Abstract
Background
Approximately 600 million children of preschool and school age are anaemic worldwide. It is estimated that half of the cases are due to iron deficiency. Consequences of iron deficiency anaemia during childhood include growth retardation, reduced school achievement, impaired motor and cognitive development, and increased morbidity and mortality. The provision of daily iron supplements is a widely used strategy for improving iron status in children but its effectiveness has been limited due to its side effects, which can include nausea, constipation or staining of the teeth. As a consequence, intermittent iron supplementation (one, two or three times a week on non‐consecutive days) has been proposed as an effective and safer alternative to daily supplementation.
Objectives
To assess the effects of intermittent iron supplementation, alone or in combination with other vitamins and minerals, on nutritional and developmental outcomes in children from birth to 12 years of age compared with a placebo, no intervention or daily supplementation.
Search methods
We searched the following databases on 24 May 2011: CENTRAL (2011, Issue 2), MEDLINE (1948 to May week 2, 2011), EMBASE (1980 to 2011 Week 20), CINAHL (1937 to current), POPLINE (all available years) and WHO International Clinical Trials Registry Platform (ICTRP). On 29 June 2011 we searched all available years in the following databases: SCIELO, LILACS, IBECS and IMBIOMED. We also contacted relevant organisations (on 3 July 2011) to identify ongoing and unpublished studies.
Selection criteria
Randomised and quasi‐randomised trials with either individual or cluster randomisation. Participants were children under the age of 12 years at the time of intervention with no specific health problems. The intervention assessed was intermittent iron supplementation compared with a placebo, no intervention or daily supplementation.
Data collection and analysis
Two authors independently assessed the eligibility of studies against the inclusion criteria, extracted data from included studies and assessed the risk of bias of the included studies.
Main results
We included 33 trials, involving 13,114 children (˜49% females) from 20 countries in Latin America, Africa and Asia. The methodological quality of the trials was mixed.
Nineteen trials evaluated intermittent iron supplementation versus no intervention or a placebo and 21 studies evaluated intermittent versus daily iron supplementation. Some of these trials contributed data to both comparisons. Iron alone was provided in most of the trials.
Fifteen studies included children younger than 60 months; 11 trials included children 60 months and older, and seven studies included children in both age categories. One trial included exclusively females. Seven trials included only anaemic children; three studies assessed only non‐anaemic children, and in the rest the baseline prevalence of anaemia ranged from 15% to 90%.
In comparison with receiving no intervention or a placebo, children receiving iron supplements intermittently have a lower risk of anaemia (average risk ratio (RR) 0.51, 95% confidence interval (CI) 0.37 to 0.72, ten studies) and iron deficiency (RR 0.24, 95% CI 0.06 to 0.91, three studies) and have higher haemoglobin (mean difference (MD) 5.20 g/L, 95% CI 2.51 to 7.88, 19 studies) and ferritin concentrations (MD 14.17 µg/L, 95% CI 3.53 to 24.81, five studies).
Intermittent supplementation was as effective as daily supplementation in improving haemoglobin (MD –0.60 g/L, 95% CI –1.54 to 0.35, 19 studies) and ferritin concentrations (MD –4.19 µg/L, 95% CI –9.42 to 1.05, 10 studies), but increased the risk of anaemia in comparison with daily iron supplementation (RR 1.23, 95% CI 1.04 to1.47, six studies). Data on adherence were scarce and it tended to be higher among those children receiving intermittent supplementation, although this result was not statistically significant.
We did not identify any differential effect of the type of intermittent supplementation regimen (one, two or three times a week), the total weekly dose of elemental iron, the nutrient composition, whether recipients were male or female or the length of the intervention.
Authors' conclusions
Intermittent iron supplementation is efficacious to improve haemoglobin concentrations and reduce the risk of having anaemia or iron deficiency in children younger than 12 years of age when compared with a placebo or no intervention, but it is less effective than daily supplementation to prevent or control anaemia. Intermittent supplementation may be a viable public health intervention in settings where daily supplementation has failed or has not been implemented. Information on mortality, morbidity, developmental outcomes and side effects, however, is still lacking.
Plain language summary
One, two or three times a week iron supplements for improving health and development among children under 12 years of age
Approximately 600 million preschool and school‐age children are anaemic worldwide. It is estimated that half of these cases are due to a lack of iron. Iron deficiency anaemia during childhood may slow down growth, reduce motor and brain development, and increase illness and death. If anaemia is not treated promptly, these problems may persist later in life. Taking supplements containing iron (sometimes combined with folic acid and other vitamins and minerals) on a daily basis has shown to improve children's health but its use has been limited because supplements may produce side effects such as nausea, constipation or staining of the teeth. It has been suggested that giving iron one, two or three times a week (known as 'intermittent' supplementation) may reduce these side effects and be easier to remember, and thus encourage children to continue taking the iron supplements.
We analysed 33 trials involving 13,314 children (49% females) from 20 countries in Latin America, Africa and Asia, to assess the effects of intermittent iron supplementation, alone or in combination with other vitamins and minerals, on nutritional and developmental outcomes in children from birth to 12 years of age compared with a placebo, no intervention.or daily supplementation.
The studies were of mixed quality. Overall, the results of this review show that giving children supplements with iron alone or in combination with other vitamins and minerals one, two or three times a week approximately halves their risk of having anaemia in comparison with receiving no iron supplements or a placebo. Giving children supplements on a intermittent basis was as effective as daily supplementation for improving haemoglobin and ferritin concentrations, although, children receiving iron supplements intermittently were at higher risk of having anaemia.
We aimed to examine the effects of intermittent supplementation on illness, death, and school and physical performance, as well as on other side effects, but there was insufficient information to draw firm conclusions.
In summary, intermittent iron supplementation is efficacious to improve haemoglobin concentrations and reduce the risk of having anaemia or iron deficiency in children younger than 12 years of age when compared with a placebo or no intervention, but it is less effective than daily supplementation to prevent or control anaemia. Intermittent supplementation may be a viable public health intervention in settings where daily supplementation has failed or has not been implemented. Information on mortality, morbidity, developmental outcomes and side effects, however, is still lacking.
Summary of findings
Summary of findings for the main comparison. Intermittent use of iron supplements versus placebo or no intervention in children younger than 12 years of age.
| Patient or population: children under 12 years of age Settings: community settings Intervention: intermittent supplementation with iron alone or with other nutrients Comparison: placebo or no intervention | |||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
|
Anaemia (haemoglobin below a cut‐off defined by trialists, taking into account the age and altitude) |
RR 0.51 (0.37–0.72) | 1824 (10 studies) | ⊕⊕⊕⊝ moderate1 |
| Haemoglobin (g/L) | MD 5.20 (2.51–7.88) | 3032 (19 studies) |
⊕⊕⊝⊝ low2,3 |
| Iron deficiency (using ferritin concentrations) |
RR 0.24
(0.06–0.91) |
431 (3 studies) | ⊝⊝⊝⊝ very low2,3,4 |
| Iron status (ferritin (μg/L) | MD 14.17 (3.53–24.81) | 550 (5 studies) | ⊕⊕⊝⊝ low2,3 |
| Iron deficiency anaemia | Not estimable | 0 (0 studies) | None of the trials reported on this outcome |
| All‐cause mortality | Not estimable | 0 (0 studies) | None of the trials reported on this outcome |
| CI, confidence interval; RR, risk ratio; MD, mean difference. *GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We have moderate confidence in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of the effect. | |||
|
1There was high statistical heterogeneity. Given the large and consistent effect (RR 0.51; 95% CI 0.37–0.72) we have refrained from downgrading even though three of the nine studies are at high risk of bias. 2 High statistical heterogeneity but results were consistent. 3 Some studies lacked blinding and clear methods of allocation. 4 Wide confidence intervals. Note: For cluster‐randomised trials the analyses only include the estimated effective sample size, after adjusting the data to account for the clustering effect. | |||
Summary of findings 2. Intermittent versus daily use of iron supplements in children younger than 12 years of age.
| Patient or population: children under 12 years of age Settings: community settings Intervention: intermittent supplementation with iron alone or with other micronutrients Comparison: daily supplementation with iron alone or with other micronutrients | |||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
| Anaemia (haemoglobin below a cut‐off defined by trialists, taking into account the age and altitude) | RR 1.23 (1.04–1.47) | 980 (6 studies) | ⊕⊕⊝⊝ low1,2 |
| Haemoglobin (g/L) | MD –0.60 (–1.54‐0.35) | 2851 (19 studies) |
⊕⊕⊝⊝ low1,3 |
| Iron deficiency (using ferritin concentrations) |
RR 4.00
(1.23–13.05) |
76 (1 study) | ⊝⊝⊝⊝ very low4 |
| Iron status (ferritin (µg/L) |
MD –4.19 (–9.42‐ 1.05) |
902 (10 studies) |
⊕⊕⊝⊝ low1 3 |
| Iron deficiency anaemia | Not estimable | 0 (0 studies) | None of the trials reported on this outcome |
| Mortality | Not estimable | 0 (0 studies) | None of the trials reported on this outcome |
| CI, confidence interval; RR, risk ratio; MD, mean difference. *GRADE Working Group grades of evidence: High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We have moderate confidence in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of the effect. | |||
|
1 Some studies lacked blinding and clear methods of allocation. 2 Wide confidence intervals. 3 High heterogeneity but results were mostly consistent. 4 Only one trial with unclear methods to generate the random sequence and conceal the allocation. Wide confidence intervals. Note: For cluster‐randomised trials the analyses only include the estimated effective sample size, after adjusting the data to account for the clustering effect. | |||
Background
Description of the condition
Iron is an essential nutrient for all body tissues and is present in the brain of the developing fetus, where it is needed for proper formation of neural tissue (Iannoti 2006) and development of brain cells (Beard 2008). Iron deficiency, a common form of nutritional deficiency, results from long‐term imbalance caused by an inadequate dietary iron intake; poor iron absorption or utilisation; increased iron requirements for growth during childhood, adolescence or pregnancy; or chronic blood losses (Moy 2006). In the later stages of iron depletion, the haemoglobin concentration decreases, resulting in a condition known as iron deficiency anaemia.
Anaemia is characterised by a reduction in the oxygen‐carrying capacity of blood such that the body's needs can no longer be met. In addition to iron deficiency, other vitamin and mineral deficiencies (for example, folate, vitamin B12 and vitamin A), chronic inflammation, parasitic infections and inherited disorders of haemoglobin structure can result in all‐cause anaemia (WHO 2001). Among females, anaemia is often exacerbated after beginning menstruation, especially if it occurs at an early age and the young females do not consume sufficient iron to offset menstrual losses (WHO 2001). Haemoglobin concentrations are used to diagnose anaemia, while serum ferritin, an iron storage protein, and serum transferrin, an iron transport protein, are commonly used as indicators of iron status in populations (WHO 2011a; WHO 2011b).
Children, particularly those younger than five years, are vulnerable to iron deficiency anaemia because of their increased needs as a result of rapid growth. It is estimated that approximately 600 million preschool and school‐aged children are anaemic worldwide, and it is calculated that at least half of the cases are due to iron deficiency (WHO/CDC 2008). In general, low‐income countries have a higher prevalence of anaemia (WHO/CDC 2008). This association is also true in high‐income countries where people of low socioeconomic status are especially susceptible to iron and other vitamin and mineral deficiencies (Cole 2010).
Consequences of iron deficiency anaemia during childhood include growth retardation, reduced school achievement, impaired motor and cognitive development, and increased morbidity from a variety of causes including diarrhoea and acute respiratory infections (WHO 2001). Specifically, iron deficiency can lead to deficits in memory and behavioural regulation as iron is required to make neurotransmitters such as dopamine, epinephrine and serotonin (Iannoti 2006; Moy 2006; Beard 2008), while impaired myelination contributes to deficits in motor function. Long‐term effects of early iron deficiency include decreased work capacity and impaired cognitive and behavioural development (Lozoff 2000; Lozoff 2007). Some of these impairments are thought to be irreversible if they occur at an early age and the consequences may continue even after treatment, reinforcing the importance of prevention (Siddiqui 2004; Iannoti 2006; Lozoff 2007).
Description of the intervention
Mass fortification of food staples with iron; dietary diversification to increase iron intake, absorption and utilisation; and iron supplementation have been used to prevent or treat iron deficiency anaemia. Mass fortification of staple foods with iron is usually not aimed at meeting the needs of young children, with the exception of targeted complementary infant feeding programmes (WHO 2009a). Dietary diversification to improve iron status in populations at risk is also difficult because of limited food access among the most vulnerable populations, the limited quantity of food that children can consume, and the fact that the strategy requires multiple behavioural changes among children and their families. To date, there are few effective dietary diversification intervention programmes at scale (Davidsson 2003). Finally, iron supplementation, which is the provision of doses of iron alone or in combination with other micronutrients in the form of tablets, syrups or capsules, is the most widespread strategy for improving iron status in children worldwide.
The World Health Organization (WHO) recommends a supplemental provision of 2 mg of elemental iron per kilogram body weight per day for three months in children less than six years of age who were born at term. Children of school age and older should receive 30 mg of elemental iron and 250 μg (0.25 mg) of folic acid daily, particularly in populations where anaemia prevalence is greater than 40% (WHO 2001). Though the current recommendations include iron alone or with folic acid, it has been suggested that administration of additional vitamins and minerals may prevent or reverse anaemia derived from one or more nutritional deficiencies (Bhutta 2009). Daily iron supplementation has proven to be effective in increasing haemoglobin concentrations in children, especially in those who are anaemic (Gera 2007). In spite of this, in real world settings the long regimen duration, the low coverage rates and insufficient tablet distribution, and side effects associated with daily iron supplementation (for example, gastrointestinal discomfort, constipation and staining of teeth with drops or syrups) limit adherence, especially in young children (ACC/SCN 1991; Stoltzfus 2011). In older children these effects may partially be controlled with the use of slow‐release iron tablets in which iron has similar bioavailability to regular iron compounds (for example, ferrous sulphate or ferrous fumarate) (Simmons 1993; Bothwell 2000), although their higher cost may be a limiting factor for wider use.
How the intervention might work
Oral iron supplementation on an intermittent basis (that is once, twice or three times a week on non‐consecutive days) has been suggested as a more efficient preventive intervention in public health programmes than the more common daily iron supplementation scheme. The basis for this iron intermittent supplementation regimen is that the absorption is maximised by provision of iron in synchrony with the turnover of the mucosal cells (that is, intestinal cells are 'fresh' to take up iron) (Wright 1990; Berger 1997; Viteri 1997; Beaton 1999; Tavil 2003). In addition, other minerals such as zinc and copper may be more readily absorbed because they are not regularly competing with iron for absorption channels, leading to an improved micronutrient status (Baqui 2005). It has been reported that intermittent supplementation may be safer than daily supplementation because intestinal cells are less exposed to an iron‐rich environment, which may cause cell damage (Casanueva 2003; Viteri 2005). Also, it has been suggested that additional iron may exacerbate malaria infection and so this reduced exposure to iron overall is particularly relevant in malaria settings as less iron is available for the parasite's growth (Ekvall 2000; NIH 2011). Though side effects may still occur with intermittent regimens, they are experienced less frequently and may be perceived as more acceptable as a result, increasing adherence to supplementation programmes (Thu 1999; Viteri 2005).
Despite the biological plausibility of this intervention to reduce anaemia, its success as a public health intervention will likely be determined by several factors such as the available resources; the existence of the appropriate policies and legislation; the production and supply of the supplements; the development of delivery systems; the development and implementation of external and internal quality control systems, and the development and implementation of strategies for information, education and communication for behaviour change among consumers. Figure 1 presents a generic logic model for micronutrient interventions that depicts the programme theory and the plausible relationships between inputs and expected changes in health and outcomes that can be adapted to the context of each setting (De‐Regil 2011; WHO/CDC 2011).
1.
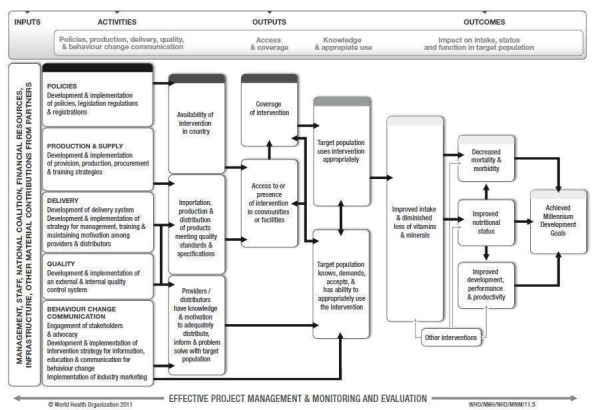
WHO/CDC logic model for micronutrients interventions in public health (with permission from WHO)
Why it is important to do this review
There are currently no international recommendations on intermittent iron supplementation regimens in children. It has been reported that the provision of an iron supplement once a week is comparable to daily supplementation in improving anaemia status (Siddiqui 2004). Other authors suggest that this effect may be enhanced when iron is given twice a week (Schultink 1995; Tavil 2003; Olsen 2006).
Weekly iron and folic acid supplementation has recently been recommended by the WHO to prevent anaemia in women of reproductive age (WHO 2009b). This intervention is currently implemented at scale in many countries around the world as part of public health programmes. It could potentially be targeted to other age groups, such as young children and school‐aged children, since the supplement can be provided at home and in schools or other institutional settings. However, to date, there has been no systematic assessment of the safety and effectiveness of weekly or any other intermittent iron supplementation regimen among children to inform policy makers.
This review complements the findings of two related Cochrane systematic reviews exploring the effects of intermittent regimens among menstruating women (Fernández‐Gaxiola 2011) and pregnant women (Peña‐Rosas 2009).
Objectives
To assess the effects of intermittent iron supplementation, alone or in combination with other vitamins and minerals, on nutritional and developmental outcomes in children less than 12 years of age compared with daily supplementation, a placebo or no supplementation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised studies with randomisation at either an individual or cluster level. We defined quasi‐randomised trials as trials which use systematic methods to allocate participants to treatment groups, such as alternation, assignment based on date of birth or case record number (Higgins 2011). We did not include cross‐over trials nor other types of evidence (for example, cohort or case‐control studies) in the meta‐analysis but we have considered such evidence in the discussion where relevant.
Types of participants
Children under the age of 12 years at the time of the trials.
We did not include studies specifically targeting premature or low birth weight infants, or children with severe infectious diseases, such as HIV, as they may metabolise iron differently and have different health and disease indicators. These topics are subject to separate Cochrane reviews (Adetifa 2009; Mills 2009).
Types of interventions
Oral supplements of iron, alone or with other vitamins and minerals, given on an intermittent basis and compared with a placebo or no supplementation, or compared with the same supplements provided daily.
Oral iron supplementation refers to the delivery of iron compounds directly to the oral cavity, either as a tablet, capsule, dispersible tablet or liquid. For the purpose of this review, intermittent supplementation is defined as the provision of iron supplements one, two or three times a week on non‐consecutive days.
We performed the following comparisons:
any intermittent iron supplementation versus no supplementation or placebo (0 to < 12 years of age);
any intermittent iron supplementation versus any daily iron supplementation (0 to < 12 years of age);
any intermittent iron supplementation versus no supplementation or placebo (0 to 59 months of age);
any intermittent iron supplementation versus any daily iron supplementation (0 to 59 months of age);
any intermittent iron supplementation versus no supplementation or placebo (5 to < 12 years of age);
any intermittent iron supplementation versus any daily iron supplementation (5 to < 12 years of age).
Any intermittent or daily supplementation with iron includes the provision of iron alone, iron plus folic acid or iron plus other vitamins and minerals.
We have included studies that examined interventions where iron supplementation was combined with co‐interventions such as deworming, education or other approaches only if the co‐interventions were the same in both the intervention and comparison groups.
We excluded studies examining tube feeding, parenteral nutrition or supplementary food‐based interventions such as mass fortification of staple or complementary foods, home fortification with micronutrient powders, lipid‐based supplements or Foodlets tablets, or biofortification.
Types of outcome measures
Primary outcomes
Anaemia (haemoglobin below a cut‐off defined by trialists, taking into account the age and altitude)*
Haemoglobin (g/L)*
Iron deficiency (as measured by trialists by using indicators of iron status, such as ferritin or transferrin)*
Iron status (ferritin in μg/L)*
Iron deficiency anaemia (defined by the presence of anaemia plus iron deficiency, diagnosed with an indicator of iron status selected by trialists)*
All‐cause mortality (number of deaths during the trial)*
* Outcomes that were included in the 'Summary of Findings' tables.
Secondary outcomes
All‐cause morbidity (number of children with at least one reported illness during the trial)
Acute respiratory infection (as measured by trialists)
Diarrhoea (as measured by trialists)
Any other adverse side effects (as measured by trialists, such as stained teeth, headache, stomach ache, discomfort, constipation)
Adherence (percentage of children who consumed more than 70% of the expected doses)
Folate status (as measured by trialists)
Mental development and motor skill development (children 0 to 59 months) (as assessed by trialists, including Bayley Mental Development Index (MDI), Bayley Psychomotor Development Index (PDI), Stanford‐Binet Test, DENVER II Developmental Screening Test)
School performance (children 60 months and older) (as measured by trialists)
Physical capacity (children 60 months and older) (as measured by trialists)
Height‐for‐age Z‐scores and weight‐for‐age Z‐scores
We planned to group the outcome time points as follows: immediately after the end of the intervention, one to six months after the end of intervention, and seven to 12 months after the end of the intervention. However, we limited our analyses to the end of the intervention as only two trials reported on continued follow‐up after the end of the intervention. We have described this in Characteristics of included studies and plan to extract this information in future updates, if available.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 2), part of The Cochrane Library (searched 24 May 2011); MEDLINE,1948 to May week 2, 2011 (searched 24 May 2011); EMBASE, 1980 to 2011 Week 20 (searched 24 May 2011); CINAHL, 1937 to current (searched 24 May 2011); ICTRP (searched 24 May 2011); POPLINE (searched 24 May 2011); SCIELO (searched 29 June 2011); LILACS (searched 29 June 2011); IBECS (searched 29 June 2011); IMBIOMED (searched 29 June 2011).
The search strategies are in Appendix 1.
We did not apply any language restrictions. For those articles written in a language other than English, we extracted the information or commissioned their translation into English.
Searching other resources
For assistance in identifying ongoing or unpublished studies, we contacted authors and known experts to identify any additional or unpublished data. We also contacted the Departments of Nutrition for Health and Development and regional offices of the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), the nutrition section of the United Nations Children's Fund (UNICEF), the World Food Programme (WFP), the Micronutrient Initiative (MI) and Sight and Life Foundation (3 July 2011).
We searched the International Clinical Trials Registry Platform (ICTRP) (searched 24 May 2011) for any ongoing or planned trials.
Data collection and analysis
Selection of studies
LMD screened all titles and abstracts for potential eligibility, while MEJ, TD and AS each assessed one‐third of the abstracts. LMD contacted relevant institutions and searched for ongoing trials. All the authors independently assessed half of the full‐text articles for inclusion according to the above mentioned criteria; each paper was therefore assessed by two review authors. We resolved any disagreement through discussion.
If studies were published only as abstracts, or the study reports contained little information on methods, we contacted the authors to obtain further details of study design and results.
Data extraction and management
For eligible studies, two authors independently extracted data using a form designed for this review. LMD extracted data from all the studies and the remaining authors each extracted a third. LMD entered data into the Review Manager 5 software (RevMan 2011). The same review author who extracted one‐third of the data in duplicate carried out checks for accuracy. We resolved any discrepancies through discussion and documented each stage of the process.
We completed the data collection form electronically and recorded information as follows.
(1) Trial methods
Study design
Unit and method of allocation
Unit of analysis
Masking of participants and outcome assessors
Exclusion of participants after randomisation and proportion of losses at follow‐up
Study power
(2) Participants
Location of the study
Sample size
Age
Sex
Socioeconomic status (as defined by trialists and where such information was available)
Baseline status of anaemia
Inclusion and exclusion criteria as described in the Criteria for considering studies for this review
(3) Intervention
Dose
Type of iron compound
Supplementation regimen
Duration of the intervention
Co‐intervention
(4) Comparison group
Type of comparison (no intervention, placebo or daily supplementation with the same nutrients)
(5) Outcomes
Primary and secondary outcomes outlined in the Types of outcome measures section
We recorded both prespecified and non‐prespecified outcomes, although we did not use the latter to underpin the conclusions of the review.
When information regarding any of the studies was unclear, we contacted authors of the original reports to provide further details. If there was insufficient information for us to be able to assess risk of bias, studies were put into the awaiting assessment section of the review until further information is published or made available to us.
Assessment of risk of bias in included studies
One author (LMD) assessed the risk of bias for all the included studies and the remaining authors each assessed one‐third of the studies so that all the trials were assessed by two authors independently, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
We reported this assessment in the 'Description of studies' and risk of bias tables. We explicitly mention when authors provided input on their trials.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence.
We assessed the method as:
low risk of bias (any truly random process, for example, random number table; computer random number generator);
high risk of bias (any non‐random process, for example, odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence and have assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (for example, telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear risk of bias.
(3) Blinding (checking for possible performance and detection bias)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. For interventions involving the provision of iron supplements it may be possible to blind children, clinical staff and outcome assessors to group allocation by providing placebo preparations.
We assessed blinding separately for different classes of outcomes and have noted where there has been an attempt at partial blinding.
We assessed the risk of performance bias associated with blinding as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
We assessed the risk of detection bias associated with blinding as:
low, high or unclear risk of bias for outcome assessors.
Whilst assessed separately, we combined the results into a single evaluation of risk of bias associated with blinding (Higgins 2011).
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion, where reported, and whether missing data were balanced across groups. We assessed methods as:
low risk of bias (less than 20% of cases lost to follow‐up and balanced in numbers across intervention groups);
high risk of bias (20% or more cases lost to follow‐up or outcome data imbalanced in numbers across intervention groups);
unclear risk of bias .
(5) Selective reporting bias
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We have described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
high risk of other bias;
low risk of other bias;
unclear risk of other bias.
(7) Overall risk of bias
We summarised the risk of bias at two levels: within studies (across domains) and across studies.
For the first, we made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. Attrition, lack of blinding and losses to follow‐up may be particular problems in studies looking at different regimens of iron supplementation and where children are followed up over time. We explored the impact of the level of bias by undertaking sensitivity analyses, see Sensitivity analysis below.
For the assessment across studies, the main findings of the review are set out in 'Table 1 and Table 2 (SoF) prepared using GRADE profiler software (GRADEpro 2008). The primary outcomes for each comparison have been listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes. For each individual outcome, the quality of the evidence has been assessed independently by two review authors using the GRADE approach (Balshem 2010), which involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias; this results in one out of four levels of quality (high, moderate, low or very low). This assessment was limited only to the trials included in this review and as we did not consider there was a serious risk of indirectness or publication bias we did not downgrade in these domains.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as average risk ratios (RR) with 95% confidence intervals (CI).
Continuous data
We present the results as mean difference (MD) with 95% confidence intervals at the end of the intervention. If trials did not provide this information but reported the mean change, we included these data as suggested by Higgins 2011. There was no need to use the standardised mean difference to combine trials as these outcomes were measured with the same methods.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials in the analyses along with individually‐randomised trials. Cluster‐randomised trials are labelled with a (C). We obtained the intra‐cluster correlation coefficient (ICC) from Hall 2002 (C) (ICC 0.0698; average cluster size (ACS): 18.55; design effect (DE) 2.22), Desai 2004 (C) (ICC 0.069; ACS: 1.5; DE 1.035) and Roschnik 2004 (C) (ICC 0.1123; ACS: 33.82; DE 4.35). We calculated the ACS from the reports and imputed the ICC from Roschnik 2004 (C) to Roschnik 2003 (C) as the study designs were very similar (ACS: 29); and from Hall 2002 (C) to Liu 1995 (C) (ACS: 27.3), Sinisterra 1997 (C) (ACS: 199.5), Yang 2004 (C) (ACS: 32), Sen 2009 (C) (ACS: 60) and Arcanjo 2011 (C) (ACS: 17.7) and then calculated each trial's effective sample size. In the case of Yang 2004 (C), the number of classes was not clear so we assumed an average cluster size of 32 based on other reports (Okebe 2011). On the other hand, Awasthi 2005 (C) reported that the sample size was calculated including a design effect of 2.0. We used this value to calculate its effective sample size and also to conduct a sensitivity analysis to examine the potential effect of clustering on the CIs of the summary estimates. As the CIs did not change significantly (5% or more), we do not report the results of the sensitivity analysis. Desai 2004 (C) and Engstrom 2008 (C) were not adjusted as the trial authors reported that the analyses accounted for the effect of clustering.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi‐arm studies), we included the directly relevant arms only. When we identified studies with various relevant arms, we combined the groups into a single pair‐wise comparison (Higgins 2011) and included the disaggregated data in the corresponding subgroup category. When the control group was shared by two or more study arms, we divided the control group (events and total population) over the number of relevant subgroup categories to avoid double counting the participants. The details are described in the Characteristics of included studies tables.
Cross‐over trials
We did not include cross‐over trials.
Dealing with missing data
For included studies, we have noted levels of attrition in the Characteristics of included studies tables. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by carrying out sensitivity analysis (these same trials were assessed as being at high risk of bias, see Sensitivity analysis below).
We carried out analyses, as far as possible, on an intention‐to‐treat basis (ITT), that is, by attempting to include all participants randomised to each group in the analyses. If this was not possible, we performed an available case analysis in which data were analysed for every participant for whom the outcome was obtained.
Assessment of heterogeneity
We visually examined the forest plots from meta‐analyses to look for any obvious heterogeneity among studies in terms of the size or direction of treatment effect. We used the I2 statistic, Tau2 and Chi2 test to quantify the level of heterogeneity among the trials in each analysis. If we identified moderate or substantial heterogeneity, we explored it by prespecified Subgroup analysis and investigation of heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We generated funnel plots (estimated differences in treatment effects against their standard error) only for haemoglobin in comparisons one and two, and ferritin in comparison two, as sufficient studies contributed data to these outcomes. Asymmetry could be due to publication bias but it can also be due to a real relationship between trial size and effect size, such as when larger trials have lower adherence and adherence is positively related to effect size.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2011). In this review we prespecified that we would use random‐effects model analyses in view of anticipated heterogeneity in the interventions, populations and methods used in different trials.
Subgroup analysis and investigation of heterogeneity
Where data were available, we carried out the following subgroup analysis:
by dose of elemental iron per week in the intermittent group: 25 mg or less; greater than 25 mg to 75 mg; greater than 75 mg;
by duration of the supplementation: 0 to three months or less; more than three months;
by type of compound: ferrous sulphate; ferrous fumarate; other;
by anaemia status at baseline (haemoglobin < 110 g/L or < 115 g/L for children 6 to 59 months or 5 to 11 years old, respectively, adjusted by altitude where appropriate): anaemic; non‐anaemic; mixed or not reported;
by intermittent supplementation regimen: one supplement a week; other intermittent regimen;
by sex: males; females; mixed or not reported; and
by micronutrient composition: iron alone; iron + folic acid; iron + other micronutrient; iron + multiple micronutrients.
We used the primary outcomes in subgroup analysis.
Pragmatically, we decided not to conduct subgroup analyses for those outcomes with three trials or fewer. We examined differences between subgroups by visual inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals suggesting a statistically significant difference in treatment effect between the subgroups. We also used the Borenstein 2008 approach to formally investigate the differences between two or more subgroups. Analyses were conducted in Revman version 5.1.1 (RevMan 2011).
Sensitivity analysis
We carried out sensitivity analysis to examine the effects of removing studies at high risk of bias (studies with poor or unclear randomisation and allocation concealment, and either blinding or high or imbalanced losses to follow‐up) from the analysis. We also examined the effect of different intra‐cluster correlation coefficients imputed to cluster‐randomised trials on the summary estimates of primary outcomes.
Results
Description of studies
Results of the search
The search strategy identified 7784 references for possible inclusion, 2453 of which were duplicate references. We assessed 81 published articles in full text, three unpublished reports, one review that contained published and unpublished data, and one abstract that has not been published in full. Nine studies were published in languages other than English: Chinese (Yang 2004 (C)), Farsi (Kargarnovin 2010), French (Nguyen 2002) and Spanish (Sinisterra 1997 (C); Rivera 1998; Sotelo‐Cruz 2002; Evangelista‐Salazar 2004; UNICEF 2006; Avila‐Jimenez 2011). Figure 2 depicts the process for assessing and selecting the studies. We included 33 trials (42 references); excluded 40 (41 references); three trials are awaiting assessment (Husseini 1999; Reid 2001; Kargarnovin 2010), and we identified one ongoing study (Zeeba Zaka‐ur‐Rab 2010).
2.
