Abstract
Background
There is a significant increase in the number of HIV-infected older adults (HOA). This population may experience functional decline at a much younger age. Little is known about the relationship between functional limitations and systemic adipokines in HOA.
Objective
Our study aimed to evaluate the relationship between functional limitations and systemic adipokine levels in HOA population.
Design
Cross-sectional.
Setting
Academic hospital-based infectious disease clinic.
Participants
The study investigated community-dwelling HIV-infected adults >50 years old and compared this group with age, gender and BMI comparable healthy controls.
Measurements
We measured functional status, body composition and plasma concentrations of adipokines.
Results
Fifty-four HOA were studied (mean: age 57 years, BMI 29 kg/m2, CD4 604, duration of HIV 17 years) and compared with thirty-two age, gender and BMI comparable healthy controls. The HOA group showed significantly higher functional limitations compared to the age, gender and BMI comparable controls (p<0.05). Levels of adipokines were significantly different between the two groups (p<0.05). Multiple regression analyses indicated that adiponectin and visfatin were significantly correlated with several physical function measures after controlling for age, sex, and metabolic comorbidities. Adiponectin was negatively correlated with functional limitations, and this relationship was stronger in the control group compared to the HOA group. Conversely, visfatin was positively correlated with functional limitations only in the HOA group.
Conclusion
HOA have significant functional limitations and alteration in adipokine levels compared to controls. Adiponectin and visfatin were associated with functional limitations. Visfatin was a correlate of physical function only in the HOA group. Prospective longitudinal studies could provide further insight on the role of adipokines in HIV-related functional decline.
Keywords: HIV, older adults, physical function, adipokines, fat redistribution
Introduction
One of the hallmark signs of healthy aging is the ability to preserve physical function. Functional limitations in older adults increase the risk of loss of independence, nursing home admissions and mortality (1). We are currently experiencing a dramatic demographic shift in the distribution of the US population towards that of older ages, and accordingly addressing physical frailty is widely recognized as a major challenge not only today, but also for many years to come (2).
HIV-infected individuals may have reduced physical function and can experience physical frailty characteristics at a much younger age (3–6). Of note, the number of HIV-infected older adults (HOA) has significantly increased over the last two decades (7). This is an important statistic because HOA are particularly vulnerable to prolonged HIV disease and antiretroviral therapy (ART) related metabolic disturbances (8, 9), which may play an important role in accelerating one’s aging and having a negative impact on a person’s quality of life (QOL).
ART toxicity has been implicated in altering the homeostatic regulation of adipokines secreted from adipose tissue (10). Adipokines have been associated with fat redistribution and metabolic abnormalities in HIV-infected patients (10). Decreased adiponectin and leptin levels have been associated with ART-induced metabolic syndrome and an increase in central adiposity whereas C-reactive protein (CRP) levels are elevated in patients on ART (11–13).
Further, in the older HIV-uninfected population, adipokines have been linked with functional limitations (14, 15). That being said, little is known about adipokines and its relationship with functional impairment in the HOA population. Our aim in this study, therefore, was to 1) investigate functional impairment and systemic adipokine levels in HOA compared to age, gender and BMI comparable controls and 2) examine whether adipokines are associated with functional impairment in HOA.
Methods
Subjects
HOA were recruited from our urban hospital-based infectious disease clinic, which provides services to over 1,000 HIV-infected patients. Healthy control participants were recruited through flyers posted in the local community. Eligibility criteria for HIV-infected patients included: age >50 years, stable ART regimen, able to ambulate without assistive devices, and free of any AIDS defining illness (ADI) for six months prior to enrollment. The exclusion criteria for controls are similar to the criteria used for the recruitment of HOA subjects and include comorbid medical conditions that would prevent the subject from safely undergoing treadmill exercise testing. Subjects with severe cardiopulmonary illness, severe anemia, significant orthopedic or neuromuscular limitations, renal failure, cirrhosis, significant cognitive or sensory limitations, untreated depression, unstable manic or psychotic disorder, and active malignancy were excluded. All subjects received a baseline medical evaluation. The medical history of subjects was obtained from self-completed questionnaires regarding the subject’s past medical history and from electronic medical records. Subjects who did not have CD4 cell count and HIV viral load documented three months prior to enrollment provided blood samples. An informed consent was obtained from all subjects prior to enrollment and the study was approved by the University of Rochester Medical Center’s Research Subjects Review Board and Clinical Research Center Scientific Advisory Committee.
