle of consumption and regeneration that prevents the accumulation of defects due to tissue ageing and fatigue. Healing and biomaterials are most commonly linked through the tissue response to the presence of an implant 1-3. Ratner has coined the term “biomaterials that heal” to describe biomaterials that actively promote wound healing 4 as opposed to those aimed at passivity or inertness. While the biology and chemistry of healing have significant impacts on biomaterial performance, biological healing does not address the physical repair of biomaterials that experience mechanical and chemical breakdown as they are subjected to loading and degradation effects in vivo. Developing synthetic biomaterials with the intrinsic ability to autonomously repair mechanical and chemical damage would be particularly important for implants that replace tissues that are also capable of self-repair.
The in situ repair of synthetic materials for engineering applications first requires the ability to detect the damage — usually by visual inspection or by non-destructive testing techniques such as ultrasonics, x-ray tomography, computerized vibro thermography, and infrared thermography — followed by time-consuming and/or expensive steps to repair or replace the damaged section 5. In situ detection and repair of biomaterials is particularly difficult due to the lack of adequate in vivo imaging techniques to detect failures and suitable minimally invasive methods to repair the damage. Overall, only substantially damaged or compromised biomaterials can be detected in situ, and if detected are generally retrieved and replaced 6-12.
The development of synthetic materials that autonomously repair in situ on the microscopic level before suffering macroscopic failures would significantly extend the lifetime of a given structure or device. This is precisely the motivation behind the newly emerging class of “self-healing materials” that are endowed with the intrinsic ability of self-repair in response to damage arising from physical and chemical stresses within its use environment 5,13-17. Generally speaking, self-healing materials are designed to sense, halt, and even reverse damage, ideally without requiring the application of external physical or chemical stimuli. These materials hold the potential for significantly extending material lifetimes by avoiding failures initiated by accumulated microcracks. To date, the majority of the research conducted on self-healing bulk materials has employed composites, adhesives, and cements proposed for applications in traditional engineering applications 5,13-18.
Here the common mechanical failure modes of existing polymeric implants are reviewed followed by a description of traditional composite approaches employed to retard these failures. Next materials intended to halt and then ultimately repair microscopic damages before they coalesce into macroscopic failures are discussed. If traditional materials that retard failure may be considered zeroth generation self-healing materials, then taxonomically-speaking, first generation self-healing materials may be used to describe approaches that “halt” and “fill” damage, whereas second generation self-healing materials describe those that strive to “fully restore” the pre-failed material structure. Note that these designations are tied only to the material design and do not refer to the quality of performance of such materials. They do not imply that one approach is necessarily “better” or “more suitable” than another. Finally, these concepts of self-healing materials are placed in the context of building self-healing biomaterials that also must possess the vital characteristics of biocompatibility and non-toxicity.
2. Mechanical failure of polymeric biomaterials
2.1. Loads seen by biomedical implants
Physiological loads experienced by implants vary according to complex loading patterns as well as the activity levels of the recipient (Table 1). For example, the stresses on artificial heart valves and TJRs fluctuate throughout daily activities such as strenuous exercise or sleep and may reach maximums in individual instances of injury. Materials used to replace these tissues are generally selected to have mechanical tolerances that exceed these physiological stresses by several orders of magnitude 19,20. However, a singular instance of normal loading rarely leads to the implant failure; rather it is the repeated normal loading of the implant that incrementally leads to failure. Therefore, mechanical failure in polymeric biomedical implants most often arises from cyclic loading that leads to microcracking, wear, fatigue, and material deformation and loss (Table 2). Furthermore, aqueous polyelectrolytic biological fluids, such as blood, interstitial fluid, and lymph that bathe polymer implants represent an aggressive use environment that exacerbates failures arising from plasticizing, crazing and crack propagation, wear, and corrosion 21. Plasticizers interfere with the interchain interactions, thereby enabling the polymer chains to slide past each other, reducing the physical entanglements that maintain polymer cohesion. Plasticization yields a polymer with a lower glass transition temperature, Tg, that is more susceptible to mechanical deformation under loading, which can promote crack formation and material failure 19.
Table 1.
Typical physiological stresses experienced by implants
Table 2.
Common implant materials and their modes of failure
| Implant | Material | Failure Mode | References |
|---|---|---|---|
| Joint replacement load-bearing surfaces (hip, knee) | UHMWPE | Wear | 9, 25 |
| HDPE | Wear and abrasion, strain softening after yield | 25 | |
| PTFE | Wear, creep under compression | 25, 26 | |
| Polyacetal | Wear, strain softening after yield | 25 | |
| Heart valves | PU, silicone polyether urethanes, polycarbonate urethanes | Biodegradation, mineralization, fatigue, wear, crack formation | 10, 11 |
| Bone cement | Acrylics (ex: PMMA) | Joint loosening, microcrack formation and accumulation through cyclic loading, creep under compression, cause third body wear in joint replacement | 9, 109, 110 |
| Dental implants | HA, ceramics, UHMWPE, PTFE, PS, PET | Fatigue, cracking and chipping, dislocation from base, wear and the formation of wear particles | 106, 111, 112, 113, 114 |
| Dental cements and sealants | Zinc phosphate, zinc polycarboxylate, resin cement, glass ionmer | Degradation due to harshness of mouth environment, cracking, renewed tooth decay | 49 |
2.2. Crazing and crack formation
Microscopic examination of cyclically loaded polymeric implants often reveals the presence of crazed microcracks characterized by a network of fibrils that span the crack edges19. Crazes and microcracks form to relieve internal or external stresses in areas of high stress concentration, such as along a pre-existing crack, at the surface of the polymer, or at a void within the polymer. A crack will propagate if the stress on the system is reduced by its growth. As propagation occurs, the fibrils joining the bulk surfaces of the polymer can either behave in a ductile manner by recruiting fresh material from the edge of the bulk surface to maintain the fibril connections, or the fibrils can behave in a brittle manner and proceed to fracture.
2.3. Wear and wear particle formation
Wear is the loss of bulk material through adhesion, abrasion, erosion, fretting, and/or fatigue 22,23. Wear damage in biomaterials is common to the articulating surfaces in TJRs and is regarded as the primary failure mode that influences the long-term performance of such implants 9,19,22-26. Particles that break off from the bulk material during wear comprise both mass loss from the original implant as well as debris that can lead to added abrasion. Attempts by the immune system to consume and remove these wear particles often leads to a state of a chronic inflammation. It has also been observed that wear debris cleared from the joint space by lymph or blood flow collects in the lymph nodes and other organs of the immune system 23,27,28.
The current gold standard for articulating surface applications is UHMWPE 25. With a molecular weight between 2 and 6 million, UHMWPE imparts toughness through crystallinity between 39 and 75% and the extensive intertwining of the extremely long molecular chains that interlock the crystalline domains, restricting motion to the amorphous polymer regions 12. The capacity of UHMWPE to resist cyclic fatigue can be augmented by direct compression molding and hot isostatic pressing to give better fatigue resistance and smooth surface finish than that afforded by extrusion techniques 6,12,29,30.
The currently available alternatives to UHMWPE in TJRs are the limited use of alumina and zirconium as the articulating surfaces 31. While these ceramics demonstrate excellent strength, stiffness, and lubricity, they have poor toughness and resistance to crack propagation. Attempts have been made to improve the surface properties of metals through the application of diamond-like, titanium nitride, and chromium nitride coatings, but poor adhesion of these coatings to the metal substrate also results in debris that compromises the implant28.
3. Zeroth generation self-healing materials: Composites that retard but do not repair mechanical damage
Composite materials are attractive because they can be designed to improve material stiffness and strength by dispersing stiffer or stronger particulates or fibers into the softer polymer matrix 19,30. By employing materials with different mechanical properties for the matrix and additive phases, the overall properties of the composite will reflect the most desirable traits of each material. Dispersed particulates and fibers of composite structures can also increase the toughness, impact strength, and wear resistance of the base matrix by absorbing a greater fraction of the load, by inhibiting pathways for crack propagation, and by resisting void formation.
Properly designed composite materials thus have the capacity to retard mechanical failure but do not have the ability to repair damage. As such, traditional composites behave like a “stuck zipper” that is hard to un-zip, but also one that cannot be re-zipped. This capacity gives traditional composite materials a “zeroth-order” self-healing status.
Huang and Ramakrishna provide an excellent and comprehensive review of biomedical composite materials 32. Table 3 summarizes the use of polymer-based composite biomedical implants. In spite of a long history of development in load-bearing applications, only a few composite devices have progressed to widespread clinical use 32. The lack of success with composite biomaterials may be attributed to the deleterious effects of placing the material in an environment that accelerates water absorption and compromises adhesion between the dispersed and matrix phases; i.e. a 37 degree, cyclically-loaded, aqueous, high-salt polyelectrolytic environment.
Table 3.
