Summary
Mature spores of the bacterium Bacillus subtilis are encased by two concentric shells: an inner shell (the ‘cortex’), made of peptidoglycan; and an outer proteinaceous shell (the ‘coat’), whose basement layer is anchored to the surface of the developing spore via a 26-amino-acid-long protein called SpoVM. During sporulation, initiation of cortex assembly depends on the successful initiation of coat assembly, but the mechanisms that co-ordinate the morphogenesis of both structures are largely unknown. Here, we describe a sporulation pathway involving SpoVM and a 37-amino-acid-long protein named ‘CmpA’ that is encoded by a previously un-annotated gene and is expressed under control of two sporulation-specific transcription factors (σE and SpoIIID). CmpA localized to the surface of the developing spore and deletion of cmpA resulted in cells progressing through the sporulation programme more quickly. Overproduction of CmpA did not affect normal growth or cell division, but delayed entry into sporulation and abrogated cortex assembly. In those cells that had successfully initiated coat assembly, CmpA was removed by a posttranslational mechanism, presumably in order to overcome the sporulation inhibition it imposed. We propose a model in which CmpA participates in a developmental checkpoint that ensures the proper orchestration of coat and cortex morphogenesis by repressing cortex assembly until coat assembly successfully initiates.
Introduction
Understanding the mechanisms that underlie how cells differentiate into genetically identical, but morphologically distinct, daughter cells is a central challenge in developmental biology. One aspect that contributes to the proper morphology of an organism is the presence of complex static structures whose assemblies must be carefully orchestrated during developmental programmes (Hartwell and Weinert, 1989; Keaton and Lew, 2006; Richman and Handrigan, 2011). A genetically tractable system to study the morphogenesis of such cellular structures is the process of bacterial endospore formation (sporulation), a simple developmental programme in which a normally growing cell differentiates into two different cell types (Stragier and Losick, 1996; Errington, 2003; Piggot and Hilbert, 2004). In the rod-shaped bacterium Bacillus subtilis, nutrient deprivation triggers sporulation, whereupon the cell responds by elaborating an asymmetrically placed division septum that results in two genetically identical, but unequally sized, daughter cells: the smaller ‘forespore’ that eventually matures into a dormant spore and the larger ‘mother cell’ that nourishes the forespore as it matures (Fig. 1A). The two daughter cells initially lie side-by-side, held together by a cell wall that surrounds both cells. Next, the mother cell swallows the forespore in a process called engulfment. As a result, the forespore becomes a double membrane-bound cell inside the mother cell cytosol. Eventually, the mother cell lyses in a programmed event and the small cell, now a dormant spore, is released into the environment.
Fig. 1. Ile15 of VM is required for cortex morphogenesis.
A. Schematic representation of sporulation in B. subtilis. After asymmetric division (top), the larger mother cell (MC) and the forespore (FS) lie side-by-side, held together by a cell wall (not shown for simplicity). Plasma membrane is depicted in black. Next, the mother cell engulfs the forespore (middle). Eventually, the forespore becomes a double membrane-bound cell inside the mother cell cytosol (bottom). The proteins that comprise the coat (Ct, dark grey) are synthesized in the mother cell and are deposited on the forespore surface. The cortex (Cx, light grey) is built in the space between the two membranes that encapsulate the forespore. For simplicity, early localization of certain coat proteins to the engulfing membrane in panel two is not shown (McKenney and Eichenberger, 2012).
B–F. Electron micrographs of negatively stained thin sections of sporulating wild type (B, strain PY79) or VM deletion (C, strain KR94) strains of B. subtilis collected 5 h after induction of sporulation. VM deletion strain in (C) was complemented with a wild-type copy (D, strain KR103) or the I15A allele (E, strain KR322) of VM at an ectopic locus (amyE) on the chromosome. (F) Strain in (E) harbouring a spontaneous suppressor mutation in the cmpA gene (strain KRC56). All images are oriented such that the engulfed forespore is to the right and the mother cell is to the left. Cortex (Cx) in the micrographs is labelled with a black bar; scale bar: 500 nm. Strain genotypes are listed in Table S1.
Mature spores of B. subtilis are about one micron in diameter and are encased in two concentric shells that protect the spore’s genetic material from various environmental insults (Setlow, 2006; Henriques and Moran, 2007). The outer shell, called the ‘coat’, is composed of some 70 different proteins and, being the outermost structure, is responsible for the spore’s characteristic appearance (Driks, 2002; 2004). Coat proteins are synthesized in the mother cell and are deposited onto the surface of the developing forespore (Driks et al., 1994; Webb et al., 1995; Kim et al., 2006; McKenney et al., 2010; McKenney and Eichenberger, 2012). Coat morphogenesis is initiated by the assembly of a basement layer around the developing forespore that is composed largely of a structural protein called SpoIVA which polymerizes to form a platform atop which coat proteins assemble (Roels et al., 1992; Driks et al., 1994; Price and Losick, 1999; Ramamurthi and Losick, 2008). SpoVM, a 26-amino-acid-long amphipathic protein, interacts with SpoIVA and the membrane, thereby anchoring SpoIVA onto the forespore surface (Levin et al., 1993; van Ooij and Losick, 2003; Ramamurthi et al., 2006). A third protein, SpoVID, is required to drive the encasement of coat proteins around the developing forespore (Driks et al., 1994; Wang et al., 2009; McKenney et al., 2010). The inner shell (the ‘cortex’), made of a specialized peptidoglycan (Imae and Strominger, 1976; Popham and Setlow, 1993; Meador-Parton and Popham, 2000; Gilmore et al., 2004), is built between the two membranes encircling the forespore and is responsible for maintaining the spore’s shape (Atrih and Foster, 1999). Cortex assembly is largely directed from the mother cell, where peptidoglycan precursors are synthesized and subsequently transported across the surface of the forespore into the intermembrane space between the double membranes that encircle the forespore (Vasudevan et al., 2007). The transported precursors are then assembled into peptidoglycan polymers by transglycosylases and transpeptidases located in this compartment (Popham and Stragier, 1991; Popham et al., 1995; 1999).
Several observations indicated that, although the coat and cortex are spatially separated by a membrane, successful initiation of cortex assembly is absolutely dependent on successful initiation of coat assembly at the forespore surface, suggesting that the morphogenesis of both structures is linked. For example, sporulation-defective mutants that display defects in initiating coat assembly are concomitantly defective in cortex assembly (Coote, 1972; Piggot and Coote, 1976). Indeed, in the absence of either SpoIVA or SpoVM not only does the coat fail to form properly, but cortex assembly also fails to initiate (Roels et al., 1992; Levin et al., 1993). Moreover, point mutations in spoIVA that disrupt coat assembly also abolish cortex assembly, and the isolation of mutant alleles of spoIVA that are defective for the assembly of one structure, but not the other, have not been reported (Catalano et al., 2001). It is important to note that removal of other major coat morphogenetic proteins [e.g. SafA, CotE, CotZ or SpoVID (Zheng et al., 1988; Beall et al., 1993; Takamatsu et al., 1999; McKenney et al., 2010)] do not abrogate the morphogenesis of the cortex, suggesting a unique role for SpoIVA and SpoVM in orchestrating coat and cortex assembly. Although many of the factors that are required for the formation of either structure have been very well characterized, the mechanism that temporally orchestrates the morphogenesis of both spatially separated structures has remained largely mysterious.
Here, we report the discovery of a sporulation pathway that links cortex morphogenesis to successful initiation of coat assembly. This pathway involves the small protein SpoVM and another small sporulation protein herein named Cortex morphogenetic protein A (CmpA), encoded by a previously un-annotated, 37-codon-long open reading frame that is expressed under the control of two mother cell-specific transcription factors (σE and SpoIIID). Deletion of cmpA suppressed the sporulation defect of a mutant allele of spoVM that was capable of initiating coat assembly, but failed to initiate cortex assembly. In contrast, artificially overproducing CmpA delayed steps of sporulation that require peptidoglycan synthesis and reduced sporulation efficiency. CmpA localized to the surface of the forespore early during sporulation while coat assembly was initiating, but was undetectable in cells that had successfully progressed through the sporulation programme. Taken together, we propose a model in which CmpA represses premature cortex assembly until coat assembly successfully initiates, whereupon its inhibition is relieved by a post-translational mechanism.