Study Measurements
Assessment of Physical Function: The physical performance test (PPT) is a global measure of physical function that assesses the ability to perform usual daily activities (4, 16, 17). The PPT includes seven standardized timed tasks (50-foot walk, putting on and removing a coat, picking up a penny, standing up from a chair, lifting a book, climbing one flight of stairs, and a progressive Romberg test) and two additional tasks (climbing four flights of stairs and performing a 360 degree turn). The score for each item ranges between 0 and 4, where a perfect score is 36 (4, 16, 17). Hand grip strength was determined using a hand grip dynamometer, while subjects held the dynamometer in the hand to be tested with the elbow positioned at 90 degrees on the side of the body. Dynamic balance was assessed as the time needed to complete an obstacle course whereby subjects stand from a standard 18-inch high chair, walk forward 6 feet, step over a 2 × 2-inch obstacle, walk forward another 6 feet, ascend a 6-inch high curb, turn around, step down off the curb, and return to the chair as quickly and safely as possible (16). Peak aerobic power (VO2peak) was assessed during a graded treadmill walking test. Specifically, during a 5-minute warm-up at 0% grade, the speed was adjusted to identify the fastest comfortable walking speed. Speed was held constant and the treadmill incline was increased by 3% every 2 minutes. Participants were allowed to lightly hold on to a handrail for the purpose of maintaining their balance during the test. Blood pressure was measured by auscultation every 2 minutes. Cardiorespiratory data were collected at 30-second intervals using a computerized system. The test was terminated when the participant becomes too fatigued to continue (16).
Assessment of Body Composition: Fat mass (FM) and lean mass (LM) were measured using Dual Energy X-ray Absorptiometry, Hologic Discovery (DXA). Appendicular fat was calculated as the total fat in the extremities. In the upper extremities, the appendicular fat included subcutaneous adipose tissue from the shoulder to the wrist. In the lower extremities, appendicular fat included subcutaneous adipose tissue from the hip to the ankle. Trunk fat was the amount of fat measured by the DXA from below the neck to the pelvis. The bone mineral-free portion of the appendicular lean mass represents skeletal muscle in the extremities (18).
Assessment of Adipokines: The quantitative determination of serum adiponectin, leptin, retinol binding protein-4 (RBP-4), resistin, visfatin, tumor necrosis factor-alpha (TNF-alpha), Interleukin 6 (IL-6) and CRP was performed by Quantikine enzyme-linked immunosorbent assay (ELISA) method using commercially available reagent kits (R&D Systems, Minneapolis, MN), according to manufacturer’s instructions. The concentration of analyte in patient samples was calculated from standard curve which was performed for each plate.
Statistical Analysis
Descriptive data are presented as mean and standard deviation (S.D.) for continuous variables and frequencies and percentages for categorical variables. Group comparison was performed using the Chi-square test in categorical variables, and two-sample Student’s t-test incontinuous variables. All of the variables underwent a normal assumption check. For those variables with non-normality, a log transformation was applied, and the results were presented in the original scales. Linear regression models with variable selection examined the association of functional limitations with all the potential predictors, and the model fitting results suggested that age, gender, adiponectin and visfatin were significant to the functional outcomes. Then we investigated the effect of adiponectin and visfatin on physical function measures, controlling for age, gender and metabolic comorbidities such as hypertension, diabetes and hyperlipidemia.
The data analysis for this paper was generated using SAS/STAT and SAS/GRAPH software, Version 9.3 of the SAS System for Windows. Copyright © 2002–2010, SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Results
Table 1 presents the demographic characteristics of the subjects. HOA and controls were carefully controlled for age, BMI and gender. The HOA group had a higher prevalence of the metabolic diseases (hypertension, hyperlipidemia and diabetes) compared to the control group.
Table 1.