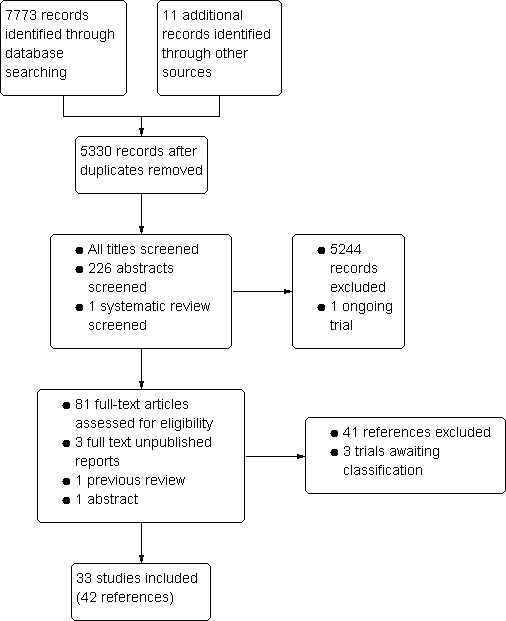
Study flow diagram.
Included studies
We included 33 trials with 13,114 children; those studies which included more than two intervention arms may have been included in more than one comparison. All included trials contributed data to the review but some studies randomised participants to intervention arms that were not relevant to the comparisons we assessed. For these studies we did not include data from all groups in the analyses. We have indicated in the Characteristics of included studies tables if any randomised arms were not included.
Three of the trials had two arms providing different regimens of intermittent supplementation (Liu 1995 (C); Faqih 2006; Sen 2009 (C)). In these cases we combined the study arms for the overall comparison and included the disaggregated information in the subgroup analyses. Levels of supervision varied among trials but most of them were unsupervised. In addition, very few studies addressed the use of co‐interventions such as health education to improve adherence or deworming prior to supplementation.
The sample size ranged between 60 and 1785 participants but overall tended to be small: 75% of the studies included fewer than 500 children. However, for cluster‐randomised trials the analyses only included the estimated effective sample size, after adjusting the data to account for the clustering effect.
Settings
The studies included in the review were carried out over the last 16 years in low‐ and middle‐income countries in Asia, Africa and Latin America: Bangladesh (Baqui 2003), Bolivia (Berger 1997; Aguayo 2000), Brazil (Da Silva 2008; Engstrom 2008 (C); Arcanjo 2011 (C)), China (Liu 1995 (C); Yang 2004 (C)), India (Awasthi 2005 (C); Sen 2009 (C)), Indonesia (Schultink 1995; Palupi 1997; Soemantri 1997), Iran (Khademloo 2009), Jordan (Faqih 2006), Kenya (Olsen 2000; Verhoef 2002; Desai 2004 (C)), Malawi (Young 2001; Roschnik 2003 (C)), Mali (Hall 2002 (C)), Mexico (Evangelista‐Salazar 2004), Pakistan (Siddiqui 2004), Panama (Sinisterra 1997 (C)), Phillipines (Roschnik 2004 (C)), South Africa (Taylor 2001), Tanzania (Ekvall 2000), Thailand (Sungthong 2002), Turkey (Ermis 2002; Tavil 2003; Yurdakok 2004) and Vietnam (Thu 1999; Nguyen 2002).
Participants
Participant ages ranged from newborn to 19 years old. While we did not include studies specifically recruiting postmenarchal females, as these are the subject of a separate review (Fernández‐Gaxiola 2011), three included studies recruited adolescents and separate data were not available for younger children (Olsen 2000; Taylor 2001; Hall 2002 (C)). Based on the age range reported in these studies, at least half of their participants fulfilled our inclusion criteria and thus we decided to retain them in the review. If the disaggregated data by age is made available to us, we will include it in future updates of the review.
In the analyses (comparisons three to six), we have set out our findings separately for studies recruiting children in these younger and older age groups. Fifteen studies included children from birth to 59 months of age only (Schultink 1995; Palupi 1997; Thu 1999; Ekvall 2000; Young 2001; Ermis 2002; Nguyen 2002; Verhoef 2002; Baqui 2003; Tavil 2003; Evangelista‐Salazar 2004; Desai 2004 (C); Yurdakok 2004; Engstrom 2008 (C); Khademloo 2009) and 11 trials included only older children 60 months of age and older (Sinisterra 1997 (C); Soemantri 1997; Aguayo 2000; Taylor 2001; Sungthong 2002; Roschnik 2003 (C); Roschnik 2004 (C); Siddiqui 2004; Da Silva 2008; Sen 2009 (C); Arcanjo 2011 (C)). Seven studies included children in both age categories (Liu 1995 (C); Berger 1997; Olsen 2000; Hall 2002 (C); Yang 2004 (C); Awasthi 2005 (C); Faqih 2006). In those cases we took into account the reported average age in allocating the trial. For example, Faqih 2006 recruited children aged two to six years of age and was included in comparisons two and four (younger children), while Olsen 2000 assessed children aged four to 19 years and was included in comparisons one and five (older children).
On average, 49% of the participants were females, with a range from 37% (Tavil 2003) to 100% (Sen 2009 (C)). Seven trials included only anaemic children (Schultink 1995; Berger 1997; Verhoef 2002; Tavil 2003; Desai 2004 (C); Faqih 2006; Siddiqui 2004); three only non‐anaemic (Aguayo 2000; Yang 2004 (C); Yurdakok 2004); and the rest of the trials had a baseline prevalence of anaemia ranging between 15% and 90%.
Participants socioeconomic status was not explicit in most of the studies although references to underprivileged populations were frequent.
Intermittent regimens, dose and type of iron compounds
Nine trials included arms where children were supplemented with iron twice a week (Liu 1995 (C); Schultink 1995; Olsen 2000; Verhoef 2002; Tavil 2003; Desai 2004 (C); Awasthi 2005 (C); Faqih 2006; Sen 2009 (C)) and in two studies children were provided with iron every other day (three times a week) (Ekvall 2000; Ermis 2002). The rest of the studies provided iron supplements once weekly.
The total weekly iron dose given to the children ranged from 7.5 to 200 mg of elemental iron per week. Evangelista‐Salazar 2004 provided 7.5 mg; Nguyen 2002 gave 15 mg; two trials provided 20 mg elemental iron (Thu 1999; Baqui 2003); in two trials children received a total weekly dose of 25 mg elemental iron (Da Silva 2008; Engstrom 2008 (C)); three trials gave 30 mg (Palupi 1997; Ekvall 2000; Yang 2004 (C)); one trial (Awasthi 2005 (C)) supplemented participants with 40 mg per week and another trial with 50 mg of iron per week (Arcanjo 2011 (C)). In five trials children received 60 mg of elemental iron per week (Schultink 1995; Sinisterra 1997 (C); Young 2001; Sungthong 2002; Siddiqui 2004); in three trials children received in total a weekly dose of 65 mg (Taylor 2001; Hall 2002 (C); Roschnik 2003 (C)); in one study the dose was 108 mg (Roschnik 2004 (C)); in another study the dose was 120 mg (Olsen 2000); and in Sen 2009 (C) the total weekly dose was 200 mg of elemental iron.
Some studies reported the provision of 1 mg to 8 mg of elemental iron per kg per day (Liu 1995 (C); Berger 1997; Soemantri 1997; Aguayo 2000; Ermis 2002; Verhoef 2002; Tavil 2003; Desai 2004 (C); Yurdakok 2004; Faqih 2006). In these cases we calculated the weekly dose by using the median or average age reported in the trial and the corresponding weight according to the WHO growth charts, percentile 50.
In almost all the studies, ferrous sulphate was the source of supplemental iron. Other iron compounds tested were ferrous polymaltose (Olsen 2000); ferrous dextran (Sen 2009 (C)) and ferrous fumarate (Taylor 2001; Verhoef 2002).
Most of the studies supplemented only with iron; one study gave iron in combination with 30 mg of vitamin C (Evangelista‐Salazar 2004) and five studies gave iron in combination with folic acid. In these trials the weekly dose of folic acid also varied: one trial gave 100 µg (0.1 mg) of folic acid per week (Taylor 2001; Awasthi 2005 (C)), in two the dose was 250 µg (0.25 mg) (Hall 2002 (C); Roschnik 2003 (C)), while in Sen 2009 (C) the dose was 500 µg (0.5 mg) folic acid per week . Four studies provided supplements containing multiple micronutrients (Thu 1999; Young 2001; Baqui 2003; Yang 2004 (C)).
Excluded studies
We excluded 40 trials (41 references) from the review. In 12 trials the evaluated population was out of the scope of this review (Beasley 2000; Kianfar 2000; Sharma 2000; Zavaleta 2000; Ahmed 2001; Februhartanty 2002; Shah 2002; Agarwal 2003; Shobha 2003; Jaleel 2004; Soekarjo 2004; Leenstra 2009). The second main reason for exclusion was that trials were not randomised (Rivera 1998; Jayatissa 1999; Perrin 2002; Sotelo‐Cruz 2002; Jackson 2003; Kapur 2003; Kanal 2005; Lima 2006; UNICEF 2006; Vir 2008; Mwanakasale 2009; Azeredo 2010). We excluded eight trials because the supplements were provided as Foodlets a (a crushable tablet that may be mixed with foods) and this intervention is outside the scope of this review (Briars 2003; Hop 2005; López de Romaña 2005; Smuts 2005; Lechtig 2006; López de Romaña 2006; Wijaya‐Erhardt 2007; Schümann 2009). Six trials were excluded because intermittent supplementation regimens were not compared with daily regimens or no treatment or placebo (Menendez 1997; Tee 1999; Tomashek 2001; Ahmed 2005; Risonar 2008; Avila‐Jimenez 2011). We excluded Hafeez 1998 because the intermittent supplements were given on consecutive days and Lin 2001 because the nutrient tested was vitamin A. We have described these studies in the Characteristics of excluded studies tables.
Risk of bias in included studies
Overall, study methods were not well described in many of the included studies and this meant that assessing risk of bias was difficult (seeFigure 3 and Figure 4). We attempted to contact the study authors for further clarifications and noted in the Characteristics of included studies when the information was provided by the authors.
3.
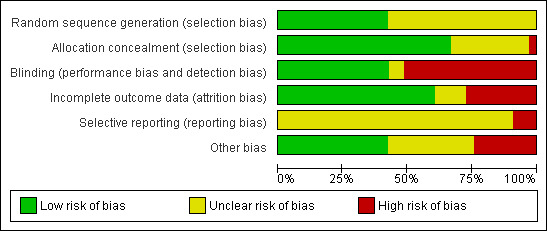
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
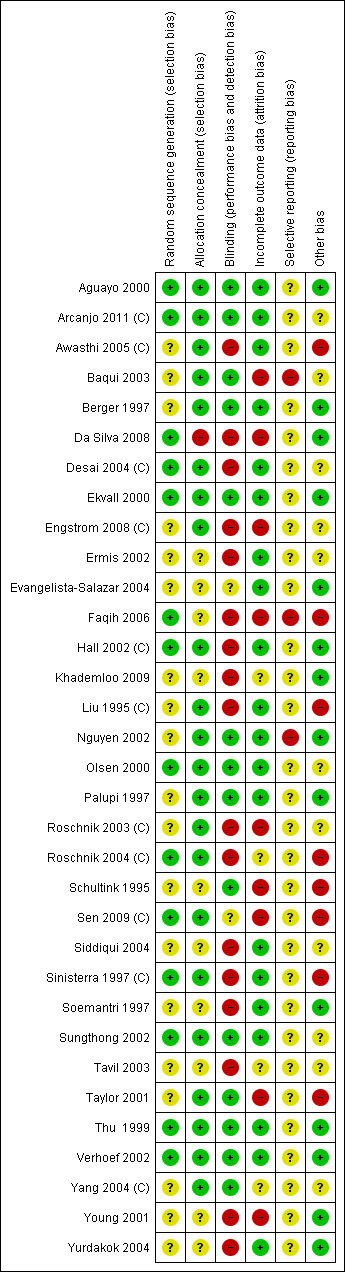
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Where we assessed methods of randomisation or allocation concealment as being at high risk of bias (or unclear), and trials were either not blinded or had high or imbalanced attrition rates, we assumed that they were at high risk of bias in the sensitivity analysis looking at the impact of study quality. Using these criteria, nine studies were assessed as being at low risk of bias (Thu 1999; Aguayo 2000; Ekvall 2000; Olsen 2000; Hall 2002 (C); Sungthong 2002; Verhoef 2002; Desai 2004 (C); Arcanjo 2011 (C)). The remaining studies were either assessed as being at high risk of bias or the methods were unclear.
Allocation
In 20 of the included trials, it was unclear how the randomisation sequence had been generated. In six studies investigators used random number tables (Thu 1999; Aguayo 2000; Verhoef 2002; Roschnik 2004 (C); Faqih 2006; Arcanjo 2011 (C)); in a further six studies computer‐generated randomisation sequences were used (Ekvall 2000; Olsen 2000; Hall 2002 (C); Sungthong 2002; Desai 2004 (C); Da Silva 2008), and in two studies the groups were assigned to the treatments by drawing lots (Sinisterra 1997 (C); Sen 2009 (C)).
Eleven of the included studies used methods of concealing group allocation that we judged were low risk of bias, for example, by providing coded supplements to treatment and control groups that appeared similar to participants and to those carrying out randomisation (Berger 1997; Palupi 1997; Thu 1999; Aguayo 2000; Ekvall 2000; Olsen 2000; Taylor 2001; Nguyen 2002; Sungthong 2002; Verhoef 2002; Baqui 2003). In the remaining trials, methods were either not described or were unclear.
Eleven trials were randomised at cluster level (Liu 1995 (C); Sinisterra 1997 (C); Hall 2002 (C); Roschnik 2003 (C); Desai 2004 (C); Roschnik 2004 (C); Yang 2004 (C); Awasthi 2005 (C); Engstrom 2008 (C); Sen 2009 (C); Arcanjo 2011 (C)) and in these cases it was judged that selection bias at individual level was unlikely (low risk of bias).
Blinding
In 14 trials, we considered that there was low risk of bias related to blinding (Schultink 1995; Berger 1997; Palupi 1997; Thu 1999; Aguayo 2000; Ekvall 2000; Nguyen 2002; Olsen 2000; Taylor 2001; Verhoef 2002; Sungthong 2002; Baqui 2003; Yang 2004 (C); Arcanjo 2011 (C)). In the remaining trials, blinding was either not attempted or not mentioned.
Incomplete outcome data
While we assessed that the majority of the included trials (20 out of 33) had acceptable levels of attrition (with loss to follow‐up and missing data being less than 20% and balanced across groups), in the remaining trials the levels of attrition were high or not balanced across groups. In these studies high levels of attrition were likely to represent an important source of bias and thus results are difficult to interpret; this is the case particularly if we consider that reasons for attrition may have been related to outcomes (for example, when children with side effects or those who developed anaemia were excluded from the analysis). In one trial (Baqui 2003) the dropout rate was considerably higher in one of the intervention groups (those receiving multi‐micronutrients lost 41% compared to a loss of 8% to 19% in other groups) and there were further missing data for some outcomes. High levels of loss to follow‐up also occurred in the studies by Engstrom 2008 (C) (20.2% attrition); Faqih 2006 (53% attrition); Young 2001 (60% attrition); Schultink 1995 (75% attrition); Sen 2009 (C) (68% missing data for some outcomes); Roschnik 2003 (C) (41.2% attrition), and Taylor 2001 (36% attrition). In one study (Da Silva 2008), 16% of participants were lost to follow up and loss was not balanced across groups; the reasons given by the authors included children developing anaemia or side effects, with no clarity about the number of children lost in each group for these reasons. In four trials losses to follow‐up were not clear as the denominators were not provided (Tavil 2003; Roschnik 2004 (C); Yang 2004 (C); Khademloo 2009).
Selective reporting
We were not able to fully assess outcome reporting bias as we only had access to published study reports. We assessed publication bias using funnel plots only for haemoglobin (in comparisons one and two) and for ferritin (comparison two), as more than 10 trials contributed data to those outcomes. We did not find clear asymmetry that may suggest publication bias (graphs not shown). In the analyses we have ordered studies by weight so that the effect of small studies is more apparent; we have drawn attention to any results where visual inspection of the forest plot seems to suggest a more pronounced treatment effect in small as compared with larger studies.
Other potential sources of bias
In a study (Awasthi 2005 (C)) some children received supervised intake of the supplement; it was not clear whether this varied depending on intervention group.
There was some baseline imbalance on outcomes or other potential confounders in terms of participant characteristics in some studies (Schultink 1995; Sinisterra 1997 (C); Taylor 2001; Siddiqui 2004; Faqih 2006; Arcanjo 2011 (C)).
A potentially important source of bias was the impact of unit of randomisation; several of the included trials did not randomise at the individual level but used classes, schools or clinics as clusters for randomisation. The impact of the cluster‐design effect was not clearly taken into account in most of the cluster‐randomised trials (Liu 1995 (C); Sinisterra 1997 (C); Hall 2002 (C); Roschnik 2003 (C); Roschnik 2004 (C); Yang 2004 (C); Awasthi 2005 (C); Engstrom 2008 (C); Arcanjo 2011 (C)). In the Engstrom 2008 (C) trial, regression analysis was carried out to try to identify possible confounding factors but unit of analysis did not appear to be part of this analysis. We were able to obtain the ICCs for three trials (Desai 2004 (C); Roschnik 2003 (C) and Hall 2002 (C)) and we imputed the last two values to other trials to obtain their effective sample size. The summary estimates obtained from cluster trials did not differ significantly from those obtained from studies randomised at an individual level.
There are three trials awaiting assessment (Husseini 1999; Reid 2001; Kargarnovin 2010). Based on the sample size of Kargarnovin 2010 and the findings reported in the abstract, we do not consider that its temporary exclusion from the analysis will bias the results of this review. Similarly, we did not consider that the omission of the data from Reid 2001 was likely to introduce serious bias due to the small sample size. On the other hand, the effect of excluding Husseini 1999 is uncertain as the only information available is published in Beaton 1999 who obtained it by personal communication. At the end of the intervention haemoglobin concentrations were higher and anaemia prevalence was lower among those children receiving daily supplements in comparison to those children receiving intermittent supplements. As we do not have access to the primary information, it is difficult to assess the quality of the study and to adjust data by the effect of clustering, which limits any assessment of its impact on our summary estimate.
Effects of interventions
We have included data from 33 trials; overall, these trials involved 13,114 children. This figure represents the number of children recruited to studies, in some studies we have not included data for all arms of the trials in the review comparisons. The analyses include only the estimated effective sample size, after adjusting the data to account for the clustering effect.
We have organised the summary of results by comparing supplementation regimens and by primary and secondary outcomes. Most of the included studies focused on haematological outcomes and few reported on any of the other outcomes pre‐specified in the review protocol. See the Data and analyses section for detailed results on primary and secondary outcomes.
Comparison 1. Intermittent iron supplementation versus no supplementation or placebo (19 trials)
Nineteen trials evaluated this comparison (Berger 1997; Palupi 1997; Thu 1999; Aguayo 2000; Ekvall 2000; Olsen 2000; Taylor 2001; Ermis 2002; Hall 2002 (C); Verhoef 2002; Sungthong 2002; Baqui 2003; Roschnik 2003 (C); Evangelista‐Salazar 2004; Roschnik 2004 (C); Yurdakok 2004; Yang 2004 (C); Sen 2009 (C); Arcanjo 2011 (C)). Seven of the trials met the prespecified criteria mentioned above for being at lower risk of bias (Thu 1999; Aguayo 2000; Ekvall 2000; Olsen 2000; Hall 2002 (C); Verhoef 2002; Sungthong 2002). In sensitivity analyses these trials were retained in the analysis whilst trials at higher risk of bias were temporarily removed to examine whether this had any impact on the overall pattern of results.
Primary outcomes
Anaemia
Ten trials with 1824 children provided data on anaemia following the interventions (Berger 1997; Palupi 1997; Thu 1999; Aguayo 2000; Hall 2002 (C); Verhoef 2002; Roschnik 2003 (C); Evangelista‐Salazar 2004; Roschnik 2004 (C); Arcanjo 2011 (C)). Those receiving intermittent iron supplementation were significantly less likely to have anaemia at follow‐up compared with children receiving no intervention (average risk ratio (RR) 0.51, 95% confidence interval (CI) 0.37 to 0.72) (Analysis 1.1). There was variation among trials in terms of the size of the treatment effect (T2 = 0.18, I2 = 81% and Chi2 test for heterogeneity P < 0.00001). The large effect remained significant even after excluding the trials at higher risk of bias (RR 0.60; 95% CI 0.42 to 0.87).
1.1. Analysis.
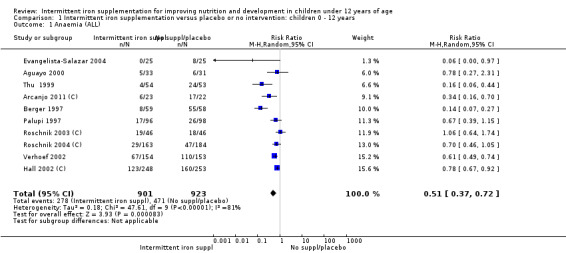
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 1 Anaemia (ALL).
Haemoglobin concentrations (g/L)
Nineteen studies with 3032 participants provided data on mean haemoglobin levels following the intervention (Berger 1997; Palupi 1997; Thu 1999; Aguayo 2000; Ekvall 2000; Olsen 2000; Taylor 2001; Ermis 2002; Hall 2002 (C); Verhoef 2002; Sungthong 2002; Baqui 2003; Roschnik 2003 (C); Evangelista‐Salazar 2004; Roschnik 2004 (C); Yang 2004 (C); Yurdakok 2004; Sen 2009 (C); Arcanjo 2011 (C)). Those receiving intermittent iron supplements on average had higher haemoglobin (Hb) levels than those receiving no intervention or a placebo; the difference was statistically significant (mean difference (MD) 5.20, 95% CI 2.51 to 7.88) (Analysis 1.9). There were high levels of heterogeneity among trials (T2 = 32.45, I2 = 93% and Chi2 test for heterogeneity P < 0.00001). The effect remained significant after removing the trials at high risk of bias (RR 5.02, 95% CI 2.01 to 8.03).
1.9. Analysis.
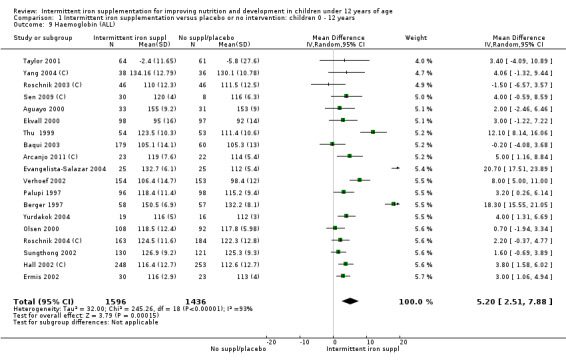
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 9 Haemoglobin (ALL).
Iron deficiency
Three trials with 431 children (Verhoef 2002; Evangelista‐Salazar 2004; Yang 2004 (C)) reported on this outcome. Findings suggested that children receiving intermittent supplements were at lower risk of having iron deficiency at the end of the intervention as those receiving nothing or a placebo (RR 0.24, 95% CI 0.06 to 0.91) (Analysis 1.17). There were high levels of heterogeneity among trials (T2 = 1.01, I2 = 88% and Chi2 test for heterogeneity P < 0.0003).
1.17. Analysis.
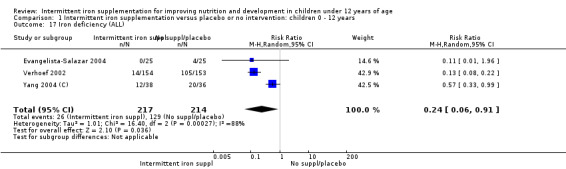
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 17 Iron deficiency (ALL).
Iron status measured by ferritin (μg/L)
Five trials with follow‐up data for 550 participants (Ermis 2002; Sungthong 2002; Baqui 2003; Yang 2004 (C); Yurdakok 2004) reported higher mean levels of ferritin among those receiving intermittent supplements compared with those receiving no treatment (MD 14.17, 95% CI 3.53 to 24.81) (Analysis 1.18). Only one trial (Sungthong 2002) was assessed as being at lower risk of bias.
1.18. Analysis.
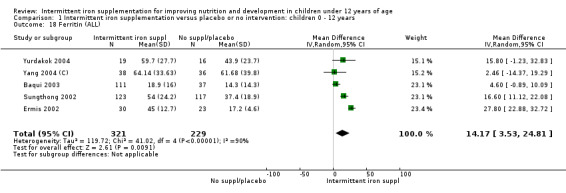
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 18 Ferritin (ALL).
Iron deficiency anaemia
No trials reported on this outcome.
All‐cause mortality
No trials reported on mortality.
Secondary outcomes
All‐cause morbidity
Information on all‐cause morbidity was reported in one trial (Palupi 1997), with data for 194 children. There was no evidence of differences between groups (Analysis 1.26).
1.26. Analysis.
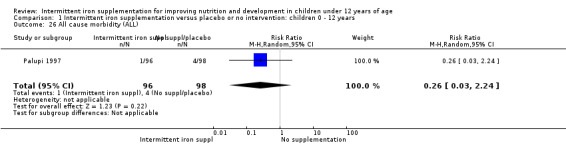
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 26 All cause morbidity (ALL).
Acute respiratory infection
No trials reported on this outcome.
Diarrhoea
No trials provided information on diarrhoea.
Any other adverse effects
One trial (Ermis 2002) reported no statistically significant difference in the total number of side effects reported by those children receiving supplements intermittently and those receiving no intervention or a placebo (Analysis 1.27). One trial (Aguayo 2000) reported on nausea and did not find differences between groups (Analysis 1.28).
1.27. Analysis.
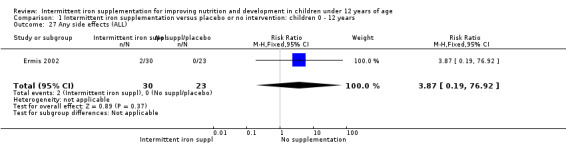
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 27 Any side effects (ALL).
1.28. Analysis.
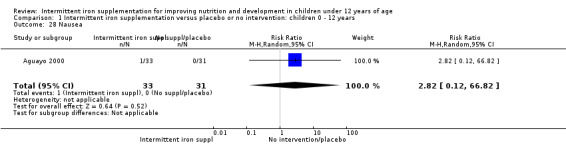
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 28 Nausea.
Adherence
Baqui 2003 and Ekvall 2000 reported that children receiving intermittent iron supplements had similar levels of adherence to intermittent iron supplementation as those children receiving a placebo or no intervention (RR 1.04, 95% CI 0.98 to 1.09) (Analysis 1.29).
1.29. Analysis.
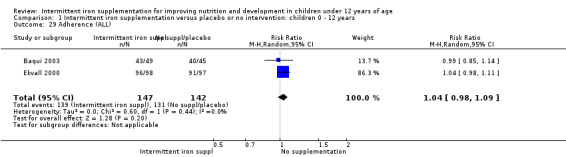
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 29 Adherence (ALL).
Folate status (as measured by trialists)
No trials reported on this outcome.
Mental development and motor skill development
Baqui 2003 reported on several measures of cognitive and physical development. There was no clear evidence of difference between groups for most of these outcomes (Analysis 1.30; Analysis 1.31; Analysis 1.32; Analysis 1.34).
1.30. Analysis.
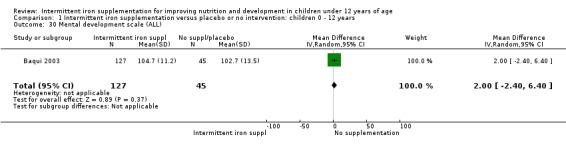
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 30 Mental development scale (ALL).
1.31. Analysis.
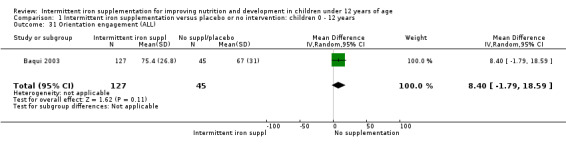
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 31 Orientation engagement (ALL).
1.32. Analysis.
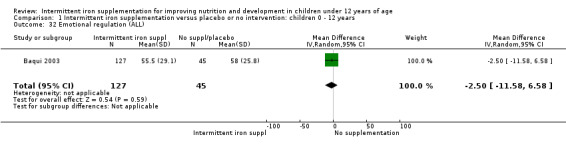
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 32 Emotional regulation (ALL).
1.34. Analysis.
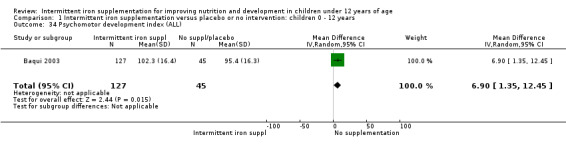
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 34 Psychomotor development index (ALL).
School performance
One study (Sungthong 2002) examined intelligence quotient (IQ), language development and mathematics performance; there were no clear differences between those receiving intermittent iron and those on no supplementation (Analysis 1.35; Analysis 1.36; Analysis 1.37).
1.35. Analysis.
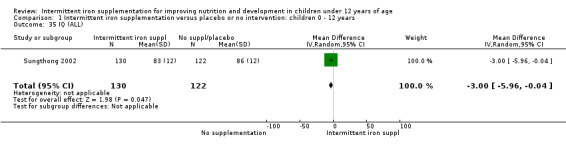
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 35 IQ (ALL).
1.36. Analysis.
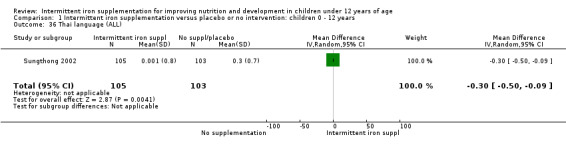
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 36 Thai language (ALL).
1.37. Analysis.
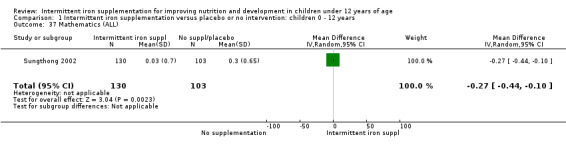
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 37 Mathematics (ALL).
Physical capacity
One trial examined (Baqui 2003) the motor quality of children, which included seven items such as motor control and tone, and expressed the results in percentile scores. Authors found that children receiving intermittent supplementation had higher percentile scores although the clinical significance of this difference was not clear (MD 15.60, 95% CI 7.66 to 23.54) (Analysis 1.33).
1.33. Analysis.
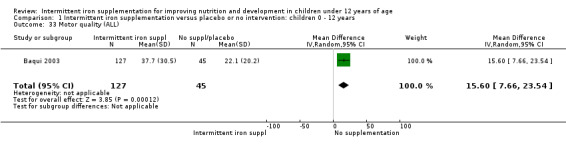
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 33 Motor quality (ALL).
Height‐for‐age and weight‐for‐age Z‐scores
Three trials (Palupi 1997; Thu 1999; Aguayo 2000) reported results for weight‐for‐age and height‐for‐age Z‐scores for school‐aged children and did not find a statistically significant effect on these outcomes (Analysis 1.38; Analysis 1.39).
1.38. Analysis.
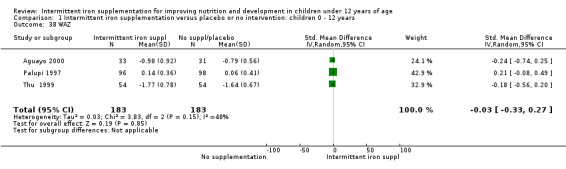
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 38 WAZ.
1.39. Analysis.
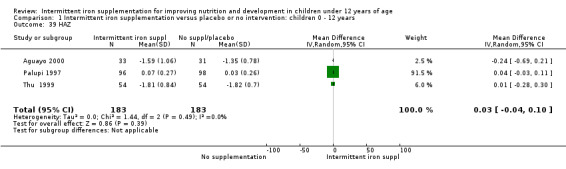
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 39 HAZ.
Comparison 2. Intermittent iron supplementation versus daily iron supplementation (21 trials)
Twenty‐one trials evaluated this comparison (Liu 1995 (C);,Schultink 1995; Berger 1997; Sinisterra 1997 (C); Soemantri 1997; Thu 1999; Young 2001; Ermis 2002; Nguyen 2002; Sungthong 2002, Tavil 2003; Desai 2004 (C); Siddiqui 2004; Yang 2004 (C); Yurdakok 2004; Awasthi 2005 (C); Faqih 2006; Da Silva 2008; Engstrom 2008 (C); Khademloo 2009; Sen 2009 (C)) and all of them contributed data to the analysis. Three of these trials were assessed as being at lower risk of bias and, where they contributed data, they were retained in the analysis when we conducted sensitivity analyses (Thu 1999; Sungthong 2002; Desai 2004 (C)).
Primary outcomes
Anaemia
Six trials with 980 participants provided data on the number of children with anaemia following the interventions (Schultink 1995; Berger 1997; Sinisterra 1997 (C); Thu 1999; Awasthi 2005 (C); Engstrom 2008 (C)). Children receiving intermittent iron supplementation had a higher risk of being anaemic at the end of the study period compared to those receiving daily iron supplementation (RR 1.23, 95% CI 1.04 to 1.47) (Analysis 2.1). Only one trial was considered at low risk of bias (Thu 1999) and found similar results (RR 1.31, 95% CI 0.31 to 5.57).