Results
VM co-ordinates coat and cortex assembly during sporulation
Deletion of spoVM (hereafter, simply ‘VM’) causes a severe reduction in sporulation efficiency due to defects in assembling the spore coat and cortex (Levin et al., 1993; Fig. 1B and C; contrast the presence of the cortex, which is seen as a white ring, labelled ‘Cx’, in the WT cell encircling the forespore which excludes the stain to the absence of such a ring in the ΔVM strain). In order to understand how VM co-ordinates the assembly of both structures we sought to first identify amino acid residues in VM, by alanine scanning mutagenesis, that would specifically abrogate cortex assembly, while still permitting the initiation of coat assembly. Substitution of Ile15 of VM with Ala resulted in a severe sporulation defect in cells harbouring VMI15A as the only copy of VM [1.2 × 10−6 relative to wild type; Table S2 (van Ooij and Losick, 2003)]. Examination of these cells by electron microscopy revealed that cortex assembly was abrogated, similar to strains in which theVM gene had been deleted (Fig. 1E; compare with Fig. 1C).
In order to ensure that initiation of coat assembly was not affected in strains harbouring VMI15A, we first examined the localization of VMI15A fused to green fluorescent protein (GFP) using fluorescence microscopy. In otherwise wild-type cells, VM–GFP localized almost exclusively around the surface of the developing forespore, whereas a previously characterized variant, VMP9A–GFP, promiscuously mis-localized to the membrane surrounding the mother cell as well (Fig. 2A and B; van Ooij and Losick, 2003; Ramamurthi et al., 2009). In comparison, localization of VMI15A–GFP was similar to that of wild-type VM, suggesting that substitution of I15 with Ala did not affect localization of VM (Fig. 2C). Because VM is responsible for anchoring the basement layer of the coat to the forespore surface, we next examined the ability of VMI15A to recruit SpoIVA (hereafter, simply ‘IVA’), which is a major structural component of the basement layer. In cells harbouring a wild-type copy of VM, GFP-IVA localized around the surface of the forespore in a pattern that was similar to the localization of VM–GFP (Price and Losick, 1999). In cells expressing VMP9A as the only copy of VM, GFP-IVA mis-localized as a cap on the mother cell-proximal side of the forespore. However, GFP-IVA localized normally in the presence of VMI15A (Fig. 2D–F). Next, we examined the localization of CotE, a later-assembling coat protein that is recruited by IVA and is in turn required for the recruitment of proteins that comprise the outer layers of the spore coat (Zheng et al., 1988). In the presence of wild-type VM, CotE–GFP encircled the forespore with a biased accumulation on the mother cell-proximal face of the forespore (Webb et al., 1995). In the presence of the mis-localizing VMP9A, CotE–GFP mis-localized as a cap on the mother cell-proximal side of the forespore, but in the presence of VMI15A, the pattern of CotE–GFP localization was similar to wild type (Fig. 2G–I). Taken together, we conclude that substitution of Ile15 of VM with Ala specifically impairs cortex assembly, but not the initiation of coat assembly.
Fig. 2. Ile15 of VM is not required for initiation of coat assembly.
A–C. Localization of VM–GFP (A, strain CVO1195), VMP9A–GFP (B, strain CVO1395) or VMI15A–GFP (C, strain SE44) in an otherwise wild-type strain.
D–F. Localization of GFP-IVA in the presence of wild-type VM (strain KR165), VMP9A (strain SE50) or VMI15A (strain SE6).
G–I. Localization of CotE–GFP in the presence of wild-type VM (strain SE55), VMP9A (strain KR603) or VMI15A (strain SE47).
Overlay of GFP fluorescence in (A)–(I) and membranes visualized with the fluorescent dye FM4-64 (added after the cells were harvested for imaging) is shown below each corresponding panel.
Allele-specific suppression of VMI15A
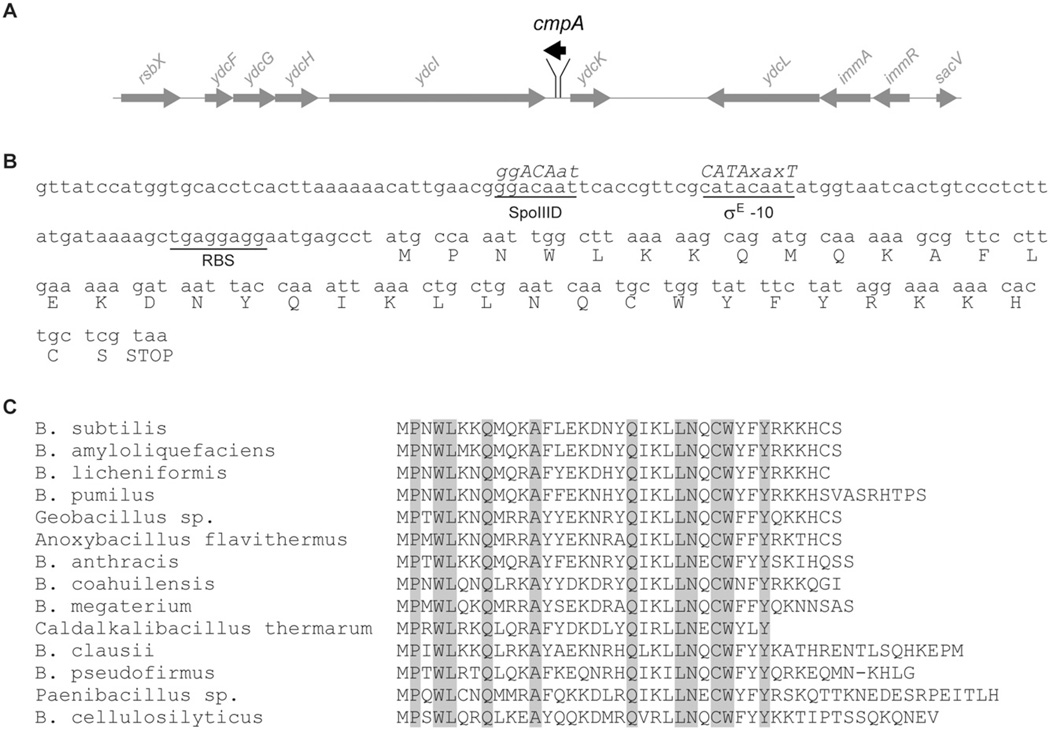
In an effort to identify other factors that participate with VM in co-ordinating cortex assembly with coat assembly, we took advantage of the strong sporulation phenotype caused by VMI15A to select for a suppressor mutation that would correct this defect. We therefore subjected cells harbouring VMI15A to repeated cycles of sporulation, followed by heat treatment to eliminate cells that were unable to sporulate, followed by germination and growth in fresh medium. Our selection yielded an extragenic suppressor mutation which not only corrected the heat resistance defect caused by VMI15A to near wild-type levels (0.28 ± 0.02 relative to wild type; Table S2, strain K) but also restored cortex assembly (Fig. 1F; compare with Fig. 1B). The mutation was found to be a single nucleotide transition of cytosine to thymine, located in an intragenic region between ydcI and ydcK, two genes of unknown function, at approximately 45° relative to the origin of the B. subtilis chromosome. Closer examination of this intragenic region revealed a previously un-annotated small putative open reading frame on the complementary strand encoding a 37-amino-acid-long protein which we named Cortex morphogenetic protein A (CmpA; Fig. 3A and B). The suppressor mutation changed the tenth codon of cmpA from CAA, specifying Gln, to TAA, specifying an ochre stop codon, which would presumably result in a truncated CmpA protein product. The upstream region of the cmpA ORF harboured a DNA sequence that conformed to a canonical B. subtilis ribosome binding site nine bases upstream of the start codon. In addition, the cmpA gene was preceded by a sequence that exactly matched the consensus −10 sequence for promoters bound by the mother cell-specific sporulation sigma factor E [σE (Eichenberger et al., 2004)], which also drives expression of VM (Levin et al., 1993). Although we were unable to find a convincing match for the −35 element recognized by σE, the upstream sequence did harbour a nearly perfect match to the consensus sequence recognized by the transcription factor SpoIIID (Eichenberger et al., 2004). Interestingly, Schmalisch et al. recently identified a small RNA transcript corresponding to this region that was specifically expressed during stationary phase in sporulation medium (Schmalisch et al., 2010). Given the possible sporulation-specific involvement of cmpA and its reported expression profile, we investigated whether the gene was conserved in other organisms. blast search revealed that cmpA is well conserved among closely related spore forming species of the Bacillales order (including Paenibacillus and Geobacillus spp.), but not among the spore forming Clostridium species (Fig. 3C). Additionally, the σE −10 consensus site and the putative SpoIIID binding site were also largely conserved among members of the Bacillales order (Fig. S1). It is worth noting that in the closely related (but non-spore-forming) Listeria monocytogenes, the surrounding chromosomal region is largely conserved, but the open reading frames of ydcI and ydcK overlap so that the intergenic region harbouring cmpA is absent. Beyond the conservation of cmpA orthologues in other spore forming bacteria, we were unable to identify any conserved motifs that would imply any cellular function.
Fig. 3. cmpA is a small open reading frame that is conserved among spore-forming bacteria.