Characteristics of HIV+ Older Adults (HOA) and Control Study Subjects
| Variable | Control (32) | HOA (54) | p-value |
|---|---|---|---|
| Age in years – Mean (S.D.) | 58.1 (6.5) | 56.7 (6.0) | 0.32 |
| BMI in kg/m2 – Mean (S.D.) | 27.9 (4.6) | 28.7 (5.3) | 0.45 |
| Gender (n% Male) | 20 (62.5%) | 41 (75.9%) | 0.19 |
| Race (n% White) | 25 (78.1%) | 31 (60.8%) | 0.20 |
| Ethnicity (n% Hispanic) | 3 (9.4%) | 3 (5.6%) | 0.50 |
| Hypertension (n% Yes) | 6 (18.7%) | 35 (64.8%) | <.01 |
| Diabetes (n% Yes) | 3 (9.4%) | 13 (24.1%) | 0.09 |
| Hyperlipidemia (n% Yes) | 2 (6.2%) | 27 (50.0%) | <.01 |
| Duration of HIV in years – Mean (S.D.) | - | 16.6 (6.2) | - |
| CD4 Count – Mean (S.D.) | - | 603.5 (270.6) | - |
| CD4 Nadir – Mean (S.D.) | - | 222.9 (175.7) | - |
| Viral Load (<50) copies – (n% Yes) | - | 41 (75.9%) | - |
| Current PI Use – (n% Yes) | - | 27 (50.0%) | - |
| Current N(t)RTI Use – (n% Yes) | - | 45 (83.3%) | - |
PI, Protease Inhibitors; N(t)RTI, Nucleoside/Nucleotide Reverse Transcriptase Inhibitors.
Table 2 presents the physical function and body composition characteristics of the subjects. Compared with the control group, the HOA group had significantly lower scores in both PPT and VO2peak, and significantly higher scores for most of the timed tasks. Moreover, specific tests of physical function (static and dynamic balance, muscle strength) were significantly lower in the HOA group compared to the control group. Although both groups had similar total fat, percent fat and lean mass, the HOA group exhibited significant (p<0.01) lipodystrophy characteristics as measured by appendicular to trunk fat, trunk to total fat and appendicular to total fat ratios compared to the control group.
Table 2.
Results of Physical Function and Body Composition Variables by Group
| Variable | Control (32) Mean 95% C.I. |
HOA (54) Mean 95% C.I. |
p-value |
|---|---|---|---|
| Lift 7 lb book to shelf (sec) | 2.16 (2.03, 2.30) | 2.15 (2.05, 2.26) | 0.93 |
| Put on/remove lab coat (sec) | 11.92 (10.89, 13.04) | 12.86 (12.03, 13.74) | 0.19 |
| Pick up a coin (sec) | 2.15 (1.98, 2.33) | 2.88 (2.59, 3.20) | <.01 |
| Walk 50 feet (sec) | 8.50 (8.04, 8.98) | 10.45 (10.00, 10.91) | <.01 |
| Stand up from chair (sec) | 7.78 (7.01, 8.65) | 11.25 (10.52, 12.03) | <.01 |
| Climb 1 flight of stairs (sec) | 3.74 (3.45, 4.04) | 4.58 (4.30, 4.88) | <.01 |
| Obstacle course (sec) | 6.99 (6.54, 7.47) | 8.60 (8.14, 9.10) | <.01 |
| One leg limb stand (sec) | 22.99 (18.64, 28.37) | 11.07 (8.25, 14.84) | <.01 |
| Hand grip strength (sec) | 35.71 (32.43, 38.98) | 30.24 (27.51, 32.98) | 0.02 |
| PPT Score | 33.50 (32.97, 34.03) | 30.94 (30.41, 31.48) | <.01 |
| Peak aerobic power (ml/kg/min) | 32.78 (28.96, 36.61) | 22.36 (20.17, 24.56) | <.01 |
| Fat mass (kg) | 23.30 (20.26, 26.33) | 23.49 (20.93, 26.05) | 0.92 |
| Lean mass (kg) | 54.88 (50.30, 59.45) | 55.38 (52.88, 57.88) | 0.84 |
| Total body mass (kg) | 80.75 (74.82, 86.68) | 82.40 (77.99, 86.82) | 0.65 |
| Fat percentage (%) | 28.82 (25.82, 31.83) | 28.27 (26.08, 30.47) | 0.76 |
| Appendicular/trunk fat ratio | 0.85 (0.75, 0.95) | 0.66 (0.60, 0.72) | <.01 |
| Trunk/total fat ratio | 0.52 (0.50, 0.55) | 0.58 (0.56, 0.60) | <.01 |
| Appendicular/total fat ratio | 0.43 (0.40, 0.45) | 0.37 (0.35,0.39) | <.01 |
PPT, Physical Performance Test
Table 3 presents the adipokine variables by group. The HOA group had showed significantly different levels of adipokines such as adiponectin, visfatin, RBP-4, and CRP compared to control group (p<0.05). Notably, leptin, resistin, IL-6 and TNF-alfa did not differ significantly between the groups. Further, controlling for metabolic comorbid conditions we found similar results as presented in Table 2 and Table 3.