2.1. Analysis.
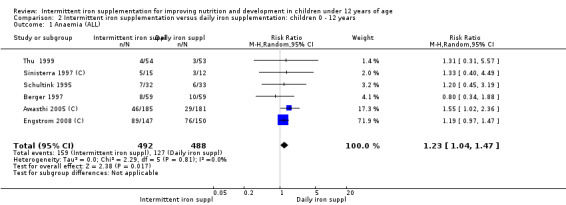
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 1 Anaemia (ALL).
Haemoglobin concentrations (g/L)
Nineteen trials with 2851 participants provided data on mean haemoglobin levels following the intervention (Liu 1995 (C); Schultink 1995; Berger 1997; Soemantri 1997; Thu 1999; Young 2001; Ermis 2002; Nguyen 2002; Sungthong 2002; Tavil 2003; Desai 2004 (C); Siddiqui 2004; Yang 2004 (C); Yurdakok 2004; Awasthi 2005 (C); Faqih 2006; Engstrom 2008 (C); Khademloo 2009; Sen 2009 (C)). The groups receiving intermittent iron supplements on average had 0.60 less grams of haemoglobin per litre than those receiving daily supplementation but the difference between groups was not statistically significant (95% CI ‐1.54 to 0.35) (Analysis 2.9). There were high levels of heterogeneity for this outcome (T2 = 2.26, I2 = 56%, and Chi2 test for heterogeneity P = 0.001). When only those trials at lower risk of bias (Sungthong 2002; Desai 2004 (C)) were retained in the analysis, the difference between groups remained statistically non‐significant (MD ‐0.87, 95% CI ‐2.77 to 1.02) (data for sensitivity analysis not shown).
2.9. Analysis.
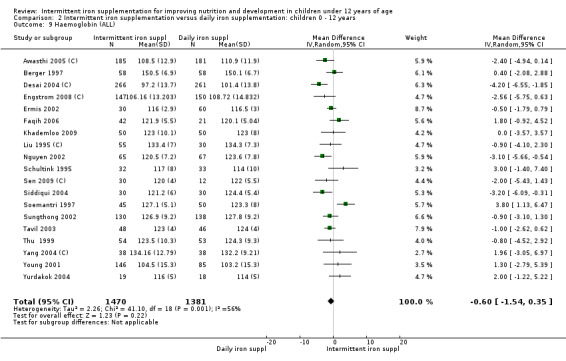
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 9 Haemoglobin (ALL).
Iron deficiency
Only one trial (Yang 2004 (C)) reported on iron deficiency and found that at the end of the intervention the number of children with iron deficiency was higher among those who received iron supplements intermittently compared to daily (RR 4.00, 95% CI 1.23 to 13.05) (Analysis 2.17).
2.17. Analysis.
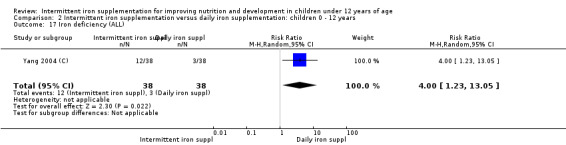
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 17 Iron deficiency (ALL).
Iron status measured by ferritin (ng/L)
Ten trials with data for 902 participants (Liu 1995 (C); Schultink 1995; Ermis 2002; Sungthong 2002; Tavil 2003; Siddiqui 2004; Yang 2004 (C); Yurdakok 2004; Faqih 2006; Khademloo 2009) reported that ferritin values were not statistically different between those receiving iron intermittently and those receiving daily iron (MD ‐4.19, 95% CI ‐9.42 to 1.05) (Analysis 2.18). Only one trial was at low risk of bias (Sungthong 2002) and found no differences between these two interventions. There was high heterogeneity for this outcome with considerable variation in mean values between trials; in addition, one of the studies reported exceptionally low standard errors for mean ferritin values (from which we calculated SDs) (Siddiqui 2004). We carried out a sensitivity analysis temporarily excluding this study from the meta‐analysis; removing this study did not change the interpretation of results (MD ‐ 5.20, 95% CI ‐10.76 to 0.35).
2.18. Analysis.
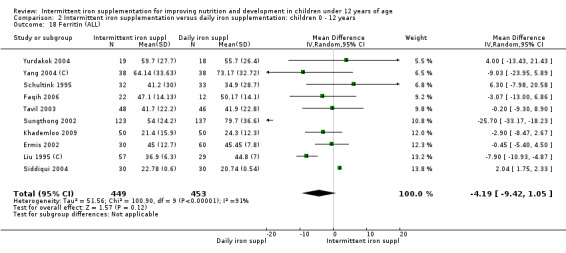
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 18 Ferritin (ALL).
Iron deficiency anaemia
No trials reported data on iron deficiency anaemia.
All‐cause mortality
No trials reported mortality by any cause.
Secondary outcomes
All‐cause morbidity
Information on all‐cause morbidity was reported in two trials (Desai 2004 (C); Da Silva 2008), with data for 601 children. There was no evidence of a difference between groups (RR 0.96, 95% CI 83 to 1.12) (Analysis 2.27).
2.27. Analysis.
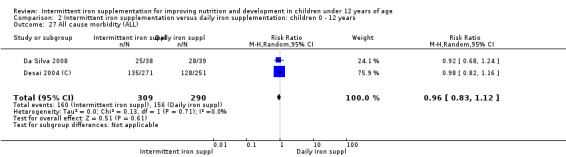
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 27 All cause morbidity (ALL).
Acute respiratory infection
No trials reported on this outcome.
Diarrhoea
Two trials (Yurdakok 2004; Da Silva 2008) had data on diarrhoea and did not find differences between groups (Analysis 2.28).
2.28. Analysis.
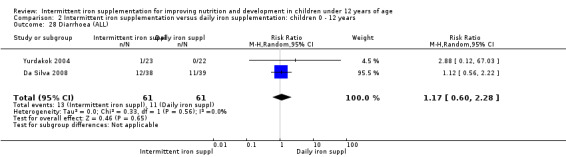
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 28 Diarrhoea (ALL).
Any other adverse effects
Four trials (Liu 1995 (C); Ermis 2002; Desai 2004 (C); Yurdakok 2004) reported side effects among 895 children. There was no evidence of differences between intermittent and daily iron supplementation (RR 0.60, 96% CI 0.19 to 1.87) (Analysis 2.29).
2.29. Analysis.
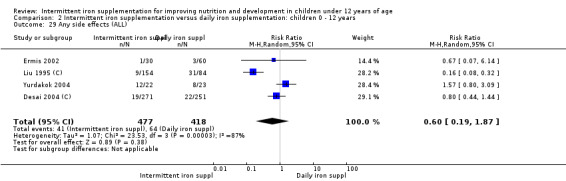
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 29 Any side effects (ALL).
Adherence
Five trials involving 1130 participants reported on this outcome (Berger 1997; Desai 2004 (C); Awasthi 2005 (C); Engstrom 2008 (C); Sen 2009 (C)). There was no statistically significant difference in adherence to the interventions between groups although it tended to be higher among those children receiving intermittent iron supplements (RR 1.23, 95% CI 0.98 to 1.54) (Analysis 2.30).
2.30. Analysis.
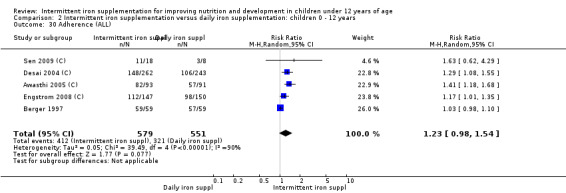
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 30 Adherence (ALL).
Folate status (as measured by trialists)
No trials reported on this outcome.
Mental development and motor skill development
No trials reported on this outcome.
School performance
One study (Sungthong 2002) examined IQ, Thai language development and mathematics performance; there were no clear differences between groups receiving intermittent iron versus no supplementation (Analysis 2.31; Analysis 2.32; Analysis 2.33).
2.31. Analysis.
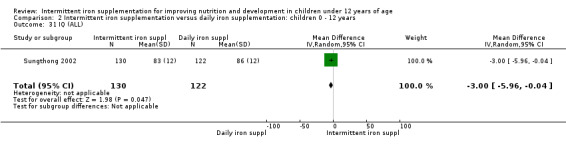
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 31 IQ (ALL).
2.32. Analysis.
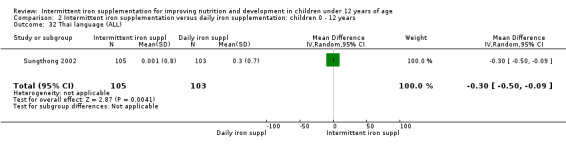
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 32 Thai language (ALL).
2.33. Analysis.
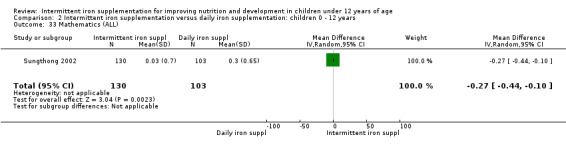
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 33 Mathematics (ALL).
Physical capacity
One trial that provided weekly and twice‐a‐week supplementation (Sen 2009 (C)) did not find statistically significant differences in the increment of steps climbed by children receiving either intermittent or daily supplementation (Analysis 2.26).
2.26. Analysis.
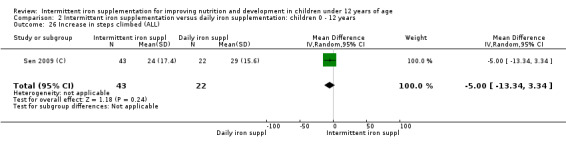
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 26 Increase in steps climbed (ALL).
Height‐for‐age and weight‐for‐age Z‐scores
Three trials reported results for height‐for‐age Z‐scores for school‐aged children and did not find an effect on this outcome (Analysis 2.34).
2.34. Analysis.
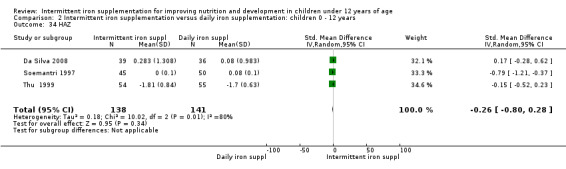
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 34 HAZ.
Subgroup comparisons
There was considerable variation among trials in terms of the populations examined and the way studies were conducted, which very likely resulted in the high statistical heterogeneity observed in some outcomes. For primary outcomes, we examined subgroups to look for possible differences between studies in terms of the duration of the intervention; children's anaemia status at baseline; higher and lower weekly doses of iron; type of iron compound provided; and supplementation regimen.
For most of the outcomes very few studies contributed data, so we limited the subgroup analysis to anaemia and haemoglobin and ferritin concentrations. In the analyses we have provided overall totals along with subtotals for subgroups, and the statistics for subgroup differences.
Intermittent iron dose per week (25 mg or less; greater than 25 mg to 75 mg; greater than 75 mg)
Most of the trials provided between 25 and 75 mg of iron per week. There was some within subgroup heterogeneity and no consistent and clear differences between subgroup categories (Analysis 1.10; Analysis 1.19; Analysis 2.2; Analysis 2.10; Analysis 2.19). It seemed that the effect of intermittent supplementation on anaemia was lost among those children receiving iron doses greater than 75 mg per week, although only two trials contributed to this subgroup (Analysis 1.2).
1.10. Analysis.
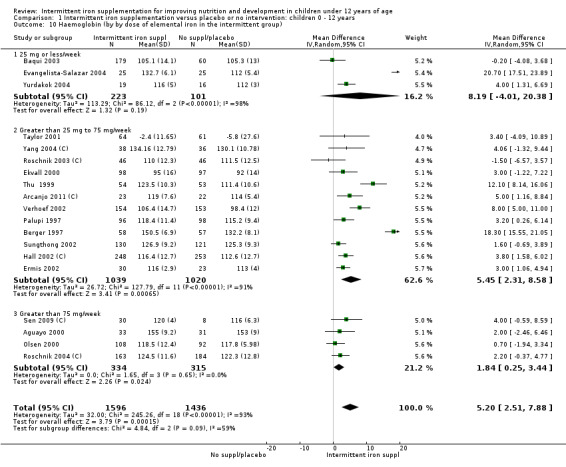
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 10 Haemoglobin (by by dose of elemental iron in the intermittent group).
1.19. Analysis.
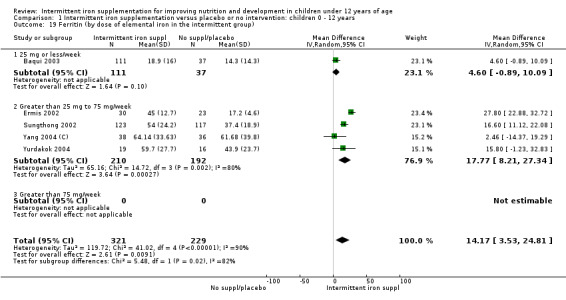
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 19 Ferritin (by dose of elemental iron in the intermittent group).
2.2. Analysis.
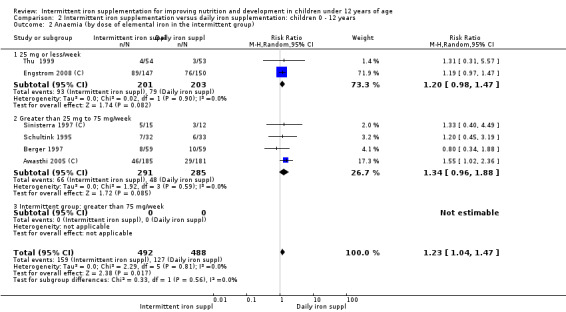
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 2 Anaemia (by dose of elemental iron in the intermittent group).
2.10. Analysis.
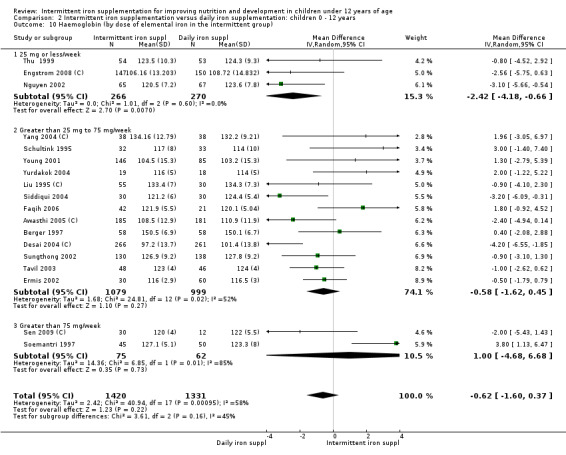
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 10 Haemoglobin (by dose of elemental iron in the intermittent group).
2.19. Analysis.
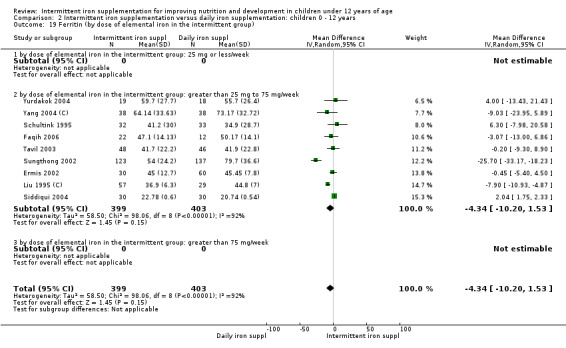
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 19 Ferritin (by dose of elemental iron in the intermittent group).
1.2. Analysis.
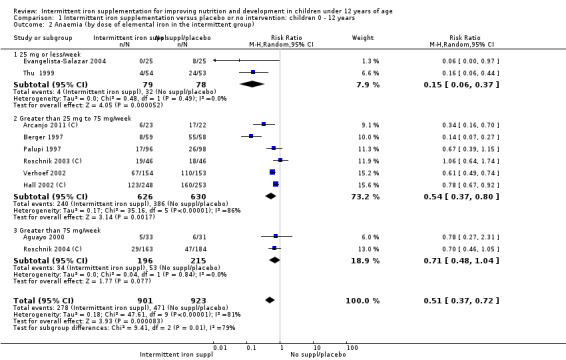
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 2 Anaemia (by dose of elemental iron in the intermittent group).
Duration of the intervention (0 to three months; more than three months)
An almost even number of trials provided iron supplements for three months or less, or for more than three months. There was no statistical evidence that the response of haematological outcomes to intermittent supplementation differed by duration of the intervention (Analysis 1.3; Analysis 1.11; Analysis 1.20; Analysis 2.3; Analysis 2.11; Analysis 2.20).
1.3. Analysis.
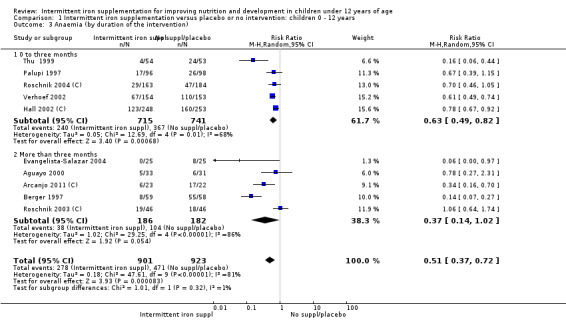
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 3 Anaemia (by duration of the intervention).
1.11. Analysis.
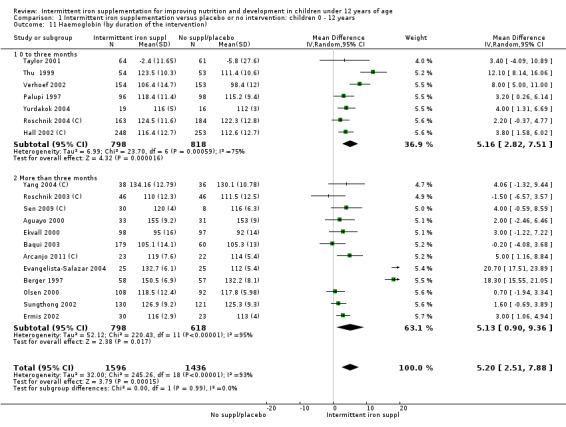
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 11 Haemoglobin (by duration of the intervention).
1.20. Analysis.
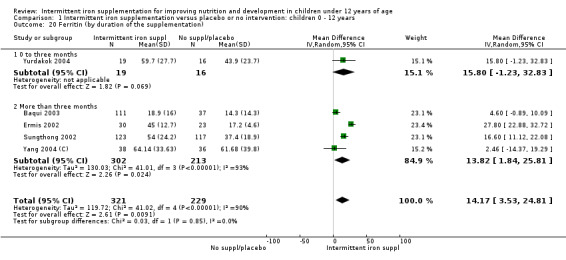
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 20 Ferritin (by duration of the supplementation).
2.3. Analysis.
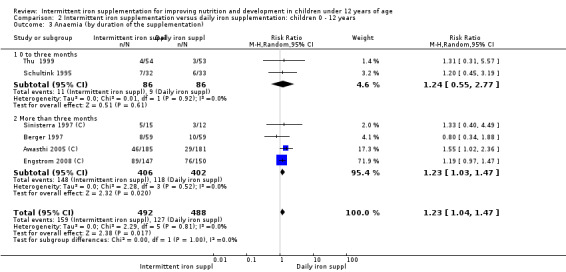
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 3 Anaemia (by duration of the supplementation).
2.11. Analysis.
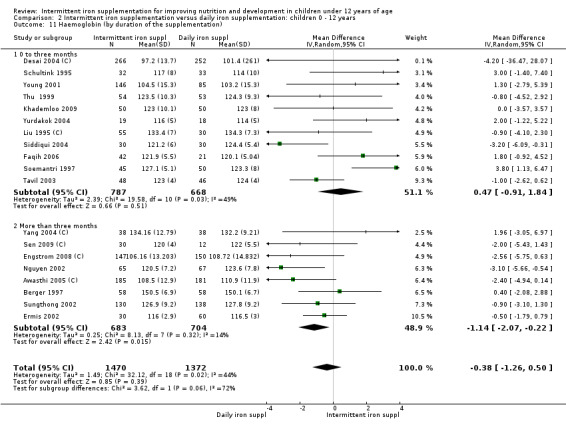
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 11 Haemoglobin (by duration of the supplementation).
2.20. Analysis.
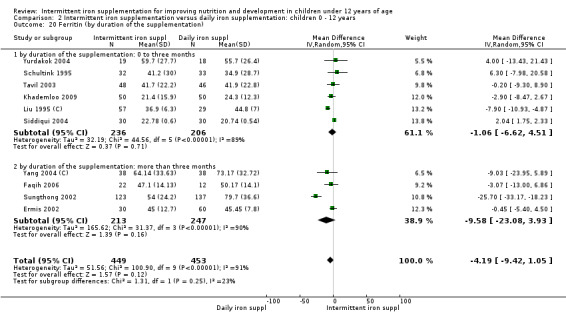
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 20 Ferritin (by duration of the supplementation).
Type of compound (ferrous sulphate; ferrous fumarate; other)
Most of the trials provided iron in the form of ferrous sulphate, but when other compounds were given there was no clear statistical evidence that they produced different results on haematological outcomes from those observed with ferrous sulphate (Analysis 1.4; Analysis 2.4; Analysis 2.12; Analysis 2.21). In one case haemoglobin responded better to supplementation with fumarate, but only one study contributed to this subgroup category and findings should be cautiously interpreted (Analysis 1.21).
1.4. Analysis.
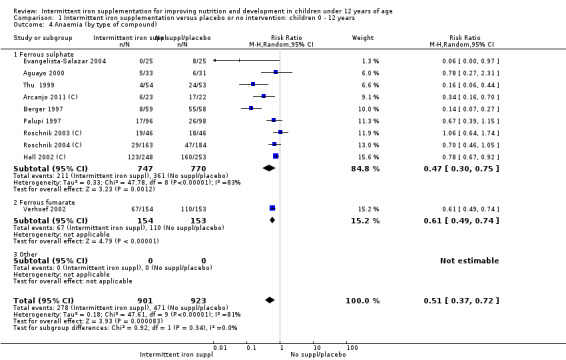
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 4 Anaemia (by type of compound).
2.4. Analysis.
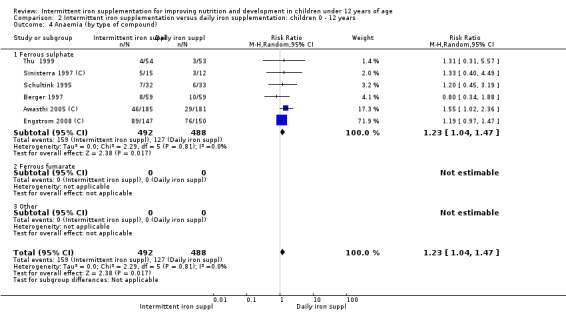
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 4 Anaemia (by type of compound).
2.12. Analysis.
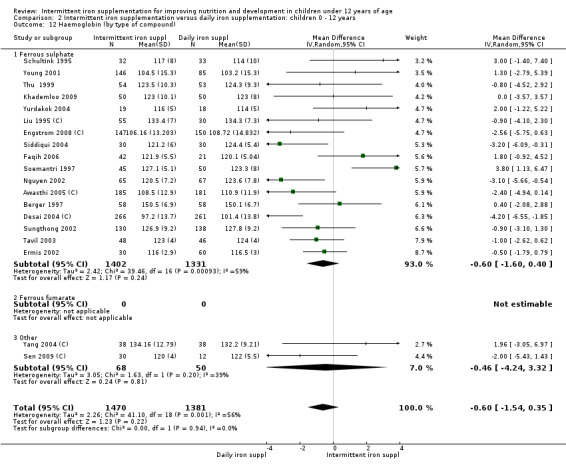
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 12 Haemoglobin (by type of compound).
2.21. Analysis.
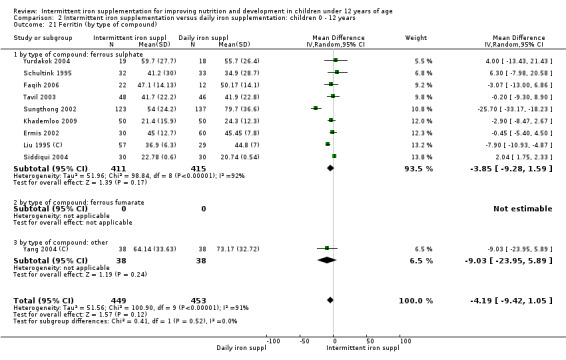
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 21 Ferritin (by type of compound).
1.21. Analysis.
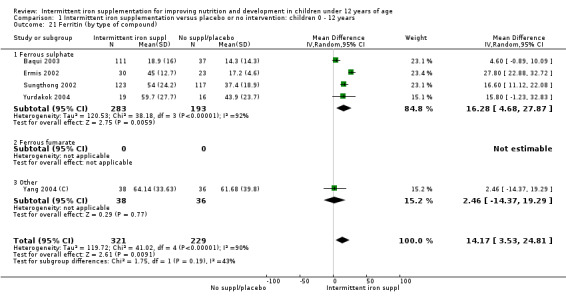
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 21 Ferritin (by type of compound).
Anaemia status at baseline (anaemic; non‐anaemic; mixed or not reported)
Intermittent supplementation appeared to be as efficacious in trials that included only anaemic children as in those studies that included populations with different degrees of anaemia (Analysis 1.5; Analysis 1.13; Analysis 1.22; Analysis 2.5; Analysis 2.13; Analysis 2.22). One study conducted in anaemic Bolivian children (Berger 1997) reported a very pronounced therapeutic effect on haematological outcomes and this trial contributed to the observed statistical heterogeneity; its results were consistent in terms of direction with the rest of the trials.
1.5. Analysis.
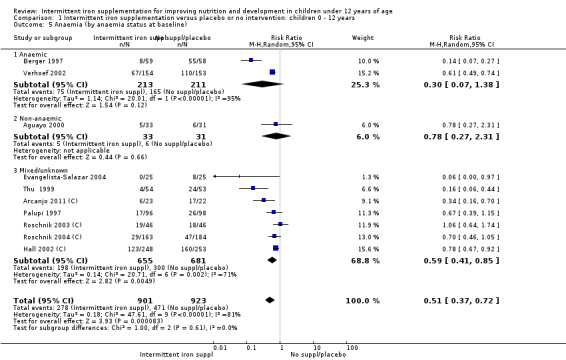
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 5 Anaemia (by anaemia status at baseline).
1.13. Analysis.
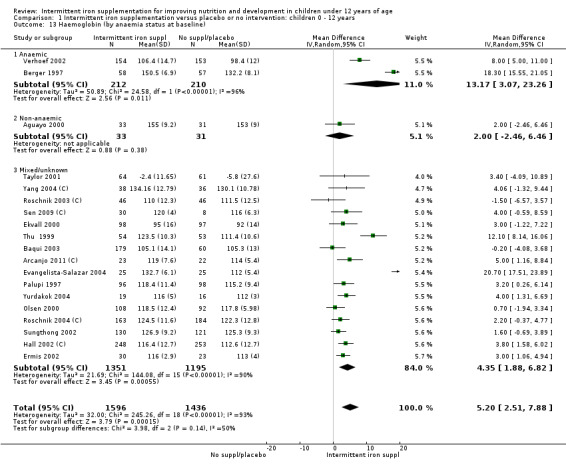
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 13 Haemoglobin (by anaemia status at baseline).
1.22. Analysis.
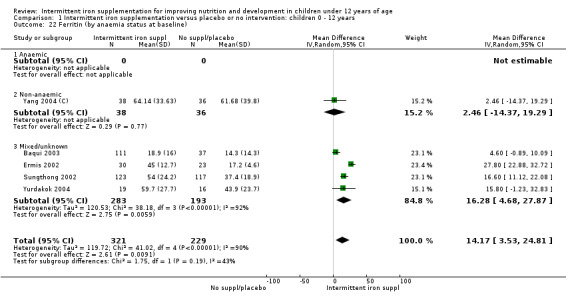
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 22 Ferritin (by anaemia status at baseline).
2.5. Analysis.
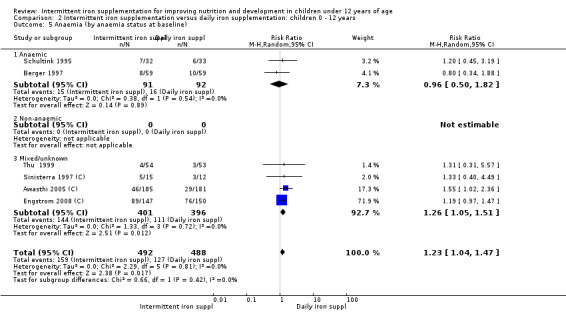
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 5 Anaemia (by anaemia status at baseline).
2.13. Analysis.
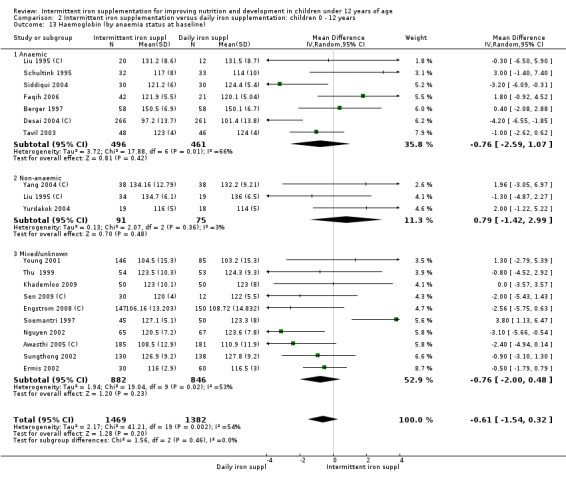
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 13 Haemoglobin (by anaemia status at baseline).
2.22. Analysis.
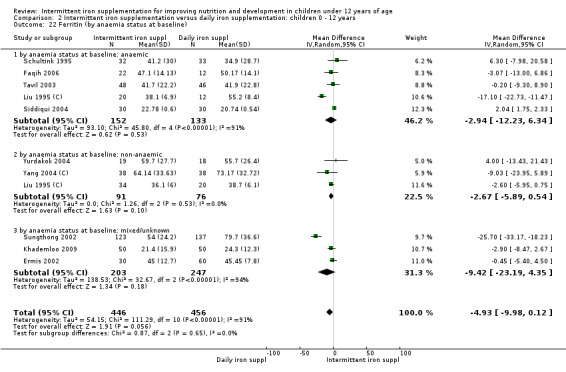
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 22 Ferritin (by anaemia status at baseline).
Intermittent regimen (one supplement a week; other intermittent regimen)
Most of the trials supplemented children on a weekly basis and in some cases only one study was included in each subgroup, which impeded the interpretation of the analyses (Analysis 1.6; Analysis 1.23). For the rest of the subgroup comparisons, there was no statistical evidence that the results of haematological outcomes differed when the supplements were given once, twice or three times a week (Analysis 1.14; Analysis 2.6; Analysis 2.14; Analysis 2.23).
1.6. Analysis.
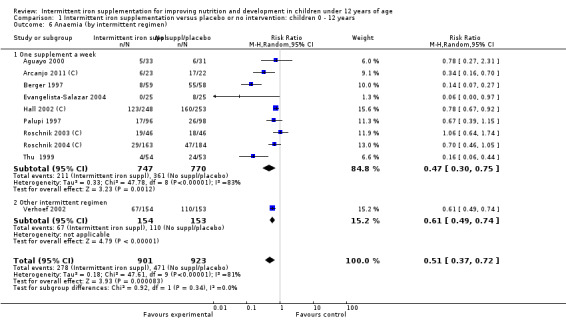
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 6 Anaemia (by intermittent regimen).
1.23. Analysis.
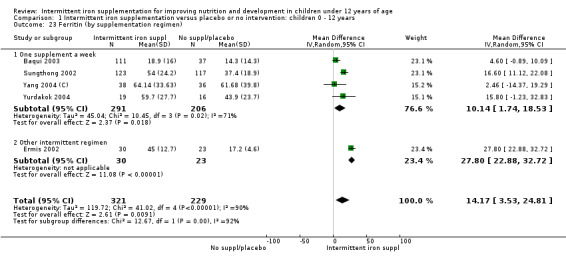
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 23 Ferritin (by supplementation regimen).
1.14. Analysis.
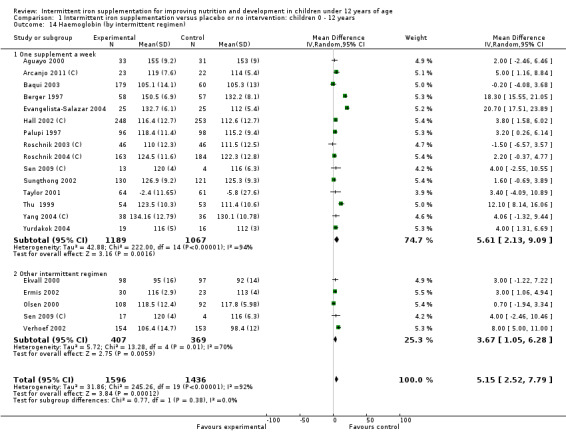
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 14 Haemoglobin (by intermittent regimen).