A. Physical map of the cmpA region of the B. subtilis chromosome. Arrows depict the direction of transcription. Map is drawn to scale, except that the relative length of the arrow depicting the cmpA open reading frame (black) has been exaggerated.
B. Nucleotide sequence of the cmpA region of the chromosome. Predicted amino acid sequence of the protein is shown below each codon. Putative ribosome binding site (RBS), −10 binding site for σE and putative SpoIIID binding site are underlined. Consensus nucleotide sequence for the −10 binding site for σE and SpoIIID are shown in italics above the chromosomal nucleotide sequence, where capital letters depict highly conserved nucleotides, and ‘x’ is any nucleotide.
C. Amino acid sequence conservation of CmpA among spore forming bacteria. Conserved amino acids are shaded in grey.
In order to test if the suppression phenotype was caused by a loss of function of a putative CmpA protein product, we constructed a complete deletion of the cmpA ORF by insertion of an antibiotic resistance cassette. Strains harbouring a complete deletion of cmpA suppressed the VMI15A sporulation defect to similar levels (0.14 ± 0.04 relative to WT; strain SE181) as the spontaneous suppressor harbouring a stop codon in cmpA, suggesting that a loss of CmpA function was responsible for the suppression phenotype. Complementation of this cmpA deletion mutation by introducing cmpA (putative cmpA ORF plus 126 nucleotides upstream of the start codon) at an ectopic locus in the chromosome (thr) restored the sporulation defect of VMI15A (1.5 × 10−5 ± 2.9 × 10−6 relative to WT; strain SE188), indicating that inactivation of the cmpA gene alone was responsible for the suppression of the VMI15A sporulation defect. Next, we wished to determine if the cmpAQ10stop mutation was bypassing the requirement for VM altogether and if it was able to suppress the sporulation defects caused by mutant alleles of VM other than VMI15A. The results in Table S2 show that the cmpAQ10stop mutation did not bypass a ΔVM mutation (strain J) and that it was unable to suppress other mutant alleles of VM that were known to cause sporulation defects (van Ooij and Losick, 2003). We conclude that truncation of cmpA at position 10 results in a loss of function of the protein it encodes and suppresses the sporulation defect of VMI15A in an allele specific manner. These results led us to hypothesize that cmpA encodes a protein that inhibits cortex assembly until coat assembly initiates, and that this inhibition is never properly relieved in cells harbouring VMI15A, despite proper initiation of spore coat assembly.
cmpA is a novel sporulation gene regulated by σE and SpoIIID
To determine how cmpA is transcriptionally regulated, we constructed a strain in which sequences upstream of the cmpA ORF (which include the putative promoter, ribosome binding site and start codon) were fused in frame to the lacZ reporter gene. Cultures of the strain harbouring this PcmpA–lacZ construct were induced to sporulate, and β-galactosidase activity in cell extracts prepared at various time points during sporulation were measured. β-Galactosidase activity was undetectable above background before the induction of sporulation, but began to increase at approximately 2.5 h after induction (Fig. 4A). Similar results were obtained for a translation fusion in which the entire cmpA ORF was fused in frame to lacZ, indicating that the open reading frame was not simply transcribed, but was also translated into a protein product. In comparison, fusion of the promoter and ORF of VM, a bona fide σE-controlled gene, to lacZ displayed a similar timing of expression, although at higher absolute levels of β-galactosidase activity, consistent with the idea that cmpA expression also may be driven by σE.
Fig. 4. cmpA is transcribed only during sporulation and depends on the mother cell-specific transcription factors σE and SpoIIID.
A. β-Galactosidase accumulation was measured in cells harbouring a spoVM–lacZ (▼; strain SE241), PcmpA–lacZ (●; strain SE222) or cmpA–lacZ (■; strain SE230) reporter fusion at various times during sporulation in wild-type cells or in cells that did not harbour a lacZ reporter [▲; strain PY79, labelled ‘(−)’].
B. β-Galactosidase accumulation was measured from a PcmpA–lacZ reporter fusion at various times during sporulation in wild type (●; strain SE222), and cells harbouring a deletion in sigK (◇; strain SE246, ‘ΔσK’), sigG (▼; strain SE236, ‘ΔσG’), spo0A (■; strain SE234, ‘Δspo0A’), sigE (▲; strain SE235, ‘ΔσE’) or spoIIID (□; strain IT232, ‘ΔspoIIID’). Symbols represent mean values of three independent measurements; error bars represent standard error of the mean.
To examine the sporulation-specific expression of cmpA, we introduced deletions of genes encoding various sporulation-specific transcription factors into the strain harbouring PcmpA–lacZ. Deletion of spo0A, which governs the entry of cells into sporulation, sigE (which encodes σE) or spoIIID (a mother cell-specific transcription factor) abolished cmpA expression (Fig. 4B). In contrast, deletion of sigG or sigK, which encode sporulation sigma factors that act downstream of σE and SpoIIID, did not eliminate cmpA expression. Taken together, we conclude that cmpA is a gene that is exclusively produced during sporulation and is regulated by the mother cell-specific transcription factors σE and SpoIIID.
CmpA localizes to the surface of the forespore
In order to study the subcellular localization of CmpA, we fused cmpA in frame to the gene encoding green fluorescent protein (gfp) under control of the cmpA promoter. When produced under control of its native promoter, though, its fluorescence signal was too faint to be detected by fluorescence microscopy. We therefore cloned the cmpA–gfp fusion under control of the IPTG-inducible Phyperspank promoter. Cells that expressed VMI15A along with cmpA–gfp as the only copy of cmpA displayed an intermediate sporulation efficiency (6.5 × 10−3 ± 1.7 × 10−3 relative to wild type; strain SE211), indicating that the CmpA–GFP construct was partially functional. To achieve mother cell-specific overexpression of cmpA–gfp during sporulation, we exploited a previously described property of sporangia wherein, after the completion of engulfment, activation of xylose-inducible promoters in the forespores was abolished after xylose was added to the growth medium, presumably because the forespore became impermeable to externally added chemicals as it began to metabolically shut down (Rudner et al., 2002; Camp and Losick, 2009; Doan et al., 2009). To test if IPTG is also unable to induce gene expression in the forespore after completion of engulfment, we examined the production of free GFP in sporulating cells harbouring Phyperspank–gfp. Engulfment was monitored using membrane-permeable (TMA-DPH) and membrane-impermeable (FM4-64) fluorescent dyes as described previously (Sharp and Pogliano, 1999) in order to measure when the forespore surface separates from the mother cell plasma membrane. Before completion of engulfment (2 h after the start of sporulation), forespores were detectable using both dyes and, when IPTG was added to cells at this stage, free GFP was uniformly produced in both compartments (Fig. S2A, C, E and G). Completion of engulfment (3.5 h after the start of sporulation) was evident when forespores could not be detected using the membrane-impermeable FM4-64, but were visible using TMA-DPH (Fig. S2D and F). When IPTG was added to these post-engulfed cells, GFP fluorescence was detected exclusively in the mother cell (Fig. S2B and H) indicating that, after engulfment, IPTG only induced gene expression from the Phyperspank promoter in the mother cell.
Three hours after initiation of sporulation, 88% of cells harbouring Phyperspank–cmpA–gfp had completed engulfment, at which time we added inducer to produce CmpA– GFP. Thirty minutes later, we observed that CmpA–GFP production could be monitored by immunoblotting (Fig. S3) and that CmpA–GFP localized largely to the forespore surface (Fig. 5A). To test if CmpA localization was dependent on VM, we observed the localization of CmpA–GFP in cells harbouring a deletion in VM. In the absence of VM, CmpA–GFP continued to localize to the forespore surface, but most of the fluorescence signal was present as a focus on the mother cell-proximal side of the forespore (Fig. 5B). In the presence of VMI15A, CmpA– GFP localized in a similar pattern as seen in otherwise wild-type cells (Fig. 5C). Taken together, we conclude that CmpA localizes to the surface of the forespore and that its proper localization either directly or indirectly depends on VM.
Fig. 5. CmpA localizes to the surface of the forespore.
A–C. Localization of CmpA–GFP in the presence of wild-type VM (strain SE364), absence of VM (strain SE367) or VMI15A (strain SE374).
D–F. Overlay, GFP fluorescence and membranes in (A)–(C) visualized with FM4–64 added after cells were harvested for imaging.
CmpA inhibits sporulation and is required for proper coat assembly
The suppression of the VMI15A cortex assembly defect by removing CmpA suggested that CmpA inhibits cortex assembly until coat assembly properly initiates. If so, would overexpression of cmpA inhibit cortex assembly? To test this, we first wished to determine if expression of cmpA during vegetative growth would also inhibit peptidoglycan assembly in the cell wall and therefore be toxic to cells. We therefore artificially induced the expression of cmpA in cells harbouring Phyperspank–cmpA at the amy locus during normal growth in CH medium (Sterlini and Mandelstam, 1969) and monitored growth by measuring the turbidity of the culture (Fig. 6A, left). Compared with wild type, induced expression of cmpA did not appear to be toxic to vegetatively growing cells, and the morphology of cells overexpressing cmpA appeared similar to that of wild-type cells (Fig. 6A, right). Similar results were obtained with cultures grown in DSM medium (Fig. S4), indicating that CmpA likely does not affect peptidoglycan assembly during vegetative growth.