Biomedical composites
| Implant | Dispersed phase/matrix phase | Used clinically? | References |
|---|---|---|---|
| Acetabular cups | CF/UHMWPE, UHMWPE/UHMWPE | No | 32, 115, 116 |
| Articular surfaces in joint applications | CF/UHMWPE, CF/PMMA, CF/PS, CF/epoxy | No | 32, 117 |
| Bone plates | CF/epoxy, CF/PMMA, CF/PP, CF/PS, CF/PE, CF/nylon, CF/PBT, CF/PEEK, CF/PLA*, glass fibers/epoxy | Yes, in part | 32, 118, 119, 120, 121, 122, 123, 124 |
| Bone grafts | Bioglass/PS, HA/PE, HA/PEG, HA/PBT, HA/PLLA, HA/PHB, CS/PEEK | Yes, in part | 32, 124, 125, 126 |
| Dental composites | Quartz, barium glass, or colloidal silica as fillers in BIS-GMA or urethane dimethacrylate matrices | Yes | 32 |
| Dental posts | Glass fibers/polyester, unidirectional carbon fiber/epoxy, braided CF/epoxy | No | 32, 127 |
| Hip stems | CF/PS, CF/carbon, CF/epoxy, CF/PEEK | Yes, in part | 32, 128, 117 |
| Intramedullary nails and screws | Glass fibers/PEEK, CF/Vectra A950, CF/PEEK, CF/PS, PLA/PLA, PGA/PGA, PLGA/PLGA | Yes, in part | 24, 32, 33, 129 |
| Lumbar interbody fusion | CF/PEEK, CF/PS | No | 32, 130, 131, 132 |
| Vascular grafts | Lycra-type PU fibers in a Pellathane-type PU/PELA mixture matrix | No | 32, 133 |
| Vertebral body replacement | Bioglass/PU | No | 32, 134, 135 |
| Vertebral disc replacement | PET/silicone rubber, PET/hydrogel | No | 32, 136, 137 |
Italicized materials denote those used clinically or currently undergoing clinical testing
Selecting a chemically inert nonpolar matrix polymer has the advantage of resisting water absorption, but it also increases the difficulty of finding a dispersed phase that bonds well with the matrix material. Strong interface bonding is necessary for efficient transferring of loads between the two phases and the prevention of voids or crack formation at the interfaces, which can serve as stress concentrators that initiate crack propagation 19. Finding suitable materials to disperse in nonpolar and chemically inert UHMWPE thus has proven to be problematic. For example, carbon fiber-reinforced UHMWPE has shown poor results clinically because the carbon fibers did not bond well with the UHMWPE matrix, serving as stress concentrators and sources of crazing 30. Consequently, fatigue cracks propagate up to eight times faster in carbon-reinforced UHMWPE than in pure UHMWPE 30.
Self-reinforcing (SR) materials are a special class of composites consisting of a matrix and reinforcing additive made from the same material 33. Ideally, the compatibility between dispersed and matrix phases made of the same material will allow for composite strengthening through improved interface bonding. SR-PGA and SR-PLLA have been shown to exhibit shear strengths of 2-3 fold higher than the <100MPa shear strengths of their injection-molded counterparts 34,35. This approach is also attractive because cytotoxic adhesion promoters such as silanes are not required 24. SR rods, plates, pins, screws, and tacks have all been produced from SR-PLA, PGA, and their copolymers and are most commonly used as osteofixation devices in craniomaxillofacial implants24,32,33.
4. First generation self-healing materials: Composites that irreversibly repair but do not restore damaged matrix
An emerging area of composite research is the development of dispersed components that respond to the presence of damage by releasing a healing agent. This “first generation” of self-healing materials employs the process of matrix repolymerization to replace the damaged original material with a cured substitute. Here, matrix repolymerization is analogous to gluing together the two sides of an open zipper, which will close the zipper but is irreversible and does not restore its original zipped structure.
Kessler and Murphy both provide excellent reviews and in-depth discussions of matrix repolymerization self-healing systems that have been investigated in recent years 13,36. Two common matrix repolymerization systems employed to date rely on a ruthenium-based Grubbs’ catalyst to induce ring-opening metathesis polymerization (ROMP) of a dicyclopentadiene (DCPD) monomer 5,13,14 or various reactive epoxy resins that induce curing of a bisphenol-A epoxy matrix 15. Microencapsulated PDMS self-healing systems have also been investigated 37,38. While many groups have successfully utilized these systems, the following is meant to discuss illustrative examples of the feasibility of the approach.
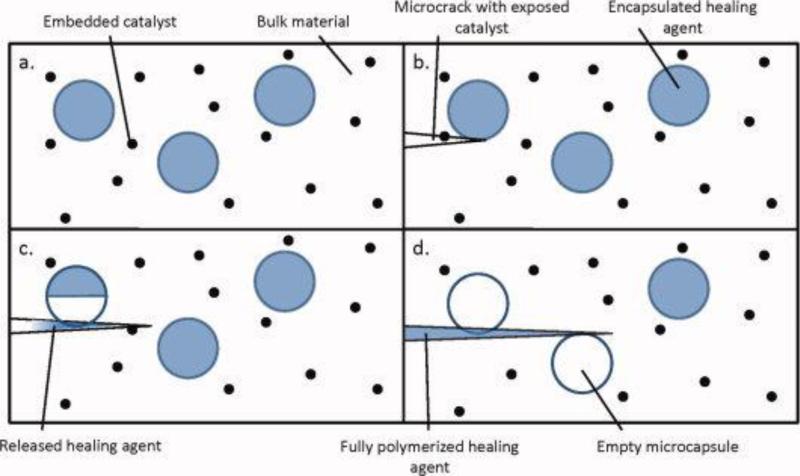
Figure 1 illustrates the matrix repolymerization method utilizing microencapsulated healing agent dispersed in a catalyst-embedded polymer matrix. When a propagating crack encounters a microcapsule, it causes the capsule shell to rupture, releasing the healing agent into the crack plane via capillary action or crack closure following unloading. Crack formation also exposes embedded catalyst that initiates curing of the healing agent released from the capsules into the crack area. The reaction binds the surfaces of the crack together, halting its progression through the material 5,13,14,37,39,40 . The reverse case of microencapsulated catalyst and phase-separated healing agent has also been explored 37,38.
Figure 1.
First generation self-healing mechanism. The undamaged matrix is shown in (a). Embedded catalyst is exposed as microcracks are generated (b). Microcapsules containing a healing agent are fractured by the microcracks, causing the release of healing agent and its subsequent reaction with the exposed catalyst (c). Following this polymerization, propagation of the microcrack is inhibited (d). Adapted from various works by M. Kessler, S. White, N. Sottos, et al.5, 13, 14, 77, 79 [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Healing agent-filled hollow cylinders and branched network structures also have been employed to increase the efficiency of healing agent delivery to the defect area. Bleay et al. investigated a variety of small diameter hollow glass fibers that were capable of simultaneously storing healing agent and providing structural reinforcement to an epoxy matrix. However, this group encountered problems with release of healing agent from smaller diameter fibers 13. Toohey et al. and Lee et al. have proposed three-dimensional networks capable of autonomously healing following repeated damage 16,17 but in both approaches the vascular network must be replenished with monomer healing agent between each damage event. Regardless of whether one employs microcapsules, hollow fibers, or network structures, the encapsulating vessel must be large enough to release a sufficient amount of healing agent to repair the defect but small enough to not negatively impact matrix material mechanical properties.
White et al. were able to successfully encapsulate DCPD within a polymer matrix containing Grubbs’ catalyst. Following the formation of microcracks and subsequent ROMP reaction, this group demonstrated up to 75% recovery of the virgin fracture toughness following 48 hours of healing time 14. Healing efficiency was assessed by comparing the critical loads at fracture of healed and virgin specimens following TDCB testing. A more detailed description of TDCB testing can be found in various references, including 5,36,41. This group also found that the average critical load for virgin self-healing matrices containing DCPD and Grubbs’ catalyst was 20% higher than the control group containing no microspheres or embedded catalyst, indicating improved matrix toughness through the inhibition of craze formation and subsequent crack propagation.
Andersson et al. described the self-healing capabilities of PDMS with UF microcapsules following tear testing 37. These investigators showed that the samples were able to consistently recover more than 70% of the original tear strength of the polymer. In research conducted by Blaiszik et al., UF microcapsules containing epoxy resins (Epon 828 and 862) and solvents for use in self-healing applications were successfully engineered 15. These microcapsules were shown to satisfy the desirable requirements for self-healing materials, including processing survivability, thermal stability, and efficient in situ rupture for the delivery of the encapsulated healing agent. Table 4 summarizes the catalyst and matrix materials previously used with repolymerization self-healing techniques.
Table 4.
Healing agents, matrix materials, and their composites studied for application with first generation self-healing techniques
| Matrix Material | Healing Agent | Catalyst | References |
|---|---|---|---|
| Vinyl ester | HOPDMS and PDES (phase separated in matrix) | DBTL in PU microcapsules | 37,38 |
| Epon 828 cured with DETA | DCPD in UF capsules | Grubbs’ catalyst in paraffin wax microspheres | 45,138 |
| PMMA | DCPD in UF capsules | Grubbs’ catalyst | 51 |
| Epon 828 cured with DETA | Epon 862 or Epon 828 diluted with cholorobenzene, phenylacetate, ethyl phenylacetate in UF capsules | None | 15 |
| Epon 828 cured with DETA | DCPD in UF capsules | Grubbs’ catalyst | 5,13,14,43,44 |
| YD-115 cured with KH-816 | DCPD in UF capsules | Grubbs’ catalyst | 43,44 |
| YD-115 cured with KH-816 | ENB in UF capsules | Grubbs’ catalyst | 43,44 |
With any self-healing system, the healing process must occur on the same time scale as the event that initiates and propagates the damage. With current systems the incorporation of mechanically stable curing periods is essential to achieve adequate healing 37. Clearly, incorporating long curing periods is not feasible with many biomedical applications of constant or semi-constant cyclic loading, such as in orthopedic, dental, and cardiovascular applications. As a result, the competition between crack propagation and self-repair represents a recurring and significant challenge for extension into biomaterials.
There are also a number of widely recognized, general technical challenges associated with implementing first generation self-healing materials in engineering applications. These include identifying catalyst and resin systems that maintain chemical reactivity while encapsulated, can be deployed in response to the presence of microcracks, will infuse adequately into these cracks upon release, will progress to a fully cured product, and will form stable chemical bonds within the matrix material. While promising results have been demonstrated with other materials, effective self-healing materials require careful optimization of the physio-chemical characteristics of the catalyst, the healing agent, the encapsulation/release vehicle materials, and the matrix. Clearly, full optimization is application-specific; however, this field is still very new and the published work to date has primarily focused on characterizing self-healing model systems rather than designing materials for specific applications.