2.6. Analysis.
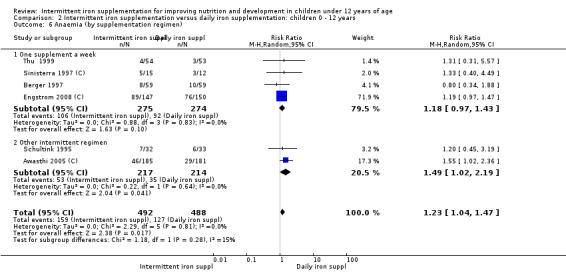
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 6 Anaemia (by supplementation regimen).
2.14. Analysis.
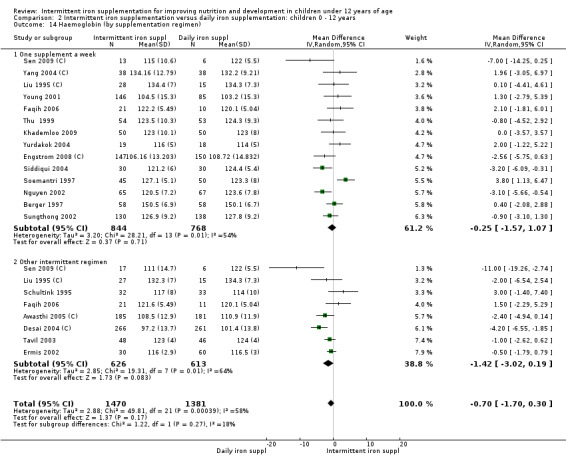
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 14 Haemoglobin (by supplementation regimen).
2.23. Analysis.
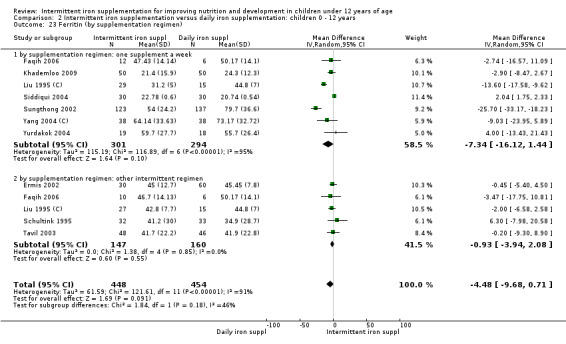
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 23 Ferritin (by supplementation regimen).
Sex (males; females; mixed or not reported)
All but one trial included males and females, although it was possible to extract the results by sex only from Hall 2002 (C). There was no statistical evidence that in this population the positive effect of intermittent supplementation on haematological outcomes differed by sex (Analysis 1.7; Analysis 1.15; Analysis 1.24; Analysis 2.7; Analysis 2.15; Analysis 2.24).
1.7. Analysis.
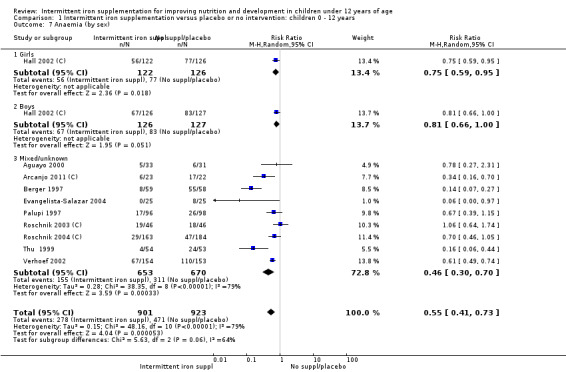
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 7 Anaemia (by sex).
1.15. Analysis.
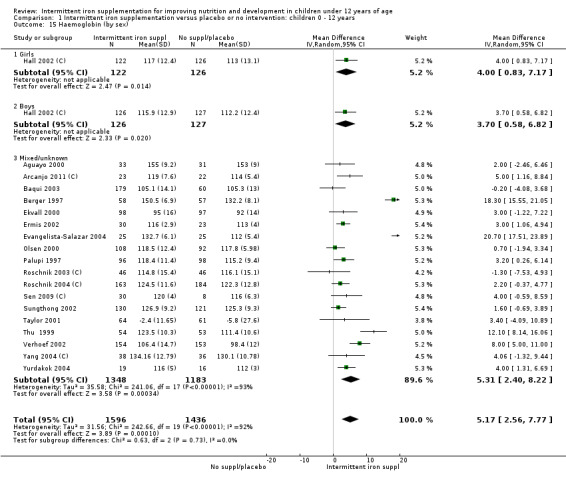
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 15 Haemoglobin (by sex).
1.24. Analysis.
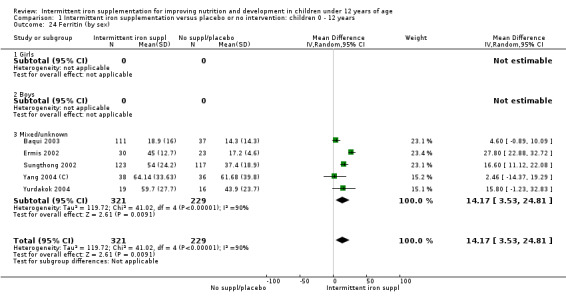
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 24 Ferritin (by sex).
2.7. Analysis.
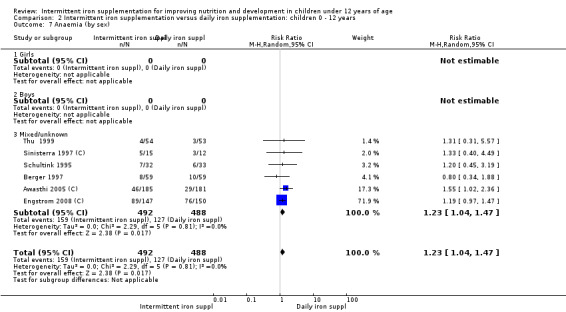
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 7 Anaemia (by sex).
2.15. Analysis.
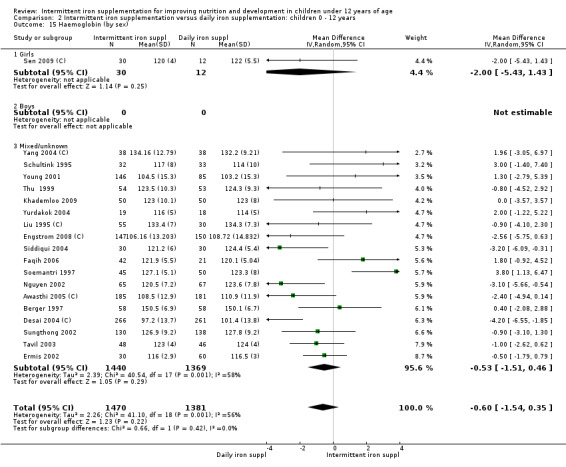
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 15 Haemoglobin (by sex).
2.24. Analysis.
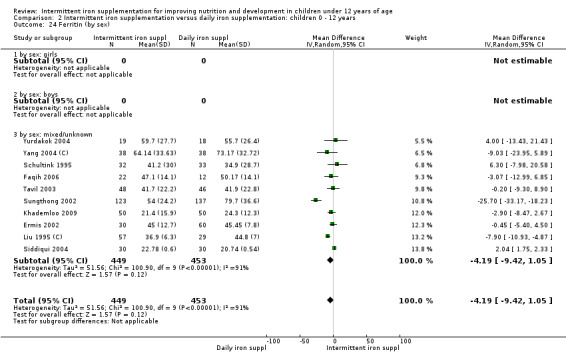
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 24 Ferritin (by sex).
Supplement's nutrient composition (iron alone; iron + folic acid; iron+other nutrient; iron + multiple micronutrients)
Most of the trials provided only iron. In the majority of the subgroup analyses there was no evidence that the provision of other nutrients in addition to iron altered the effects of intermittent supplementation on haematological outcomes (Analysis 1.16; Analysis 1.25; Analysis 2.8; Analysis 2.16; Analysis 2.25). However, it seemed that the effect of intermittent supplementation on anaemia was higher among those children receiving iron + vitamin C (Analysis 1.8), although this result should be interpreted cautiously as only one trial assessed the joint effect of these micronutrients.
1.16. Analysis.
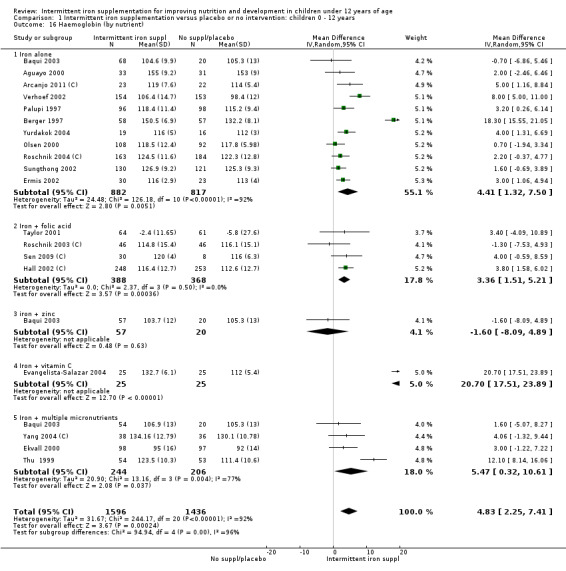
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 16 Haemoglobin (by nutrient).
1.25. Analysis.
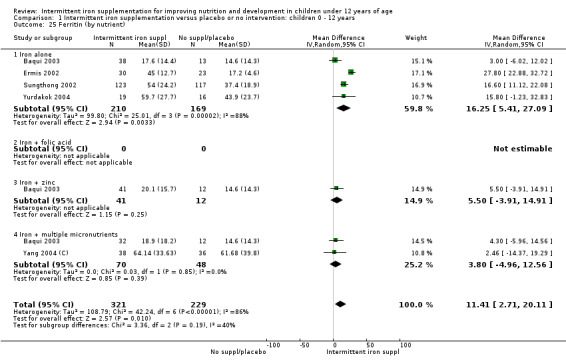
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 25 Ferritin (by nutrient).
2.8. Analysis.
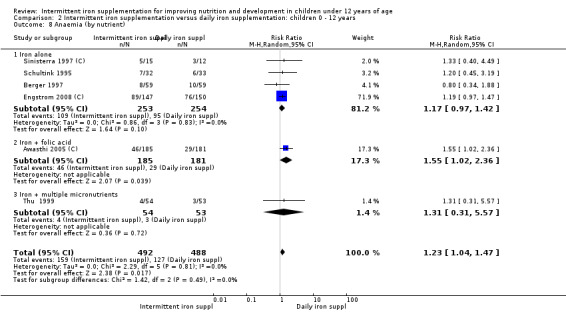
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 8 Anaemia (by nutrient).
2.16. Analysis.
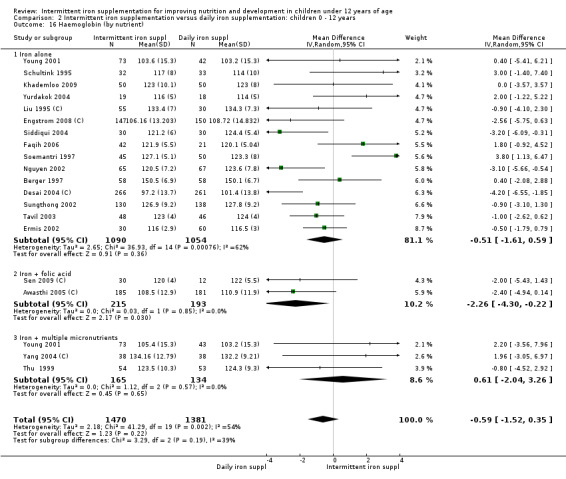
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 16 Haemoglobin (by nutrient).
2.25. Analysis.
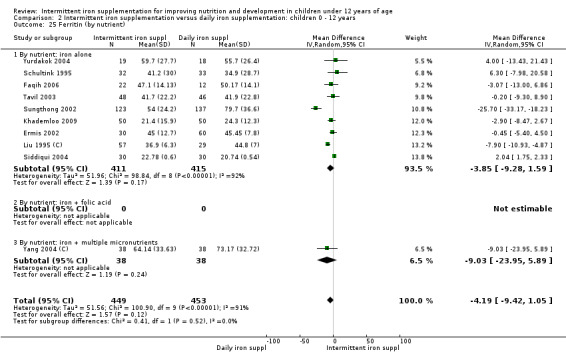
Comparison 2 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years, Outcome 25 Ferritin (by nutrient).
1.8. Analysis.
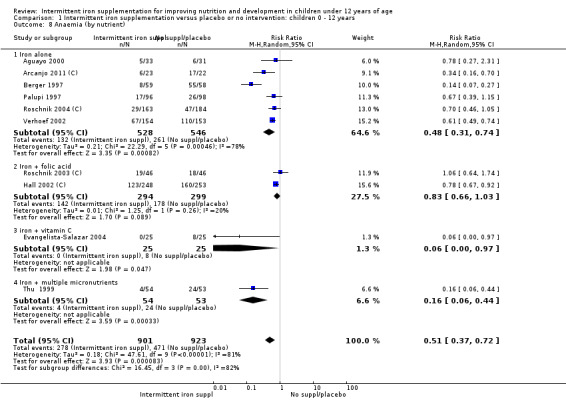
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 8 Anaemia (by nutrient).
Comparisons 3 to 6. Analysis by age group: children younger than 60 months versus 60 months and older
We have summarised the results of comparisons 3 to 6 in Table 3 and Table 4.
1. Intermittent iron supplementation versus placebo or no intervention by age group.
| Outcome |
Comparison 3 Children 0 to 59 months Relative effect (95% CI) Number of trials and effective sample size |
Comparison 5 Children 60 months and older Relative effect (95% CI Number of trials and effective sample size |
| Anaemia | RR 0.43 (0.23 to 0.80) 4 trials, 658 children |
RR 0.54, (0.33 to 0.90) 6 trials, 1166 children |
| Haemoglobin (g/L) | MD 6.45 (2.36 to 10.55) 9 trials, 1254 children |
MD 4.04 (0.30 to 7.78) 10 trials, 1778 children 4.04 [0.30, 7.78] |
| Iron deficiency (using ferritin concentrations) | RR 0.24 (0.06 to 0.91) 3 trials, 431 children |
None of the trials reported on this outcome |
| Ferritin (μg/L) | MD 13.15 (‐2.28 to 28.59) 4 trials, 310 children |
MD 16.60 (11.12 to 22.08) 1 trial, 240 children |
| Adherence | RR 1.04 (0.98 to 1.09) 2 trials, 289 children |
None of the trials reported on this outcome |
2. Intermittent versus daily iron supplementation by age group.
| Outcome |
Comparison 4 Children 0 to 59 months Relative effect (95% CI) Number of trials and effective sample size |
Comparison 6 Children 60 months and older Relative effect (95% CI) Number of trials and effective sample size |
| Anaemia | RR 1.26 (1.05 to 1.51) 3 trials, 770 children |
RR 0.95 (0.47 to 1.91) 2 trials, 145 children |
| Haemoglobin (g/L) | MD ‐0.75 (‐1.80 to 0.29) 14 trials, 2270 children |
MD ‐0.31 (‐2.59 to 1.97) 5 trials, 581 children |
| Iron deficiency | RR 4.00 (1.23 to 13.05) 1 trial, 76 children |
None of the trials reported on this outcome |
| Ferritin (μg/L) | MD ‐3.10 (‐6.59 to 0.39) 8 trials, 582 children |
MD ‐11.57 (‐38.75 to 15.61) 2 trials, 320 children |
| Adherence | RR 1.29 (1.15 to 1.45) 3 trials, 1185 children |
RR 1.29 (0.44 to 3.75) 2 trials, 245 children |
The visual examination of the confidence intervals suggests that the haematological effects produced by intermittent supplementation are similar between young (0 to 59 months) and older children (60 months and older), although the statistical power may be an issue in assessing the consistency among results.
Discussion
Summary of main results
Available data indicate that among children less than 12 years of age, intermittent supplementation with iron (alone or in combination with other nutrients) effectively increases haemoglobin and ferritin concentrations and reduces the prevalence of anaemia compared to placebo or no intervention. Overall, this positive response does not differ between once, biweekly or three times weekly supplementation; nor does it depend on child's sex or age or the duration of the intervention.
In comparison to daily iron supplementation, children receiving intermittent iron supplementation are more likely to develop anaemia but their haemoglobin and ferritin concentrations are similar.
Adherence tends to be higher in children receiving intermittent iron supplementation compared with those receiving daily iron supplements, although the results were not statistically significant.
Information on morbidity, mortality, adverse side effects, neurocognitive and motor outcomes is scarce and therefore no clear conclusions can be drawn.
Overall completeness and applicability of evidence
A total of 33 randomised trials were included in this review, with data for 13,114 children included in the analysis. Seventy‐five per cent of the included trials had a sample size of less than 500 children and the trials often lacked blinding and a clear description of randomisation methods. The trials were published in a wide variety of journals (and the level of quality of the journals might vary) and were mostly written in English. The diversity of publications may also reflect the range of settings in which studies were carried out: Latin America, Africa and Asia.
No studies were conducted in high‐income countries and it is uncertain whether the results would be similar in those settings. On the one hand, the prevalences of anaemia and iron deficiency are lower in high income countries and there is an inverse relationship between initial iron status and response to iron supplementation. On the other hand, intermittent supplementation for children in high income countries could, however, be successful because of potentially strong institutional infrastructure and high attendance rates at schools that could support sustained high coverage and use of this intervention.
We decided to include only randomised and quasi‐randomised trials in this review. Whilst randomisation reduces the risk of bias, this approach also limited the inclusion of large scale pre‐post trials with no comparison groups. Such studies are more likely to be affected by external circumstances, such as famines, and it is possible that the magnitude of the effect of intermittent iron supplementation might be different under programmatic conditions.
The baseline anaemia and iron deficiency status varied across studies; most were conducted in settings with a high prevalence of anaemia. The studies included in this review largely examined this intervention for prevention as a public health strategy and not treatment of anaemia and iron deficiency as part of clinical practice. However, seven of the 33 trials included only anaemic children and subgroup analysis suggested that weekly supplementation was efficacious compared with daily supplementation. The efficacy of the intermittent supplementation schemes on haematological outcomes also seemed similar across different age groups, with few inconsistencies.
There were insufficient studies to allow us to evaluate in detail all the outcomes of interest, and by subgroups. Particularly, there were insufficient trials and a lack of comparable measures to examine mortality, morbidity, cognitive and developmental outcomes.
In addition, there was a lack of data to meaningfully examine adherence and adverse effects specifically related to intensity and frequency of dosing. These last two are critical limitations considering that these are primary justifications for the use of weekly over daily supplementation.
Quality of the evidence
1. Quality of the evidence across within studies. Less than one third of the trials were assessed as having a low risk of bias after considering the methods for allocating the treatment, the blinding and the attrition rates, with many studies being at high risk of bias (see Risk of bias in included studies). In most of the included trials, the methods used to randomly assign participants and conceal allocation were not described. Blinding of participants, care providers and outcome assessors was not generally attempted, although in some studies technical staff carrying out laboratory investigations were reported to be unaware of group allocation. The lack of blinding may represent a potentially serious source of bias. Attrition was also a problem in many of these studies.
2. Quality of the evidence across studies. We used the GRADE methodology for this assessment and set out the results for primary outcomes in the Table 1 and the Table 2. We considered that indirectness or publication bias was unlikely but the quality of the trials and inconsistency (or the lack of studies) were potentially important factors in the overall assessment of the evidence. When intermittent supplementation was compared with a placebo or no intervention, the overall quality of the available evidence was found to be moderate for anaemia, whereas for haemoglobin and ferritin concentrations it was low and very low for iron deficiency. When compared with daily supplementation, the quality of the available evidence with regard to anaemia, haemoglobin and ferritin concentrations was found to be low and for iron deficiency it was very low.
Potential biases in the review process
There were a number of potential biases in the review process. We attempted to be as inclusive as possible in the search strategy and found publications in different languages in journals from all the continents, although the literature identified was predominantly written in English. We were also able to obtain unpublished information.
We attempted to minimise bias in several ways: two review authors independently assessed eligibility for inclusion and two review authors checked data extraction, assessments of risk of bias and data entry. However, carrying out reviews is not an exact science and may require a number of subjective judgements; it is possible that a different review team may have reached different decisions regarding assessments of eligibility and risk of bias. We would encourage readers to examine the Characteristics of included studies tables to assist in the interpretation of results.
In addition to the individual assessments of the study risk of bias, we included 'Summary of findings' tables to assess the overall quality of the evidence for primary outcomes. We attempted to produce the tables using a transparent process with two review authors independently assessing the evidence for each outcome for each quality domain and discussing any disagreements.
Agreements and disagreements with other studies or reviews
To our knowledge, only one meta‐analysis of randomised controlled trials has been conducted on the efficacy of intermittent iron supplementation in the control of iron deficiency anaemia (Beaton 1999). It includes the results of 22 trials completed before 1999 in different age groups. In some cases authors were able to obtain the full data sets but in the rest of the cases summary statistics were collected from abstracts, final reports or directly supplied by investigators. Of the included studies, four were carried out among preschool‐aged children (age range five months to five years), 10 among school‐aged children and adolescents (age range three years to 21 years) and eight among pregnant women. All of the preschool and school children or adolescent trials compared once or twice a week versus daily supplementation, and most included control groups. All the studies reported results for haemoglobin; two studies in preschool children and three in schoolchildren or adolescents also measured ferritin. All the studies that Beaton 1999 included involving preschool and school‐aged children were also included in this review.
The authors found that intermittent supplementation was efficacious compared to no treatment and that it increased haemoglobin and ferritin levels and reduced anaemia. In contrast to the present review, they found that daily supplementation was more efficacious than intermittent supplementation in improving haemoglobin and ferritin levels. The authors concluded that weekly supplementation should be considered for preschool and school‐aged children only in situations where there is strong assurance of supervision and high adherence.
The larger number of trials included in this Cochrane review, conducted in different settings and with different levels of supervision, suggest that intermittent supplementation is an efficacious public health intervention in children younger than 12 years of age that may be implemented in a various contexts. It may be a viable approach to consider, particularly where daily supplementation has failed, is operationally complex or unfeasible or in settings where it has not been implemented yet.
The results of the present review are only applicable to children 12 years and younger. However, other systematic review assessing the benefits and safety of this intervention in menstruating women (Fernández‐Gaxiola 2011) concur with our findings. From the programme implementation perspective, a recent narrative review reports that weekly iron and folic acid supplementation has been successfully implemented in Cambodia, Egypt, India, Laos, Philippines and Vietnam, reaching over half a million menstruating women (WHO 2009).
Authors' conclusions
Implications for practice.
The findings from this review show that intermittent supplementation with iron (alone or in combination with other nutrients) is efficacious in improving haemoglobin concentrations and ferritin levels and reducing anaemia among children younger than 12 years of age in settings with moderate to high prevalence of anaemia. The effects of intermittent supplementation on haemoglobin and ferritin outcomes were similar to those achieved with daily supplementation although children receiving intermittent supplements were at higher risk of anaemia.
Most of the evidence in this review is derived from trials providing weekly doses between 25 and 75 mg of elemental iron, either alone, with folic acid or with other micronutrients. The positive effect of intermittent supplementation was observed in populations of males and females, with different anaemia backgrounds, and seemed not to be affected by the duration of the intervention, although a minimum of three months seems reasonable to trigger the haematological response and build some iron stores. Very few trials reported on the level of supervision or the use of a communication or education strategy to improve the use of supplements. An integrated approach with a strong behaviour change communication component that targets different audiences may be necessary to adequately support adherence and appropriate use for any supplementation regimen. Intermittent supplementation for children might be an option for countries with strong institutional infrastructures for delivery that facilitate wide and sustained coverage, for example, where school attendance is high; although it is clear that efforts should be made to also reach those children not covered by the school or health systems.
This review attempted to examine several of the primary justifications for choosing intermittent over daily supplementation, including improved adherence, reduced side effects and improved efficiency in absorption. Surprisingly, very few trials reported on these outcomes and they did not show that the children receiving supplements intermittently adhere better to the intervention or have fewer side effects that those receiving daily supplements. Clearly, more research is needed in this area. Other rationales for intermittent supplementation include diminished exposure to an iron‐rich environment, which may exacerbate oxidative stress in the gut lumen and intestinal mucosal cells, as well as decreased competition with other minerals such as zinc and copper for absorption channels. Unfortunately, few trials reported on other indicators of vitamin and mineral status and therefore no conclusions can be drawn.
In summary, intermittent supplementation is efficacious at improving haemoglobin and ferritin concentrations and reducing anaemia prevalence, although children receiving daily supplements were less likely to present anaemia compared to those receiving intermittent supplements. These results suggest that in settings where daily supplementation is likely to be unsuccessful or not feasible, intermittent supplementation could be an effective public health strategy to improve iron status and reduce anaemia in children under 12 years of age.
Implications for research.
Important research is needed at different levels before we can fully assess the effects and safety of intermittent iron supplementation regimens on anaemia, iron status and development in children less than 12 years of age. Future research should focus on the following.
Clinical research
Examining the efficacy of intermittent iron regimens on neurocognitive and developmental outcomes and growth. In addition, attempts should be made to use comparable measures across studies, when possible.
Reporting the side effects in greater detail to acknowledge not only the presence of a side effect but also its intensity and frequency.
Expanding the evidence on the provision of multiple micronutrients on an intermittent basis and their effect on iron status and other indicators of vitamin and mineral status, such as retinol or zinc.
Reporting comprehensively the effects of the intermittent supplementation on anaemia, haemoglobin concentrations or ferritin to better understand the clinical significance of haemoglobin changes.
Programme implementation
Establishing the periodicity of this intervention over a year, taking into account both its biological and programmatic feasibility.
Improving reporting of adherence and addressing the relevance of direct and continued supervision.
Exploring the factors which may influence adherence (such as behaviour change communication (BCC)) and the types of support needed to improve adherence in supplementation interventions. BCC and supporting adherence may be important components of an effective supplementation programme but trials rarely provide detailed information about them. This limits the ability to understand the intensity of these activities needed to achieve the effects found in the trials.
Examining the cost effectiveness of intermittent compared with daily supplementation, taking into account more than just the differential cost of pills.
Acknowledgements
We would like to thank the trial authors and organisations who contributed additional information for this review and Ms Xiaoting Huo for her help in translating Yang 2004 (C) into English. Thanks are due to Deidre Thomas from the US CDC Public Health Library and Information Center and Margaret Anderson from the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG) for their help in devising the search strategy. We would also like to thank the staff at the editorial office of the CDPLPG for their support in the preparation of this review.
As part of the prepublication editorial process the text has been commented on by three peers (an editor and two referees who are external to the editorial team) and one of CDPLPG's statistical editors. We are grateful for their feedback.