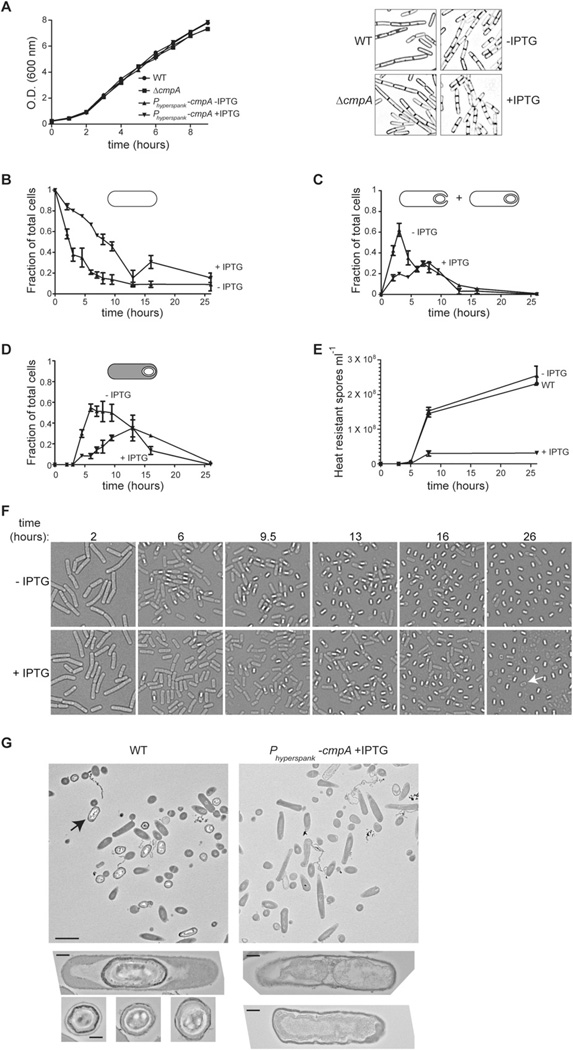
Fig. 6. Overproduction of CmpA arrests sporulation, but not vegetative growth.
A. Left: Representative growth curves of wild-type (strain PY79), ΔcmpA (strain SE178) or cells (strain SE191) that had (+ IPTG) or had not (− IPTG) been induced to overproduce CmpA grown in CH medium from a single experiment. Right: Morphology of the same cells examined by fluorescence microscopy at t = 2.5 h using the membrane stain TMA-DPH.
B–D. Kinetics of sporulation in cultures of cells (strain SE191) that had (▼; + IPTG) or had not (▲; − IPTG) been induced to overproduce CmpA. Cells were induced to sporulate by resuspension in SM medium, stained with the fluorescent dye TMA-DPH to visualize membranes, and examined by differential interference contrast microscopy (DIC) and epifluorescence microscopy. Fraction of total cells in given fields at various time points that were at Stage 0–1 (B) or Stage II–III (C) of sporulation as determined by membrane staining (cell morphology at each stage is represented by the cartoon above each graph). (D) Fraction of cells at various time points that had elaborated phase bright forespores as determined by DIC. Symbols in (B)–(D) represent mean values of between 230 and 646 total scored cells from three independent sporulating cultures; error bars represent standard error of the mean.
E. Kinetics of production of heat-resistant spores of wild-type cells (●; strain PY79), or cells (strain SE191) induced (▼; + IPTG) or not induced (▲; − IPTG) to overproduce CmpA. Symbols represent mean values obtained from three independent measurements; error bars represent standard error of the mean.
F. Change in morphology of cells over time that had not (− IPTG) or had (+ IPTG) been induced to overproduce CmpA as measured by differential interference contrast microscopy. The white arrow at 26 h indicates a spore-like particle that was not phase bright.
G. Top: Electron micrographs of negatively stained thin sections of wild type (left, strain PY79; arrow indicates a mature spore) or cells overproducing CmpA (right, strain SE191) examined 24 h after induction of sporulation by nutrient depletion in DSM medium at lower magnification to show a large field of view (scale bar: 2 µm). Below, a representative image of a late stage sporangium and three representative images of mature released spores (left) of wild-type cells; on the right, two representative images of cells overproducing CmpA that had been arrested during sporulation (scale bar: 250 nm).
To determine if overproduction of CmpA would inhibit cortex assembly, cells harbouring Phyperspank–cmpA at the amy locus (in addition to the copy of wild-type cmpA at its native locus) were induced to sporulate either in the presence or in the absence of inducer and sporulation efficiency was measured by calculating the number of heat-resistant spores produced in DSM medium as compared with wild-type cells. As mentioned above, whereas native levels of CmpA(when fused to GFP) could not be detected by immunoblotting, the fusion was easily detected when expression of cmpA–gfp was induced with IPTG, suggesting that the hyperspank promoter indeed drives overproduction of CmpA (Fig. S3). The results in Table S3 show that, whereas the sporulation efficiency of wild-type cells was not diminished by addition of inducer, introducing an extra copy of cmpA, even in the absence of inducer, reduced sporulation efficiency twofold to 0.48 ± 0.05 relative to wild type, presumably due to leaky expression from the hyperspank promoter [a true diploid strain harbouring a second copy of cmpA under control of its native promoter (strain SE174) sporulated with an efficiency of 0.74 ± 0.05 relative to wild type]. When cells were grown in the presence of 1 mM IPTG, sporulation efficiency was lowered over threefold to 0.29 ± 0.12 relative to wild type (Table S3). To determine the stage at which these cells were impaired, we used a combination of fluorescence and differential interference contrast microscopy to monitor cells harbouring Phyperspank–cmpA at various time points when sporulation was induced by resuspension either in the presence or in the absence of IPTG, and measured the number of cells that had not yet elaborated polar septa (comprising vegetative cells, and sporangia at Stage 0 or Stage I of sporulation); cells that had elaborated polar septa, were engulfing or had finished engulfment (sporangia at Stage II or Stage III); or sporangia that had elaborated phase bright forespores (Fig. 6B–D). Whereas cells that did not receive IPTG rapidly began to enter sporulation (evidenced by the decrease in the number of Stage 0 and Stage I cells; Fig. 6B, ‘− IPTG’), those cells that were induced to overexpress cmpA were delayed in entering sporulation. Concomitantly, the majority of cells that did not receive IPTG had either begun or finished engulfment by hour 3. In contrast, cells that received IPTG displayed a delayed entry into the engulfment stage, with a wide peak centred around hour 8 of sporulation (Fig. 6C). Similarly, cells that did not receive IPTG began to elaborate phase bright forespores from hour 5 to hour 8 of sporulation, whereas for IPTG-induced cells, the peak number of cells harbouring phase bright forespores did not occur until hour 13 of sporulation (Fig. 6D). Finally, heat-resistant spores accumulated at a similar rate in cultures un-induced with IPTG compared with the accumulation of spores in cultures of wild-type cells (final sporulation efficiency of strain SE191, un-induced with IPTG 26 h after induction of sporulation by resuspension was 1.1 ± 0.2 relative to wild type; Fig. 6E). In contrast, cells that were induced to overproduce CmpA accumulated heat-resistant spores with an efficiency of only 0.13 ± 0.02 relative to wild type in resuspension medium (Fig. 6E). Examining the morphology of these cells at various time points by differential interference contrast microscopy revealed that the elaboration of phase bright spores and released spores was both reduced and delayed when cells were induced to overproduce CmpA (Fig. 6F). Interestingly, in cultures that received IPTG we noticed an additional peak in the number of cells that we scored as ‘Stage 0’ or ‘Stage I’ cells at t = 16 (Fig. 6B) which coincided with a drop in the number of cells harbouring phase bright forespores (Fig. 6D). This drop was not accompanied by an increase in heat-resistant spores at t = 16 (Fig. 6E), suggesting that some cells that initially elaborated phase bright forespores failed to progress through the sporulation programme and became eventually unaccounted for (perhaps due to lysis), resulting in a relative increase in cells scored as ‘Stage 0–1’ in the population. Additionally, we noticed that cells overproducing CmpA released many bodies that were similar in size and shape to mature spores, but were not phase bright (Fig. 6F, t = 26 h, indicated with an arrow). Examination of CmpA-overproducing cells by electron microscopy also revealed that far fewer mature spores were produced. Additionally, those cells that were present largely failed to assemble a cortex (Fig. 6G, right; Fig. S5). Taken together, we conclude that overexpression of cmpA results in a delay in engulfment (and likely, polar septation), a delay in the appearance of phase bright forespores, and an almost eightfold decrease in the production of heat resistance spores, apparently due to defects in the initiation of cortex assembly. Curiously, although vegetatively growing cells are largely unaffected by artificially produced CmpA, overexpression of cmpA at the onset of sporulation arrests or delays sporulation at stages that are known to require peptidoglycan synthesis (Henriques and Moran, 2007; Meyer et al., 2010), suggesting that CmpA specifically affects peptidoglycan assembly during sporulation.