The only self-healing material of this design nearing commercial application is not polymer-based, but a self-healing concrete being developed at the University of Michigan 18,42. This self-healing concrete is designed to bend under tensile strain via the formation of tiny microcracks while, alternatively, existing concrete forms large cracks under the same forces. The mixture contains dispersed depots of dry cement within the concrete matrix that are exposed to water and carbon dioxide as cracks form. The reaction of these components forms a calcium carbonate “scar” that fills the defect and halts crack propagation. It was demonstrated that self-healing concrete is capable of withstanding strains up to 5% (compared to 0.01% tensile strain that causes failure of standard concrete) and will recover most, if not all, of its mechanical strength after deformation 18.
While first generation systems are conceptually straightforward and have received the greatest attention for engineering applications to date, there still remain a number of concerns that have limited their more widespread usage. Clever solutions for many of these challenges have been demonstrated for non-biological, ex vivo materials, but the constraints are much more severe for in vivo applications. In the next section, we discuss the near-term prospects for self-healing biomaterials in the context of specific candidate systems.
Technical concerns associated with first generation approaches:
Healing agent must infiltrate crack plane readily but not diffuse away too quickly 44
Uneven healing agent/catalyst distribution or ratios in matrix 5,17,45-47
Superimposed upon the above concerns are the following considerations specific to biomaterials:
5. Candidate systems for first generation self-healing biomaterials
Clotting and scar formation follow a first generation self-healing paradigm; i.e. both are intended to halt and repair damage but do not restore the tissue to its original undamaged state. For example, a defect (wound) initiates the catalytic system (thrombin formation) that causes the healing agent (fibrinogen) to cure and fill the wound site with healing matrix (crosslinked fibrin) 3,48. As such, fibrin glue is a commercial topical adhesive that consists of fibrinogen and thrombin that form a fibrin coagulum within one minute of mixing. It is used to arrest bleeding and for sealing tears in delicate tissues where sutures or staples are not appropriate. Fibrin glue is non-toxic, tissue compatible, and promotes wound healing; however, fibrin coagulum is a soft material with poor mechanical properties 49. This limits the use of fibrin glue to very soft tissues (e.g. lung, spleen, kidney, etc) that are typically not subject to mechanical loading. The more practical challenge in biomaterials is to identify non-toxic and mechanically robust self-healing systems that can lessen the wear and degradation of mechanically loaded implants, such as orthopedic, cardiovascular, and dental materials (Table 1). To our knowledge, no such self-healing biomaterials system has been reported.
Arguably, self-healing bone cement represents the simplest and most straightforward mechanically loaded first generation self-healing biomaterial to design and test. PMMA bone cement is a space-filling matrix that forms mechanical interlocks between the stem of the implant and the surrounding boney tissue 50. Bone cement consists of two components: low molecular weight PMMA powder plus an initiator (e.g. benzoyl peroxide), and liquid MMA monomer. Mixing the two components forms a slurry that initiates polymerization yielding a workable dough that is applied to the implant, which next hardens into a solid mass after the implant is inserted into the boney tissue 50. Cemented total joint replacements are commonly used to provide superior long-term survival of the implant through bone integration but lose effectiveness following wear and microcracking 7,8. Minimizing the generation of wear particles associated with cemented joint replacements through development of a first generation self-healing bone cement would contribute significantly to the extension of the implant lifetime and improve patient quality of life.
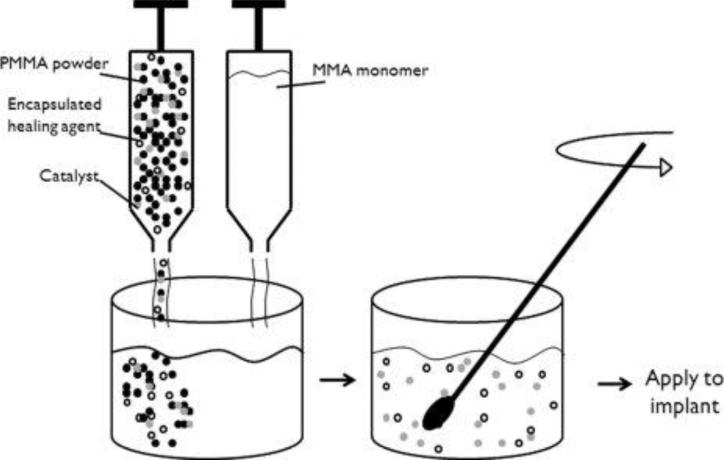
Following the matrix repolymerization paradigm outlined previously (Figure 1), self-healing bone cement would consist of the PMMA matrix plus an embedded catalyst and a dispersed microencapsulated healing agent. Figure 2 illustrates how one might incorporate a self-healing capability into the two-component bone cement; e.g. incorporating encapsulated healing agent into the liquid monomer component, and the healing agent catalyst into the powder component. Mixing the two components polymerizes the PMMA and distributes the encapsulated healing agent and healing agent catalyst throughout the resultant PMMA matrix. Because the cement is mixed in the operating room and then applied directly to the implant there are also no manufacturing or machining processes that must be followed to produce implants of specific sizes and geometries, further simplifying material design.
Figure 2.
Potential design for a self-healing bone cement utilizing the first generation self-healing approach. The catalyst and encapsulated healing agent to be embedded are packaged with the PMMA powder component of the bone cement. Mixture of the two bone cement components. Mixture of the two bone cement components will disperse the solid catalyst and encapsulated healing agent within the bone cement. The cement can then be applied to the implant following current protocols.
The only report to date of developing a self-healing biomaterial of any sort came from Biggs et al.51 who incorporated the matrix repolymerization formulation of White et al.14 directly into a commercial two-component PMMA bone cement. The Biggs study consisted of encapsulating DCPD monomer in UF microcapsules52, and blending the microcapsules and organo-metallic Grubbs’ catalyst with the PMMA powder component. Mechanical testing of the cured cement was used to demonstrate that the self-healing material formulation increased fracture crack resistance by a substantial 4-8 fold compared to the unmodified formulation. Clearly, Grubbs’ catalyst was efficient in catalyzing ROMP of DCPD, and thus effective in sealing microcracks in PMMA matrix; however, DCPD and Grubbs’ catalyst are mildly and acutely toxic, respectively53,54. UF may also be a problem due to the potential for formaldehyde leaching. These toxicity considerations would render the formulation of White et al. undesirable for biomaterials applications. That said, the prospect of self-healing bone cements remains a straightforward and attractive biomaterial prospect, but only if an appropriately non-toxic formulation can be identified.
Table 5 lists some less toxic healing agent systems that could be incorporated into PMMA bone cement. Depending on the system chosen, it may make more sense to separate the placement of the healing agent and catalyst, or possibly to put both healing agent and catalyst in the same component of the bone cement system (Figure 2). Each of these systems requires an embedded catalyst except for the cyanoacrylates, for which the catalyst would be moisture that infuses into the matrix upon microcrack formation.
Table 5.
Possible healing agent systems for self-healing bone cement
| Healing agent | Catalyst | Advantages | Disadvantages |
|---|---|---|---|
| Fibrin glue | Thrombin | Non-toxic, tissue compatible | Biodegradable, mechanically weak with lower adhesion strengths |
| MMA monomer | Benzoyl peroxide | Same base as PMMA bone cement, mechanically strong | Both components are toxic, MMA immiscible with water |
| Medical grade epoxy resin | Polyamine monomers | Good mechanical strength, bonds well with PMMA, proven system | Unreacted components are toxic, resin immiscible with water |
| Medical grade cyanoacrylate tissue adhesive | Moisture | Tissue compatible, bonds quickly to PMMA, good mechanical strength | Unreacted monomer may be toxic, cyanoacrylate subject to moisture intrusion |
6. Second generation self-healing materials: Materials that reversibly restore damaged matrix
A “second generation” self-healing material, as defined here, reversibly restores a damaged material to its original, undamaged state. This is analogous to the act of opening (introducing damage) and then closing (initiating repair) a zipper. Except in the melt, reversible repair of most polymers is kinetically inaccessible because the energy barrier to molecular rearrangement is high and the molecular dynamics are slow. Consequently, all second generation self-healing polymer systems currently under investigation concern either the chemistry of weak bonds that are reversible at low temperature or techniques that input the energy necessary for molecular rearrangement. Regardless of temperature and absolute time scale, however, the two approaches are united in that local reversibility (i.e. bond reforming reactions) must be significantly faster than global processes (e.g. polymer flow and macroscopic deformation).
Table 6 provides a summary of a variety of different second generation systems that have been explored. The examples discussed here are again meant to provide a brief introduction to the breadth of the field.
Table 6.
Second generation self-healing systems
| Trigger mechanism | Autonomous healing? | Healing mechanism | References |
|---|---|---|---|
| Hydrogen bonding | Yes | Quadruple hydrogen bonds dimerized with 2-ureido-4-pyrimidone derivatives | 62 |
| Hydrogen bonding | Yes | Multiple hydrogen bonds formed between amidoethyl imidazolidone, di(amido ethyl) urea, and diamido tetraethyl triurea | 86 |
| Metal-ligand coordination | Yes | Bifunctional palladium or platinum coordinating with pyridine in hydrogel matrix | 77 |
| Heating | No | PPE repair achieved through oxygen and a copper catalyst resulting in polymerization | 16 |
| Steam | No | Polymerization of the PC backbone catalyzed by weak alkali | 16,139 |
| UV light | No | Recombination of free radicals to form PU crosslinks | 80 |
| Heat | Yes | Heat from projectile puncture repairs thin films of EMAA, ethylene-octene, and PB-g-PMA-co-PAN | 74 |
| Heat | No | Thermally reversible Diels-Alder cycloaddition of multi-furan and multi-maleimide | 78,79 |
| Heat | No | Thermoreversibility via denaturing double-stranded DNA crosslinks | 69,87 |
| Competitive binding | No | Competitive binding of a removal strand with DNA crosslink results in dissolution of DNA crosslinks | 69,87 |
| Electric field | No | Enhanced hydrogen bonding under positive electric field | 140 |
6.1. Second generation self-healing materials based on shape restoration
Second generation strategies for self-healing materials might involve hybrid architectures that possess both reversible and irreversible interactions. Upon application of a force, the ensemble of weak reversible interactions preferentially yields (providing deformability), while the stronger irreversible interactions remain intact (providing scaffolding). Ideally, when the force is removed, the scaffold of irreversible interactions guides the reassembly of the reversible interactions, restoring the material to its original undeformed state (Figure 3). Shape restoration materials therefore differ from “shape memory materials” because they are driven by end group associations rather than by an energy-consuming transition between two states. The successful implementation of the shape restoration strategy might allow for energy dissipation and structural recovery occur on length and time scales such that damage (and the subsequent repair) is never observed. In many situations, the distinction between self-repair, as described here, and highly efficient energy dissipation eventually becomes rather fine to the point of being moot.