Appendices
Appendix 1. Search strategies
CENTRAL #1MeSH descriptor Iron, this term only #2MeSH descriptor Iron, Dietary, this term only #3MeSH descriptor Anemia, Iron‐Deficiency, this term only #4MeSH descriptor Folic Acid, this term only #5MeSH descriptor Dietary Supplements, this term only #6MeSH descriptor Trace Elements, this term only #7iron* #8folic* or folate* or folvite* or folacin* or pteroylglutamic* #9diet* NEAR/3 supplement* #10micro‐nutrient* or micronutrient* or multi‐nutrient* or multinutrient* #11MeSH descriptor Ferric Compounds, this term only #12MeSH descriptor Ferrous Compounds, this term only #13ferrous* or ferric* or fe #14MeSH descriptor Micronutrients, this term only #15(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16MeSH descriptor Drug Administration Schedule, this term only #17MeSH descriptor Dose‐Response Relationship, Drug explode all trees #18MeSH descriptor Time Factors, this term only #19week* or biweek* or bi NEXT week* or intermittent* or alternat* #20(#16 OR #17 OR #18 OR #19) #21(#15 AND #20) #22(iron NEAR/3 (dose* or dosage or administer* or administration or frequency)) #23(#21 OR #22) #24 (baby or babies or newborn* or neonat* or toddler* or child* or preschool* or schoolchild* or boy* or girl* or pre‐school* or teen* or adolescen* or preteen* or youth* or young person* or young people) #25(#23 AND #24) MEDLINE 1 Iron/ or Anemia, Iron‐Deficiency/ or Iron, Dietary/ 2 Folic Acid/ 3 micronutrients/ 4 Dietary Supplements/ 5 iron$.tw. 6 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw. 7 Trace Elements/ 8 (diet$ adj3 supplement$).tw. 9 (micro‐nutrient$ or micronutrient$ or multi‐nutrient$ or multinutrient$).tw. 10 Ferric Compounds/ 11 Ferrous Compounds/ 12 (ferrous$ or ferric$ or fe).tw. 13 or/1‐12 14 Drug Administration Schedule/ 15 Dose‐Response Relationship, Drug/ 16 Time Factors/ 17 (week$ or biweek$ or bi‐week$ or intermittent$ or alternat$).tw. 18 or/14‐17 19 13 and 18 20 (iron adj3 (dose$ or dosage or administer$ or administration or frequency)).tw. 21 19 or 20 22 exp Infant/ 23 exp Child/ 24 Adolescent/ 25 (baby or babies or newborn$ or neonat$ or toddler$ or child$ or preschool$ or schoolchild$ or boy$ or girl$ or pre‐school$ or teen$ or adolescen$ or preteen$ or youth$ or young person$ or young people).tw. 26 or/22‐25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomi#ed.ab. 30 placebo$.ab. 31 drug therapy.fs. 32 randomly.ab. 33 trial.ab. 34 groups.ab. 35 or/27‐34 36 exp animals/ not humans.sh. 37 35 not 36 38 21 and 26 and 37 EMBASE 1 iron/ 2 iron intake/ 3 iron deficiency anemia/ 4 folic acid/ 5 exp trace element/ 6 diet supplementation/ 7 iron$.tw. 8 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw. 9 (diet$ adj3 supplement$).tw. 10 (micro‐nutrient$ or micronutrient$ or multi‐nutrient$ or multinutrient$).tw. 11 ferric ion/ 12 ferrous ion/ 13 or/1‐12 14 drug administration/ 15 drug dose regimen/ 16 time/ 17 (week$ or biweek$ or bi‐week$ or intermittent$ or alternat$).tw. 18 or/14‐17 19 13 and 18 20 (iron adj3 (dose$ or dosage or administer$ or administration or frequency)).tw. 21 19 or 20 22 exp infant/ 23 exp child/ 24 adolescent/ 25 (baby or babies or newborn$ or neonat$ or toddler$ or child$ or preschool$ or schoolchild$ or boy$ or girl$ or pre‐school$ or teen$ or adolescen$ or preteen$ or youth$ or young person$ or young people).tw. 26 or/22‐25 27 21 and 26 28 exp Clinical trial/ 29 Randomization/ 30 Single blind procedure/ 31 Double blind procedure/ 32 Crossover procedure/ 33 Placebo/ 34 Randomi#ed.tw. 35 RCT.tw. 36 (random$ adj3 (allocat$ or assign$)).tw. 37 randomly.ab. 38 groups.ab. 39 trial.ab. 40 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 41 Placebo$.tw. 42 prospective study/ 43 (crossover or cross‐over).tw. 44 prospective.tw. 45 or/28‐44 46 27 and 45
CINAHL
S43 S24 and S42 S42 S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 S41 (MH "Evaluation Research") OR (MH "Summative Evaluation Research") OR (MH "Program Evaluation") S40 (MH "Treatment Outcomes") S39 (MH "Comparative Studies") S38 TI (evaluat* study or evaluat* research) or AB (evaluat* study or evaluat* research) or TI (effectiv* study or effectiv* research) or AB (effectiv* study or effectiv* research) OR TI (prospectiv* study or prospectiv* research) or AB(prospectiv* study or prospectiv* research) or TI (follow‐up study or follow‐up research) or AB (follow‐up study or follow‐up research) S37 placebo* S36 crossover* or "cross over*" S35 (MH "Crossover Design") S34 (tripl* N3 mask*) or (tripl* N3 blind*) S33 (trebl* N3 mask*) or (trebl* N3 blind*) S32 (doubl* N3 mask*) or (doubl* N3 blind*) S31 (singl* N3 mask*) or (singl* N3 blind*) S30 (clinic* N3 trial*) or (control* N3 trial*) S29 (random* N3 allocat*) or (random* N3 assign*) S28 randomis* or randomiz* S27 (MH "Meta Analysis") S26 (MH "Clinical Trials+") S25 MH random assignment S24 S19 and S23 S23 S20 or S21 or S22 S22 baby or babies or newborn* or neonat* or toddler* or child or preschool* or schoolchild* or boy* or girl* or pre‐school* or teen* or adolescen* or preteen* or youth* or young person* or young people S21 AG adolescent S20 AG infant or child S19 S17 or S18 S18 (iron N3 dose*) or (iron N3 dosage) or (iron N3 administer*) or (iron N3 administration) or (iron N3 frequency) S17 S11 and S16 S16 S12 or S13 or S14 or S15 S15 (week* or biweek* or bi‐week*or bi week* or intermittent* or alternat*) S14 (MH "Time Factors") S13 (MH "Dose‐Response Relationship, Drug") S12 (MH "Drug Administration Schedule") S11 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 S10 micro‐nutrient* or micronutrient* or micro nutrient* multi‐nutrient* or multinutrient* or multi nutrient* S9 ferrous* or ferric* or "fe" S8 diet* N3 supplement* S7 folic* or folate* or folvite* or folacin* or pteroylglutamic* S6 iron* S5 (MH "Micronutrients") S4 (MH "Trace Elements") S3 (MH "Dietary Supplements") S2 (MH "Folic Acid") S1 (MH "Iron") OR (MH "Anemia, Iron Deficiency") OR (MH "Iron Compounds") OR (MH "Ferric Compounds") OR (MH "Ferrous Compounds") POPLINE (iron* /folic* / folate* /supplement*/micronutrient*/micro‐nutrient*) & (week* /bi‐week* / bi week* / biweek* / intermittent / alternat*) ICTRP Intervention: iron or folic or folate or micronutrient* limited to Clinical trials in children
IMBIOMED Intervention: suplementacion hierro
LILACS Intervention: suplementacion hierro
IBECS Intervention: suplementacion hierro
Scielo Intervention: suplementacion hierro
Data and analyses
Comparison 1. Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (ALL) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 2 Anaemia (by dose of elemental iron in the intermittent group) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 2.1 25 mg or less/week | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.06, 0.37] |
| 2.2 Greater than 25 mg to 75 mg/week | 6 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.37, 0.80] |
| 2.3 Greater than 75 mg/week | 2 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.48, 1.04] |
| 3 Anaemia (by duration of the intervention) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 3.1 0 to three months | 5 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.82] |
| 3.2 More than three months | 5 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.14, 1.02] |
| 4 Anaemia (by type of compound) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 4.1 Ferrous sulphate | 9 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.75] |
| 4.2 Ferrous fumarate | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.49, 0.74] |
| 4.3 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Anaemia (by anaemia status at baseline) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 5.1 Anaemic | 2 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.07, 1.38] |
| 5.2 Non‐anaemic | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.31] |
| 5.3 Mixed/unknown | 7 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.41, 0.85] |
| 6 Anaemia (by intermittent regimen) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 6.1 One supplement a week | 9 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.75] |
| 6.2 Other intermittent regimen | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.49, 0.74] |
| 7 Anaemia (by sex) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 7.1 Girls | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 7.2 Boys | 1 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 1.00] |
| 7.3 Mixed/unknown | 9 | 1323 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.30, 0.70] |
| 8 Anaemia (by nutrient) | 10 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.72] |
| 8.1 Iron alone | 6 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.31, 0.74] |
| 8.2 Iron + folic acid | 2 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.03] |
| 8.3 iron + vitamin C | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.97] |
| 8.4 Iron + multiple micronutrients | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.06, 0.44] |
| 9 Haemoglobin (ALL) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 5.20 [2.51, 7.88] |
| 10 Haemoglobin (by by dose of elemental iron in the intermittent group) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 5.20 [2.51, 7.88] |
| 10.1 25 mg or less/week | 3 | 324 | Mean Difference (IV, Random, 95% CI) | 8.19 [‐4.01, 20.38] |
| 10.2 Greater than 25 mg to 75 mg/week | 12 | 2059 | Mean Difference (IV, Random, 95% CI) | 5.45 [2.31, 8.58] |
| 10.3 Greater than 75 mg/week | 4 | 649 | Mean Difference (IV, Random, 95% CI) | 1.84 [0.25, 3.44] |
| 11 Haemoglobin (by duration of the intervention) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 5.20 [2.51, 7.88] |
| 11.1 0 to three months | 7 | 1616 | Mean Difference (IV, Random, 95% CI) | 5.16 [2.82, 7.51] |
| 11.2 More than three months | 12 | 1416 | Mean Difference (IV, Random, 95% CI) | 5.13 [0.90, 9.36] |
| 12 Haemoglobin (by type of compound) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 5.20 [2.51, 7.88] |
| 12.1 Ferrous sulphate | 14 | 2288 | Mean Difference (IV, Random, 95% CI) | 5.57 [2.21, 8.92] |
| 12.2 Ferrous fumarate | 2 | 432 | Mean Difference (IV, Random, 95% CI) | 7.03 [3.36, 10.71] |
| 12.3 Other | 3 | 312 | Mean Difference (IV, Random, 95% CI) | 2.03 [‐0.26, 4.33] |
| 13 Haemoglobin (by anaemia status at baseline) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 5.20 [2.51, 7.88] |
| 13.1 Anaemic | 2 | 422 | Mean Difference (IV, Random, 95% CI) | 13.17 [3.07, 23.26] |
| 13.2 Non‐anaemic | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐2.46, 6.46] |
| 13.3 Mixed/unknown | 16 | 2546 | Mean Difference (IV, Random, 95% CI) | 4.35 [1.88, 6.82] |
| 14 Haemoglobin (by intermittent regimen) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 5.15 [2.52, 7.79] |
| 14.1 One supplement a week | 15 | 2256 | Mean Difference (IV, Random, 95% CI) | 5.61 [2.13, 9.09] |
| 14.2 Other intermittent regimen | 5 | 776 | Mean Difference (IV, Random, 95% CI) | 3.67 [1.05, 6.28] |
| 15 Haemoglobin (by sex) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 5.17 [2.56, 7.77] |
| 15.1 Girls | 1 | 248 | Mean Difference (IV, Random, 95% CI) | 4.0 [0.83, 7.17] |
| 15.2 Boys | 1 | 253 | Mean Difference (IV, Random, 95% CI) | 3.70 [0.58, 6.82] |
| 15.3 Mixed/unknown | 18 | 2531 | Mean Difference (IV, Random, 95% CI) | 5.31 [2.40, 8.22] |
| 16 Haemoglobin (by nutrient) | 19 | 3032 | Mean Difference (IV, Random, 95% CI) | 4.83 [2.25, 7.41] |
| 16.1 Iron alone | 11 | 1699 | Mean Difference (IV, Random, 95% CI) | 4.41 [1.32, 7.50] |
| 16.2 Iron + folic acid | 4 | 756 | Mean Difference (IV, Random, 95% CI) | 3.36 [1.51, 5.21] |
| 16.3 iron + zinc | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.09, 4.89] |
| 16.4 Iron + vitamin C | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 20.70 [17.51, 23.89] |
| 16.5 Iron + multiple micronutrients | 4 | 450 | Mean Difference (IV, Random, 95% CI) | 5.47 [0.32, 10.61] |
| 17 Iron deficiency (ALL) | 3 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.91] |
| 18 Ferritin (ALL) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 19 Ferritin (by dose of elemental iron in the intermittent group) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 19.1 25 mg or less/week | 1 | 148 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐0.89, 10.09] |
| 19.2 Greater than 25 mg to 75 mg/week | 4 | 402 | Mean Difference (IV, Random, 95% CI) | 17.77 [8.21, 27.34] |
| 19.3 Greater than 75 mg/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Ferritin (by duration of the supplementation) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 20.1 0 to three months | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 15.80 [‐1.23, 32.83] |
| 20.2 More than three months | 4 | 515 | Mean Difference (IV, Random, 95% CI) | 13.82 [1.84, 25.81] |
| 21 Ferritin (by type of compound) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 21.1 Ferrous sulphate | 4 | 476 | Mean Difference (IV, Random, 95% CI) | 16.28 [4.68, 27.87] |
| 21.2 Ferrous fumarate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Other | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐14.37, 19.29] |
| 22 Ferritin (by anaemia status at baseline) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 22.1 Anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Non‐anaemic | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐14.37, 19.29] |
| 22.3 Mixed/unknown | 4 | 476 | Mean Difference (IV, Random, 95% CI) | 16.28 [4.68, 27.87] |
| 23 Ferritin (by supplementation regimen) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 23.1 One supplement a week | 4 | 497 | Mean Difference (IV, Random, 95% CI) | 10.14 [1.74, 18.53] |
| 23.2 Other intermittent regimen | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 27.80 [22.88, 32.72] |
| 24 Ferritin (by sex) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 24.1 Girls | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Mixed/unknown | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 14.17 [3.53, 24.81] |
| 25 Ferritin (by nutrient) | 5 | 550 | Mean Difference (IV, Random, 95% CI) | 11.41 [2.71, 20.11] |
| 25.1 Iron alone | 4 | 379 | Mean Difference (IV, Random, 95% CI) | 16.25 [5.41, 27.09] |
| 25.2 Iron + folic acid | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Iron + zinc | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 5.50 [‐3.91, 14.91] |
| 25.4 Iron + multiple micronutrients | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐4.96, 12.56] |
| 26 All cause morbidity (ALL) | 1 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.24] |
| 27 Any side effects (ALL) | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [0.19, 76.92] |
| 28 Nausea | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.12, 66.82] |
| 29 Adherence (ALL) | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.98, 1.09] |
| 30 Mental development scale (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐2.40, 6.40] |
| 31 Orientation engagement (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 8.40 [‐1.79, 18.59] |
| 32 Emotional regulation (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐11.58, 6.58] |
| 33 Motor quality (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 15.60 [7.66, 23.54] |
| 34 Psychomotor development index (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 6.90 [1.35, 12.45] |
| 35 IQ (ALL) | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐5.96, ‐0.04] |
| 36 Thai language (ALL) | 1 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.50, ‐0.09] |
| 37 Mathematics (ALL) | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.44, ‐0.10] |
| 38 WAZ | 3 | 366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.33, 0.27] |
| 39 HAZ | 3 | 366 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
1.12. Analysis.
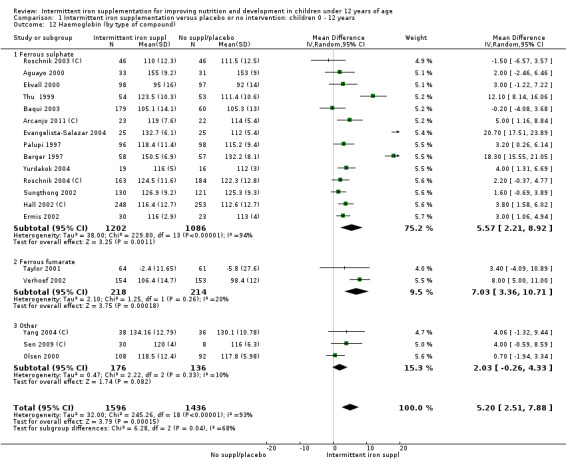
Comparison 1 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 12 years, Outcome 12 Haemoglobin (by type of compound).
Comparison 2. Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 12 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (ALL) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 2 Anaemia (by dose of elemental iron in the intermittent group) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 2.1 25 mg or less/week | 2 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.98, 1.47] |
| 2.2 Greater than 25 mg to 75 mg/week | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.96, 1.88] |
| 2.3 Intermittent group: greater than 75 mg/week | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Anaemia (by duration of the supplementation) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 3.1 0 to three months | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.55, 2.77] |
| 3.2 More than three months | 4 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.03, 1.47] |
| 4 Anaemia (by type of compound) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 4.1 Ferrous sulphate | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 4.2 Ferrous fumarate | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Anaemia (by anaemia status at baseline) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 5.1 Anaemic | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.50, 1.82] |
| 5.2 Non‐anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Mixed/unknown | 4 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.05, 1.51] |
| 6 Anaemia (by supplementation regimen) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 6.1 One supplement a week | 4 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.97, 1.43] |
| 6.2 Other intermittent regimen | 2 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.02, 2.19] |
| 7 Anaemia (by sex) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 7.1 Girls | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Boys | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mixed/unknown | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 8 Anaemia (by nutrient) | 6 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.04, 1.47] |
| 8.1 Iron alone | 4 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.97, 1.42] |
| 8.2 Iron + folic acid | 1 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.02, 2.36] |
| 8.3 Iron + multiple micronutrients | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.31, 5.57] |
| 9 Haemoglobin (ALL) | 19 | 2851 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.54, 0.35] |
| 10 Haemoglobin (by dose of elemental iron in the intermittent group) | 18 | 2751 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.60, 0.37] |
| 10.1 25 mg or less/week | 3 | 536 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐4.18, ‐0.66] |
| 10.2 Greater than 25 mg to 75 mg/week | 13 | 2078 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.62, 0.45] |
| 10.3 Greater than 75 mg/week | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐4.68, 6.68] |
| 11 Haemoglobin (by duration of the supplementation) | 19 | 2842 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.26, 0.50] |
| 11.1 0 to three months | 11 | 1455 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.91, 1.84] |
| 11.2 More than three months | 8 | 1387 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.07, ‐0.22] |
| 12 Haemoglobin (by type of compound) | 19 | 2851 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.54, 0.35] |
| 12.1 Ferrous sulphate | 17 | 2733 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.60, 0.40] |
| 12.2 Ferrous fumarate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Other | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐4.24, 3.32] |
| 13 Haemoglobin (by anaemia status at baseline) | 19 | 2851 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.54, 0.32] |
| 13.1 Anaemic | 7 | 957 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.59, 1.07] |
| 13.2 Non‐anaemic | 3 | 166 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐1.42, 2.99] |
| 13.3 Mixed/unknown | 10 | 1728 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.00, 0.48] |
| 14 Haemoglobin (by supplementation regimen) | 19 | 2851 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.70, 0.30] |
| 14.1 One supplement a week | 14 | 1612 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.57, 1.07] |
| 14.2 Other intermittent regimen | 8 | 1239 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐3.02, 0.19] |
| 15 Haemoglobin (by sex) | 19 | 2851 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.54, 0.35] |
| 15.1 Girls | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐5.43, 1.43] |
| 15.2 Boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Mixed/unknown | 18 | 2809 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.51, 0.46] |
| 16 Haemoglobin (by nutrient) | 19 | 2851 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.52, 0.35] |
| 16.1 Iron alone | 15 | 2144 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.61, 0.59] |
| 16.2 Iron + folic acid | 2 | 408 | Mean Difference (IV, Random, 95% CI) | ‐2.26 [‐4.30, ‐0.22] |
| 16.3 Iron + multiple micronutrients | 3 | 299 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐2.04, 3.26] |
| 17 Iron deficiency (ALL) | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [1.23, 13.05] |
| 18 Ferritin (ALL) | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐9.42, 1.05] |
| 19 Ferritin (by dose of elemental iron in the intermittent group) | 9 | 802 | Mean Difference (IV, Random, 95% CI) | ‐4.34 [‐10.20, 1.53] |
| 19.1 by dose of elemental iron in the intermittent group: 25 mg or less/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 by dose of elemental iron in the intermittent group: greater than 25 mg to 75 mg/week | 9 | 802 | Mean Difference (IV, Random, 95% CI) | ‐4.34 [‐10.20, 1.53] |
| 19.3 by dose of elemental iron in the intermittent group: greater than 75 mg/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Ferritin (by duration of the supplementation) | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐9.42, 1.05] |
| 20.1 by duration of the supplementation: 0 to three months | 6 | 442 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐6.62, 4.51] |
| 20.2 by duration of the supplementation: more than three months | 4 | 460 | Mean Difference (IV, Random, 95% CI) | ‐9.58 [‐23.08, 3.93] |
| 21 Ferritin (by type of compound) | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐9.42, 1.05] |
| 21.1 by type of compound: ferrous sulphate | 9 | 826 | Mean Difference (IV, Random, 95% CI) | ‐3.85 [‐9.28, 1.59] |
| 21.2 by type of compound: ferrous fumarate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 by type of compound: other | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐23.95, 5.89] |
| 22 Ferritin (by anaemia status at baseline) | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.93 [‐9.98, 0.12] |
| 22.1 by anaemia status at baseline: anaemic | 5 | 285 | Mean Difference (IV, Random, 95% CI) | ‐2.94 [‐12.23, 6.34] |
| 22.2 by anaemia status at baseline: non‐anaemic | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐2.67 [‐5.89, 0.54] |
| 22.3 by anaemia status at baseline: mixed/unknown | 3 | 450 | Mean Difference (IV, Random, 95% CI) | ‐9.42 [‐23.19, 4.35] |
| 23 Ferritin (by supplementation regimen) | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.48 [‐9.68, 0.71] |
| 23.1 by supplementation regimen: one supplement a week | 7 | 595 | Mean Difference (IV, Random, 95% CI) | ‐7.34 [‐16.12, 1.44] |
| 23.2 by supplementation regimen: other intermittent regimen | 5 | 307 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐3.94, 2.08] |
| 24 Ferritin (by sex) | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐9.42, 1.05] |
| 24.1 by sex: girls | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 by sex: boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 by sex: mixed/unknown | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐9.42, 1.05] |
| 25 Ferritin (by nutrient) | 10 | 902 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐9.42, 1.05] |
| 25.1 By nutrient: iron alone | 9 | 826 | Mean Difference (IV, Random, 95% CI) | ‐3.85 [‐9.28, 1.59] |
| 25.2 By nutrient: iron + folic acid | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 By nutrient: iron + multiple micronutrients | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐23.95, 5.89] |
| 26 Increase in steps climbed (ALL) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐13.34, 3.34] |
| 27 All cause morbidity (ALL) | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.12] |
| 28 Diarrhoea (ALL) | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.60, 2.28] |
| 29 Any side effects (ALL) | 4 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.87] |
| 30 Adherence (ALL) | 5 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.98, 1.54] |
| 31 IQ (ALL) | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐5.96, ‐0.04] |
| 32 Thai language (ALL) | 1 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.50, ‐0.09] |
| 33 Mathematics (ALL) | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.44, ‐0.10] |
| 34 HAZ | 3 | 279 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.80, 0.28] |
Comparison 3. Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (ALL) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 2 Anaemia (by dose of elemental iron in the intermittent group) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 2.1 25 mg or less/week | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.06, 0.37] |
| 2.2 Greater than 25 mg to 75 mg/week | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.51, 0.74] |
| 2.3 Greater than 75 mg/week | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Anaemia (by duration of the supplementation) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 3.1 0 to three months | 3 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.85] |
| 3.2 More than three months | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.97] |
| 4 Anaemia (by type of compound) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 4.1 Ferrous sulphate | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.03] |
| 4.2 Ferrous fumarate | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.49, 0.74] |
| 4.3 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Anaemia (by anaemia status at baseline) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 5.1 Anaemic | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.49, 0.74] |
| 5.2 Non‐anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Mixed/unknown | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.03] |
| 6 Anaemia (by intermittent regimen) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 6.1 One supplement a week | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 1.03] |
| 6.2 Other intermittent regimen | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.49, 0.74] |
| 7 Anaemia (by sex) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 7.1 Girls | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Boys | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mixed/unknown | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 8 Anaemia (by nutrient) | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.80] |
| 8.1 Iron alone | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.51, 0.74] |
| 8.2 Iron + folic acid | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 iron + vitamin C | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.97] |
| 8.4 Iron + multiple micronutrients | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.06, 0.44] |
| 9 Haemoglobin (ALL) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 10 Haemoglobin (by dose of elemental iron in the intermittent group) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 10.1 25 mg or less/week | 3 | 324 | Mean Difference (IV, Random, 95% CI) | 8.19 [‐4.01, 20.38] |
| 10.2 Greater than 25 mg to 75 mg/week | 6 | 930 | Mean Difference (IV, Random, 95% CI) | 5.50 [2.64, 8.36] |
| 10.3 Greater than 75 mg/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Haemoglobin (by duration of the supplementation) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 11.1 0 to three months | 4 | 643 | Mean Difference (IV, Random, 95% CI) | 6.64 [3.01, 10.27] |
| 11.2 More than three months | 5 | 611 | Mean Difference (IV, Random, 95% CI) | 6.16 [‐1.55, 13.87] |
| 12 Haemoglobin (by type of iron compound) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 12.1 Ferrous sulphate | 7 | 873 | Mean Difference (IV, Random, 95% CI) | 6.54 [1.44, 11.63] |
| 12.2 Ferrous fumarate | 1 | 307 | Mean Difference (IV, Random, 95% CI) | 8.0 [5.00, 11.00] |
| 12.3 Other | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 4.06 [‐1.32, 9.44] |
| 13 Haemoglobin (by anaemia status at baseline) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 13.1 Anaemic | 1 | 307 | Mean Difference (IV, Random, 95% CI) | 8.0 [5.00, 11.00] |
| 13.2 Non‐anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Mixed/unknown | 8 | 947 | Mean Difference (IV, Random, 95% CI) | 6.25 [1.60, 10.90] |
| 14 Haemoglobin (by supplementation regimen) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 14.1 One supplement a week | 6 | 699 | Mean Difference (IV, Random, 95% CI) | 7.35 [0.92, 13.77] |
| 14.2 Other intermittent regimen | 3 | 555 | Mean Difference (IV, Random, 95% CI) | 4.68 [1.28, 8.08] |
| 15 Haemoglobin (by sex) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 15.1 Girls | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Mixed/unknown | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.45 [2.36, 10.55] |
| 16 Haemoglobin (by nutrient) | 9 | 1254 | Mean Difference (IV, Random, 95% CI) | 6.01 [2.13, 9.89] |
| 16.1 Iron alone | 5 | 744 | Mean Difference (IV, Random, 95% CI) | 3.81 [1.61, 6.01] |
| 16.2 Iron + folic acid | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Iron + multiple micronutrients | 5 | 510 | Mean Difference (IV, Random, 95% CI) | 8.46 [0.60, 16.32] |
| 17 Iron deficiency (ALL) | 3 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.91] |
| 18 Ferritin (ALL) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 19 Ferritin (by dose of iron in the intermittent group) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 19.1 25 mg or less/week | 1 | 148 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐0.89, 10.09] |
| 19.2 Greater than 25 mg to 75 mg/week | 3 | 162 | Mean Difference (IV, Random, 95% CI) | 16.91 [0.99, 32.82] |
| 19.3 Greater than 75 mg/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Ferritin (by duration of the supplementation) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 20.1 0 to three months | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 15.80 [‐1.23, 32.83] |
| 20.2 More than three months | 3 | 275 | Mean Difference (IV, Random, 95% CI) | 12.34 [‐6.19, 30.87] |
| 21 Ferritin (by type of iron compound) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 21.1 Ferrous sulphate | 3 | 236 | Mean Difference (IV, Random, 95% CI) | 16.12 [‐1.81, 34.05] |
| 21.2 Ferrous fumarate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Other | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐14.37, 19.29] |
| 22 Ferritin (by anaemia status at baseline) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 22.1 by anaemia status at baseline: anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 by anaemia status at baseline: non‐anaemic | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐14.37, 19.29] |
| 22.3 by anaemia status at baseline: mixed/unknown | 3 | 236 | Mean Difference (IV, Random, 95% CI) | 16.12 [‐1.81, 34.05] |
| 23 Ferritin (by supplementation regimen) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 23.1 One supplement a week | 3 | 257 | Mean Difference (IV, Random, 95% CI) | 5.37 [0.39, 10.36] |
| 23.2 Other intermittent regimen | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 27.80 [22.88, 32.72] |
| 24 Ferritin (by sex) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 24.1 Girls | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Mixed/unknown | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 13.15 [‐2.28, 28.59] |
| 25 Ferritin (by nutrient) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 11.15 [‐1.92, 24.22] |
| 25.1 Iron alone | 3 | 144 | Mean Difference (IV, Random, 95% CI) | 15.70 [‐2.68, 34.08] |
| 25.2 Iron + folic acid | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Iron + multiple micronutrients | 2 | 166 | Mean Difference (IV, Random, 95% CI) | 4.58 [‐2.27, 11.43] |
| 26 All cause morbidity (ALL) | 1 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.24] |
| 27 Any side effects (ALL) | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 3.87 [0.19, 76.92] |
| 28 Adherence (ALL) | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.98, 1.09] |
| 29 Mental development scale (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐2.40, 6.40] |
| 30 Orientation engagement (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 8.40 [‐1.79, 18.59] |
| 31 Emotional regulation (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐11.58, 6.58] |
| 32 Motor quality (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 15.60 [7.66, 23.54] |
| 33 Psychomotor development index (ALL) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 6.90 [1.35, 12.45] |
| 34 HAZ | 2 | 302 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
3.1. Analysis.
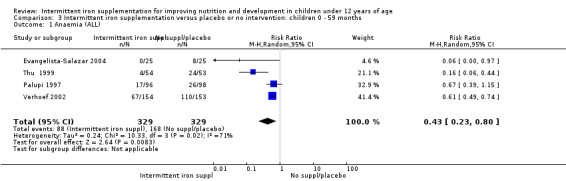
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 1 Anaemia (ALL).
3.2. Analysis.
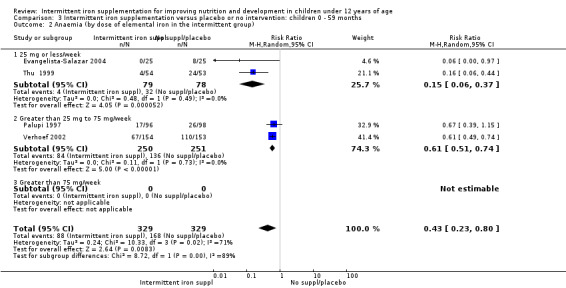
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 2 Anaemia (by dose of elemental iron in the intermittent group).
3.3. Analysis.
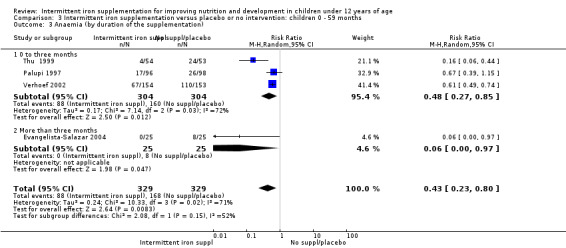
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 3 Anaemia (by duration of the supplementation).
3.4. Analysis.
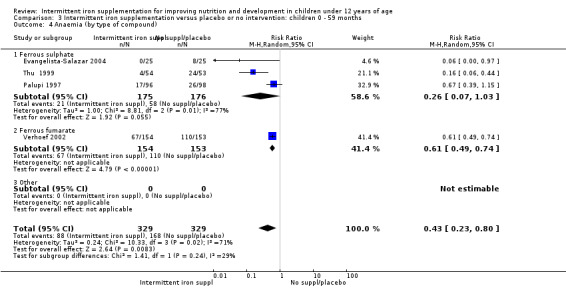
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 4 Anaemia (by type of compound).
3.5. Analysis.
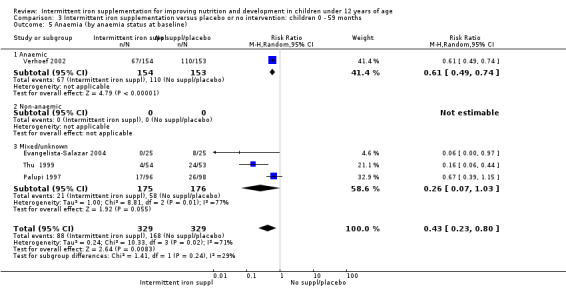
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 5 Anaemia (by anaemia status at baseline).
3.6. Analysis.
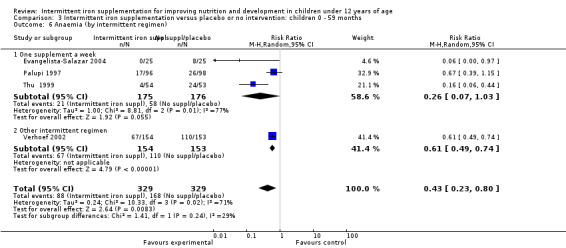
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 6 Anaemia (by intermittent regimen).
3.7. Analysis.
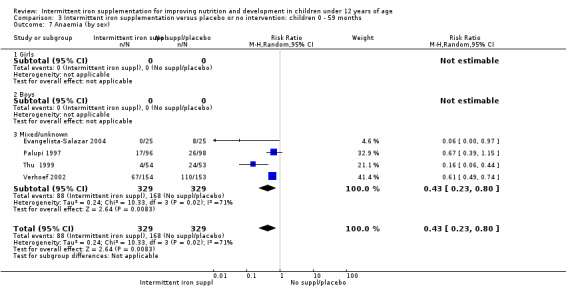
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 7 Anaemia (by sex).
3.8. Analysis.
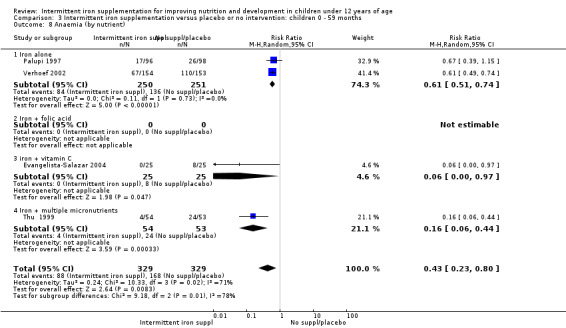
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 8 Anaemia (by nutrient).
3.9. Analysis.
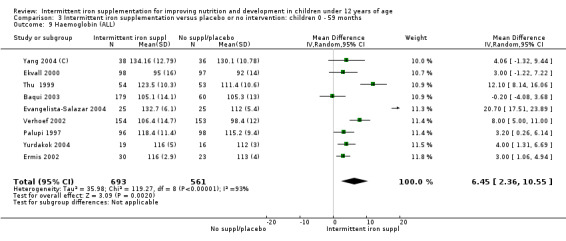
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 9 Haemoglobin (ALL).
3.10. Analysis.
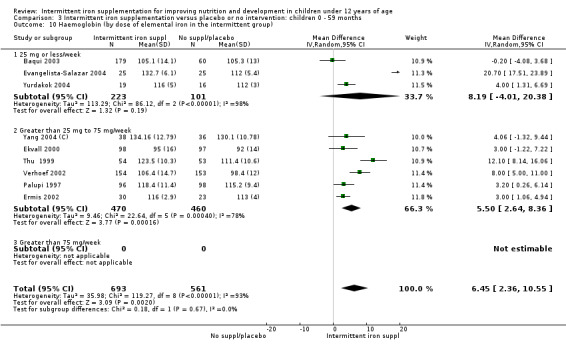
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 10 Haemoglobin (by dose of elemental iron in the intermittent group).
3.11. Analysis.
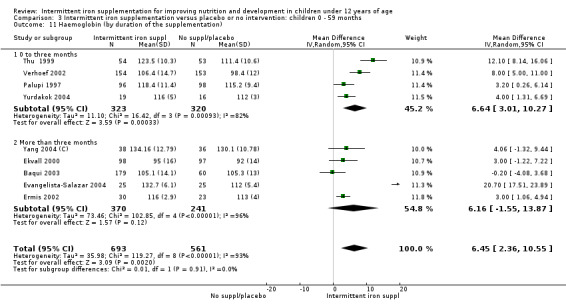
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 11 Haemoglobin (by duration of the supplementation).
3.12. Analysis.
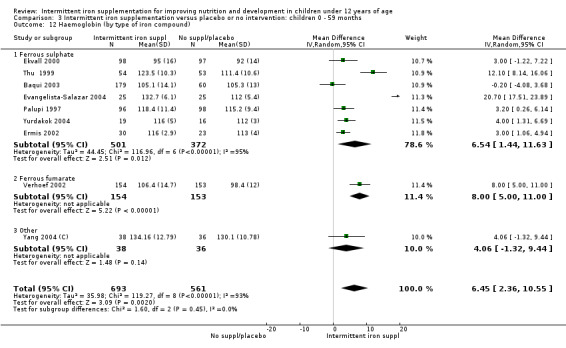
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 12 Haemoglobin (by type of iron compound).
3.13. Analysis.
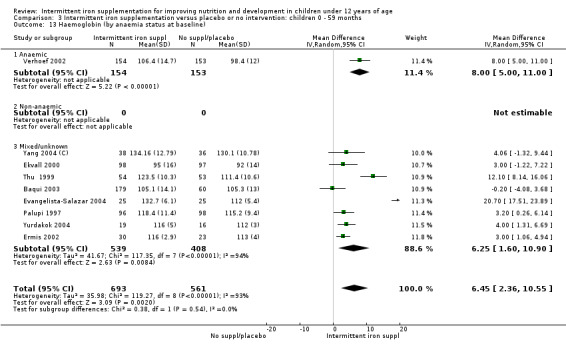
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 13 Haemoglobin (by anaemia status at baseline).
3.14. Analysis.
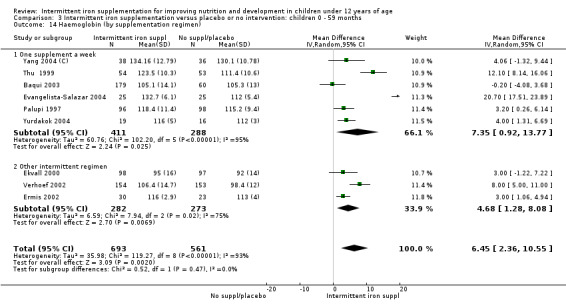
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 14 Haemoglobin (by supplementation regimen).