Table 3.
Adipokine Variables by Group
| Variable | Control (32) Mean 95% C.I. |
HOA (54) Mean 95% C.I. |
p-value |
|---|---|---|---|
| RBP (μg/ml) | 80.3 (71.3, 90.4) | 100.3 (93.2, 108.0) | <.01 |
| Adiponectin (ng/ml) | 4072.3 (3291.3, 5038.7) | 2573.3 (2199.1, 3011.3) | <.01 |
| Leptin (pg/ml) | 4098.4 (3250.7, 5167.2) | 4302.7 (3483.4, 5314.7) | 0.76 |
| CRP (ng/ml) | 1148.2 (801.4, 1645.2) | 1948.9 (1420.0, 2674.7) | 0.03 |
| Resistin (ng/ml) | 11.4 (9.6, 13.5) | 12.4 (10.0, 15.2) | 0.57 |
| TNF alpha (pg/ml) | 1.5 (1.1, 2.0) | 1.8 (1.5, 2.2) | 0.20 |
| IL-6 (pg/ml) | 1.1 (0.7, 1.6) | 1.5 (1.2, 1.9) | 0.13 |
| Visfatin (ng/ml) | 6.7 (4.7, 9.6) | 12.0 (9.5, 15.2) | <.01 |
RBP = Retinol Binding Protein; CRP = C - reactive protein; IL-6 = Interleukin 6
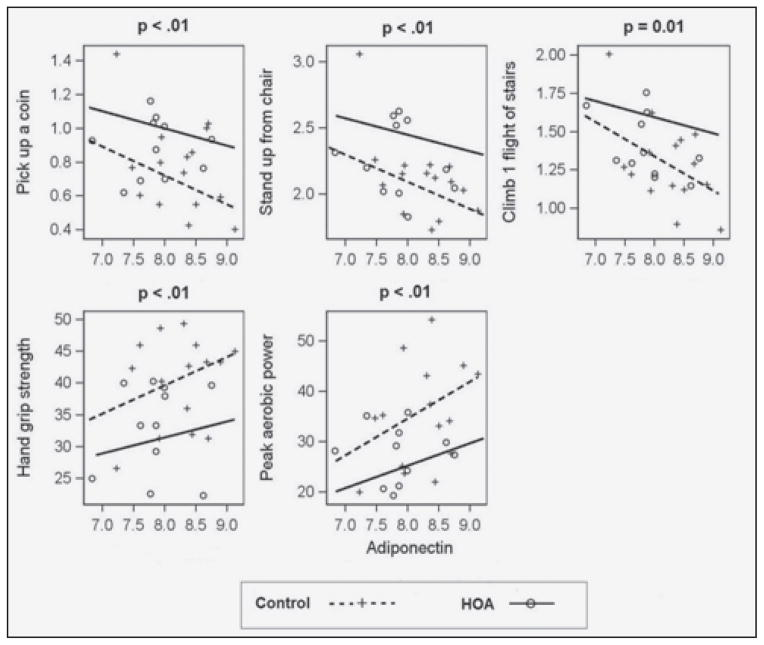
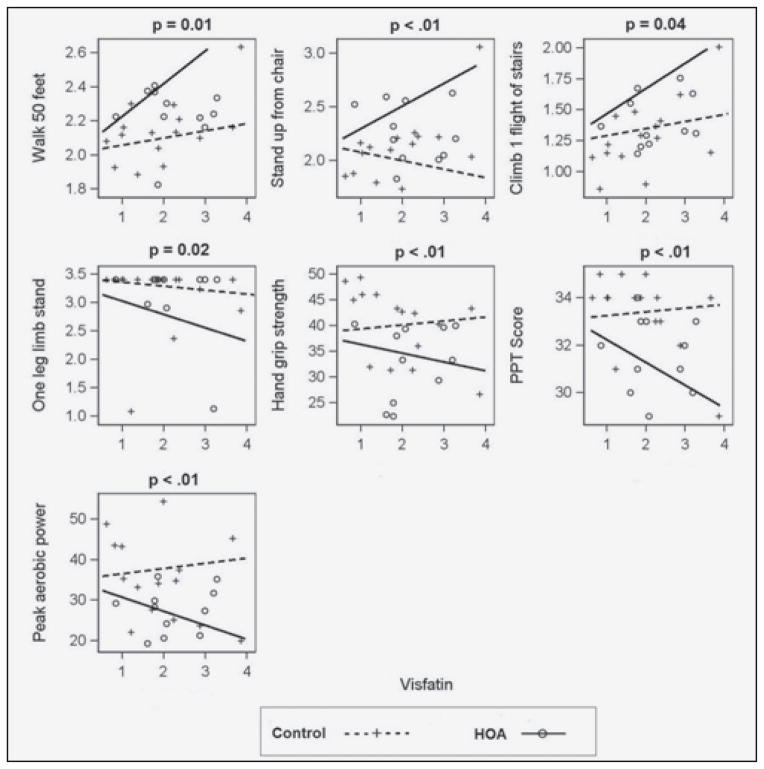
Among the adipokines studied, adiponectin and visfatin were significantly correlated with several physical function measures after controlling for demographics and comorbidities. Figure 1 and Figure 2 presents the association of physical function measures with adiponectin and visfatin, respectively. We present the association curves only when the association was significant (p<0.05) in at least one group (control or HOA) and the association curves were significantly different between the two groups. Adiponectin was negatively correlated with functional limitations (Figure1), and this relationship was stronger in the control group compared to the HOA group. Conversely, visfatin was positively correlated with functional limitations, only in the HOA group (Figure 2). There was no significant association of visfatin and functional limitations in the control group.
Figure 1.
Comparison of Physical Function Outcomes for Adiponectin after controlling for age, gender and metabolic comorbidity
Figure 2.
Comparison of Physical Function Outcomes for Visfatin after controlling for age, gender and metabolic comorbidity
Discussion