Figure 3.
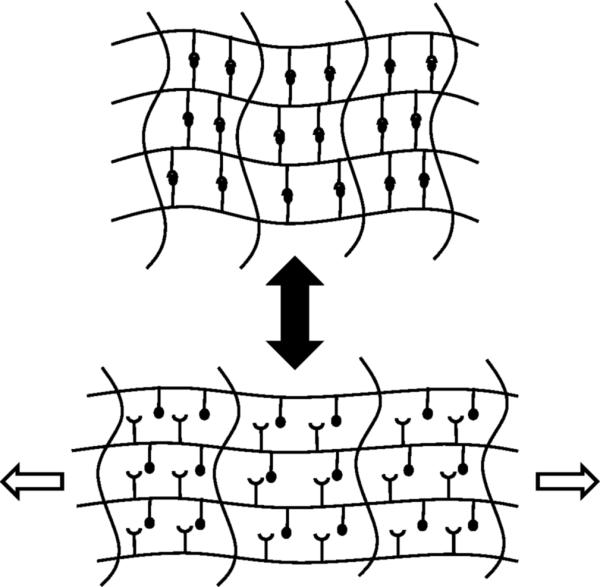
Autonomous second generation materials. Following the application of a force, the reversible crosslinks dissociate while the covalent crosslinks remain intact. When the force is removed, the material returns to its original structure.
The molecular basis for this strategy finds its roots in dynamic covalent chemistry, which comprises the reversible making and breaking of covalent bonds, and supramolecular chemistry, which imparts the capability of self-assembly or self-organization using highly directional and reversible non-covalent interactions that dictate the overall mechanical properties of a material 55. The dynamic dissociation and reassociation of stress-bearing bonds allow for rapid conformational changes that affect the properties of shape restoration materials56,57. These properties depend on the characteristics of the association, the overall flexibility of the molecule, and the environment of the system57. Current dynamic covalent methods comprise a wide range of well-known reaction types58, and retro Diels-Alder reactions40, disulfide exchange reactions59,60, and hydrazone linkages61 are all potentially compatible with biological environments. Reversible supramolecular interactions in polymers are generally achieved through numerous mechanisms56,62,63, including: hydrogen bonding, exemplified by the ureidopyrimidinone unit of Sijbesma and Meijer62; metal coordination chemistry, for example silver complexation by tridentate heterocyclic peptides64 or calcium-bridged carboxylates65; or π-stacking, such as that seen with triphenylene motifs66. Other synthetic supramolecular motifs are known to function in biological environments, but an even greater repository of useful structures is likely found in biology itself; for example, peptide-peptide, ligand-receptor, and nucleic acid-based interactions 67-72.
6.2. Second generation self-healing materials based on application of heat or light
A somewhat exotic but interesting example of heat-induced defect repair is polymer flow caused by projectile puncture. Research conducted at the NASA Langley Research Center described self-healing characteristics of EMAA (Surlyn), ethylene-octene (Affinity EG 8200), and PB-g-PMA-co-PAN that utilize the heat produced by a penetrating projectile (e.g. a bullet) to induce localized melting and rapid reassociation of polymer chains, inducing repair 13,73,74. While such materials may have military and space exploration applications, this approach does not seem well-suited for biomaterials applications 75. Interested readers are encouraged to view a short NASA Real World Mathematics eClip, accessed online on May 19, 2010, at: http://www.nasa.gov/audience/foreducators/nasaeclips/realworld/aeronautics.html.
In contrast to repair by localized polymer flow are self-healing systems that repair defects by reforming bonds upon the application of heat, radiation, or electric fields (Figure 4). As with the shape restoration strategies in the preceding section, a primary challenge is to identify chemistries in which localized bond forming takes place on time scales that are much shorter than both material flow/deformation and crack propagation. One demonstrated example of such chemistry is the Diels-Alder reaction, in which a diene and a dienophile undergo cycloaddition to form the Diels-Alder adduct that can dissociate at high temperatures via the retro-Diels-Alder reaction 76. As such, polymers containing Diels-Alder chemistries have reversible covalent bonds that can be formed and broken by thermal cycling. These polymers are particularly useful because they minimize free radical formation that could lead to undesirable chain reactions resulting in improper structural recovery 77. Chen et al. were able to design a matrix able to repeatedly heal under mild conditions (i.e. 75°C for 3 hours) without requiring a catalyst, additional monomer, or special surface treatments of the fracture plane 78,79. This group found that the energy needed to break the Diels-Alder adducts was much lower than the energy required to break other covalent bonds, demonstrating the preferential failure of these interactions. Recovery of about 57% of the original fracture load was reported. The group proposed using this material for electronic packaging applications where cracking occurs due to differences in the thermal index of expansion 78.
Figure 4.
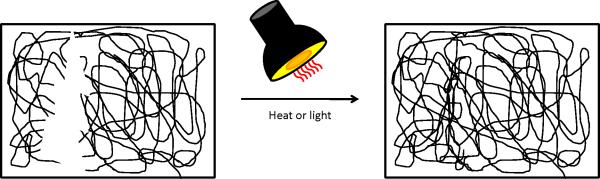
Second generation materials requiring energy input to achieve healing. These materials are capable of restoring the original material structure following the application of an external force such as heat or light. As depicted, a polymer matrix is damaged and following the application of heat, the polymer chains are able to flow and restore matrix integrity. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Repair by the application of light is also of considerable interest. The Urban research group has developed a self-healing material consisting of oxetane-substituted chitosan precursors incorporated into polyurethane networks 80. These systems use ultraviolet light to recombine free radicals to form crosslinks and have been found to repair surface scratch damage in less than one hour. These materials have been proposed for use in automotive coatings that are both damaged and exposed to ultraviolet light during common usage. This system also may be employed regardless of ambient temperature or humidity, further enabling its widespread use. Unfortunately, development of self-healing biomaterials based on energy-dependent mechanisms will be limited to surface-accessible environments such as cutaneous repair unless it can be tied to a deeply-penetrating heat or radiation source.
In comparison to first generation self-healing materials, the second generation approach is more compatible with “soft”, low-modulus material applications. In addition, there exists the possibility of pseudo-infinite cycling and potential advantages that come with a single-component (as opposed to composite) system. Nevertheless, the utility of second generation materials currently lags that of first generation materials, and a number of significant challenges exist.
Technical concerns associated with second generation approaches:
As with first generation systems, there are also unique concerns with second generation approaches that are associated with biocompatibility constraints:
Toxicity of materials
Diffusion of reversible crosslink materials out of scaffold
7. Candidate systems for second-generation self-healing biomaterials
Shape restoration exists in a number of biological systems. For example, the native double helical structure of DNA is reversibly restored during DNA synthesis and repair by the breaking and reforming of paired, hydrogen-bonding purines and pyrimidines 82. Similarly, many denatured proteins can “renature” to their native conformation, given proper conditions of ionic strength and temperature, by reforming hydrogen, ionic, and hydrophobic bonds between specific amino acid residues 82. A third example of reversible shape restoration behavior is the protein elastin where the deformed (i.e. extended) state strains the kinked peptide chains that generate the entropy-driven shape restoration force of elastic tissues 83. In each case the native structure is deformed and then restored (if the damage is not too extensive) by disassociation and then reassociation of an ensemble of individually weak interactions. The key to biological shape restoration is that the native state is both thermodynamically favorable and kinetically accessible. Following the concept of weak reversible interactions incorporated within stronger irreversible networks, Thompson et al. have reported that bone contains certain “sacrificial bonds” that protect the biopolymer backbone and dissipate energy. These sacrificial bonds are found within or between the collagen molecules and contribute to the toughness of the bone by acting as reversible crosslinks that undergo predictable and sequential failure 84. Synthetic materials with similar properties are also known85.
Work by Leibler's group has utilized supramolecular assembly to create a self-healing and thermoreversible rubber that demonstrates remarkable recoverable extensibility and minimal creep under applied load 86. The material network was generated from fatty dimer acids from vegetable oils and urea. The group found that the strain at break exceeded 500%, confirming the rubber-like properties of the material. Samples were also able to completely recover their dimensions following the application of a stress of 5kPa for more than 22 hours. These mechanical properties illustrate that the material behaves like a rubber although it is made of oligomers. Furthermore, when the sample was cut into pieces, the individual pieces could self-heal when brought into contact at room temperature. The material is capable of undergoing many cycles of stretching, breaking, and healing; contact times as short as 15 minutes were found sufficient to achieve healing.
This supramolecular material contains three types of functional groups, amidoethyl imidazolidone, di(amido ethyl) urea, and diamido tetraethyl triurea, that associate via multiple hydrogen bonds. Self-healing is therefore efficient because there are a large number of groups available to restore linkages at the fracture plane. Although the Leibler work is conducted by looking at macroscopic failure that requires the broken interfaces to be held together to achieve healing, similar processes could happen internally to repair microcrack damage. An approach similar to this one could produce a material capable of “healing” damage before it is even detectable.