3.15. Analysis.
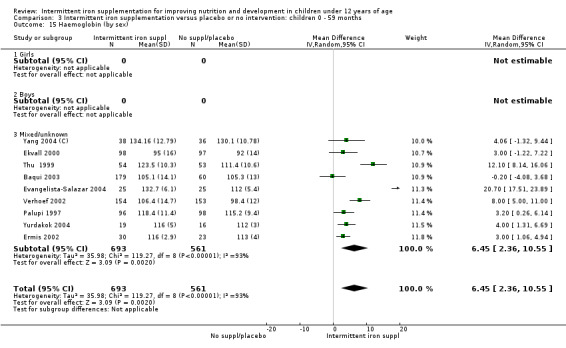
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 15 Haemoglobin (by sex).
3.16. Analysis.
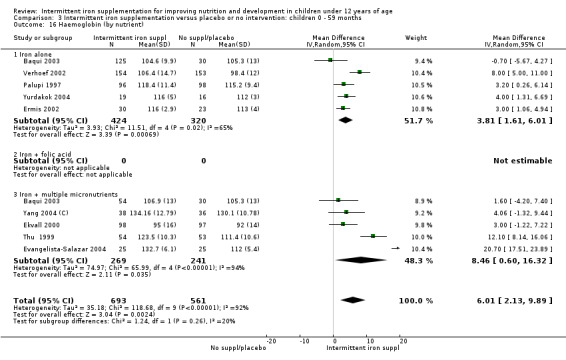
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 16 Haemoglobin (by nutrient).
3.17. Analysis.
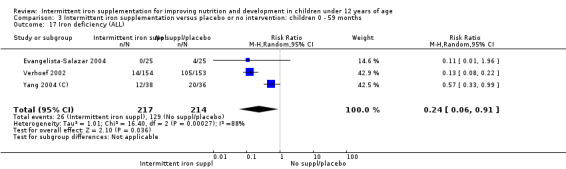
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 17 Iron deficiency (ALL).
3.18. Analysis.
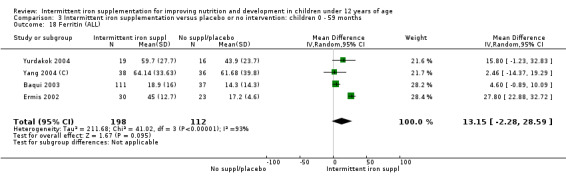
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 18 Ferritin (ALL).
3.19. Analysis.
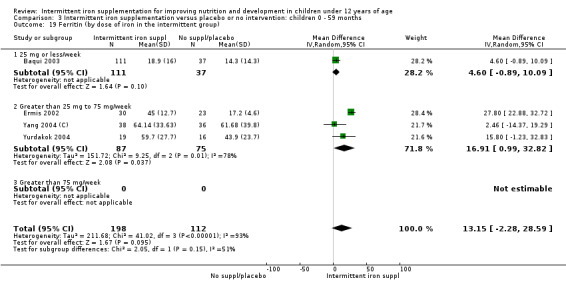
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 19 Ferritin (by dose of iron in the intermittent group).
3.20. Analysis.
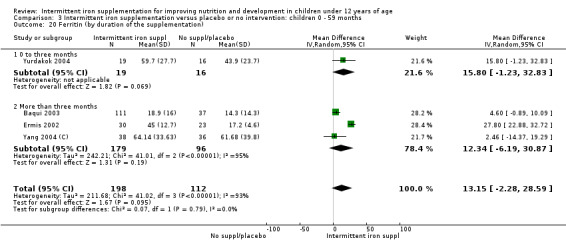
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 20 Ferritin (by duration of the supplementation).
3.21. Analysis.
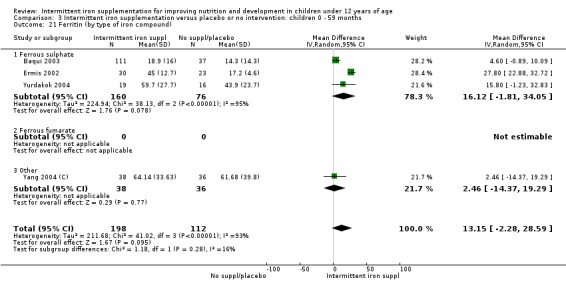
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 21 Ferritin (by type of iron compound).
3.22. Analysis.
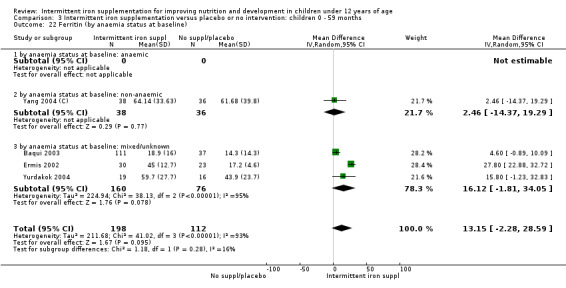
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 22 Ferritin (by anaemia status at baseline).
3.23. Analysis.
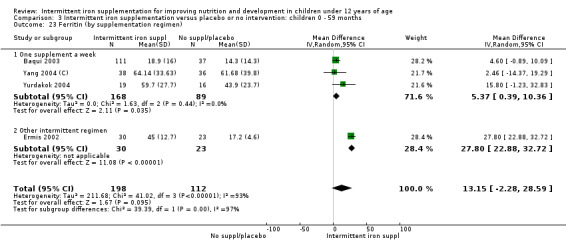
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 23 Ferritin (by supplementation regimen).
3.24. Analysis.
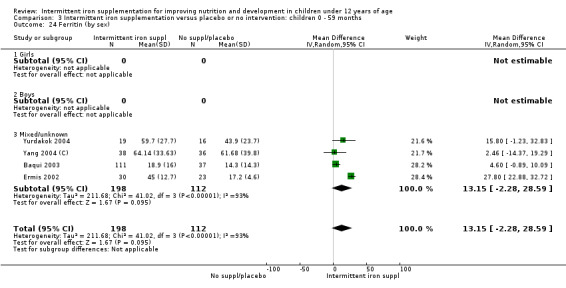
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 24 Ferritin (by sex).
3.25. Analysis.
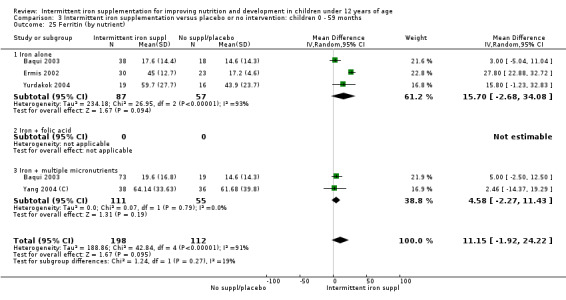
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 25 Ferritin (by nutrient).
3.26. Analysis.
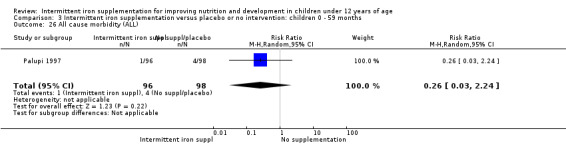
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 26 All cause morbidity (ALL).
3.27. Analysis.
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 27 Any side effects (ALL).
3.28. Analysis.
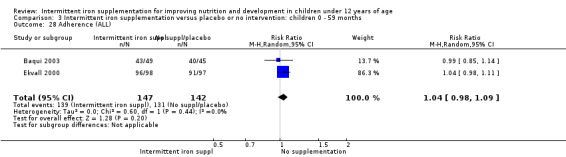
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 28 Adherence (ALL).
3.29. Analysis.
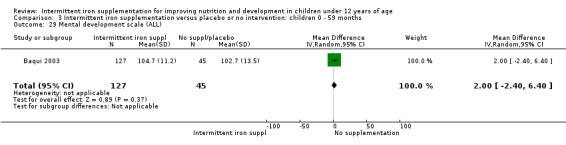
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 29 Mental development scale (ALL).
3.30. Analysis.
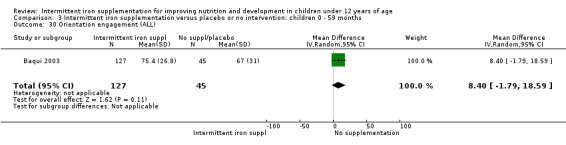
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 30 Orientation engagement (ALL).
3.31. Analysis.
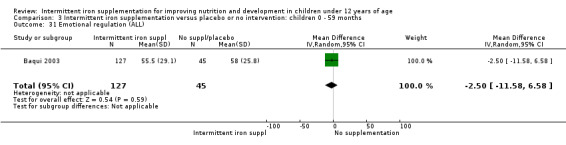
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 31 Emotional regulation (ALL).
3.32. Analysis.
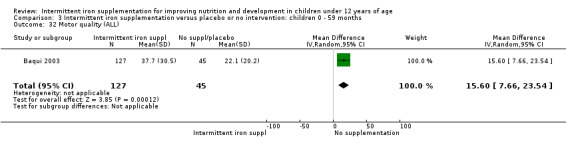
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 32 Motor quality (ALL).
3.33. Analysis.
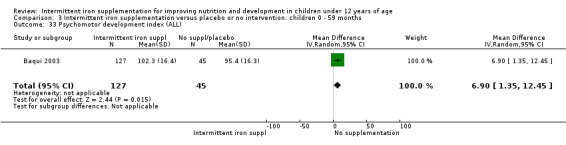
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 33 Psychomotor development index (ALL).
3.34. Analysis.
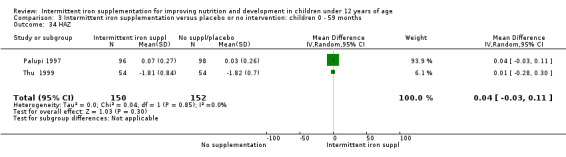
Comparison 3 Intermittent iron supplementation versus placebo or no intervention: children 0 ‐ 59 months, Outcome 34 HAZ.
Comparison 4. Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (ALL) | 3 | 770 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.05, 1.51] |
| 2 Haemoglobin (ALL) | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.80, 0.29] |
| 3 Haemoglobin (by dose of elemental iron in the intermittent group) | 14 | 2438 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.82, 0.18] |
| 3.1 25 mg or less/week | 3 | 536 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐4.18, ‐0.66] |
| 3.2 Greater than 25 mg to 75 mg/week | 11 | 1902 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.59, 0.68] |
| 3.3 Greater than 75 mg/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Haemoglobin (by duration of supplementation) | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.80, 0.29] |
| 4.1 0 to three months | 9 | 1309 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.66, 1.36] |
| 4.2 More than three months | 5 | 961 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.95, ‐0.11] |
| 5 Haemoglobin (by type of compound) | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.80, 0.29] |
| 5.1 Ferrous sulphate | 13 | 2194 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.91, 0.21] |
| 5.2 Ferrous fumarate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Other | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 1.96 [‐3.05, 6.97] |
| 6 Haemoglobin (by anaemia status at baseline) | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.80, 0.29] |
| 6.1 Anaemic | 5 | 834 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐2.81, 1.68] |
| 6.2 Non‐anaemic | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 1.99 [‐0.72, 4.70] |
| 6.3 Mixed/unknown | 7 | 1323 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.22, ‐0.19] |
| 7 Haemoglobin (by supplementation regimen) | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.71, 0.27] |
| 7.1 One supplement a week | 9 | 1054 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.67, 1.21] |
| 7.2 Other intermittent regimen | 7 | 1216 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.57, 0.29] |
| 8 Haemoglobin (by sex) | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.80, 0.29] |
| 8.1 Girls | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed/unknown | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.80, 0.29] |
| 9 Haemoglobin (by nutrient) | 14 | 2270 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.80, 0.29] |
| 9.1 Iron alone | 10 | 1490 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.05, 0.46] |
| 9.2 Iron + folic acid | 1 | 366 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐4.94, 0.14] |
| 9.3 Iron + multiple micronutrients | 3 | 414 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐1.84, 2.98] |
| 10 Iron deficiency (ALL) | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [1.23, 13.05] |
| 11 Ferritin (ALL) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.59, 0.39] |
| 12 Ferritin (by dose of elemental iron in the intermittent subgroup) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐2.22 [‐6.03, 1.59] |
| 12.1 25 mg or less/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Greater than 25 mg to 75 mg/week | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐2.22 [‐6.03, 1.59] |
| 12.3 Greater than 75 mg/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Ferritin (by duration of supplementation) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.59, 0.39] |
| 13.1 0 to three months | 5 | 382 | Mean Difference (IV, Random, 95% CI) | ‐3.02 [‐7.91, 1.87] |
| 13.2 More than three months | 3 | 200 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐5.88, 2.62] |
| 14 Ferritin (by type of compound) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.59, 0.39] |
| 14.1 Ferrous sulphate | 7 | 506 | Mean Difference (IV, Random, 95% CI) | ‐2.69 [‐6.42, 1.05] |
| 14.2 Ferrous fumarate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Other | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐23.95, 5.89] |
| 15 Ferritin (by anaemia status at baseline) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐8.25, 0.86] |
| 15.1 Anaemic | 4 | 225 | Mean Difference (IV, Random, 95% CI) | ‐4.47 [‐15.45, 6.52] |
| 15.2 Non‐anaemic | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐2.67 [‐5.89, 0.54] |
| 15.3 Mixed/unknown | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐5.23, 2.17] |
| 16 Ferritin (by supplementation regimen) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.27 [‐7.87, 1.33] |
| 16.1 One supplement a week | 5 | 291 | Mean Difference (IV, Random, 95% CI) | ‐6.21 [‐12.98, 0.55] |
| 16.2 Other intermittent regimen | 4 | 291 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐3.89, 2.27] |
| 17 Ferritin (by sex) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.58, 0.39] |
| 17.1 Girls | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Mixed/unknown | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.58, 0.39] |
| 18 Ferritin (by nutrient) | 8 | 582 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐6.59, 0.39] |
| 18.1 Iron alone | 7 | 506 | Mean Difference (IV, Random, 95% CI) | ‐2.69 [‐6.42, 1.05] |
| 18.2 Iron + folic acid | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Iron + multiple micronutrients | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐9.03 [‐23.95, 5.89] |
| 19 All cause morbidity (ALL) | 1 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.16] |
| 20 Diarrhoea (ALL) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [0.12, 67.03] |
| 21 Any side effects (ALL) | 4 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.87] |
| 22 Adherence (ALL) | 3 | 1185 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.15, 1.45] |
| 23 HAZ | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.52, 0.23] |
| 24 WAZ | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.82, ‐0.06] |
4.1. Analysis.
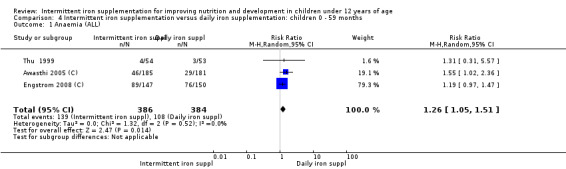
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 1 Anaemia (ALL).
4.2. Analysis.
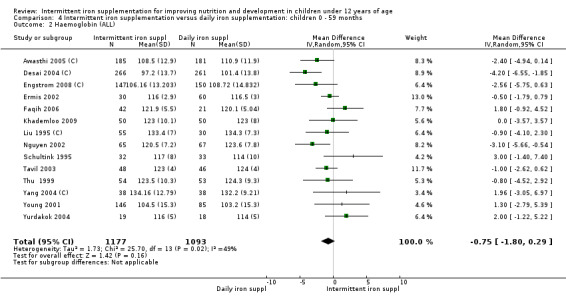
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 2 Haemoglobin (ALL).
4.3. Analysis.
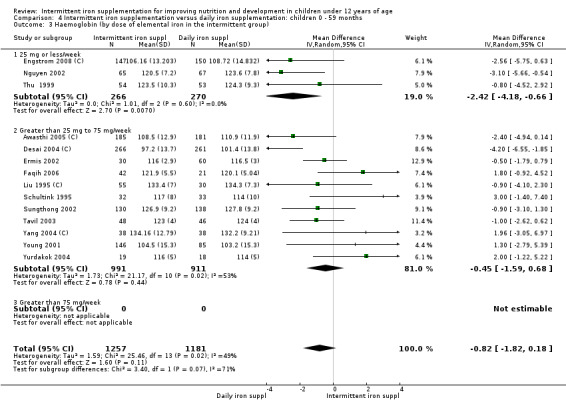
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 3 Haemoglobin (by dose of elemental iron in the intermittent group).
4.4. Analysis.
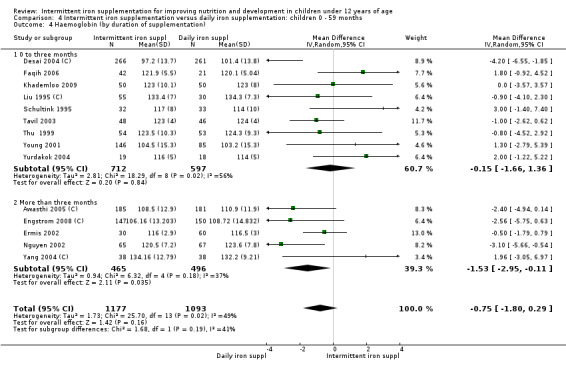
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 4 Haemoglobin (by duration of supplementation).
4.5. Analysis.
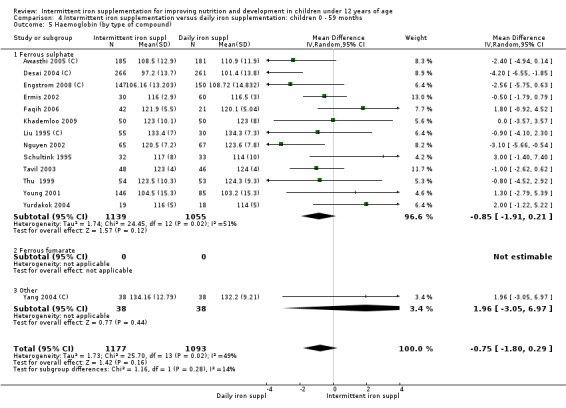
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 5 Haemoglobin (by type of compound).
4.6. Analysis.
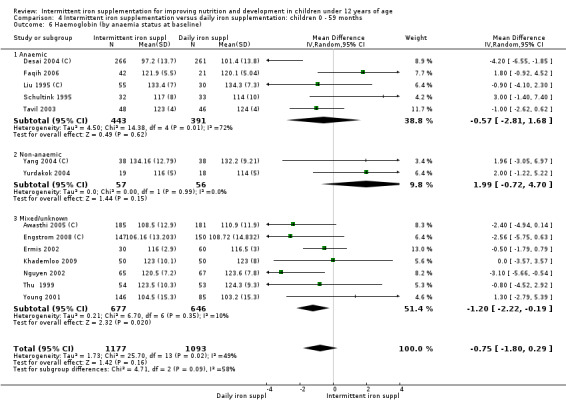
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 6 Haemoglobin (by anaemia status at baseline).
4.7. Analysis.
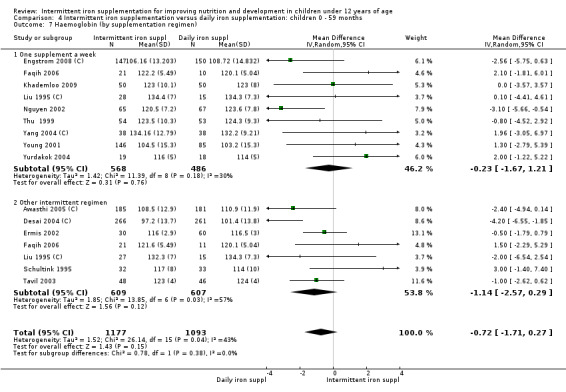
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 7 Haemoglobin (by supplementation regimen).
4.8. Analysis.
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 8 Haemoglobin (by sex).
4.9. Analysis.
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 9 Haemoglobin (by nutrient).
4.10. Analysis.
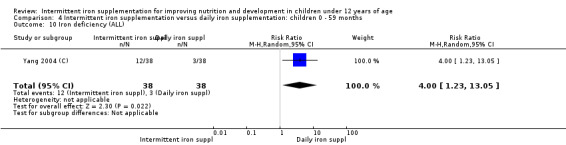
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 10 Iron deficiency (ALL).
4.11. Analysis.
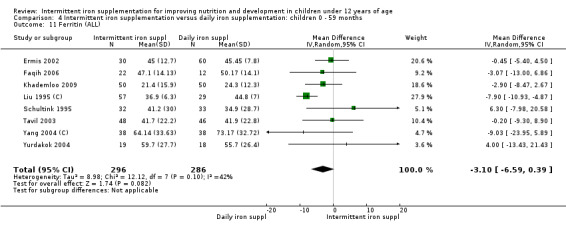
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 11 Ferritin (ALL).
4.12. Analysis.
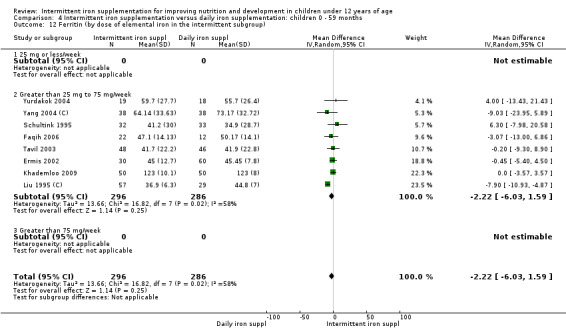
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 12 Ferritin (by dose of elemental iron in the intermittent subgroup).
4.13. Analysis.
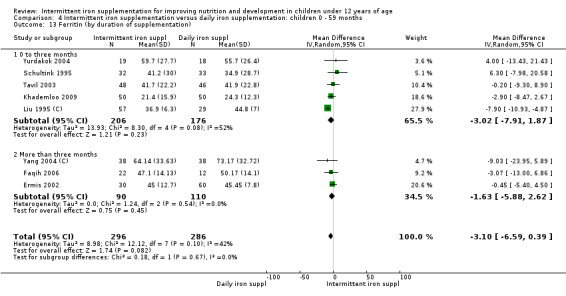
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 13 Ferritin (by duration of supplementation).
4.14. Analysis.
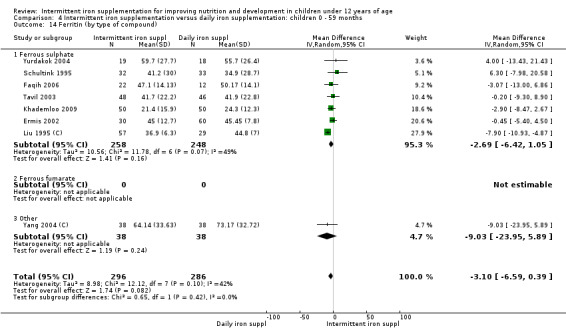
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 14 Ferritin (by type of compound).
4.15. Analysis.
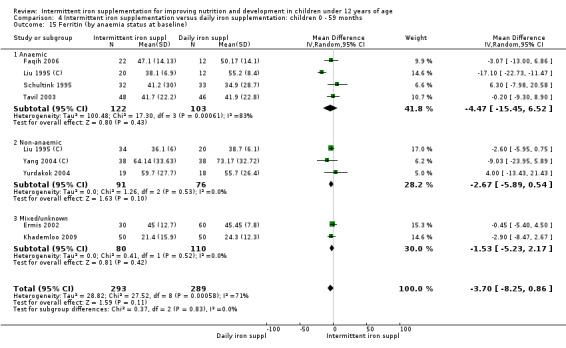
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 15 Ferritin (by anaemia status at baseline).
4.16. Analysis.
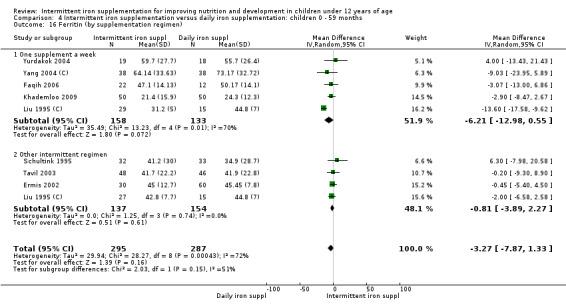
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 16 Ferritin (by supplementation regimen).
4.17. Analysis.
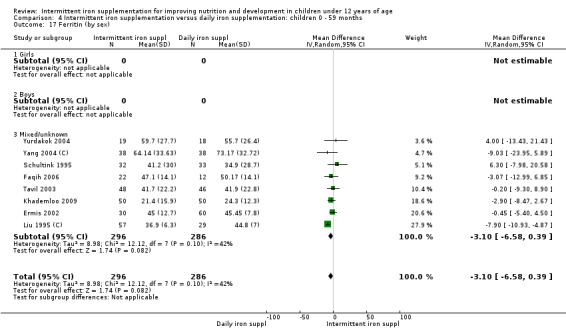
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 17 Ferritin (by sex).
4.18. Analysis.
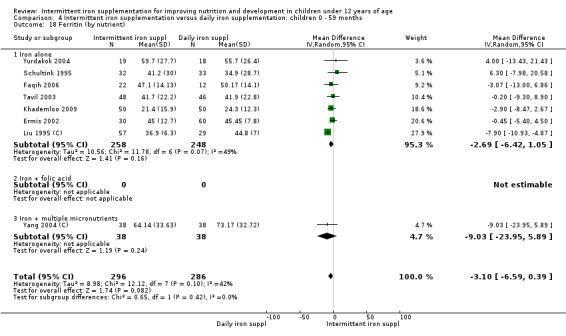
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 18 Ferritin (by nutrient).
4.19. Analysis.
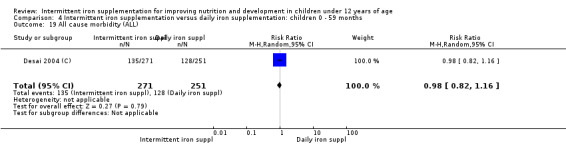
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 19 All cause morbidity (ALL).
4.20. Analysis.
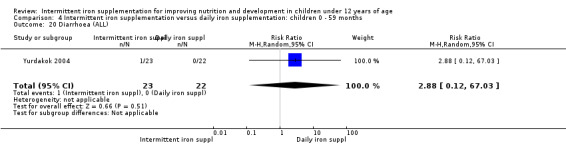
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 20 Diarrhoea (ALL).
4.21. Analysis.
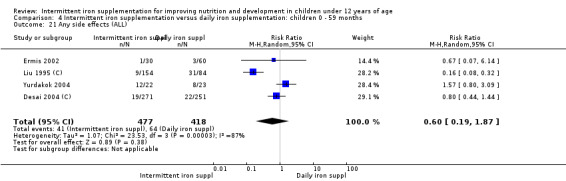
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 21 Any side effects (ALL).
4.22. Analysis.
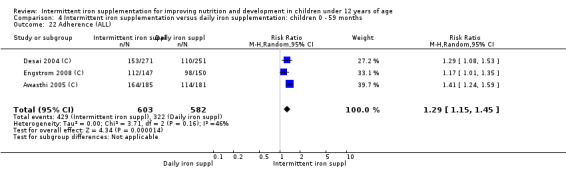
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 22 Adherence (ALL).
4.23. Analysis.
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 23 HAZ.
4.24. Analysis.
Comparison 4 Intermittent iron supplementation versus daily iron supplementation: children 0 ‐ 59 months, Outcome 24 WAZ.
Comparison 5. Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (ALL) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 2 Anaemia (by dose) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 2.1 25 mg or less/week | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Greater than 25 mg to 75 mg/week | 4 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.02] |
| 2.3 Greater than 75 mg/week | 2 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.48, 1.04] |
| 3 Anaemia (by duration) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 3.1 0 to three months | 2 | 848 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.89] |
| 3.2 More than three months | 4 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| 4 Anaemia (by type of compound) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 4.1 Ferrous sulphate | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 4.2 Ferrous fumarate | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Anaemia (by anaemia status at baseline) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 5.1 Anaemic | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.27] |
| 5.2 Non‐anaemic | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.31] |
| 5.3 Mixed/unknown | 4 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.54, 0.98] |
| 6 Anaemia (by intermittent regimen) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 6.1 One supplement a week | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 6.2 Other intermittent regimen | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Anaemia (by sex) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.86] |
| 7.1 Girls | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 7.2 Boys | 1 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 1.00] |
| 7.3 Mixed/unknown | 5 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.24, 1.01] |
| 8 Anaemia (by nutrient) | 6 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.90] |
| 8.1 Iron alone | 4 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.17, 0.90] |
| 8.2 Iron + folic acid | 2 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.03] |
| 8.3 Iron + multiple micronutrients | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Haemoglobin (ALL) | 10 | 1778 | Mean Difference (IV, Random, 95% CI) | 4.04 [0.30, 7.78] |
| 10 Haemoglobin (by dose of elemental iron in the intermittent group) | 10 | 1778 | Mean Difference (IV, Random, 95% CI) | 4.04 [0.30, 7.78] |
| 10.1 25 mg or less/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Greater than 25 mg to 75 mg/week | 6 | 1129 | Mean Difference (IV, Random, 95% CI) | 5.24 [‐0.78, 11.26] |
| 10.3 Group: greater than 75 mg/week | 4 | 649 | Mean Difference (IV, Random, 95% CI) | 1.84 [0.25, 3.44] |
| 11 Haemoglobin (by duration of the supplementation) | 10 | 1778 | Mean Difference (IV, Random, 95% CI) | 4.04 [0.30, 7.78] |
| 11.1 0 to three months | 3 | 973 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.49, 4.77] |
| 11.2 More than three months | 7 | 805 | Mean Difference (IV, Random, 95% CI) | 4.38 [‐1.20, 9.96] |
| 12 Haemoglobin (by type of iron compound) | 10 | 1778 | Mean Difference (IV, Random, 95% CI) | 4.04 [0.30, 7.78] |
| 12.1 Ferrous sulphate | 7 | 1415 | Mean Difference (IV, Random, 95% CI) | 4.59 [‐0.30, 9.47] |
| 12.2 Ferrous fumarate | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 3.4 [‐4.09, 10.89] |
| 12.3 Other | 2 | 238 | Mean Difference (IV, Random, 95% CI) | 1.79 [‐1.25, 4.84] |
| 13 Haemoglobin (by anaemia status at baseline) | 10 | 1778 | Mean Difference (IV, Random, 95% CI) | 4.04 [0.30, 7.78] |
| 13.1 Anaemic | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 18.30 [15.55, 21.05] |
| 13.2 Non‐anaemic | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐2.46, 6.46] |
| 13.3 Mixed/unknown | 8 | 1599 | Mean Difference (IV, Random, 95% CI) | 2.37 [1.17, 3.57] |
| 14 Haemoglobin (by supplementation regimen) | 10 | 1868 | Mean Difference (IV, Random, 95% CI) | 4.04 [0.45, 7.62] |
| 14.1 One supplement a week | 9 | 1647 | Mean Difference (IV, Random, 95% CI) | 4.43 [0.21, 8.65] |
| 14.2 Other intermittent regimen | 2 | 221 | Mean Difference (IV, Random, 95% CI) | 1.17 [‐1.27, 3.61] |
| 15 Haemoglobin (by sex) | 10 | 1778 | Mean Difference (IV, Random, 95% CI) | 4.03 [0.51, 7.55] |
| 15.1 Girls | 1 | 248 | Mean Difference (IV, Random, 95% CI) | 4.0 [0.83, 7.17] |
| 15.2 Boys | 1 | 253 | Mean Difference (IV, Random, 95% CI) | 3.70 [0.58, 6.82] |
| 15.3 Mixed/unknown | 9 | 1277 | Mean Difference (IV, Random, 95% CI) | 4.05 [‐0.37, 8.46] |
| 16 Haemoglobin (by nutrient) | 10 | 1778 | Mean Difference (IV, Random, 95% CI) | 4.04 [0.30, 7.78] |
| 16.1 Iron alone | 6 | 1022 | Mean Difference (IV, Random, 95% CI) | 4.98 [‐0.71, 10.68] |
| 16.2 Iron + folic acid | 4 | 756 | Mean Difference (IV, Random, 95% CI) | 2.91 [0.65, 5.16] |
| 16.3 Iron + multiple micronutrients | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Ferritin (ALL) | 1 | 240 | Mean Difference (IV, Random, 95% CI) | 16.6 [11.12, 22.08] |
| 18 All cause morbidity (ALL) | 1 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.24] |
| 19 Any side effects (ALL) | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 3.87 [0.19, 76.92] |
| 20 Nausea | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.12, 66.82] |
| 21 IQ (ALL) | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐5.96, ‐0.04] |
| 22 Thai language (ALL) | 1 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.50, ‐0.09] |
| 23 Mathematics (ALL) | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.44, ‐0.10] |
| 24 Increase in steps climbed (ALL) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 8.0 [‐0.72, 16.72] |
| 25 WAZ | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.74, 0.25] |
| 26 HAZ | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.69, 0.21] |
5.1. Analysis.
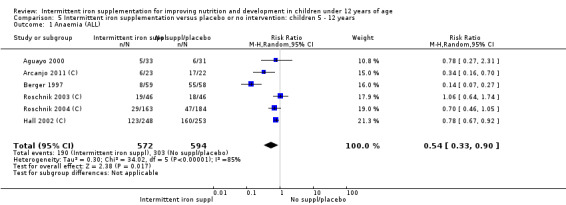
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 1 Anaemia (ALL).