Our cross-sectional study suggests that HOA on ART have lower physical function and altered adipokine levels compared to age, gender and BMI comparable controls. This is an important finding because there is a growing number of people living with HIV disease who are aging (7). The physical function results of this study are consistent with the findings of previous studies which examined HIV patients (5, 19). It should be noted, however, that the control groups for the previous studies were not controlled for their BMI. Controlling for BMI is an important element in this study’s methodology because not only is obesity a predictor of frailty and functional limitations(20), but also the HIV disease has become a manageable chronic illness and one which has been progressively accompanied with an increased prevalence of overweight and obesity (21). To our knowledge, this is the first study which is able to demonstrate lower physical function in a HOA group compared to a control group, despite having similar body mass.
Our study also suggests that HOA had more lipodystrophy and central obesity compared to age, gender and BMI comparable controls. This is an important finding because central adiposity and adipokines have been linked with cardiovascular disease in HIV-infected patients (22). Our results are consistent with previous studies conducted in young and middle-aged HIV patients (23, 24). Moreover, our study found that the HOA group has significantly different levels of adipokines compared to the controls group. Previous studies have reported that adipocyte differentiation was perturbed as a direct effect of ART (25, 26). Further, ART has also been shown to decrease steady states levels of adiponectin messenger RNA levels in adipocytes (27). To that end, and consistent with previous studies conducted in young HIV-infected patients, we found that adiponectin levels are lower and CRP levels are higher in the HOA group compared to the control group (11, 12). Finally, our study also examined visfatin and RBP-4 levels in the HOA population. We found that the HOA group had elevated levels of visfatin and RBP-4 compared to the control group. While previous studies have shown lower levels of leptin and higher levels of IL-6 and TNF- alpha in the HOA group, we did not find any significant difference in these adipokines between the two groups (10, 28).