How, then, does the cell relieve the inhibition of sporulation imposed by CmpA? In order to investigate this, we monitored the presence of CmpA–GFP either in cells harbouring wild-type VM or in those harbouring VMI15A, which are unable to progress beyond the CmpA block. After the majority of cells had completed engulfment, we added inducer to produce CmpA–GFP exclusively in the mother cell (because the CmpA–GFP fusion is largely nonfunctional, it did not inhibit sporulation despite its overproduction). At an early stage of sporulation, before the elaboration of phase bright spores, CmpA–GFP was present around the forespores of cells that harboured either VMWT or VMI15A (Fig. 7A and B, arrows). However, 5.5 h after the induction of sporulation, CmpA–GFP was undetectable in greater than 91% of wild-type cells that had elaborated a phase bright forespore, despite the continued presence of inducer in the medium that drove transcription of cmpA–gfp (n = 144; the remaining 9% of cells harboured only residual amounts of CmpA–GFP that did not encircle the forespore; Fig. 7C, arrowhead). Interestingly, those few cells that had not yet elaborated a phase bright forespore at this later time point still retained CmpA–GFP around the forespore (Fig. 7C, arrow). In contrast, in greater than 80% of cells (n = 184) expressing VMI15A that elaborated a phase bright forespore, CmpA–GFP was still present at the later time point and completely encircled the forespore (the remaining approximately 20% retained significant amounts CmpA–GFP, but the fluorescence signal did not completely encircle the forespore; Fig. 7D). Although fusion of GFP to CmpA could artificially stabilize the protein in the presence of VMI15A, our observation that in cells harbouring wild-type VM CmpA–GFP eventually becomes undetectable suggests that the fusion is able to be removed. Taken together, we conclude that once cells progress beyond the stage of sporulation at which they begin to elaborate phase bright spores, CmpA is post-translationally removed, perhaps to overcome its inhibitory effects. Conversely, in those cells that fail to progress beyond this stage, CmpA remains present. The data are therefore consistent with a model in which the inhibitory effect of CmpA must be removed before cells can progress through the sporulation programme.
Fig. 7. CmpA is undetectable in cells that progress through sporulation.
A and B. CmpA–GFP localization in cells harbouring either wild-type VM (A, strain SE381) or VMI15A (B, strain SE374) 3.5 h after the induction of sporulation, when expression of cmpA–gfp was induced by addition of 1 mM IPTG at 3 h after induction of sporulation. Arrows indicate representative cells that have not elaborated a phase bright forespore.
C and D. CmpA–GFP localization in cells harbouring either wild-type VM (C) or VMI15A (D) 5.5 h after the induction of sporulation. Arrowheads indicate representative cells that have elaborated a phase bright forespore.
E–H. DIC images corresponding to (A)–(D).
I–L. Overlay, GFP fluorescence and DIC.
M. Kinetics of sporulation, as measured by production of heat-resistant spores, of either wild-type (●; strain PY79) or ΔcmpA (■; strain SE178) cells induced to sporulate by resuspension.
Would premature removal of CmpA, then, have an effect on the sporulation programme? Deletion of cmpA did not reduce sporulation efficiency when cells were grown in DSM medium (Table S2, strain I), but we wondered if sporulation would proceed faster in the absence of inhibitory activity of CmpA. To test this, we measured the appearance of heat-resistant spores at various time points after the induction of sporulation by resuspension in wild-type cells and cells harbouring a cmpA deletion. At hour 26 after the induction of sporulation, both strains produced a similar number of heat-resistant spores (approximately 2.5 × 108 ml−1; Fig. 7M). However, examination of earlier time points revealed that at hour 5, the ΔcmpA strain had produced about 4.6 × 106 heat-resistant spores ml−1, whereas the wild-type strain had only produced 3.0 × 106 spores. This difference was more apparent by hour 7, when the ΔcmpA strain had already produced 1.4 × 108 heat-resistant spores, whereas wild-type cells had only produced about 7.5 × 107 spores. Taken together, we conclude that when the CmpA-mediated inhibition of sporulation is removed, cells progress more quickly through the sporulation programme.
The remarkable conservation of cmpA exclusively among other spore forming bacteria led us to wonder what selective pressure assured the maintenance of this gene and what the consequences of unchecked progression through sporulation could be. Heat resistance of Bacillus spores correlates with cortex integrity, whereas resistance to lysozyme treatment correlates with coat integrity (Gould and Hitchins, 1963; Melly et al., 2002). Thus, it is conceivable that deletion of cmpA, a cortex inhibitor, would result in unrestricted cortex assembly and would therefore not affect heat resistance. Could unrestricted cortex assembly in the absence of CmpA result in coat maturation defects? ΔcmpA mutants were not impaired in their ability to correctly localize CotE–GFP to the forespore surface, nor did their mature spores display any obvious gross morphology defects as measured by electron microscopy (Fig. S6). We therefore sought to employ a more sensitive technique to assess coat assembly by measuring the ability of ΔcmpA spores to resist lysozyme treatment, a resistance property conferred by the coat. Deletion of cmpA affected the rate of sporulation as measured by heat resistance (Fig. 7M), but had little or no effect on the absolute number of spores produced. In contrast, ΔcmpA spores were more than threefold more sensitive to lysozyme treatment than wild-type cells (0.29 ± 0.21; Table S4), suggesting that they harboured a coat maturation defect that was undetectable by examining the localization of early coat proteins by fluorescence microscopy.
Discussion
Spores of B. subtilis are encased in two concentric shells: an outer proteinaceous ‘coat’ and an inner ‘cortex’ made of peptidoglycan. Assembly of the cortex is dependent on the successful initiation of coat assembly and therefore the morphogenesis of both structures must be carefully co-ordinated during sporulation. Significant progress has been made in understanding how coat assembly initiates and continues self-assembling, how the synthesis of peptidoglycan precursors that comprise the cortex is regulated, and how these precursors are incorporated into the assembling cortex. However, despite the identification of factors that are required for the assembly of both structures, the mechanisms that mediate communication between the coat and cortex during spore morphogenesis are largely unknown. Here, we describe a pathway whereby two small proteins [‘sproteins’ (Hobbs et al., 2011)] produced in the mother cell, the 26-amino-acidlong VM that initiates coat assembly, and a newly discovered 37-amino-acid-long protein which we have named CmpA that seems to repress cortex assembly, ensure that the morphogenesis of both cellular structures is temporally co-ordinated.
Our model that CmpA is a repressor of cortex assembly is based on three observations. First, deletion of the cmpA gene suppressed the cortex assembly defect in cells harbouring the VMI15A allele of spoVM, which were otherwise arrested at a stage of sporulation immediately preceding cortex assembly. Cells in which cmpA was deleted produced heat-resistant spores more quickly than wild-type cells, but were sensitive to lysozyme (suggesting that the coat was somehow improperly assembled). We interpret these results to mean that unchecked cortex assembly results in coat assembly defects. Second, overproduction of CmpA before the onset of sporulation caused a three to eightfold decrease in the production of heat-resistant spores, consistent with a block in cortex assembly. Examining the kinetics of sporulation in these cells revealed that they were delayed in polar septation, engulfment, and the production of phase bright spores, steps that either require peptidoglycan synthesis or are coincident with cortex assembly (Henriques and Moran, 2007; Meyer et al., 2010). Furthermore, electron microscopy revealed that overproduction of CmpA abrogated cortex assembly. Third, in wild-type cells CmpA–GFP was detectable during stages of sporulation preceding the elaboration of phase bright spores, but was undetectable after phase bright spores were evident. In contrast, in cells harbouring VMI15A, which were arrested at a stage immediately preceding cortex assembly, CmpA–GFP remained localized around the forespore despite becoming phase bright.