5.2. Analysis.
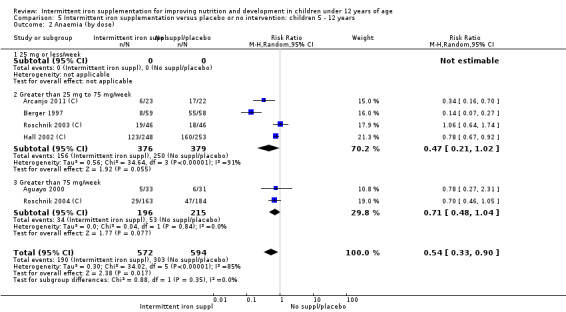
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 2 Anaemia (by dose).
5.3. Analysis.
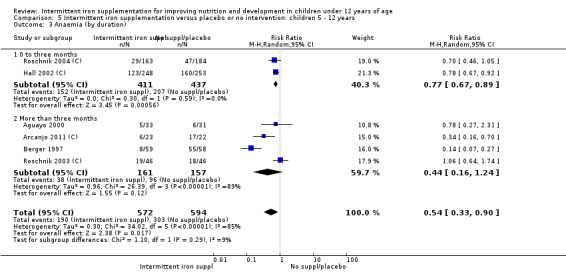
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 3 Anaemia (by duration).
5.4. Analysis.
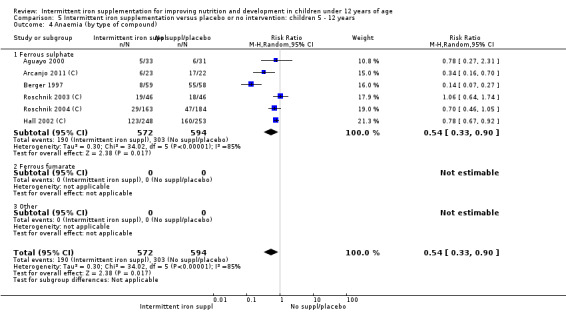
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 4 Anaemia (by type of compound).
5.5. Analysis.
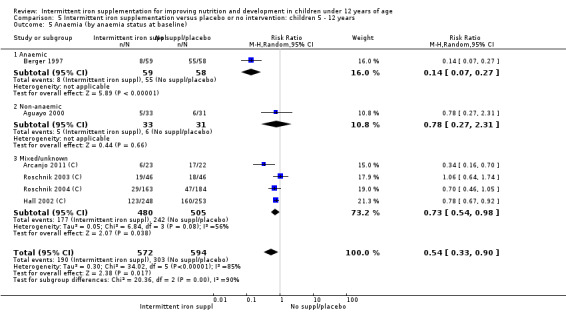
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 5 Anaemia (by anaemia status at baseline).
5.6. Analysis.
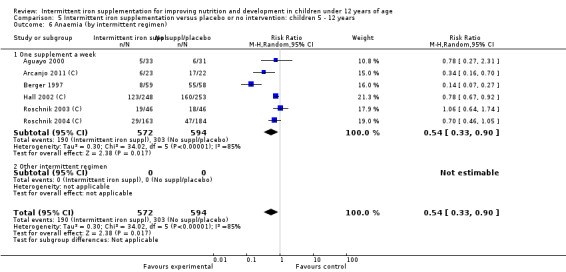
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 6 Anaemia (by intermittent regimen).
5.7. Analysis.
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 7 Anaemia (by sex).
5.8. Analysis.
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 8 Anaemia (by nutrient).
5.9. Analysis.
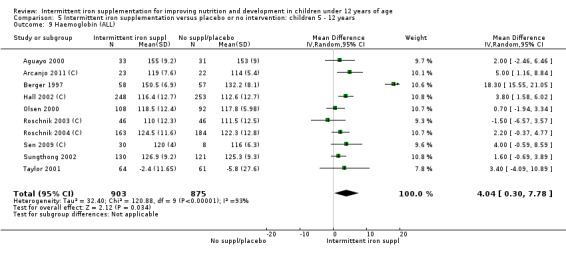
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 9 Haemoglobin (ALL).
5.10. Analysis.
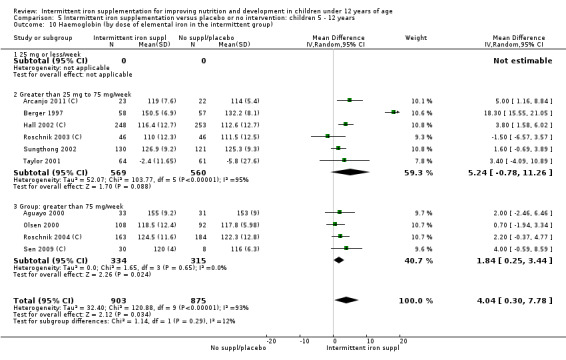
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 10 Haemoglobin (by dose of elemental iron in the intermittent group).
5.11. Analysis.
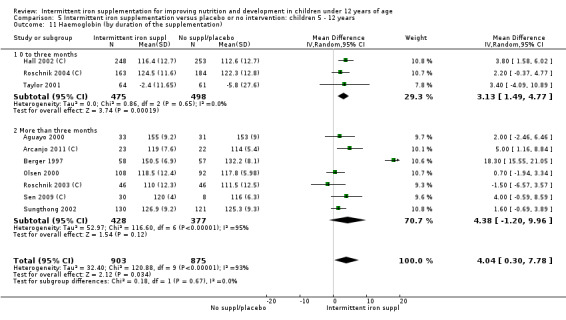
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 11 Haemoglobin (by duration of the supplementation).
5.12. Analysis.
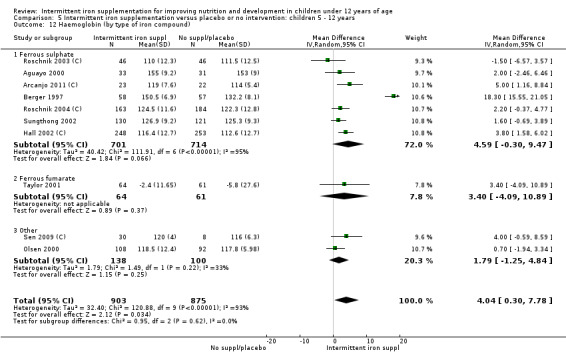
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 12 Haemoglobin (by type of iron compound).
5.13. Analysis.
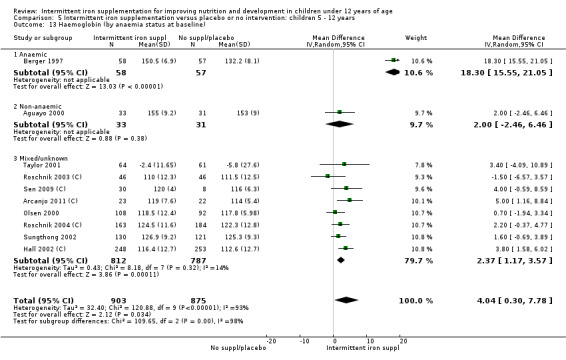
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 13 Haemoglobin (by anaemia status at baseline).
5.14. Analysis.
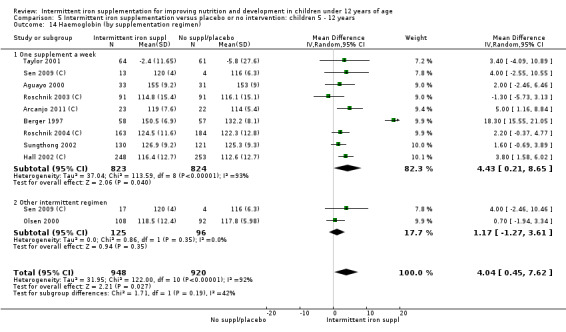
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 14 Haemoglobin (by supplementation regimen).
5.15. Analysis.
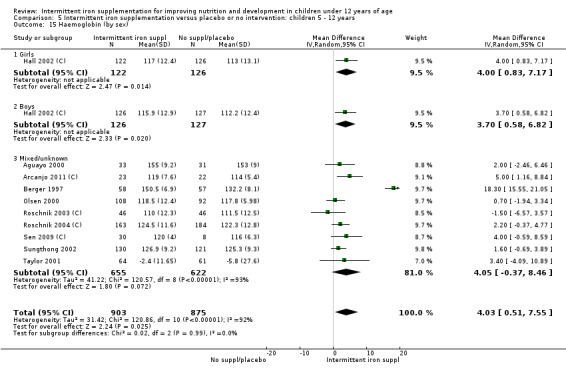
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 15 Haemoglobin (by sex).
5.16. Analysis.
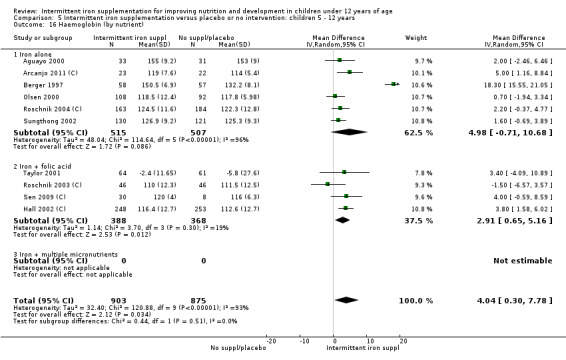
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 16 Haemoglobin (by nutrient).
5.17. Analysis.
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 17 Ferritin (ALL).
5.18. Analysis.
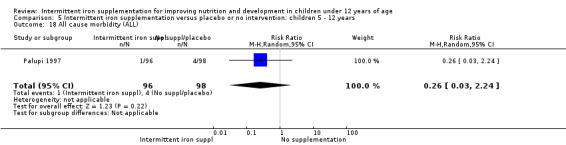
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 18 All cause morbidity (ALL).
5.19. Analysis.
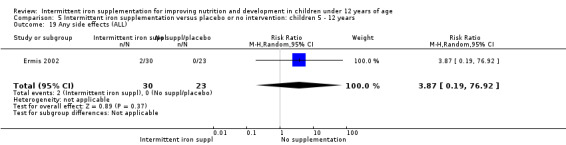
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 19 Any side effects (ALL).
5.20. Analysis.
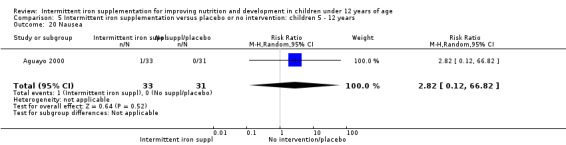
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 20 Nausea.
5.21. Analysis.
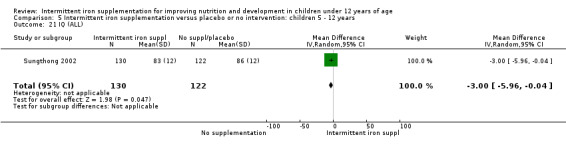
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 21 IQ (ALL).
5.22. Analysis.
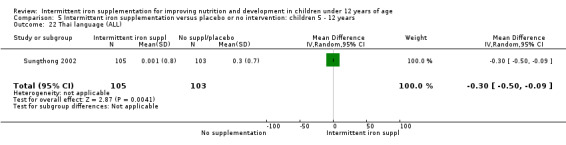
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 22 Thai language (ALL).
5.23. Analysis.
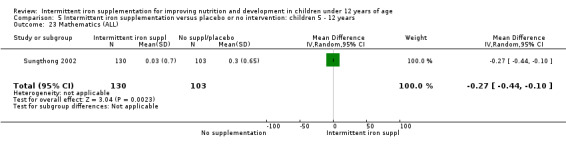
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 23 Mathematics (ALL).
5.24. Analysis.
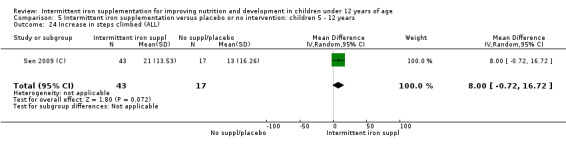
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 24 Increase in steps climbed (ALL).
5.25. Analysis.
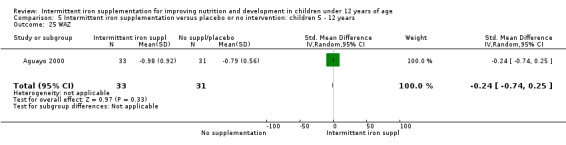
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 25 WAZ.
5.26. Analysis.
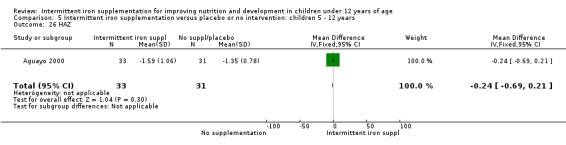
Comparison 5 Intermittent iron supplementation versus placebo or no intervention: children 5 ‐ 12 years, Outcome 26 HAZ.
Comparison 6. Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (ALL) | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.47, 1.91] |
| 2 Haemoglobin (ALL) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 3 Haemoglobin (by dose of elemental iron) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 3.1 25 mg or less/week | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Greater than 25 mg to 75 mg/week | 3 | 444 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐3.01, 0.80] |
| 3.3 Intermittent group: greater than 75 mg/week | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐4.68, 6.68] |
| 4 Haemoglobin (by duration of the supplementation) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 4.1 0 to three months | 2 | 155 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐6.54, 7.18] |
| 4.2 More than three months | 3 | 426 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐2.12, 0.84] |
| 5 Haemoglobin (by type of compound) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 5.1 Ferrous sulphate | 4 | 539 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐2.63, 2.71] |
| 5.2 Ferrous fumarate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Other | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐5.43, 1.43] |
| 6 Haemoglobin (by baseline prevalence of anaemia) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 6.1 Anaemic | 3 | 271 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐3.44, 4.17] |
| 6.2 Non‐anaemic | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mixed/unknown | 2 | 310 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐3.08, 0.63] |
| 7 Haemoglobin (by supplementation regimen) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 7.1 by supplementation regimen: one supplement a week | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 7.2 by supplementation regimen: other intermittent regimen | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Haemoglobin (by sex) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 8.1 Girls | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐5.43, 1.43] |
| 8.2 Boys | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Mixed/unknown | 4 | 539 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐2.63, 2.71] |
| 9 Haemoglobin (by nutrient) | 5 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐2.59, 1.97] |
| 9.1 Iron alone | 4 | 539 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐2.63, 2.71] |
| 9.2 Iron + folic acid | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐5.43, 1.43] |
| 9.3 By nutrient: iron + multiple micronutrients | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Ferritin (ALL) | 2 | 320 | Mean Difference (IV, Random, 95% CI) | ‐11.57 [‐38.75, 15.61] |
| 11 All cause morbidity (ALL) | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.24] |
| 12 Diarrhoea (ALL) | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.56, 2.22] |
| 13 Adherence (ALL) | 2 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.44, 3.75] |
| 14 IQ (ALL) | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐5.96, ‐0.04] |
| 15 Thai language (ALL) | 1 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.50, ‐0.09] |
| 16 Mathematics (ALL) | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.44, ‐0.10] |
| 17 Increase in steps climbed (ALL) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐13.34, 3.34] |
| 18 HAZ | 2 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.26, 0.63] |
| 19 WAZ | 2 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.21, 0.39] |
| 20 WAZ | 2 | 302 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.34, 0.41] |
6.1. Analysis.
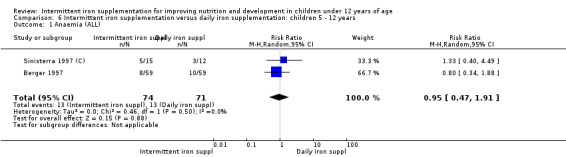
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 1 Anaemia (ALL).
6.2. Analysis.
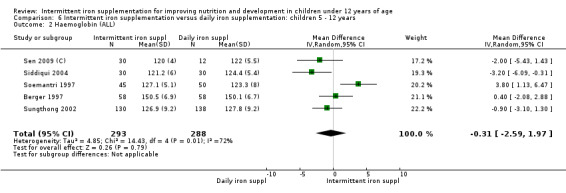
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 2 Haemoglobin (ALL).
6.3. Analysis.
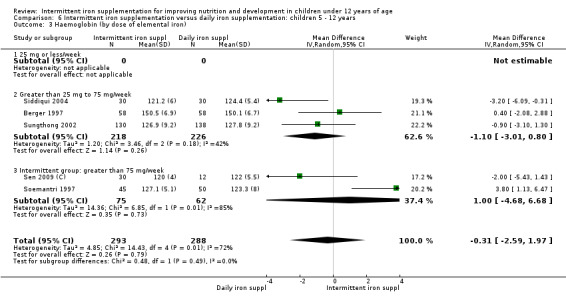
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 3 Haemoglobin (by dose of elemental iron).
6.4. Analysis.
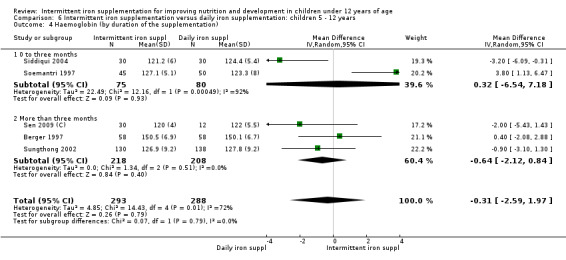
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 4 Haemoglobin (by duration of the supplementation).
6.5. Analysis.
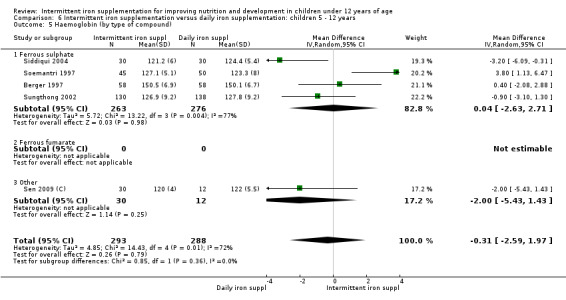
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 5 Haemoglobin (by type of compound).
6.6. Analysis.
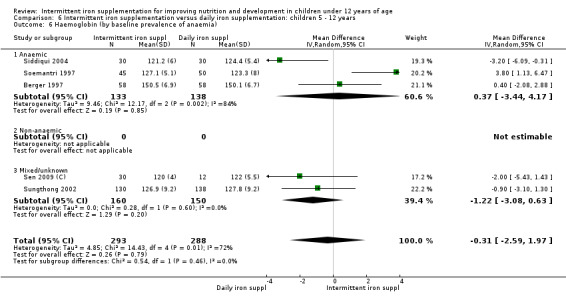
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 6 Haemoglobin (by baseline prevalence of anaemia).
6.7. Analysis.
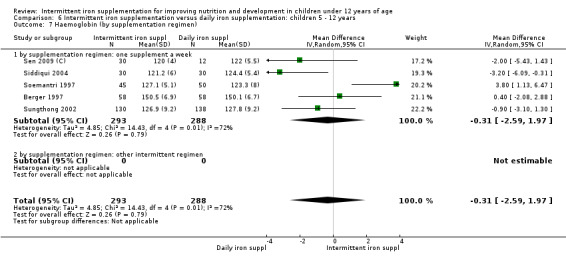
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 7 Haemoglobin (by supplementation regimen).
6.8. Analysis.
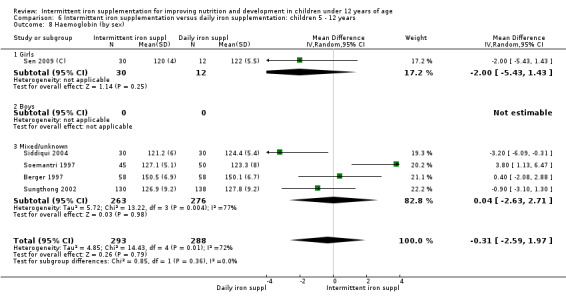
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 8 Haemoglobin (by sex).
6.9. Analysis.
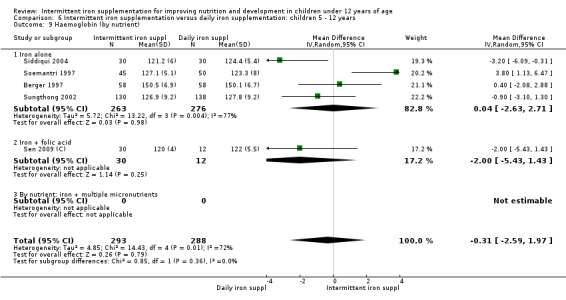
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 9 Haemoglobin (by nutrient).
6.10. Analysis.
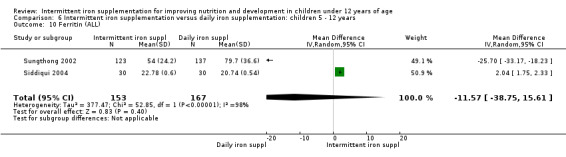
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 10 Ferritin (ALL).
6.11. Analysis.
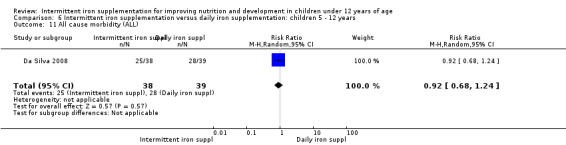
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 11 All cause morbidity (ALL).
6.12. Analysis.
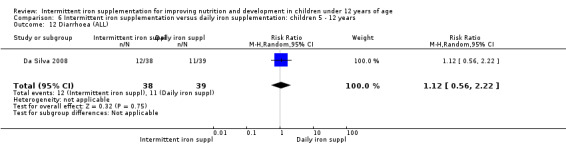
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 12 Diarrhoea (ALL).
6.13. Analysis.
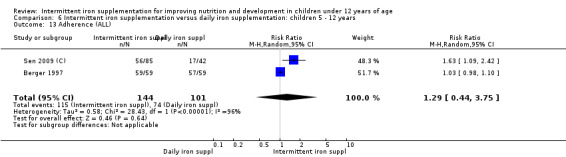
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 13 Adherence (ALL).
6.14. Analysis.
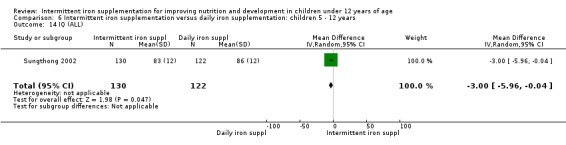
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 14 IQ (ALL).
6.15. Analysis.
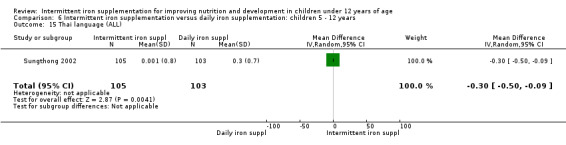
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 15 Thai language (ALL).
6.16. Analysis.
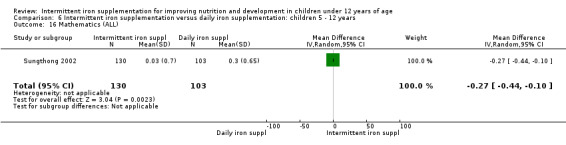
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 16 Mathematics (ALL).
6.17. Analysis.
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 17 Increase in steps climbed (ALL).
6.18. Analysis.
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 18 HAZ.
6.19. Analysis.
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 19 WAZ.
6.20. Analysis.
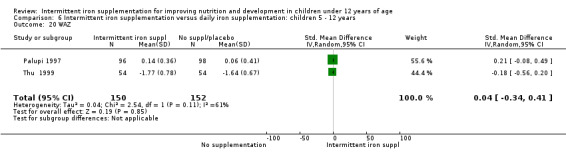
Comparison 6 Intermittent iron supplementation versus daily iron supplementation: children 5 ‐ 12 years, Outcome 20 WAZ.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aguayo 2000.
| Methods | Randomised double‐blind placebo‐controlled trial. 2‐arm design with individual randomisation. | |
| Participants | 73 children (64 children followed up), both sexes (30 females (47%)), aged 6–11.9 years (9 years in average), from outskirts of La Paz, Bolivia (4000 m above sea level). Inclusion criterion: non‐anaemic. Socioeconomic status not reported. | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 37): children received weekly tablets containing iron. The iron dose was calculated to provide children with 3 mg of elemental iron per kg of body weight (approximately 85 mg of iron per week). The supplement consisted of two types of tablets containing either 20 mg or 36 mg of elemental iron (as ferrous sulphate). These tablets were used in combination to adjust the dose to the child’s weight; Group 2 (n = 36): children received a placebo similar in colour and appearance to the iron supplement. Length of the intervention: 18 weeks |
|
| Outcomes | Haemoglobin, mean haemoglobin change, anaemia, anthropometric measurements (weight for age Z‐score, height for age Z‐score and mid‐upper arm circumference), and side effects. | |
| Notes | A teacher trained by the principal investigator was responsible for delivering the iron tablets in the classrooms. All children completed at least 17 doses. Pills were administered on Wednesday and students who were not in school on Wednesday were administered the supplements on Thursday. Z‐scores used the National Center for Health Statistics data as a reference. Non‐malaria area. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomly assigned to the treatment or the control group using a table with randomly assorted digits. |
| Allocation concealment (selection bias) | Low risk | A teacher trained by the principal investigator was responsible for the delivery of the iron tablets in the classrooms. The teacher was provided with a list of the names of the children and the number and kind of pills (colour coded) each child should take every week. Neither the teacher nor the assistant were aware of the composition of the tablets delivered to the children and tablets were similar in appearance. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Tablets were similar in appearance. Participants:Children were not aware of the treatment. Personnel: Neither the teacher nor his assistant were aware of the composition of the tablets delivered to the children Outcome assessors: not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A complete set of data was obtained for 33 children in the treatment group (89.2 %) and for 31 children (86.1 %) in the control group |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | No significant differences at baseline in the variables studied,and females/males ratio. No differences at baseline between those that completed the study and those who dropped out. |
Arcanjo 2011 (C).
| Methods | Cluster‐randomised, placebo‐controlled double‐blind trial. 2 arm design with randomisation at classroom level. | |
| Participants | 106 preschool children, both sexes (56 females (52.8%), aged 5 years. The study was conducted in a public school located in the City of Sobral, in the northeast of Brazil between September and December 2009. Exclusion criteria: current supplement intake. Baseline prevalence of anaemia: 58.5%. Forty per cent of the families had an income <300 USD. | |
| Interventions | Classrooms were allocated to one of the following groups: Group 1 (3 classrooms, 52 children): children received once a week 50 mg of elemental iron (as ferrous sulphate heptahydrate) once a week; Group 2 (3 classrooms, 54 children): children received once a week a placebo (on Wednesdays). The placebo contained 2 ml of natural colour additive, annatto, which is odourless and tasteless, providing a yellow–orange colour similar to that of the elemental iron used in the study. Length of the intervention: 14 weeks. |
|
| Outcomes | Haemoglobin, hematocrit and anaemia (Hb less than 115 g/L) | |
| Notes | The supplements were administered on Wednesdays. The supplement was administered by a teacher using a plastic medical syringe with scale to squirt the composition into the child’s mouth. The syringes were prepared on an individual basis by medical staff. We adjusted the results of this study to account for the effect of clustering in data; the estimated effective sample size was used in the analyses. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An allocation code was generated with a table of random numbers for randomizations of schools and classes. |
| Allocation concealment (selection bias) | Low risk | The study used a placebo. Since randomisation occurred at classroom level, it is unlikely a selection bias at individual level. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: were not aware of different interventions. Personnel: the teacher was not aware of the treatment nor involved in data collection. Ouctome assessors: the staff involved in data collection was blinded with regard to the intervention and placebo groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | During the study, there were 2 (3.8%) dropouts in group 1, and 5 (9.2%) dropouts in group 2. Intention to treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | The data was not adjusted by the effect of clustering. Anaemia prevalence at baseline was not balanced between groups: 48% in group 1 and 69% in group 2 (but similar concentrations of haemoglobin). |
Awasthi 2005 (C).
| Methods | Cluster‐randomised community effectiveness trial. 2 arm design with randomisation at subcentre level. | |
| Participants | 803 children, both sexes (730 females (45.4%)), aged 3‐6 years, living in sub centres of Shahpur Baxolia and Sipa Hidayatpur from Nindura Block, Barabanki district, North India. Exclusion criteria: those without written informed consent, or those likely to move within the next three months. Children identified as severely anaemic were given iron and folic acid in therapeutic doses under close supervision (but does not say they were excluded). Baseline prevalence of anaemia in children was 53.79 %. Socioeconomic status not reported. | |
| Interventions | Sub centres were allocated to one of the following groups: Group 1 (n = 403): children in Shahpur Baxolia sub centre received tablets containing 20 mg elemental iron (presumably in form of ferrous sulphate) iron and 100 μg (0.1 mg) folic acid twice a week, on fixed days (Wednesday and Saturday); Group 2 (n = 400): children in Sipa Hidayatpur sub centre received one tablet daily. Length of the intervention: one year. |
|
| Outcomes | Haemoglobin, haemoglobin mean change, anaemia, and adherence. | |
| Notes | Iron and folic acid was given to the children either by the Anganwadi worker, if they were registered and used the informal education services of the Integrated Child Health Development Services, or by the mother for non‐registered children. Mothers could pick up monthly supplies for their children one day a month from an Anganwadi centre. A monitoring in‐charge was responsible for each intervention type. He visited each Anganwadi centre every 15 days to take an account of the IFA distributed to registered children. The monitor in‐charge also visited 20 randomly selected houses of non‐registered children and collected information about the IFA tablet intake, including the number of pills consumed. Sample size was calculated taking into consideration a design effect of 2. We adjusted the results of this study to account for the effect of clustering in data; the estimated effective sample size was used in the analyses. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | For this study all sub centres were listed alphabetically, serially numbered, and two were selected by random for assessment of the interventional strategies, one per sub‐centre. It is unclear whether the allocation to the treatment was at random. |
| Allocation concealment (selection bias) | Low risk | Since the intervention was allocated at sub‐centre level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported. Personnel: Not reported. Outcome assessors: Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow up 8.34% at one year with no difference between groups (biweekly 8.1% versus daily 8.5%). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Some children had directly observed intake and others were given the pills by the mother. About 1/3 of the children are registered to obtain services of the Anganwadi centre (under the ICDS services) and this had a differential effect on supplementation (favouring registered children). Results did not account for the cluster effect. |
Baqui 2003.
| Methods | Randomised, double‐blinded community‐based trial. 5‐arm design with individual randomisation. | |
| Participants | 799 Bangladeshi children, both sexes (406 females (50.8%)), enrolled at 5‐6 months of age for a 6 month study (12 mo old when completed). Potential families were identified through ongoing health and demographic surveillance system. Participants were eligible if did not receive infant formula, were not severely malnourished (mid‐upper arm circumference >110mm), not severely anaemic (haemoglobin >90 g/L), with no obvious neurologic disorders, physical disabilities, or chronic illnesses that might affect feeding, activity, and cognitive development. There were no differences in monthly income, household size or father's education across the arms. Approximately two‐thirds of the children were mildly anaemic at recruitment. | |
| Interventions | Infants were randomly allocated to one of the following groups: Group 1 (n = 154): Infants received once a week multiple micronutrients in a dose that doubled the recommended dietary allowance (WHO standards) of thiamine, niacin, folic acid, pantothenic acid, iodine, copper, manganese, selenium, and vitamins C, D, E, B6 and B12. It contained 20 mg elemental iron (as ferrous sulphate), 20 mg elemental zinc (as zinc acetate), and 1 mg riboflavin. Group 2 (n = 161): Infants received once a week 20 mg elemental iron and 1 mg riboflavin. Group 3 (n = 161): Infants received once a week 20 mg of elemental zinc and 1 mg riboflavin. Group 4 (n = 162): Infants received once a week 20 mg of elemental zinc, 20 mg elemental iron and 1 mg riboflavin. Group 5 (n = 157): Infants received riboflavin (control). For the purpose of this review, groups 1, 2 & 4 were merged and compared with group 5. Length of the intervention: 6 months. |
|
| Outcomes | Ferritin, diarrhoea, ALRI, physical growth, mental, motor, behavioral development from 6 to 12 month (measured using Bayley II scales of infant development), adherence. Data on diarrhoea and ALRI was not combined as it is reported in incidence rate/(child‐y) | |
| Notes | Supplements were prepared as capsules, which were mixed with flavoured syrup and fed to infants by community health workers. All supplements had similar taste and appearance and all groups also received 100,000 IU of vitamin A at the beginning of the study, in line with national policy in Bangladesh. Trial with sub‐studies with different sample sizes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to the study groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Each study infant received the assigned supplement in the same type of capsules and labelled in such a way that the various types of supplements could not be differentiated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as doubled‐blinded clinical trial. Each study infant received the assigned supplement in the same type of capsules and labelled in such a way that the various types of supplements could not be differentiated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop out rate much higher (41%) in the MM group than in other groups (8‐19%). Motor/Cognitive outcomes: 125 kids (36%) did not complete 12 mo‐assessment, leaving 221 children in final sample. There were no differences among arms or major sociodemographic variables for dropouts. 16.3% did not undergo evaluation with HOME scale. 5% did not have haemoglobin data at 12 mo, 1.8% did not have anthropometric data at 12 mo but did for other measures. |
| Selective reporting (reporting bias) | High risk | Trial with sub studies with different sample sizes. |
| Other bias | Unclear risk | No discussion of adjustment or exclusion for inflammation for iron status analysis. |
Berger 1997.