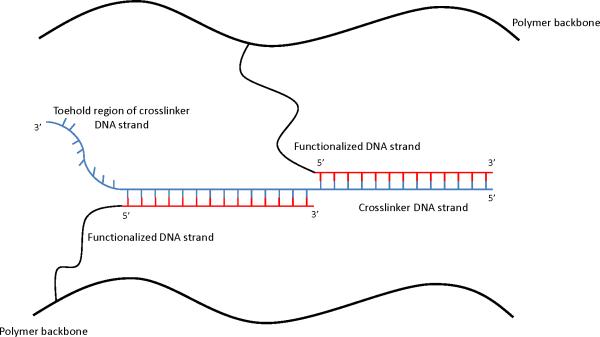
Many groups have also investigated polymers that incorporate oligonucleotide sequences that hybridize to form reversible crosslinks (Figure 5)68,69,87-98. Changing the specificity, affinity, density, and accessibility of the reversible crosslinks allows them to tailor the mechanical and shape restoration properties of these materials. However, when DNA crosslinks are disrupted, additional crosslinking DNA must be provided, but no catalyst is required to restore lost linkages 87. These approaches demonstrate that there is a robust toolkit of interactions both from nature and through synthesis that could be utilized in future work to develop self-healing biomaterials. “Softer” biological materials, such as synovial fluid or cartilage, may provide the ideal introductory pathway for second generation approaches to biomaterials.
Figure 5.
Second generation materials based on DNA crosslinks. The toehold region of the crosslinker DNA strand can be used to eliminate the crosslinks. Addition of a DNA strand complementary to this toehold region results in competitive binding between the removal strand and the crosslinker DNA strand without requiring the application of further external forces. Adapted from Lin et al.103[Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
8. Design criteria: Requirements for self-repair
All biomaterials experience a wide variety of degrading conditions throughout the lifetime of the implant, often losing viability as time progresses. Titanium and titanium alloys are the only clinically used biomaterials that demonstrate any capacity to self-repair in situ 21. These materials have the ability to spontaneously form a tightly adherent and corrosion-resistant titanium oxide under physiological conditions. This property enables titanium-based biomaterials to re-passivate areas where the protective oxide layer is damaged through wear or impact, thus intrinsically resisting corrosion-induced failures. That said, these metals are not capable of repairing mechanical damage, nor are any other ceramic, polymeric, or composite biomaterials currently in use.
Currently, all materials used in implants are incapable of effectively preventing or delaying the advance of mechanical damage, much less managing or even reversing damage. The following list summarizes the basic design requirements for all generations of self-healing materials as well as the considerations for biocompatible self-healing materials. Most importantly, these materials must address the issues associated with the formation and coalescence of microcracks within the bulk material using non-toxic approaches.
General requirements for self-healing materials:
Suitable mechanical and structural properties for intended application
Processed using existing or inexpensive techniques without losing healing capabilities
Must not be prohibitively expensive
Capable of healing repeatedly, autonomously, and rapidly
Capable of sensing damage without external intervention
Able to repair defects of various sizes
Additional constraints for biocompatible self-healing materials:
Sterilized using existing or inexpensive techniques
Low cytotoxicity to minimize immune responses and potential tissue damage
Produce benign byproducts during the healing reaction to reduce potential of leaching of toxic materials
Implanted with the same level of ease as existing biomaterials and implant
Shelf-lives similar to or better than those of existing biomaterials
Capable of withstanding the body's harsh environment
It has been well-documented that microcracks propagating through a polymer matrix will cause the release of healing agent capable of polymerizing to restore the material strength 13-15,37,38. Self-healing implants designed using the first generation approach will eventually deplete the supply of healing agent or embedded catalyst, but a reasonable goal for a first generation self-healing biomaterial is to extend implant lifetime rather than providing sustainable healing capabilities.
Second generation approaches to self-healing are theoretically capable of fully restoring a damaged matrix. Using reversible chemical linkages to achieve self-healing in materials is particularly intriguing because this could allow for sustained healing capabilities not possible with first generation approaches (i.e. there are is no longer the issue of healing agent or catalyst depletion) 68,81. Modifications of stimuli-responsive polymers could also yield healing systems that respond to specific triggers seen with particular biomedical applications (i.e. pH, temperature, specific forces or loading patterns). Whereas the first generation approach is somewhat limited in its uses and primarily combats microcrack propagation, reversible chemistries could be used to treat various types of damage, both mechanical and chemical in nature. Interestingly, such an approach might ultimately manifest itself as a more highly efficient zeroth generation material, in that damage might be so localized and repaired so quickly that it is never observed. Sustained self-repair has the potential for unprecedented implant success through tailored specificity, but also may make suitable chemistries extremely complicated to develop.
One of the most significant problems associated with any self-healing approach is developing a material with the necessary properties required for load-bearing applications. Self-healing polymer gels currently under development clearly lack the necessary mechanical durability 77. Increasing the molecular weight of the self-healing gel would enhance material durability at the expense of reversibility.
9. Conclusions
Self-healing materials are an exciting new area that may broadly benefit materials science in applications ranging from aerospace engineering to construction materials. While several approaches are currently in development, none specifically address the need for biocompatible self-healing materials that could be employed for implants damaged via cyclic loading patterns.
A number of first generation and shape restoration techniques already exist that are feasible, realistic, and reasonably effective. With certain innovations it should be possible to adapt an existing mechanism to design biocompatible self-healing materials that irreversibly extend implant lifetimes. It is also possible to envision a genuinely self-healing biomaterial that employs a reversible self-healing chemistry designed to yield and reassociate under the precise loading patterns seen with specific implants. The requirement for sufficient molecular mobility also may limit second generation systems to mechanically weak hydrogels. Similar to the self-healing materials field in general, the first self-healing, load-bearing biomaterials to arise will likely employ an irreversible healing agent system rather than a more complex reversible system.
Acknowledgements
We would like to thank Dr. Bruce Klitzman and Ms. Leslie Andriani of Duke University for their helpful discussions and reviewing of the manuscript. Financial support from HL-44972 (WMR) and an NIH NRSA Fellowship (ABWB) is much appreciated.
Glossary of Terms
- BIS-GMA
Bis-phenol A glycidyl methacrylate
- CF
Carbon fibers
- CS
Calcium silicate
- DBTL
Di-n-butyltin dilaurate
- DCPD
Dicyclopentadiene
- DETA
Diethylenetriamine
- EGDMA
Ethylene glycol dimethacrylate
- EMAA
Ethylene-co-methacrylic acid
- ENB
5-ethylidene-2-norbornene
- Epon 682
Diglycidyl ether of bisphenol-F
- Epon 828
Diglycidyl ether of bisphenol-A
- HA
Hydroxyapatite
- HEMA
Hydroxyethylmethacrylate
- HOPDMS
Hydroxyl end-functionalized polydimethylsiloxane
- KH-816
Cycloalipathic amine
- MMA-MEA
Poly(methyl methacrylate-co-methyl ethylacrylate)
- OMs
Oligonucleotide-based monomers
- PB-g-PMA-co-PAN
Poly(butadiene)-graft-poly(methyl acrylate-co-acrylonitrile)
- PBT
Polybutylene terephthalate
- PC
Polycarbonate
- PDES
Polydiethoxysiloxane
- PDMS
Polydimethylsiloxane
- PE
Polyethylene
- PEEK
Polyetheretherketone
- PEG
Polyethylene glycol
- PELA
Block copolymer of lactic acid and polyethylene glycol
- PET
Polyethylene terephthalate
- PHB
Polyhydroxybutyrate
- PLA
Polylactic acid
- PMMA
Poly(methyl methacrylate)
- PP
Polypropylene
- PPE
Poly(phenylene ether)
- PS
Polysulfone
- PTFE
Polytetrafluoroethylene
- PU
Polyurethane
- ROMP
Ring-opening metathesis polymerization
- RP
Reversible polymer
- SR
Self-reinforced
- TDCB
Tapered-double cantilever beam
- TJR
Total joint replacement
- UF
Urea-formaldehyde
- UHMWPE
Ultra high molecular weight polyethylene
- YD-115
Butyl diglycidyl ether of bisphenol-A
Footnotes
No benefit of any kind will be received either directly or indirectly by the authors.