Taken together, our results suggest a ‘checkpoint’ model whereby VM and CmpA orchestrate coat and cortex assembly (Fig. 8). We propose that, as engulfment proceeds, VM is synthesized in the mother cell, localizes to the surface of the developing forespore, and anchors IVA, an ATPase that polymerizes to form the basement layer of the coat. CmpA, which is produced in the mother cell under the control of the same sigma factor that governs expression of VM and IVA (albeit at much lower levels), also localizes to the forespore surface, uniformly surrounds it, and represses the initiation of cortex assembly. It is important to note that transcription of the cmpA gene is additionally regulated by SpoIIID, which would likely delay synthesis of CmpA until the completion of engulfment and after coat assembly had commenced, since, as we observed (Fig. 6), premature synthesis of CmpA delayed the onset of engulfment. Conversely, in the absence of CmpA, the production of heat-resistant spores proceeds more quickly. As a consequence, coat assembly exhibited subtle defects, suggesting that carefully orchestrating the assembly of the cortex is required for proper coat assembly. The precise mechanism by which CmpA localizes is unknown, but its uniform localization pattern depends directly or indirectly on the presence of VM. Successful formation of the basement layer of the coat (which comprises localization of VM, recruitment of IVA and subsequent polymerization of IVA), then triggers the inactivation of CmpA repression by the eventual removal of CmpA. Since transcription of the cmpA gene was driven by an inducible promoter in the experiments presented here, our preliminary evidence suggests that the inactivation of CmpA is a post-transcriptional event, perhaps repression of cmpA mRNA translation and/or proteolysis of CmpA protein (Fig. 7).
Fig. 8.
CmpA is a checkpoint protein that orchestrates coat and cortex morphogenesis. A sporulating B. subtilis cell is depicted (below) in which membranes are yellow and peptidoglycan is grey Top: magnification of the outer and inner forespore membranes and cortex (grey mesh), in which VM (red) has inserted into the phospholipid bilayer. CmpA (blue) localizes to the surface of the forespore and participates in the inhibition of cortex assembly. Recruitment of IVA (green) and successful initiation of assembly of the basement layer of the coat results in depletion of CmpA, thereby relieving cortex assembly inhibition.
The model immediately raises two outstanding questions that remain to be answered. First, what downstream targets are directly or indirectly inhibited by CmpA? Deletion of three other sporulation genes, spoVB, spoVD or spoVE results in a phenotype similar to that caused by the VMI15A allele, in which coat assembly proceeds normally, but cortex assembly is blocked. SpoVB is a putative lipid II flippase that has been proposed to transport peptidoglycan precursors synthesized in the mother cell into the intermembrane space between the double membranes surrounding the forespore to be incorporated into the assembling cortex (Popham and Stragier, 1991). SpoVD is a membrane-bound class B penicillin-binding protein that is required for cortex peptidoglycan synthesis (Daniel et al., 1994; Fay et al., 2010). Its activity was recently shown to be controlled by a thioredoxin-like protein called StoA (Liu et al., 2010), which is also required for efficient cortex assembly (Erlendsson et al., 2004). SpoVE is an integral membrane protein that is also required for cortex peptidoglycan synthesis and belongs to the ‘SEDS’ family of proteins that have been implicated in bacterial shape determination (Real et al., 2008; Fay et al., 2010). Recently, Mohammadi et al. demonstrated that at least one SEDS family member, Escherichia coli FtsW, displays lipid II flippase activity in vitro (Mohammadi et al., 2011). All three proteins therefore represent excellent candidate targets for direct or indirect inhibition by CmpA. The observation that induced, premature expression of cmpA was not toxic to vegetative cells, but delayed several sporulation-specific steps that are thought to require peptidoglycan synthesis (polar septation, engulfment and cortex morphogenesis) suggests that the target of CmpA inhibition may be a sporulation-specific factor.
Second, what signals the relief of CmpA inhibition of cortex assembly? The presence of an inhibitory element that prevents progression through a developmental programme is reminiscent of cell cycle checkpoint proteins, defined as extrinsic control elements in which loss-of-function mutations may be isolated that allow a ‘relief of dependence’ on that factor (Hartwell and Weinert, 1989), and indeed, deletion of cmpA not only permits the progression of sporulation, but even permits it to proceed faster. In one well-studied example, the eukaryotic spindle assembly checkpoint in which exit from metaphase does not occur until chromosome segregation proceeds correctly, entry into anaphase is restricted by the checkpoint if sister kinetochores attached to the mitotic spindle fail to exhibit physical tension (indicating that they are being pulled in opposite directions (Musacchio and Salmon, 2007). The checkpoint is relieved only after sister chromatid cohesion is lost. During sporulation, successful initiation of cortex assembly appears to rely not only on proper localization of VM and subsequent recruitment of IVA, but also an additional event that is mediated by the VM Ile15 residue. Perhaps a physical event that conveys the completion of the basement layer of the coat such as polymerization of IVA is monitored by the checkpoint to allow progression through the sporulation programme.
The discovery of such a small, previously un-annotated, ORF that plays a critical role during a developmental programme highlights the importance of sproteins (gene products that arise from ORFs containing fewer than 50 codons) in biology (Hobbs et al., 2011). Indeed, it is worth noting that VM, despite its diminutive size, plays at least four separate roles during sporulation. First, VM recognizes membrane curvature and, along with IVA, marks this patch of forespore membrane as the site for coat assembly (Ramamurthi et al., 2009). Second, VM anchors IVA (and by extension, the entire assembling coat) to the surface of the forespore (Ramamurthi et al., 2006). Third, along with SpoVID, VM is required for the ‘encasement’ step of the spore coat assembly around the developing forespore (Wang et al., 2009). Finally, as we have described here, VM participates in a pathway that co-ordinates the assembly of two supramolecular structures that are separated by a phospholipid bilayer. Although, as in the case of VM and cmpA, genes encoding such proteins were discovered using classical genetics due to strong deletion or suppression phenotypes, improved bioinformatics approaches may enable more facile discovery of previously ignored genes that affect various important cellular functions. Indeed, an important challenge for the future remains to identify, using a combination of methods, other factors that participate in the developmental pathway that links coat morphogenesis to cortex assembly.
Experimental procedures
Strain construction and growth
Strains are otherwise congenic derivatives of B. subtilis PY79 (Youngman et al., 1984). B. subtilis competent cells were prepared as described previously (Wilson and Bott, 1968). spoVMI15A at amyE was created using the Quikchange site-directed mutagenesis kit (Agilent Technologies) using complementary primers that harboured the mutation flanked by 15 bases on either side and using plasmid pKC2 (Ramamurthi et al., 2006) as the template to create plasmid pKR59. cmpA at thrC was created by PCR amplification of the cmpA ORF and 126 nucleotides immediately upstream of the ORF using primers ‘ydcJprom5′Eco’ (5′-aaagaattcttttgaagctctttgttatcca tg) and ‘ydcJprom3′Bam’ (5′-aaaggatcctgatgtcgtatctgtcggcg), digesting the PCR product with EcoRI and BamHI, and cloning into the integration vector pDG1731 (Guerout-Fleury et al., 1996). PcmpA–lacZ at amyE was created by PCR-amplifying 300 nucleotides upstream of the cmpA ORF using primers ‘ydcJ5′promEco’ (5′-aaagaattcttgatgatgccaatcagttcc) and ‘ydcJ3′startHind’ (5′-aaaagcttcataggctcattcctcctcag), digesting with EcoRI and HindIII, and cloning into plasmid pAH124 (Camp and Losick, 2009) to create pSE23. cmpA–lacZ at amyE was created by PCR-amplifying the cmpA ORF and upstream sequences using primers ‘ydcJ5′promEco’ and ‘ydcJnostop3′Hind’ (5′-aaaaagcttcgagcagtgttttttcctatag), and cloning into pSE23 as described above to create pSE27. spoVM–lacZ at amyE was created by cloning the EcoRI/HindIII fragment of pKC13 (Ramamurthi et al., 2006) into pSE27 to create pSE31. Phyperspank–cmpA–gfp at amyE was created by cloning cmpA and gfp into a vector and introducing a HindIII restriction site at the site of fusion. cmpA–gfp was then PCR-amplified from the resulting construct using primers ‘5′SalI(RBS)ydcJ’ (5′ aaagtcgactaaggaggaatgagcctatgcc) and ‘3′GfpNheI’ (5′-aaagctagcttatttgtatagttcatc), digested with SalI and NheI, and cloned into pDR111 (David Rudner). Phyperspank–cmpA at amyE was created by PCR-amplifying the cmpA ORF, along with 9 nucleotides upstream (specifying the RBS) and 24 nucleotides downstream, using primers ‘ydcJ5′RBSHind’ (5′-aaaaagctttaaggaggaatgagcctatgcc) and ‘ydcJ3′Nhe’ (5′-aaagctagctctatggtaaaataaaagcactg). The fragment was then digested with HindIII and NheI and cloned into pDR111. All plasmids were integrated into the B. subtilis chromosome by double recombination at the specified ectopic locus. The cmpA deletion was created using the Long Flanking Homology PCR (LFH-PCR) technique (Wach, 1996) using primers ‘ydcJ_KO_P1’ (5′-ggacagtatcagcatgacgtcagcc) and ‘ydcJ_KO_P2’ (5′-caattcgccctatagtgagtcgtgcagtgcttttattttacc atag); and ‘ydcJ_KO_P3’ (5′-ccagcttttgttccctttagtgagctgct ttttaagccaatttggc) and ‘ydcJ_KO_750P4’ (5′-gcgcgtccatt ccgccacgaccgcgtgatc). Underlined sequences correspond to DNA sequence in plasmid pAH52 (Ferguson et al., 2007) used as a template to amplify the erythromycin antibiotic resistance cassette. spoVM–gfp, gfp–spoIVA (Ramamurthi et al., 2006) and cotE–gfp (Webb et al., 1995) fusions, and the spo0A, sigE, sigG, sigK and spoIIID deletions (Kenney and Moran, 1987; Kunkel et al., 1989; Eichenberger et al., 2004; Chu et al., 2008) were described previously.