Our study demonstrates that adipokines are significantly correlated with functional limitations. This finding is important because HOA have been identified as at risk of frailty and its associated negative consequences, however little is known about the mechanism of HIV-related frailty. While this relationship has been evaluated in the general aging population (14, 15), it has not been similarly evaluated in the HIV population. There has been mixed conclusions with respect to studies that examined adiponectin in the general older adult population. One such study shows a negative relationship (14) with respect to adiponectin and functional limitations, while another study shows a positive correlation (15). A recent study which investigated middle-aged HIV patients demonstrated that functional impairment was associated with higher IL-6 levels (29). It should be noted, however, that study’s methodology did not include a control group to compare the results with HIV patients.
Our study investigated the relationship between functional limitations and adipokine in HOA. We observed that adiponectin has a negative correlation with functional limitations in both the groups, while visfatin has a positive correlation only in the HOA group. To our knowledge, this is the first study to observe a relationship between visfatin and physical function. We observed significant slope differences in the relationship between adipokine and functional limitations between the two groups. This slope difference could elucidate a key mechanism of accelerated aging and functional decline in HOA compared to controls. We speculate that disrupted adipokine signaling pathway and receptor gene expressions in HOA due to long-term ART or persistent HIV replication may explain this finding (10, 30). Future studies are needed to elucidate these important questions.
Limitations of the present study may be the small sample size and a sampling bias because only those who were willing and able to come to our facility represent the subjects who participated in the study. It should be noted, however, that we have characterized our subjects comprehensively with respect to their physical function and have compared our HOA with age, gender and BMI comparable controls. We realize that this cross-sectional study design can only identify associations. Nonetheless, our findings offer some insight on the relationships between adipokines and physical function in the HOA population. These findings are particularly important because the epidemic of HIV and aging continues to have a striking impact worldwide. That being said, future prospective and large scale studies should not only investigate the role of adipokines in HIV-related functional decline and frailty, but also evaluate interventions that can prevent or ameliorate frailty in this population with the end goal of successful aging.
Acknowledgments
We thank the participants and Dr. Stephen Dewhurst for his encouragement and support of this work.
Funding source: NIH grants P30 AI078498, AG020493 and National Institute of Aging K23AG043319-01A1
Sponsor’s Role: None
Footnotes
Conflict of Interest: None.
Author Contributions: Study concept and design: AEL, WJH, KS; Acquisition of participants and data: ZM, AEL, KS, TNH. Analysis and interpretation of data: KS, JG, HY, OP. Preparation of manuscript. KS, ZM, AEL.