References
- 1.Silverthorn D. Human Physiology. Pearson/Benjamin Cummings; San Francisco: 2006. [Google Scholar]
- 2.Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surface Science. 2002;500(1-3):PII S0039–6028(01)01587-4. [Google Scholar]
- 3.Kindt T, Goldsby RA, Osborne BA. Kuby Immunology. W.H. Freeman and Company; 2007. [Google Scholar]
- 4.Ratner BD. A paradigm shift: biomaterials that heal. Polymer International. 2007;56:1183–1185. [Google Scholar]
- 5.Kessler MR, Sottos NR, White SR. Self-healing structural composite materials. Composites Part a-Applied Science and Manufacturing. 2003;34(8):743–753. [Google Scholar]
- 6.Teoh SH. Fatigue of biomaterials: a review. International Journal of Fatigue. 2000;22(10):825–837. [Google Scholar]
- 7.Crawford RE, Murray DW. Total hip replacement: indications for surgery and risk factors for failure. Annals of the Rheumatic Diseases. 1997;56(8):455–457. doi: 10.1136/ard.56.8.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malchau H, Herberts P, Ahnfelt L. Prognosis of Total Hip-Replacement in Sweden - Follow-up of 92,675 Operations Performed 1978-1990 Acta Orthopaedica Scandinavica. 1993;64(5):497–506. doi: 10.3109/17453679308993679. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt H, Goswami T. Implant wear mechanisms-basic approach. 2008 doi: 10.1088/1748-6041/3/4/042001. [DOI] [PubMed] [Google Scholar]
- 10.Dabagh M, Abdekhodaie MJ, Khorasani MT. Effects of polydimethylsiloxane grafting on the calcification, physical properties, and biocompatibility of polyurethane in a heart valve. Journal of Applied Polymer Science. 2005;98(2):758–766. [Google Scholar]
- 11.Kidane AG, Burriesci G, Cornejo P, Dooley A, Sarkar S, Bonhoeffer P, Edirisinghe M, Seifalian AM. Current Developments and Future Prospects for Heart Valve Replacement Therapy. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2009;88B(1):290–303. doi: 10.1002/jbm.b.31151. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz SM. The UHMWPE handbook: ultra-high molecular weight polyethylene in total joint replacement. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- 13.Kessler MR. Self-healing: a new paradigm in materials design. Proceedings of the Institution of Mechanical Engineers Part G-Journal of Aerospace Engineering. 2007;221:479–495. [Google Scholar]
- 14.White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S. Autonomic healing of polymer composites. Nature. 2001;409(6822):794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 15.Blaiszik BJ, Caruso MM, McIlroy DA, Moore JS, White SR, Sottos NR. Microcapsules filled with reactive solutions for self-healing materials. Polymer. 2009;50(4):990–997. [Google Scholar]
- 16.Yuan YC, Yin T, Rong MZ, Zhang MQ. Self healing in polymers and polymer composites. Concepts, realization and outlook: A review. Express Polymer Letters. 2008;2(4):238–250. [Google Scholar]
- 17.Toohey KS, Sottos NR, Lewis JA, Moore JS, White SR. Self-healing materials with microvascular networks. Nature Materials. 2007;6:581–585. doi: 10.1038/nmat1934. [DOI] [PubMed] [Google Scholar]
- 18.Moore NC. Self-healing Concrete for Safer, More Durable Infrastructure. ScienceDaily. 2009;2009 [Google Scholar]
- 19.McCrum NG, Buckley CP, Bucknall CB. Principles of Polymer Engineering. Oxford University Press; New York: 1997. [Google Scholar]
- 20.Alexander H. Composites. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. Academic Press; San Diego: 1996. [Google Scholar]
- 21.Williams DFaRLW. Degradative Effects of the Biological Environment on Metals and Ceramics. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. Academic Press; San Diego: 1996. [Google Scholar]
- 22.Rabinowicz E. Friction and Wear of Materials. John Wiley and Sons, Inc; 1995. [Google Scholar]
- 23.McMillin C. Mechanical Breakdown in the Biological Environment. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. Academic Press; San Diego: 1996. [Google Scholar]
- 24.Yaszemski M. Biomaterials in orthopedics. Marcel Dekker, Inc.; 2004. [Google Scholar]
- 25.Edidin AA, Kurtz SM. Influence of mechanical behavior on the wear of 4 clinically relevant polymeric biomaterials in a hip simulator. Journal of Arthroplasty. 2000;15(3):321–331. doi: 10.1016/s0883-5403(00)90647-8. [DOI] [PubMed] [Google Scholar]
- 26.Ovcharenko A, Halperin G, Etsion I. Experimental Study of a Creeping Polymer Sphere in Contact With a Rigid Flat. Journal of Tribology-Transactions of the Asme. 2009;131(1) [Google Scholar]
- 27.Kranz I, Gonzalez JB, Dorfel I, Gemeinert M, Griepentrog M, Klaffke D, Knabe C, Osterle W, Gross U. Biological response to micron- and nanometer-sized particles known as potential wear products from artificial hip joints: Part II: Reaction of murine macrophages to corundum particles of different size distributions. Journal of Biomedical Materials Research Part A. 2009;89A(2):390–401. doi: 10.1002/jbm.a.32121. [DOI] [PubMed] [Google Scholar]
- 28.Gemeinert M, Dorfel I, Griepentrog M, Gross U, Klaffke D, Knabe C, Kranz I, Osterle W. Biological response to micron- and nanometer-sized particles known as potential wear products from artificial hip joints: Part I: Selection and characterization of model particles. Journal of Biomedical Materials Research Part A. 2009;89A(2):379–389. doi: 10.1002/jbm.a.31952. [DOI] [PubMed] [Google Scholar]
- 29.Furmanski J, Anderson M, Bal S, Greenwald AS, Halley D, Penenberg B, Ries M, Pruitt L. Clinical fracture of cross-linked UHMWPE acetabular liners. Biomaterials. 2009;30(29):5572–5582. doi: 10.1016/j.biomaterials.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials. 1999;20(18):1659–1688. doi: 10.1016/s0142-9612(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 31.Hench LL. Ceramics, Glasses, and Glass-Ceramics. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. Academic Press; San Diego: 1996. [Google Scholar]
- 32.Huang Z-MaSR. Composites in Biomedical Applications. In: Hin TS, editor. Engineering Materials for Biomedical Applications. World Scientific Publishing Co. Pte. Ltd.; Hackensack, NJ: 2004. [Google Scholar]
- 33.Wnek GaGB. Encyclopedia of biomaterials and biomedical engineering. Informa Health Care USA; New York: 2008. [Google Scholar]
- 34.Ashammakhi N, Rokkanen P. Absorbable polyglycolide devices in trauma and bone surgery. Biomaterials. 1997;18(1):3–9. doi: 10.1016/s0142-9612(96)00107-x. [DOI] [PubMed] [Google Scholar]
- 35.Tormala P. Biodegradable Self-Reinforced Composite Materials Manufacturing Structure and Mechanical Properties. Clinical Materials. 1992;10(1-2):29–34. doi: 10.1016/0267-6605(92)90081-4. [DOI] [PubMed] [Google Scholar]
- 36.Murphy EB, Wudl F. The world of smart healable materials. Progress in Polymer Science. 2010;35(1-2):223–251. [Google Scholar]
- 37.Andersson HM, Keller MW, Moore JS, Sottos NR, White SR. Springer; AA Dordrecht, The Netherlands: 2007. Self Healing Polymers and Composites. In: Zwaag Svd, editor. Self Healing Materials: an Alternative Approach to 20 Centuries of Materials Science. pp. 19–44. [Google Scholar]
- 38.Cho SH, Andersson HM, White SR, Sottos NR, Braun PV. Polydimethylsiloxane-based self-healing materials. Advanced Materials. 2006;18(8):997. [Google Scholar]
- 39.Kessler MR, White SR. Self-activated healing of delamination damage in woven composites. Composites Part a-Applied Science and Manufacturing. 2001;32(5):683–699. [Google Scholar]
- 40.Bergman SD, Wuld F. Re-Mendable Polymers. In: Zwaag Svd., editor. Self Healing Materials: an Alternative Approach to 20 Centuries of Materials Science. Springer; AA Dordrecht, the Netherlands: 2007. pp. 45–68. [Google Scholar]
- 41.Jones AS, Rule JD, Moore JS, Sottos NR, White SR. Life extension of self-healing polymers with rapidly growing fatigue cracks. Journal of the Royal Society Interface. 2007;4(13):395–403. doi: 10.1098/rsif.2006.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YZ, Lepech MD, Yang EH, Li VC. Autogenous healing of engineered cementitious composites under wet-dry cycles. Cement and Concrete Research. 2009;39(5):382–390. [Google Scholar]
- 43.Liu X, Lee JK, Yoon SH, Kessler MR. Characterization of diene monomers as healing agents for autonomic damage repair. Journal of Applied Polymer Science. 2006;101(3):1266–1272. [Google Scholar]
- 44.Lee JK, Hong SJ, Liu X, Yoon SH. Characterization of dicyclopentadiene and 5-ethylidene-2-norbornene as self-healing agents for polymer composite and its microcapsules. Macromolecular Research. 2004;12(5):478–483. [Google Scholar]
- 45.Rule JD, Brown EN, Sottos NR, White SR, Moore JS. Wax-protected catalyst microspheres for efficient self-healing materials. Advanced Materials. 2005;17(2):205. [Google Scholar]
- 46.Wool RP. A material fix. Nature. 2001;409(6822):773–774. doi: 10.1038/35057412. [DOI] [PubMed] [Google Scholar]
- 47.Therriault D, White SR, Lewis JA. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nature Materials. 2003;2(4):265–271. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 48.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science; New York: 2002. [Google Scholar]
- 49.Smith DC. Adhesives and Sealants. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. Academic Press; San Diego: 1996. [Google Scholar]
- 50.Stryker . Simplex P Bone Cement Products. Vol. 2010. Stryker; 2006. [Google Scholar]