General methods
β-Galactosidase activity was measured as previously described (Nicholson and Setlow, 1990). Sporulation efficiency was measured by inducing sporulation by nutrient depletion in Difco sporulation medium (DSM) for at least 24 h at 37°C. The number of colony-forming units (cfu) that survived heat treatment (80°C for 20 min) or lysozyme treatment (250 µg ml−1 for 10 min at 37°C) was determined and reported relative to the cfu obtained in a parallel culture of the wild-type PY79 strain. For cmpA overexpression experiments, cells were induced to sporulate by resuspension in SM medium (Sterlini and Mandelstam, 1969) containing 1 mM IPTG at the time of resuspension as described below. For microscopy, cells from the IPTG-induced and uninduced cultures were removed at various time points and imaged as described below.
Fluorescence microscopy
Overnight cultures grown at 22°C in CH medium (Sterlini and Mandelstam, 1969) were diluted 1:20 into fresh CH medium and grown for approximately 2.5 h at 37°C. Cells were induced to sporulate by resuspension in SM medium. If necessary, IPTG was added (1 mM final concentration) to induce expression of cmpA–gfp 3 h after resuspension (after most cells had completed engulfment). Cells were harvested, resuspended in PBS containing 1 µg ml−1 of the fluorescent dye FM4-64 or 46 µg ml−1 TMA-DPH to visualize membranes, and placed on a glass bottom culture dish (Mattek Corp.). A 1% agarose pad made with distilled water was cut to size and placed on top of the cell suspension. Cells were viewed with a DeltaVision Core microscope system (Applied Precision) equipped with an environmental control chamber. Images were captured with a Photometrics CoolSnap HQ2 camera. Seventeen planes were acquired every 0.2 µm at 22°C and the data were deconvolved using SoftWorx software.
Electron microscopy
For the images in Fig. 1, cells were induced to sporulate by resuspension in SM medium as described above and were harvested 5 h after induction of sporulation. For the images in Fig. 6G, cells were induced to sporulate by nutrient depletion in DSM for 24 h. Three millilitres of the culture was harvested, resuspended in 3 ml of PBS and fixed using 4% formaldehyde, 2% glutaraldehyde (final concentration) for at least 2 h at room temperature. Fixed cells were collected by centrifugation and processed for thin-sectioned TEM analysis. Briefly, the cell pellet was post-fixed in 1% Osmium tetroxide in 0.1 M Cacodylate buffer for 1 h at room temperature, en bloc stained with 0.5% uranyl acetate in 0.1 M acetate buffer for 1 h, then dehydrated sequentially in 35%, 50%, 75%, 95% and 100% ethanol followed by 100% propylene oxide. Cells were infiltrated in an equal volume of 100% propylene oxide and epoxy resin overnight and embedded in pure resin the following day. The epoxy resin was cured at 55°C for 48 h. The cured block was thin-sectioned and stained in uranyl acetate and lead citrate. The sample was imaged with a Hitachi H7600 TEM equipped with a CCD camera.
Isolation of spontaneous suppressors
Spontaneous suppressor mutations of spoVMI15A were isolated as described previously (Ramamurthi et al., 2006). Briefly, strain KR322, harbouring spoVMI15A as the only copy of spoVM, was grown in 100 ml of DSM supplemented with 5 µg ml−1 chloramphenicol (Cm) for 24 h at 37°C in order to accumulate spontaneous mutations and sporulate. Forty millilitres of this culture was removed and incubated at 80°C for 20 min in order to kill any cells that had not completed sporulation successfully. Thirty-three millilitres of the heat-killed culture was then diluted into 300 ml of fresh DSM/Cm and allowed to sporulate for 24 h as above. The procedure was repeated two more times and candidate survivors from each round were collected for further characterization. The suppressor mutation was mapped by generating a mini-Tn10 transposon library (Steinmetz and Richter, 1994) using strain KRC56 and isolating a clone in which suppression of the VMI15A sporulation defect was genetically linked (by transformation) to the antibiotic resistance conferred by the transposon.
Supplementary Material
Acknowledgements
We thank S. Gottesman, D. Popham, V. Lee and members of the laboratory for comments on the manuscript; R. Losick for helpful discussion and advice; N. Majdalani and A. Battesti for training; K. Nagashima of the Electron Microscopy Laboratory (NCI Frederick) for assistance with electron microscopy; and the anonymous reviewer who notified us about the SpoIIID binding site upstream of cmpA. This work was funded by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Atrih A, Foster SJ. The role of peptidoglycan structure and structural dynamics during endospore dormancy and germination. Antonie Van Leeuwenhoek. 1999;75:299–307. doi: 10.1023/a:1001800507443. [DOI] [PubMed] [Google Scholar]
- Beall B, Driks A, Losick R, Moran CP., Jr Cloning and characterization of a gene required for assembly of the Bacillus subtilis spore coat. J Bacteriol. 1993;175:1705–1716. doi: 10.1128/jb.175.6.1705-1716.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp AH, Losick R. A feeding tube model for activation of a cell-specific transcription factor during sporulation in Bacillus subtilis. Genes Dev. 2009;23:1014–1024. doi: 10.1101/gad.1781709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano FA, Meador-Parton J, Popham DL, Driks A. Amino acids in the Bacillus subtilis morphogenetic protein SpoIVA with roles in spore coat and cortex formation. J Bacteriol. 2001;183:1645–1654. doi: 10.1128/JB.183.5.1645-1654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F, Kearns DB, McLoon A, Chai Y, Kolter R, Losick R. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;68:1117–1127. doi: 10.1111/j.1365-2958.2008.06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JG. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J Gen Microbiol. 1972;71:1–15. doi: 10.1099/00221287-71-1-1. [DOI] [PubMed] [Google Scholar]
- Daniel RA, Drake S, Buchanan CE, Scholle R, Errington J. The Bacillus subtilis spoVD gene encodes a mother-cell-specific penicillin-binding protein required for spore morphogenesis. J Mol Biol. 1994;235:209–220. doi: 10.1016/s0022-2836(05)80027-0. [DOI] [PubMed] [Google Scholar]
- Doan T, Morlot C, Meisner J, Serrano M, Henriques AO, Moran CP, Jr, Rudner DZ. Novel secretion apparatus maintains spore integrity and developmental gene expression in Bacillus subtilis. PLoS Genet. 2009;5:e1000566. doi: 10.1371/journal.pgen.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driks A. Maximum shields: the assembly and function of the bacterial spore coat. Trends Microbiol. 2002;10:251–254. doi: 10.1016/s0966-842x(02)02373-9. [DOI] [PubMed] [Google Scholar]
- Driks A. The bacillus spore coat. Phytopathology. 2004;94:1249–1251. doi: 10.1094/PHYTO.2004.94.11.1249. [DOI] [PubMed] [Google Scholar]
- Driks A, Roels S, Beall B, Moran CP, Jr, Losick R. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 1994;8:234–244. doi: 10.1101/gad.8.2.234. [DOI] [PubMed] [Google Scholar]
- Eichenberger P, Fujita M, Jensen ST, Conlon EM, Rudner DZ, Wang ST, et al. The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol. 2004;2:e328. doi: 10.1371/journal.pbio.0020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlendsson LS, Moller M, Hederstedt L. Bacillus subtilis StoA is a thiol-disulfide oxidoreductase important for spore cortex synthesis. J Bacteriol. 2004;186:6230–6238. doi: 10.1128/JB.186.18.6230-6238.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol. 2003;1:117–126. doi: 10.1038/nrmicro750. [DOI] [PubMed] [Google Scholar]
- Fay A, Meyer P, Dworkin J. Interactions between late-acting proteins required for peptidoglycan synthesis during sporulation. J Mol Biol. 2010;399:547–561. doi: 10.1016/j.jmb.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CC, Camp AH, Losick R. gerT, a newly discovered germination gene under the control of the sporulation transcription factor sigmaK in Bacillus subtilis. J Bacteriol. 2007;189:7681–7689. doi: 10.1128/JB.01053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore ME, Bandyopadhyay D, Dean AM, Linnstaedt SD, Popham DL. Production of muramic delta-lactam in Bacillus subtilis spore peptidoglycan. J Bacteriol. 2004;186:80–89. doi: 10.1128/JB.186.1.80-89.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GW, Hitchins AD. Sensitization of bacterial spores to lysozyme and to hydrogen peroxide with agents which rupture disulphide bonds. J Gen Microbiol. 1963;33:413–423. doi: 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- Guerout-Fleury AM, Frandsen N, Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Henriques AO, Moran CP., Jr Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol. 2007;61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- Hobbs EC, Fontaine F, Yin X, Storz G. An expanding universe of small proteins. Curr Opin Microbiol. 2011;14:167–173. doi: 10.1016/j.mib.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imae Y, Strominger JL. Cortex content of asporogenous mutants of Bacillus subtilis. J Bacteriol. 1976;126:914–918. doi: 10.1128/jb.126.2.914-918.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaton MA, Lew DJ. Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Curr Opin Microbiol. 2006;9:540–546. doi: 10.1016/j.mib.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kenney TJ, Moran CP., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Hahn M, Grabowski P, McPherson DC, Otte MM, Wang R, et al. The Bacillus subtilis spore coat protein interaction network. Mol Microbiol. 2006;59:487–502. doi: 10.1111/j.1365-2958.2005.04968.x. [DOI] [PubMed] [Google Scholar]