References
- 1.Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310(11):1168–77. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22(11):1113–21. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 4.Shah K, Hilton TN, Myers L, et al. A New Frailty Syndrome: Central Obesity and Frailty in Older Adults with the Human Immunodeficiency Virus. J Am Geriatr Soc. 2012 doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desquilbet L, Margolick JB, Fried LP, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009;50(3):299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Önen NFPP, Baker J, Conley L, Brooks JT, Bush T, Henry K, Hammer J, Kojic EM, et al. Overton Frailty and pre-frailty in a contemporary cohort of HIV-infected adults. J Frailty Aging. 2014 doi: 10.14283/jfa.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47(4):542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calmy A, Hirschel B, Cooper DA, Carr A. A new era of antiretroviral drug toxicity. Antivir Ther. 2009;14(2):165–79. doi: 10.1177/135965350901400203. [DOI] [PubMed] [Google Scholar]
- 9.Moyle G, Moutschen M, Martinez E, et al. Epidemiology, assessment, and management of excess abdominal fat in persons with HIV infection. AIDS Rev. 2010;12(1):3–14. [PubMed] [Google Scholar]
- 10.Tsiodras S, Perelas A, Wanke C, Mantzoros CS. The HIV-1/HAART associated metabolic syndrome - novel adipokines, molecular associations and therapeutic implications. J Infect. 2010;61(2):101–13. doi: 10.1016/j.jinf.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Luo L, Zhang L, Tao M, et al. Adiponectin and leptin levels in Chinese patients with HIV-related lipodystrophy: a 30-month prospective study. AIDS Res Hum Retroviruses. 2009;25(12):1265–72. doi: 10.1089/aid.2009.0072. [DOI] [PubMed] [Google Scholar]
- 12.Borato DC, Parabocz GC, Ribas SR, et al. Changes of metabolic and inflammatory markers in HIV infection: glucose, lipids, serum Hs-CRP and myeloperoxidase. Metabolism. 2012;61(10):1353–60. doi: 10.1016/j.metabol.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Gallego-Escuredo JM, Del Mar Gutierrez M, Diaz-Delfin J, et al. Differential effects of efavirenz and lopinavir/ritonavir on human adipocyte differentiation, gene expression and release of adipokines and pro-inflammatory cytokines. Curr HIV Res. 2010;8(7):545–53. doi: 10.2174/157016210793499222. [DOI] [PubMed] [Google Scholar]
- 14.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16(8):679–86. doi: 10.1007/s12603-012-0066-4. [DOI] [PubMed] [Google Scholar]
- 15.Tsai JS, Wu CH, Chen SC, et al. Plasma adiponectin levels correlate positively with an increasing number of components of frailty in male elders. PLoS One. 2013;8(2):e56250. doi: 10.1371/journal.pone.0056250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55(6):M350–M5. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 18.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of Weight Loss and Exercise on Frailty in Obese Older Adults. Arch Intern Med. 2006;166(8):860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 19.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009;18(12):1965–74. doi: 10.1089/jwh.2008.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes Res. 2004;12(6):913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 21.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketlogetswe KS, Post WS, Li X, et al. Lower adiponectin is associated with subclinical cardiovascular disease among HIV-infected men. AIDS. 2014;28(6):901–9. doi: 10.1097/QAD.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosmiski L, Kuritzkes D, Hamilton J, et al. Fat distribution is altered in HIV-infected men without clinical evidence of the HIV lipodystrophy syndrome. HIVMed. 2003;4(3):235–40. doi: 10.1046/j.1468-1293.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown TT, Xu X, John M, et al. Fat distribution and longitudinal anthropometric changes in HIV-infected men with and without clinical evidence of lipodystrophy and HIV-uninfected controls: a substudy of the Multicenter AIDS Cohort Study. AIDS Res Ther. 2009;6:8. doi: 10.1186/1742-6405-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim RJ, Wilson CG, Wabitsch M, Lazar MA, Steppan CM. HIV protease inhibitor-specific alterations in human adipocyte differentiation and metabolism. Obesity (Silver Spring) 2006;14(6):994–1002. doi: 10.1038/oby.2006.114. [DOI] [PubMed] [Google Scholar]
- 26.Caron M, Vigouroux C, Bastard JP, Capeau J. Adipocyte dysfunction in response to antiretroviral therapy: clinical, tissue and in-vitro studies. Curr Opin HIV AIDS. 2007;2(4):268–73. doi: 10.1097/COH.0b013e32814b1638. [DOI] [PubMed] [Google Scholar]
- 27.Chaparro J, Reeds DN, Wen W, et al. Alterations in thigh subcutaneous adipose tissue gene expression in protease inhibitor-based highly active antiretroviral therapy. Metabolism. 2005;54(5):561–7. doi: 10.1016/j.metabol.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutevedzi PC, Rodger AJ, Kowal P, Nyirenda M, Newell ML. Decreased chronic morbidity but elevated HIV associated cytokine levels in HIV-infected older adults receiving HIV treatment: benefit of enhanced access to care? PLoS One. 2013;8(10):e77379. doi: 10.1371/journal.pone.0077379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erlandson KM, Allshouse AA, Jankowski CM, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis. 2013;208(2):249–59. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011;25(3):487–99. doi: 10.1016/j.beem.2010.12.001. [DOI] [PubMed] [Google Scholar]