- 51.Biggs P, Jones L, II, Wellborn B, Lewis G. McGoron CL A, Lin W-C, editors. A Self-healing PMMA Bone Cement: Influence of Crystal Size of Grubbs' Catalyst. 2009:147–150. [Google Scholar]
- 52.Brown EN, Kessler MR, Sottos NR, White SR. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. Journal of Microencapsulation. 2003;20(6):719–730. doi: 10.1080/0265204031000154160. [DOI] [PubMed] [Google Scholar]
- 53.Westhus M, Gonthier E, Brohm D, Breinbauer R. An efficient and inexpensive scavenger resin for Grubbs catalyst. Tetrahedron Letters. 2004;45(15):3141–3142. [Google Scholar]
- 54.Dicyclopentadiene. Vol. 2010. NOVA Chemicals; 2008. [Google Scholar]
- 55.Lehn JM. Supramolecular chemistry: from molecular information towards self-organization and complex matter. Reports on Progress in Physics. 2004;67(3):249–265. [Google Scholar]
- 56.Brunsveld L, Folmer BJB, Meijer EW, Sijbesma RP. Supramolecular polymers. Chemical Reviews. 2001;101(12):4071–4097. doi: 10.1021/cr990125q. [DOI] [PubMed] [Google Scholar]
- 57.Serpe MJ, Craig SL. Physical organic chemistry of supramolecular polymers. Langmuir. 2007;23(4):1626–1634. doi: 10.1021/la0621416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corbett PT, Leclaire J, Vial L, West KR, Wietor JL, Sanders JKM, Otto S. Dynamic combinatorial chemistry. Chemical Reviews. 2006;106(9):3652–3711. doi: 10.1021/cr020452p. [DOI] [PubMed] [Google Scholar]
- 59.Otto S, Furlan RLE, Sanders JKM. Selection and amplification of hosts from dynamic combinatorial libraries of macrocyclic disulfides. Science. 2002;297(5581):590–593. doi: 10.1126/science.1072361. [DOI] [PubMed] [Google Scholar]
- 60.Carnall JMA, Waudby CA, Belenguer AM, Stuart MCA, Peyralans JJP, Otto S. Mechanosensitive Self-Replication Driven by Self-Organization. Science. 327(5972):1502–1506. doi: 10.1126/science.1182767. [DOI] [PubMed] [Google Scholar]
- 61.Lam RTS, Belenguer A, Roberts SL, Naumann C, Jarrosson T, Otto S, Sanders JKM. Amplification of acetylcholine-binding catenanes from dynamic combinatorial libraries. Science. 2005;308(5722):667–669. doi: 10.1126/science.1109999. [DOI] [PubMed] [Google Scholar]
- 62.Sijbesma RP, Beijer FH, Brunsveld L, Folmer BJB, Hirschberg J, Lange RFM, Lowe JKL, Meijer EW. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science. 1997;278(5343):1601–1604. doi: 10.1126/science.278.5343.1601. [DOI] [PubMed] [Google Scholar]
- 63.Rudkevich DM. Supramolecular Polymers. Journal of the American Chemical Society. (2nd Edition) 2006;128(21):7110–7110. [Google Scholar]
- 64.Modder JF, Vrieze K, Spek AL, Challa G, Vankoten G. Stereoregular Coordination Polymers Formed on Binding of Peptide-based Polydentate Ligands to Silver (I) and Copper (I) - X-Ray Structure of ([AG(N-[N-((5-Methyl-2-Thienyl)Methylidene)-L Methionyl]Histamine)]+[O3S CF3]-.MeOH) Infinity and a Solution Structure Study. Inorganic Chemistry. 1992;31(7):1238–1247. [Google Scholar]
- 65.Kong HJ, Wong E, Mooney DJ. Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules. 2003;36(12):4582–4588. [Google Scholar]
- 66.Markovitsi D, Bengs H, Ringsdorf H. Charge-Transfer Absorption in Doped Columnar Liquid-Crystals. Journal of the Chemical Society-Faraday Transactions. 1992;88(9):1275–1279. [Google Scholar]
- 67.Kersey FR, Lee G, Marszalek P, Craig SL. Surface-to-surface bridges formed by reversibly assembled polymers. Journal of the American Chemical Society. 2004;126(10):3038–3039. doi: 10.1021/ja0499501. [DOI] [PubMed] [Google Scholar]
- 68.Fogleman EA, Yount WC, Xu J, Craig SL. Modular, well-behaved reversible polymers from DNA-based monomers. Angewandte Chemie-International Edition. 2002;41(21):4026–4028. doi: 10.1002/1521-3773(20021104)41:21<4026::AID-ANIE4026>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 69.Lin DC, Yurke B, Langrana NA. Inducing reversible stiffness changes in DNA-crosslinked gels. Journal of Materials Research. 2005;20(6):1456–1464. [Google Scholar]
- 70.Miyata T. Preparation of smart soft materials using molecular complexes. Polymer Journal. 42(4):277–289. [Google Scholar]
- 71.Gibbs-Davis JM, Schatz GC, Nguyen ST. Sharp melting transitions in DNA hybrids without aggregate dissolution: Proof of neighboring-duplex cooperativity. Journal of the American Chemical Society. 2007;129(50):15535–15540. doi: 10.1021/ja073034g. [DOI] [PubMed] [Google Scholar]
- 72.Sivakova S, Rowan SJ. Nudeobases as supramolecular motifs. Chemical Society Reviews. 2005;34(1):9–21. doi: 10.1039/b304608g. [DOI] [PubMed] [Google Scholar]
- 73.Becker T. ‘Self-Healing’ Material Seen in Movies is Real Possibility. Vol. 2010. Dow Jones & Company; 2003. [Google Scholar]
- 74.Gordon KL, Working DC, Wise KE, Bogert PB, Britton SM, Topping CC, Smith JY, Siochi EJ. Recent Advances in Thermoplastic Puncture-Healing Polymers. NASA-Langley Research Center; Hampton, VA: 2009. [Google Scholar]
- 75.Real World: Self Healing Materials. NASA; 2009. [Google Scholar]
- 76.Bansal RK. Synthetic Approaches in Organic Chemistry. Jones and Bartlett Publishers; Sudbury: 1996. [Google Scholar]
- 77.Kersey FR, Loveless DM, Craig SL. A hybrid polymer gel with controlled rates of cross-link rupture and self-repair. Journal of the Royal Society Interface. 2007;4:373–380. doi: 10.1098/rsif.2006.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen XX, Dam MA, Ono K, Mal A, Shen HB, Nutt SR, Sheran K, Wudl F. A thermally re-mendable cross-linked polymeric material. Science. 2002;295(5560):1698–1702. doi: 10.1126/science.1065879. [DOI] [PubMed] [Google Scholar]
- 79.Chen XX, Wudl F, Mal AK, Shen HB, Nutt SR. New thermally remendable highly cross-linked polymeric materials. Macromolecules. 2003;36(6):1802–1807. [Google Scholar]
- 80.Ghosh B, Urban MW. Self-Repairing Oxetane-Substituted Chitosan Polyurethane Networks. Science. 2009;323(5920):1458–1460. doi: 10.1126/science.1167391. [DOI] [PubMed] [Google Scholar]
- 81.Xu J, Fogleman EA, Craig SL. Structure and properties of DNA-based reversible polymers. Macromolecules. 2004;37:1863–1870. [Google Scholar]
- 82.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science; New York: 2008. [Google Scholar]
- 83.MacEwan SR, Chilkoti A. Elastin-Like Polypeptides: Biomedical Applications of Tunable Biopolymers. Biopolymers. 2010;94(1):60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- 84.Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414(6865):773–776. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 85.Kushner AM, Vossler JD, Williams GA, Guan ZB. A Biomimetic Modular Polymer with Tough and Adaptive Properties. Journal of the American Chemical Society. 2009;131(25):8766. doi: 10.1021/ja9009666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cordier P, Tournilhac F, Soulie-Ziakovic C, Leibler L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature. 2008;451(7181):977–980. doi: 10.1038/nature06669. [DOI] [PubMed] [Google Scholar]
- 87.Lin DC, Yurke B, Langrana NA. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. Journal of Biomechanical Engineering-Transactions of the Asme. 2004;126(1):104–110. doi: 10.1115/1.1645529. [DOI] [PubMed] [Google Scholar]
- 88.Jiang FX, Du YZ, Chippada U, Li LL, Firestein BL, Yurke B, Shreiber DI, Schloss RS, Langrana NA. Neurite elongation and branching on DNA crosslinked polyacrylamide hydrogels. Proceeding of the Asme Summer Bioengineering Conference. 2007;2007:991–992. [Google Scholar]
- 89.Kuzyk A, Yurke B, Toppari JJ, Linko V, Torma P. Dielectrophoretic trapping of DNA origami. Small. 2008;4(4):447–450. doi: 10.1002/smll.200701320. [DOI] [PubMed] [Google Scholar]
- 90.Liedl T, Dietz H, Yurke B, Simmel F. Controlled trapping and release of quantum dots in a DNA-Switchable hydrogel. Small. 2007;3:1688–1693. doi: 10.1002/smll.200700366. [DOI] [PubMed] [Google Scholar]
- 91.Lin DC, Yurke B, Langrana NA. Use of rigid spherical inclusions in Young's moduli determination: Application to DNA-crosslinked gels. Journal of Biomechanical Engineering-Transactions of the Asme. 2005;127(4):571–579. doi: 10.1115/1.1933981. [DOI] [PubMed] [Google Scholar]
- 92.Seelig G, Yurke B, Winfree E. Catalyzed relaxation of a metastable DNA fuel. Journal of the American Chemical Society. 2006;128:12211–12220. doi: 10.1021/ja0635635. [DOI] [PubMed] [Google Scholar]
- 93.Seelig G, Yurke B, Winfree E. DNA hybridization catalysts and catalyst circuits. DNA Computing. 2005;3384:329–343. [Google Scholar]
- 94.Yurke B. Using DNA to power the nanoworld. Controlled Nanoscale Motion. 2007;711:331–347. [Google Scholar]
- 95.Yurke B. Using DNA to assemble and power the nanoworld. Nanofabrication: Technologies, Devices and Applications. 2004;5592:82–90. [Google Scholar]
- 96.Yurke B, Lin DC, Langrana NA. Use of DNA nanodevices in modulating the mechanical properties of polyacrylamide gels. DNA Computing. 2006;3892:417–426. [Google Scholar]
- 97.Yurke B, Zhang D. A clocked DNA-based replicator. DNA Computing. 2005;3384:445–457. [Google Scholar]
- 98.Zhang DY, Turberfield AJ, Yurke B, Winfree E. Engineering entropy-driven reactions and networks catalyzed by DNA. Science. 2007;318(5853):1121–1125. doi: 10.1126/science.1148532. [DOI] [PubMed] [Google Scholar]