- Kunkel B, Kroos L, Poth H, Youngman P, Losick R. Temporal and spatial control of the mother-cell regulatory gene spoIIID of Bacillus subtilis. Genes Dev. 1989;3:1735–1744. doi: 10.1101/gad.3.11.1735. [DOI] [PubMed] [Google Scholar]
- Levin PA, Fan N, Ricca E, Driks A, Losick R, Cutting S. An unusually small gene required for sporulation by Bacillus subtilis. Mol Microbiol. 1993;9:761–771. doi: 10.1111/j.1365-2958.1993.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Carlsson Moller M, Petersen L, Soderberg CA, Hederstedt L. Penicillin-binding protein SpoVD disulphide is a target for StoA in Bacillus subtilis forespores. Mol Microbiol. 2010;75:46–60. doi: 10.1111/j.1365-2958.2009.06964.x. [DOI] [PubMed] [Google Scholar]
- McKenney PT, Eichenberger P. Dynamics of spore coat morphogenesis in Bacillus subtilis. Mol Microbiol. 2012;83:245–260. doi: 10.1111/j.1365-2958.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney PT, Driks A, Eskandarian HA, Grabowski P, Guberman J, Wang KH, et al. A distance-weighted interaction map reveals a previously uncharacterized layer of the Bacillus subtilis spore coat. Curr Biol. 2010;20:934–938. doi: 10.1016/j.cub.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Parton J, Popham DL. Structural analysis of Bacillus subtilis spore peptidoglycan during sporulation. J Bacteriol. 2000;182:4491–4499. doi: 10.1128/jb.182.16.4491-4499.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly E, Genest PC, Gilmore ME, Little S, Popham DL, Driks A, Setlow P. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J Appl Microbiol. 2002;92:1105–1115. doi: 10.1046/j.1365-2672.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Meyer P, Gutierrez J, Pogliano K, Dworkin J. Cell wall synthesis is necessary for membrane dynamics during sporulation of Bacillus subtilis. Mol Microbiol. 2010;76:956–970. doi: 10.1111/j.1365-2958.2010.07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi T, van Dam V, Sijbrandi R, Vernet T, Zapun A, Bouhss A, et al. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. EMBO J. 2011;30:1425–1432. doi: 10.1038/emboj.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Setlow P. Sporulation, Germination, and Outgrowth. New York: John Wiley & Sons; 1990. pp. 391–450. [Google Scholar]
- van Ooij C, Losick R. Subcellular localization of a small sporulation protein in Bacillus subtilis. J Bacteriol. 2003;185:1391–1398. doi: 10.1128/JB.185.4.1391-1398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot PJ, Coote JG. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis. Curr Opin Microbiol. 2004;7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Popham DL, Setlow P. The cortical peptidoglycan from spores of Bacillus megaterium and Bacillus subtilis is not highly cross-linked. J Bacteriol. 1993;175:2767–2769. doi: 10.1128/jb.175.9.2767-2769.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham DL, Stragier P. Cloning, characterization, and expression of the spoVB gene of Bacillus subtilis. J Bacteriol. 1991;173:7942–7949. doi: 10.1128/jb.173.24.7942-7949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham DL, Illades-Aguiar B, Setlow P. The Bacillus subtilis dacB gene, encoding penicillin-binding protein 5*, is part of a three-gene operon required for proper spore cortex synthesis and spore core dehydration. J Bacteriol. 1995;177:4721–4729. doi: 10.1128/jb.177.16.4721-4729.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham DL, Gilmore ME, Setlow P. Roles of low-molecular-weight penicillin-binding proteins in Bacillus subtilis spore peptidoglycan synthesis and spore properties. J Bacteriol. 1999;181:126–132. doi: 10.1128/jb.181.1.126-132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KD, Losick R. A four-dimensional view of assembly of a morphogenetic protein during sporulation in Bacillus subtilis. J Bacteriol. 1999;181:781–790. doi: 10.1128/jb.181.3.781-790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Losick R. ATP-driven self-assembly of a morphogenetic protein in Bacillus subtilis. Mol Cell. 2008;31:406–414. doi: 10.1016/j.molcel.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Clapham KR, Losick R. Peptide anchoring spore coat assembly to the outer forespore membrane in Bacillus subtilis. Mol Microbiol. 2006;62:1547–1557. doi: 10.1111/j.1365-2958.2006.05468.x. [DOI] [PubMed] [Google Scholar]
- Ramamurthi KS, Lecuyer S, Stone HA, Losick R. Geometric cue for protein localization in a bacterium. Science. 2009;323:1354–1357. doi: 10.1126/science.1169218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real G, Fay A, Eldar A, Pinto SM, Henriques AO, Dworkin J. Determinants for the subcellular localization and function of a nonessential SEDS protein. J Bacteriol. 2008;190:363–376. doi: 10.1128/JB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JM, Handrigan GR. Reptilian tooth development. Genesis. 2011;49:247–260. doi: 10.1002/dvg.20721. [DOI] [PubMed] [Google Scholar]
- Roels S, Driks A, Losick R. Characterization of spoIVA a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:575–585. doi: 10.1128/jb.174.2.575-585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner DZ, Pan Q, Losick RM. Evidence that subcellular localization of a bacterial membrane protein is achieved by diffusion and capture. Proc Natl Acad Sci USA. 2002;99:8701–8706. doi: 10.1073/pnas.132235899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalisch M, Maiques E, Nikolov L, Camp AH, Chevreux B, Muffler A, et al. Small genes under sporulation control in the Bacillus subtilis genome. J Bacteriol. 2010;192:5402–5412. doi: 10.1128/JB.00534-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- Sharp MD, Pogliano K. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc Natl Acad Sci USA. 1999;96:14553–14558. doi: 10.1073/pnas.96.25.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M, Richter R. Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J Bacteriol. 1994;176:1761–1763. doi: 10.1128/jb.176.6.1761-1763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini JM, Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969;113:29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–241. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Kodama T, Nakayama T, Watabe K. Characterization of the yrbA gene of Bacillus subtilis involved in resistance and germination of spores. J Bacteriol. 1999;181:4986–4994. doi: 10.1128/jb.181.16.4986-4994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan P, Weaver A, Reichert ED, Linnstaedt SD, Popham DL. Spore cortex formation in Bacillus subtilis is regulated by accumulation of peptidoglycan precursors under the control of sigma K. Mol Microbiol. 2007;65:1582–1594. doi: 10.1111/j.1365-2958.2007.05896.x. [DOI] [PubMed] [Google Scholar]
- Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wang KH, Isidro AL, Domingues L, Eskandarian HA, McKenney PT, Drew K, et al. The coat morphogenetic protein SpoVID is necessary for spore encasement in Bacillus subtilis. Mol Microbiol. 2009;74:634–649. doi: 10.1111/j.1365-2958.2009.06886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CD, Decatur A, Teleman A, Losick R. Use of green fluorescent protein for visualization of cell-specific gene expression and subcellular protein localization during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:5906–5911. doi: 10.1128/jb.177.20.5906-5911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GA, Bott KF. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968;95:1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P, Perkins JB, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposonborne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]
- Zheng LB, Donovan WP, Fitz-James PC, Losick R. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 1988;2:1047–1054. doi: 10.1101/gad.2.8.1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.