- 99.Kumar GVP, Mathew L. Biomaterial optimization in a percutaneous aortic valve stent using finite element analysis. Cardiovasc Revasc Med. 2009;10(4):247–51. doi: 10.1016/j.carrev.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Ge L, Dasi LP, Sotiropoulos F, Yoganathan AP. Characterization of hemodynamic forces induced by mechanical heart valves: Reynolds vs. viscous stresses. Annals of Biomedical Engineering. 2008;36(2):276–297. doi: 10.1007/s10439-007-9411-x. [DOI] [PubMed] [Google Scholar]
- 101.Sacks MS, Yoganathan AP. Heart valve function: a biomechanical perspective. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362(1484):1369–1391. doi: 10.1098/rstb.2007.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramos A, Fonseca F, Simoes JA. Simulation of physiological loading in total hip replacements. Journal of Biomechanical Engineering-Transactions of the Asme. 2006;128(4):579–587. doi: 10.1115/1.2205864. [DOI] [PubMed] [Google Scholar]
- 103.Sol C, Mitchell K, Torok DJ, Banks S, Graves S, Welsh R. Impact forces at the knee joint u: A comparative study on running styles. Medicine and Science in Sports and Exercise. 2001;33(5 Supplement):S128. [Google Scholar]
- 104.Anderson DJ. Measurement of stress in mastication. I. J Dent Res;1956;35(5):664–70. doi: 10.1177/00220345560350050201. [DOI] [PubMed] [Google Scholar]
- 105.Chai H, Lee JJW, Kwon JY, Lucas PW, Lawn BR. A simple model for enamel fracture from margin cracks. Acta Biomaterialia. 2009;5(5):1663–1667. doi: 10.1016/j.actbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 106.Lee JJW, Kwon JY, Chai H, Lucas PW, Thompson VP, Lawn BR. Fracture Modes in Human Teeth. Journal of Dental Research. 2009;88(3):224–228. doi: 10.1177/0022034508330055. [DOI] [PubMed] [Google Scholar]
- 107.Ingraham P. Massage Therapy for Bruxism, Jaw Clenching, and TMJ Syndrome. Vol. 2009. Regeneration Training; 2008. [Google Scholar]
- 108.Myoung S, Lee J, Constantino P, Lucas P, Chai H, Lawn B. Morphology and fracture of enamel. Journal of Biomechanics. 2009;42(12):1947–1951. doi: 10.1016/j.jbiomech.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 109.Perez MA, Garcia-Aznar JM, Doblare M. Does Increased Bone-Cement Interface Strength have Negative Consequences for Bulk Cement Integrity? A Finite Element Study. Annals of Biomedical Engineering. 2009;37(3):454–466. doi: 10.1007/s10439-008-9616-7. [DOI] [PubMed] [Google Scholar]
- 110.Hoey D, Taylor D. Quantitative analysis of the effect of porosity on the fatigue strength of bone cement. Acta Biomaterialia. 2009;5(2):719–726. doi: 10.1016/j.actbio.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 111.Tomasi C, Wennstrom JL, Berglundh T. Longevity of teeth and implants - a systematic review. Blackwell Publishing; 2008. pp. 23–32. [DOI] [PubMed] [Google Scholar]
- 112.Taylor TD, Agar JR. Twenty years of progress in implant prosthodontics. The Journal of Prosthetic Dentistry. 2002;88(1):89–95. [PubMed] [Google Scholar]
- 113.Lewis R, Dwyer-Joyce RS. Wear of human teeth: a tribological perspective. Proceedings of the Institution of Mechanical Engineers Part J-Journal of Engineering Tribology. 2005;219(J1):1–18. [Google Scholar]
- 114.Lemons JE. Dental Implants. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. Academic Press; San Diego: 1996. [Google Scholar]
- 115.Deng M, Shalaby SW. Properties of self-reinforced ultra-high-molecular-weight polyethylene composites. Biomaterials. 1997;18(9):645–655. doi: 10.1016/s0142-9612(96)00194-9. [DOI] [PubMed] [Google Scholar]
- 116.Rushton N, Rae T. The Intra-Articular Response to Particulate Carbon-Fiber Reinforced High-Density Polyethylene and its Constituents - An Experimental Study in Mice Biomaterials. 1984;5(6):352–356. doi: 10.1016/0142-9612(84)90034-6. [DOI] [PubMed] [Google Scholar]
- 117.St. John KR. Applications of Advanced Composites in Orthopedic Implants. In: Szycher M, editor. Biocompatible Polymers, Metals, and Composites. Technomic Publishing Co., Inc.; Lancaster: 1983. [Google Scholar]
- 118.Bradley JS, Hastings GW, Johnsonnurse C. Carbon-fiber Reinforced Epoxy as a High-strength, Low Modulus Material for Internal Fixation Plates. Biomaterials. 1980;1(1):38–40. doi: 10.1016/0142-9612(80)90057-5. [DOI] [PubMed] [Google Scholar]
- 119.McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical Properties of Some Fiber Reinforced Polymer Composites After Implantation as Fracture Fixation Plates. Biomaterials. 1980;1(4):189–192. doi: 10.1016/0142-9612(80)90015-0. [DOI] [PubMed] [Google Scholar]
- 120.Tayton K, Johnsonnurse C, McKibbin B, Bradley J, Hastings G. The Use of Semirigid Carbon-fiber Reinforced Plastic Plates for Fixation of Human Fractures - Results of Preliminary Trials. Journal of Bone and Joint Surgery-British Volume. 1982;64(1):105–111. doi: 10.1302/0301-620X.64B1.7040407. [DOI] [PubMed] [Google Scholar]
- 121.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Composites Science and Technology. 2001;61(9):1189–1224. [Google Scholar]
- 122.Fujihara K, Huang ZM, Ramakrishna S, Satkunanantham K, Hamada H. Development of braided carbon/peek composite bone plates. Advanced Composites Letters. 2001;10(1):13–20. [Google Scholar]
- 123.Zimmerman M, Parsons JR. The Design and Analysis of a Laminated Partially Degradable Composite Bone Plate for Fracture Fixation. Journal of Biomedical Materials Research-Applied Biomaterials. 1987;21(A3):345–361. [PubMed] [Google Scholar]
- 124.Tormala P, Vainionpaa S, Kilpikari J, Rokkanen P. The Effects of Fiber Reinforcement and Gold Plating on the Flexural and Tensile Strength of PGA-PLA Copolymer Materials In Vitro Biomaterials. 1987;8(1):42–45. doi: 10.1016/0142-9612(87)90027-5. [DOI] [PubMed] [Google Scholar]
- 125.Lin TW, Corvelli AA, Frondoza CG, Roberts JC, Hungerford DS. Glass peek composite promotes proliferation and osteocalcin production of human osteoblastic cells. Journal of Biomedical Materials Research. 1997;36(2):137–144. doi: 10.1002/(sici)1097-4636(199708)36:2<137::aid-jbm1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 126.Kim IY, Sugino A, Kikuta K, Ohtsuki C. Bioactive Composites Consisting of PEEK and Calcium Silicate Powders. Journal of Biomaterials Applications. 2009;24(2):105–118. doi: 10.1177/0885328208094557. [DOI] [PubMed] [Google Scholar]
- 127.Isidor F, Odman P, Brondum K. Intermittent loading of teeth restored using prefabricated carbon fiber posts. International Journal of Prosthodontics. 1996;9(2):131–136. [PubMed] [Google Scholar]
- 128.Christel P, Meunier A, Leclercq S, Bouquet P, Buttazzoni B. Development of a Carbon- Carbon Hip Prosthesis. Journal of Biomedical Materials Research-Applied Biomaterials. 1987;21(A2):191–218. [PubMed] [Google Scholar]
- 129.Kettunen J, Makela A, Miettinen H, Nevalainen T, Heikkila M, Tormala P, Rokkanen P. Fixation of femoral shaft osteotomy with an intramedullary composite rod: An experimental study on dogs with a two-year follow-up. Journal of Biomaterials Science-Polymer Edition. 1999;10(1):33–45. doi: 10.1163/156856299x00261. [DOI] [PubMed] [Google Scholar]
- 130.Brantigan JW, Steffee AD. A Carbon-fiber Implant to Aid Interbody Lumbar Fusion-2 Year Clinical Results in the First 26 Patients Spine. 1993;18(14):2106–2117. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 131.Brantigan JW, Steffee AD, Geiger JM. A Carbon-fiber Implant to Aid Interbody Lumbar Fusion - Mechanical Testing Spine. 1991;16(6):S277–S282. doi: 10.1097/00007632-199106001-00020. [DOI] [PubMed] [Google Scholar]
- 132.Ciappetta P, Boriani S, Fava GP. A carbon fiber reinforced polymer cage for vertebral body replacement: Technical note. Neurosurgery. 1997;41(5):1203–1206. doi: 10.1097/00006123-199711000-00040. [DOI] [PubMed] [Google Scholar]
- 133.Gershon B, Cohn D, Marom G. Compliance and Ultimate Strength of Composite Arterial Protheses Biomaterials. 1992;13(1):38–43. doi: 10.1016/0142-9612(92)90093-4. [DOI] [PubMed] [Google Scholar]
- 134.Ignatius A, Unterricker K, Wenger K, Richter M, Claes L, Lohse P, Hirst H. A new composite made of polyurethane and glass ceramic in a loaded implant model: a biomechanical and histological analysis. Journal of Materials Science-Materials in Medicine. 1997;8(12):753–756. doi: 10.1023/a:1018508511787. [DOI] [PubMed] [Google Scholar]
- 135.Claes L, Schultheiss M, Wolf S, Wilke HJ, Arand M, Kinzl L. A new radiolucent system for vertebral body replacement: Its stability in comparison to other systems. Journal of Biomedical Materials Research. 1999;48(1):82–89. doi: 10.1002/(sici)1097-4636(1999)48:1<82::aid-jbm14>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 136.Urbaniak JR, Bright DS, Hopkins JE. Replacement of Intervertebral Disks in Chimpanzees by Silicone Dacron Implants - Preliminary Report. Journal of Biomedical Materials Research. 1973;7(3):165–186. doi: 10.1002/jbm.820070311. [DOI] [PubMed] [Google Scholar]
- 137.Ambrosio L, Netti PA, Iannace S, Huang SJ, Nicolais L. Composite hydrogels for intervertebral disc prostheses. Journal of Materials Science-Materials in Medicine. 1996;7(5):251–254. [Google Scholar]
- 138.Rule JD, Sottos NR, White SR. Effect of microcapsule size on the performance of self-healing polymers. Polymer. 2007;48(12):3520–3529. [Google Scholar]
- 139.Takeda K, Unno H, Zhang M. Polymer reaction in polycarbonate with Na2CO3. Journal of Applied Polymer Science. 2004;93(2):920–926. [Google Scholar]
- 140.Liu W, Zhao YH, Nguyen J, Li Y, Jiang Q, Lavernia EJ. Electric field induced reversible switch in hydrogen storage based on single-layer and bilayer graphenes. Carbon. 2009;47(15):3452–3460. [Google Scholar]