| Methods | Double‐blind randomised controlled trial. 3‐arm design with individual randomisation. | |
| Participants | 176 children, both sexes (91 females (52%)), aged 3.3‐8.3 years (69 months old in average), attending the schools administered by the non‐governmental organization "Fe y Alegria" located in a socio‐economically disadvantaged district of La Paz, Bolivia (altitude of 4000 m above sea level). Inclusion criterion: anaemia (haemoglobin concentration equal to or lower than 144 g/L). No additional exclusion criteria listed. Socioeconomic status not reported. | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 59): children received every Tuesday 3‐4 mg of iron per kg of body weight (approximately 60‐80 mg per week); Group 2 (n = 59): children received a daily dose of 3‐4 mg of iron per kg of body weight, 5 days per week, Monday to Fri. Daily group received 5 times as much iron as weekly; Group 3 (n = 58): children received a placebo, once a week, every Tuesday. Placebo consisted of same tablets without iron. Supplements given to groups 1 and 2 consisted of two types of tablets containing either 20 mg or 36 mg of elemental iron in form of ferrous sulphate. These tablets were used in combination to adjust the dose to the child’s weight Length of the intervention: 16 weeks |
|
| Outcomes | Haemoglobin, change in haemoglobin, anaemia, zinc erythrocyte protoporphyrin, adherence. | |
| Notes | Tablets were given to children at school, with clean, boiled water, at mid morning, by trained school assistants, under the supervision of a member of the research team. Same tablets were used for weekly, daily, and same tablets without iron were used for placebo. Non malaria area. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly assigned to one of three groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Method of concealment not described, but the study reported as double blind. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind trial. Same tablets were used for weekly, daily, and same tablets without iron were used for placebo. Participants: children were not aware of the treatment Personnel: personnel were not aware of the treatment Outcome assessors: not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one person lost to follow‐up in each group, 3 people total. Dropouts were due to migration of the family out of the area of study |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Da Silva 2008.
| Methods | Randomised controlled trial. 3‐arm design with randomisation at individual level. | |
| Participants | 135 children (114 followed up, 54 female (47%)), both sexes, aged 5 to 6.9 months, from Vicosa, the Southeast of Brazil. Children were identified from live birth forms and parents were interviewed; parents who were interested in participating were recruited (213 children were screened, 78 infants with anaemia were excluded and treated). Inclusion criteria: non‐anaemic infants (Hb equal to or greater than 110 g/L), living in urban area; full term, singleton births; birth weight > 2500 g; mother aged > 19 years old; no neonatal abnormalities or chronic disease; no previous iron supplements; non‐exclusive breastfeeding. Maternal years of education ranged between 4 and 11 years (mean approximately 8 years). | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 51): infants received 1 mg of elemental iron/kg/day (as liquid ferrous sulphate); Group 2 (n = 42): infants received 2 mg of elemental iron/kg/day (as liquid ferrous sulphate); Group 3 (n = 42): infants received 25 mg elemental iron once a week (as liquid ferrous sulphate). Length of the intervention: 16 weeks For the purpose of this review only groups 2 and 3 were compared as the overall dose of iron given to the children was similar between them. |
|
| Outcomes | Height, weight and change scores for height and weight (with Z‐scores), morbidity (diarrhoea, fever, cough, nasal congestion, wheezing). | |
| Notes | Supplements were provided free to all groups and participants were advised to take 1 hour before meals. Z scores used the World Health Organization data as a reference. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number list (method communicated by the author). |
| Allocation concealment (selection bias) | High risk | Open random allocation schedule. Children were enrolled to the study in a row; there was a list showing the sequence in which children would be allocated to the groups (method communicated by the author). |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported. Personnel: Not reported. Outcome assessors: Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 135 children were randomised. 114 completed the intervention (84%). Loss was not balanced across groups: 12/51 lost from group 1, 6/42 from group 2, 3/42 from group 3. Reasons for loss included patient withdrawal (7) supplement intolerance (6) anaemia (2) and other reasons. It was not clear how many withdrew from each group for these reasons. It was stated that analysis was based on an intention to treat principle, irrespective of adherence, but those lost to follow up did not appear to be included in the analysis, although denominators were not clear in the data tables. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Desai 2004 (C).
| Methods | Cluster‐randomised trial. 2x2 factorial design in which housing compounds were the unit of randomisation. | |
| Participants | 1049 children, both sexes (519 females (49.5%)), aged 2‐59 months (27 months in average), living in 14 villages in Asembo, Bondo district, Nyanza Province, western Kenya. Inclusion criteria: haemoglobin 50‐109 g/L (anaemic); asexual parasite count <20,000/mm; no history of intake of iron, sulphadoxine‐pyrimethamine or amodiaquine use, or blood transfusion within the last 2 weeks, no known sickle cell disease Baseline prevalence of anaemia in children was 74%. Caretakers had a median of 6 or more years of education across all arms and 48.6% of households had a wealth score above the median. |
|
| Interventions | Compounds were allocated to one of the following groups at baseline: Group 1 (n = 266): children received two doses of 3‐6 mg/kg each, separated by 3‐4 days (total dose per week: 6‐12 mg/kg; approximately 36‐72 mg of iron per week). Supervised; Group 2 (n = 271): children received two doses of 3‐6 mg/kg each, separated by 3‐4 days (total dose per week: 6‐12 mg/kg). Unsupervised; Group 3 (n = 261): children received one daily dose of 3‐6 mg/(kg per day). Supervised; Group 4 (n = 251): children received one daily dose of 3‐6 mg/(kg per day). Unsupervised Target iron dose was ferrous sulphate syrup 40 g/L, 27.5% elemental iron. Iron doses were based on body weight (<5 kg: 1.25 mL/d, 5‐10 kg: 2.5 mL/d, >10 kg: 5.0 mL/d). No folic acid was given. Supervised arms (Groups 1 and 3) were used to assess the haematological response while unsupervised groups (2 and 4) provided data on adherence and side effects. Length of the intervention: 6 weeks. |
|
| Outcomes | Haemoglobin, haemoglobin mean change, hematological recovery, microcytosis, all‐cause morbidity, clinical malaria, malaria parasitaemia, adherence. | |
| Notes | All parents received the 6‐week supply of oral iron and received identical instructions in the local language about use, expected side effects, safety and correct dose of iron supplementation. To determine differences in the duration of any treatment effect on Hb levels, children were seen again at 12 wk (1 d) The mean cluster size was 1.5 children per compound, and the reported design effect was 1.035. Standard errors were adjusted for clustering at the compound level. Malaria‐endemic area. All arms were given single treatment dose of sulfadoxine‐pyrimethamine (SP). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number listing was used to sequentially assign eligible children to 1 of 4 treatment groups, using the housing compound as the randomisation unit. |
| Allocation concealment (selection bias) | Low risk | Plastic screw top bottles used, labelled with personal identifiers and dosing instructions. Since the intervention was allocated at compounds level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants:were aware of the treatment assigned. Personnel: no blinding Outcome assessors: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.9% (n = 93) and equally divided among the four arms. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Children lost to follow up had lower (P=0.01) haemoglobin concentrations at enrolment than those successfully followed for 6 wk, but were not different for other characteristics. None of the characteristics differed among the groups after excluding children lost to follow up. 6 children (4 compounds) excluded from analyses at 6 wk follow up due to missing haemoglobin values. No discussion on adjustment/exclusion for inflammation. |
Ekvall 2000.
| Methods | Randomised trial. 2‐arm design with individual randomisation. | |
| Participants | 207 children, both sexes (sex distribution unknown), 5 months‐3 years of age, living in Fukayosi village, Bagamoyo district of coastal Tanzania, from June to November 1995, during the seasonal peak of perennial malaria transmission. Exclusion criteria: migration plans, the presence of congenital malformations and Hb concentration, 50 g/L at baseline, requiring immediate treatment. Baseline prevalence of anaemia in children was 89% (Hb lower than 110 g/L). Socioeconomic status not reported. | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 104): children received three times a week 1 mL of a micronutrient preparation containing 10 mg iron (as ferrous sulphate), 1500 IU vitamin A, 400 IU vitamin D, 5 IU vitamin E, 35 mg vitamin C, 0.5 mg vitamin B1, 0.6 mg vitamin B2, 8 mg niacin and 0.4 mg vitamin B6; Group 2 (n = 103): children received three times a week 1 mL of a placebo (1 mg of promethazine hydrochloride). Iron compound and weekly dose: 30 mg of elemental iron (as ferrous sulphate) per week. Length of the intervention: 5 months. |
|
| Outcomes | Haemoglobin, mean cell volume as an indicator of iron status, clinical malaria, fever, adherence. | |
| Notes | All children were to receive a total of 56 doses over 5 months administered during home visits by six research assistants who were assigned 30–35 children each. Malaria holoendemic area. For active case detection of clinical malaria episodes, all children were seen fortnightly by the research team at the village dispensary for axillary temperature measurement. Children with malaria received chloroquine syrup (25 mg/kg over three days), and additional treatment with sulphadoxine pyrimethamine (SP) was given if a child showed clinical signs of treatment failure. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The children were randomly allocated to the supplement group or the placebo group by a computer‐generated number table. |
| Allocation concealment (selection bias) | Low risk | The supplement and placebo had different colours to facilitate correct administration. However, neither the research assistants involved in the project nor the mothers of the children knew the treatment code. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The supplement and placebo had different colours to facilitate correct administration. Participants: mothers did not know the treatment code Personnel: research assistants did not know the treatment code Ouctome assessors: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 children were lost to follow up in each group (6%). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Engstrom 2008 (C).
| Methods | Cluster‐randomised trial. 3 arm design in which health facilities were the unit of randomisation. | |
| Participants | 391 children, both sexes (184 females (47%)), 6 months old. Study carried out through primary healthcare units in Rio de Janeiro, Brazil. 15 health care centres (6 intervention, 9 control). Inclusion criteria: absence of iron supplementation in the month preceding recruitment and negative for sickle cell anaemia. Baseline prevalence of anaemia (taken from the control group): 60.4%. Socioeconomic status:approximately 30% of the mothers worked outside the home; most families (> 90%) had access to radio and television, but < 20% had access to a car. |
|
| Interventions | Health facilities were allocated to one of the following groups: Group 1 (n = 188): children received weekly supplementation with 25 mg of elemental iron (as oral ferrous sulphate) per week in syrup and education on anaemia and diet; Group 2 (n = 188): children received daily supplements containing 12.5 mg elemental iron dailyand education on anaemia and diet; Group 3 (n = 94): children received received no intervention and was recruited retrospectively. Length of the intervention: 24 weeks. For the purposes of this review we only compared groups 1 and 2. |
|
| Outcomes | Haemoglobin, anaemia (Hb <110 g/L) and adherence. | |
| Notes | Analyses were performed taking into account cluster sampling. Non‐malaria area. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Healthcare units were randomly selected. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Not reported. Since the intervention was allocated at health care unit level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Mothers were aware of supplements Personnel: Clinic staff were aware of supplements Outcome assessors: Unlikely Control group identified retrospectively so they were not aware of trial during treatment phase. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 38/188 (20.2%) were lost to follow up the daily group and 41/188 (21.8%) in the weekly group. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Baseline characteristics were similar for most variables. Regression analysis was carried out to identify possible confounders and where possible confounders accounted for at least 10% of variation they were entered into the final model. However for anaemia no confounders were maintained in the final regression analysis. |
Ermis 2002.
| Methods | Randomised placebo‐controlled trial. 4‐arm design with individual randomisation. | |
| Participants | 113 infants, both sexes (56 females (50%)), 5‐month old, receiving routine paediatric care at the Research hospital of Karaelmas University in Zonguldak, Turkey. Inclusion criteria: no gestational problems (hypertension, preeclampsia, infection), no congenital anomalies, no neonatal complications, no emergency caesarian delivery, no jaundice requiring phototherapy, no hospitalisation, no chronic illness, no iron therapy, no formula feeding. Must have been exclusively breasted, birthweight > 3.0 kg and gestational age of > 37 weeks. Exclusion: Hb < 95 g/L, serum ferritin <12 ng/mL, MCV < 74 fl or infection during iron supplementation. Children were eliminated from the study if compliance was lower than 75%. 58.6%‐74. Baseline prevalence of anaemia not reported. One percent of the mothers of participants included in the study graduated from high school or university. | |
| Interventions | Infants were allocated to one of the following groups: Group 1 (n = 30): infants were given a supplement containing 1 mg iron/kg (as ferrous sulphate) daily; Group 2 (n = 30): infants were given a supplement of 2 mg iron/kg (as ferrous sulphate) daily; Group 3 (n = 30): infants were given a supplement of 2 mg iron/kg (as ferrous sulphate)) every other day (approximately 42 mg of iron per week); Group 4 (n = 23): infants received a placebo. Length of the intervention: 4 months. Groups 1 and 2 were combined and compared with group 3. |
|
| Outcomes | Haemoglobin, MCV, ferritin, side effects. | |
| Notes | Supplements were given by mothers just before or just after breastfeeding and at least one hour before or after any other food intake. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomised to the different groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported. Personnel: Not reported. Outcome assessors: Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two, three and one cases were eliminated because of low compliance(<75%), in group 1, 2 and 3, respectively. The causes of non‐compliance were infection during iron usage, refusing iron droplets due to unpleasant taste, or mothers forgetting to use the iron drops. |
| Selective reporting (reporting bias) | Unclear risk | Cases with less than 75% of adherence were excluded. |
| Other bias | Unclear risk | Cases with less than 75% of adherence were excluded. It is unclear why the control group has 25% less participants. |
Evangelista‐Salazar 2004.
| Methods | Randomised controlled trial. 4‐arm design with individual randomisation. | |
| Participants | 100 newborns, both sexes (50 females), living in Urban areas in Colima, Mexico. Incluson criteria: healthy, term, single‐born babies during their first year of life. Exclusion criteria: low birth weight, unknown date of last menses to calculate term pregnancy, twins, bleeding disorder or other medical conditions that may be associated with anaemia (i.e., malabsorption). Baseline prevalence of anaemia: unknown. Socioeconomic status not reported but children were born to parents that were receiving a salary. | |
| Interventions | Neonates were randomly allocated at one of the following groups: Group 1 (n = 25): infants were given weekly a supplement of 7.5 mg elemental iron (as ferrous sulphate), and 30 mg vitamin C; Group 2 (n = 25): infants were given fortnightly a supplement of 7.5 mg elemental iron (as ferrous sulphate), and 30 mg vitamin C; Group 3 (n = 25): infants were given monthly a supplement of 7.5 mg elemental iron (as ferrous sulphate) and 30 mg vitamin C; Group 4 (n = 25): received no intervention. Length of the intervention: 12 months. During the first 6 months children received 7.5 mg and after that the dose was double. We only included the first period of evaluation in our analysis. For the purposes of this review we only compared groups 1 and 4. |
|
| Outcomes | Anaemia, iron deficiency, haemoglobin, ferritin. Neurocognitive development (Brazelton score at birth, Bayley mental and motor assessment) and growth. The latter data were not extracted as no measures of dispersion are reported. | |
| Notes | Trained personnel visited families to assess illness incidence and adherence. Ferritin data for the group receiving intermittent supplementation was 201.2 ± 51.08 and 120.0 ± 56.63 ng/mL (or μg/L). Although the results are consistent in terms of direction, these concentrations are much higher than those observed in the rest of the trials included in this review. The corresponding author was contacted to verify this information and we decided not include this information while we await for the response. Malaria‐free area. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children randomly allocated to the study groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants: unclear Personnel: unclear Outcome assessors: Not reported. Trial reported as single blind but the use of placebos is not described, so it is not clear who was not aware of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apparently there were no losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Faqih 2006.
| Methods | Randomised clinical effectiveness trial. 3‐arm design with randomisation at individual level. | |
| Participants | 134 children, both sexes (38.1% female at follow up), aged 2 to 6 years (in average 43 months), attending Prince Hashim Military Hospital of the Royal Medical Services in Zarqa, Jordan. This clinic is open to children from families affiliated with the army who are not medically insured and have generally low income. Inclusion criteria: Iron deficiency anaemia at baseline (Hb ≤ 105 g/L and mean corpuscular volume ≤ 75), born at full term with birthweight equal or higher than 2.5 kg and exhibited normal growth with no signs of thalassaemia, chronic illness, congential abnormalities, or chronic and repeated infections. Baseline prevalence of anaemia not reported. Socioeconomic status not reported. | |
| Interventions | Children were allocated to one of the following groups: Group 1 (n = 45): children received a daily dose of 5 mg elemental iron per kilogram of body weight; Group 2 (n = 45): children received once a week 5 mg of elemental iron per kg of body weight on Fridays (approximately 45 mg of iron per week); Group 3 (n = 44): children received 5 mg of elemental iron per kilogram of body weight twice a week, Friday and Monday (approximately 90 mg of iron per week). Parents were instructed to give the ferrous sulphate supplement in 2 portions between 30 to 60 minutes before breakfast and dinner. Parents were advised to mix the supplement with water, orange juice or lemonade if the child refused the supplement. Length of the intervention: three months. All the groups were analysed in this review. Groups 2 and 3 were combined and only reported separately for the subgroup analysis by regimen. |
|
| Outcomes | Weight, height, haemoglobin, mean corpuscular value, hematocrit, ferritin. | |
| Notes | The dose was administered by either of the parents who were advised to mix the supplement with water, orange juice,or lemonade if the child refused to take the supplement on an empty stomach. Families also counselled on nutritional causes of IDA, consequences if not treated, iron rich foods, enhancers and inhibitors. Families also received home check up visits every two weeks. In Jordan, malaria, hookworm, and schistosoma do not constitute a problem. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated randomly to one of three groups according to a table of random digits. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported Personnel: Not reported Outcome assessors: Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 71 of 134 children (53%) did not complete the study. Children lost because 1) refused to take the iron, 2) parents did not administer iron for 3 months, 3) parents did not return to clinic for follow up visits. Final number of participants did not differ across groups. |
| Selective reporting (reporting bias) | High risk | Only 34 children had ferritin values. |
| Other bias | High risk | Very large age range and small sample size for the outcomes, age is important for risk of anaemia and iron deficiency. Baseline haemoglobin higher in group 2 than in group 1. |
Hall 2002 (C).
| Methods | Cluster‐randomised trial. 2‐arm design with randomisation at school level (60 schools, 30 per arm). | |
| Participants | Children (1201 randomised, 1113 followed up), both sexes (613 female (51%)), aged 6‐19 years (mean of 11.4 years), attending rural informal community schools in the Kolondieba district of Mali. Approximately 20 randomly children (10 boys and 10 females) attending 2nd or 4th grade were selected from each school. Any child with severe anaemia (Hb ≤80 g/L) were excluded. Baseline prevalence of anaemia: approximately 55%. Socioeconomic status not reported. | |
| Interventions | Schools were allocated to one of the following groups: Group 1 (n = 551 at follow up, number randomised not clear): children received 65 mg elemental iron (as 200 mg of ferrous sulphate) and 250 μg (0.25 mg) of folic acid once a week; Group 2 (n = 562 at follow up, number randomised not clear): No intervention. Length of the intervention: 10 weeks |
|
| Outcomes | Anaemia, haemoglobin. Results by sex are included in the corresponding subgroup analysis. | |
| Notes | All children in every school were treated for parasitic infections at baseline using albendazole, and vitamin A to treat night blindness. Supplements were given by the teachers and 83% of children were given all 10 tablets and 91% received at least nine tablets. Malaria is endemic in Mali, although the study was done in the dry season when transmission is less intense than in the wet season. Authors provided the ICC (0.0698) and design effect (2.22) to adjust data by the effect of clustering; the estimated effective sample size was used in the analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 60 schools were randomly assigned to either a treatment or a comparison group by using a computer‐generated random number list (information communicated by the author). |
| Allocation concealment (selection bias) | Low risk | Not reported. Since the intervention was allocated at health care unit level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported Personnel: Not reported Outcome assessors: Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1201 children at baseline, 1113 followed up at 14‐16 weeks. (93% followed up). 88 children who did not provide second samples had similar Hb levels at baseline than as those children remaining in the study. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other bias. |
Khademloo 2009.
| Methods | Randomised controlled trial. 2‐arm design with individual randomisation | |
| Participants | 100 Infants, both sexes (sex distribution not reported), aged 6‐24 months referred to the public health care centre in Sari, Iran. Urban area. Inclusion and exclusion criteria were not adequately described. Baseline prevalence of anaemia not reported. Socioecomic status: although information on sex and mothers' educational level were collected this information was not reported. | |
| Interventions | Children were allocated to one of the following groups: Group 1 (n = 50): infants received fifteen drops containing elemental iron (as ferrous sulphate) given daily. Group 2 (n = 50): infants received thirty drops of iron once a week. Length of the intervention: 12 weeks |
|
| Outcomes | Ferritin, haemoglobin. | |
| Notes | Trial not included in the subgroup analysis by dose Malaria endemicity not reported. The total dose of iron per week is unknown. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Babies "randomly divided in two equal groups". Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported Personnel: Not reported Outcome assessors: Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. Denominators not provided in the results tables. |
| Selective reporting (reporting bias) | Unclear risk | Groups were described as similar at baseline, but information on methods and results was scarce. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Liu 1995 (C).
| Methods | Randomised clinical trial. 3 arm design with randomisation at classroom level. | |
| Participants | 246 healthy children, both sexes (131 females (57%)), aged 3 to 6 years, attending Kindergarten in Changxi, China, an autonomous region of China. Kindergarten has 9 large classrooms and two meals and two snacks are provided daily. Exclusion criteria were chronic infectious diseases, cardiopathies, or respiratory diseases, and intake during the previous month of supplements or drugs containing iron or specially prescribed iron‐rich and absorption‐promoting foods for the month prior to entering the study. Approximately 29 % of the children were anaemic at baseline. Socioeconomic status not reported. | |
| Interventions | Classrooms were allocated to one of the following groups: Group 1 (n = 89): children received 5‐6 mg of elemental iron per kilogram (as ferrous sulphate) daily; Group 2 (n = 74): children received 5‐6 mg of elemental iron per kilogram (as ferrous sulphate) twice a week (approximately 170 ‐204 mg of iron per week); Group 3 (n=83): children received 5‐6 mg of elemental iron per kilogram (as ferrous sulphate) tablet once a week (approximately 75 ‐120 mg of iron per week);. Iron tablets were administered by teachers under direct supervision 1 hour after breakfast, making sure that the child swallowed it. Length of the intervention: 3 months Group 2 and 3 were combined and compared with group 1; their individual results are presented in the subgroup analyses by regimen and by anaemia status. |
|
| Outcomes | Haemoglobin and ferritin. | |
| Notes | We adjusted the results of this study to account for the effect of clustering in data; the estimated effective sample size was used in the analyses. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly allocated to the classroom according to their age and then classrooms were randomised to each of the three intervention groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Not reported. Since randomisation occurred at classroom level, it is unlikely a selection bias at individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported Personnel: Not reported Outcome assessors: Results were tabulated, without knowledge of the children's supplementation regimen, by two nurses in charge of the clinic at the kindergarten with the assistance of a nonparticipating physician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 238 children completed the study. 5 left the kindergarten during the study and 3 children from daily group discontinued supplementation due to persistent nausea. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Data not adjusted by the effect of clustering in data |
Nguyen 2002.
| Methods | Randomised trial. 4 arm design with individual randomisation. | |
| Participants | 280 children, both sexes (133 females (47.5%), aged 5 to 12 months, living in one of four communes in the rural district of Bac Ninh, Vietnam. Inclusion criteria: Hb< 70 g/L, no pathologies after a clinical examination and not receiving any iron supplements. Baseline prevalence of anaemia: ˜60%.Socioeconomic status: ˜95% dedicated to agriculture. | |
| Interventions | Two communes were allocated to one of the following groups: Group 1 (n = 70): children received a placebo (2.5 ml of syrup without iron) every day; Group 2 (n = 70): children received a daily dose of 15 mg elemental iron (2.0 ± 0.3 mg iron/day/kg body weight) (as ferrous sulphate). Participants from other two communes were randomly allocated to one of the following groups: Group 3 (n = 70): children received a daily dose of 15 mg elemental iron (2.0 ± 0.3 mg iron/day/kg body weight) (as ferrous sulphate); Group 4 (n = 70): children received a weekly dose of 15 mg elemental iron (as ferrous sulphate). Length of the intervention: 3 months (groups 1 and 2) and 6 months (groups 3 and 4). For the purposes of this review only groups 3 and 4 were compared. |
|
| Outcomes | Haemoglobin, anthropometric measurements (height for age, weight, age and weight for height were Z‐scores) | |
| Notes | Article translated from French. The supplements were administered between 8 and 10 am by local auxiliaries, under regular supervision of a member of the research team. 98% and 95% of the infants in group 3 and 4, respectively, received more than 90% of the expected total dose of iron. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only children from groups 3 and 4 were randomly allocated to either daily or weekly supplementation. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Not described, but the trial included the provision of a placebo and multiple blinding. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: the nature of the treatment was unknown to the family of the infant; all the infants received identical looking syrups (with or without iron). Personnel: community auxiliaries were not aware of the treatments. Outcome assessors: Neither the people in charge of measurements researcher nor the data analysts were aware of the treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 children did not complete the study, 4 because parents refused to continue, 3 due to address change and 4 because of low compliance (consumed less than 80% of the doses). |
| Selective reporting (reporting bias) | High risk | Results on growth not reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Olsen 2000.
| Methods | Randomised, placebo‐controlled, double‐blind study. 2‐arm design with individual randomisation. | |
| Participants | 231 children, both sexes (99 females (43%)), aged 4‐15 years (8.6 years in average), living in Luo villages of Asino, Ohala, and Pith‐Kodhiambo in Kisumu district of Nyanza Province in western Kenya. Participants had moderately low blood haemoglobin concentrations (80‐130 g/L for children 4‐14 years of age or non‐pregnant female >14 years of age and 80‐135 g/Lif male and >14 years of age). Exclusion criteria: severe anaemia (Hb <80 g/L) or pregnant. Baseline prevalence of anaemia: 47.5%. Socioeconomic status not reported | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 121): children received treatment twice weekly with a 60 mg of elemental iron (total of 120 mg of iron per week, as 200 mg of ferrous dextran); Group 2 (n = 110): children received a placebo. Length of the intervention: 12 months. |
|
| Outcomes | Haemoglobin, serum ferritin (median and interquartile range, could not be extracted), reinfection rates and intensities of hookworm, Ascaris lumbricoides, Trichuris trichiura, and Schistosoma mansoni, compliance ("reasonable"). | |
| Notes | At baseline, any individual infected with any intestinal helminth. S. mansoni, and malaria were treated (only abstract says treated with malaria). After baseline examination, each subject was given a container (labelled with the subject's name, study number and identification sticker) containing 50 tablets. At the end of each 4‐month period, the number of tablets taken was registered, based on the number of remaining tablets. In order to encourage intake, field assistants visited every participant at least once a month. Tablet intake for the whole study period was 98.9% of the scheduled value, and 90.1% of the children each appeared to take between 80% and 120% of the scheduled number of tablets. Iron supplementation had no effect on either reinfection rates or intensities in children. Multiple logistic regression analyses controlling for baseline infection status confirmed the effect in adults of Malaria endemic area. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation using a programme written in advance. |
| Allocation concealment (selection bias) | Low risk | The tablets were coded by the manufacturer and sealed envelopes containing the codes were kept closed until the end of the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: received identical pills and instructions Personnel: envelopes revealing randomizations code not opened until analysis was complete. Outcome assessors: envelopes revealing randomizations code not opened until analysis was complete. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 231 randomised, one became pregnant and 30 lost to follow up. Lost equally distributed across both arms. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Baseline intensity of A. Lumbricoides infection was higher in the placebo group than in the iron group. HIV not assessed at baseline, but at 4 months, and assumed to reflect baseline status; it is unclear what treatment was available for participants. |
Palupi 1997.
| Methods | Double‐masked, randomised controlled field trial. 3‐arm design with individual randomisation. | |
| Participants | 299 children, both sexes (sex distribution not reported), aged 2‐5 years, who were registered at the West Javanese village of Setia Asih. Of 344 potential subjects, parental permission was obtained for 299 children. No further inclusion or exclusion criteria mentioned. Baseline prevalence of anaemia: 36.7%. Socioeconomic status not reported. | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 98): children received 30 mg elemental iron (as ferrous sulphate) once per week and anthelminthic treatment; Group 2 (n = 96): children received 30 mg elemental iron (as ferrous sulphate) once per week and placebo for anthelminthic treatment; Group 3 (n = 98): children received placebos for both iron supplements and anti‐helminthic treatment. The placebo syrup did not contain ferrous sulphate, but was similar in taste and appearance to the iron‐containing syrup. Length of the intervention: 9 weeks For the purpose of this review only groups 2 and 3 were compared. |
|
| Outcomes | Haemoglobin, haemoglobin mean change, anaemia, anthropometric measurements (Height‐for‐age Z‐score, Weight‐for‐age Z‐score, Weight‐for‐height Z‐score change), helminthic infection. | |
| Notes | The anthelminthic tablets as well as the placebos were ingested under supervision of the researcher one week before iron supplementation started. The supplements were given to the children by their mothers and intake was not supervised by health centre staff or the researchers, but compliance was controlled by checking the iron content in the stool. Z‐scores used the National Center for Health Statistics data as a reference. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly divided into three, equal‐sized treatment groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Mothers received a bottle with 100 mL glucose syrup. Although the concealment is not clearly described, this is a double‐blind trial and its unlikely that there was a selection bias. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported as double‐masked trial. Participants: were not aware of the treatment Personnel: all mothers received a bottle with 100 mL glucose syrup containing or not iron. Outcome assessors: unclear but probably blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 289 (out of 299) children remained; 10 (3%) dropped out because they had either moved or had become ill. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Roschnik 2003 (C).
| Methods | Cluster‐randomised trial. 2‐arm design with randomisation at school level and stratified by sponsorship status. | |
| Participants | 1,160 children (752 followed up), both sexes (371 females (49.5%)), aged 7–8 years and 12‐14 y. The study included 40 primary schools in the Mangochi District, Malawi. Baseline prevalence of anaemia: around 54%. Socioeconomic status not reported. | |
| Interventions | Schools were randomly allocated to one of the following treatments: Group 1 (20 schools, n = 640): children received 65 mg of elemental iron (as 200 mg ferrous sulphate) and 250 μg (0.25 mg) of folic acid once a week. Group 2 (20 schools, n = 640): children received no intervention. Length of the intervention: 15 weeks |
|
| Outcomes | Haemoglobin concentration, bilharzia infection, school attendance, test scores and drop‐out rate and repetition rate (at the school level). |
|
| Notes | Results were stratified by age (<10 y, 10‐14 y and 15+). For the purposes of this review we only included those data from children <10 years of age (192 in the intervention group and 190 in the control group), until we can obtain the data for all children <12 years. A famine occurred in the region at the time of the study. Each study group included 10 sponsorship schools and 10 non‐sponsorship schools, 10 coastal and 10 upland schools. All children in Coastal intervention and comparison schools, where the prevalence of bilharzia was over 50%, were dewormed with Praziquantel (600mg) just after the baseline survey. A vitamin A capsule (200,000 IU) was given to all children in standard 2 and below 63% of children took 10 iron tablets or more. Analysis originally not adjusted by the effect of clustering. The effective sample was calculated by imputing the ICC from Roschnik 2004 (C), which has a similar study design; the estimated effective sample size was used in the analyses. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 40 primary schools in the Mangochi District were randomly divided into the intervention (1st iron group) and comparison group (2nd iron group). Each group includes 10 sponsorship schools and 10 non‐sponsorship schools. Method of sequence generation not specified. |
| Allocation concealment (selection bias) | Low risk | Not reported. Since the intervention was allocated at school level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: not reported. Personnel: not reported Outcome assessors: not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1280 were randomised, 1160 had haemoglobin levels at baseline and 752 were followed up: 41.2% children lost to follow up |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Children attending sponsored schools responded better to the treatment. |
Roschnik 2004 (C).
| Methods | Cluster‐randomised trial. 2‐arm design with randomisation at school level. | |
| Participants | 1785 children (1510 followed up), both sexes (747 females (49.5%), aged 7–12 years. The study included 51 primary schools: 20 in Iloilo and 31 in Guimaras, Philippines. Baseline prevalence of anaemia: ˜15%. Socioeconomic status not reported. | |
| Interventions | Schools were randomly allocated to one of the following treatments: Group 1 (25 schools, unclear the number of children randomised): children received 108 mg of elemental iron (as 325 mg ferrous sulphate); Group 2 (26 schools, unclear the number of children randomised): children received no intervention. Length of the intervention: 10 weeks |
|
| Outcomes | Anaemia, haemoglobin, haemoglobin change. | |
| Notes | Supplementation started between 1 and 7 weeks after the baseline survey and the second survey took place between 5 and 18 weeks after the end of the iron supplementation. The consumption of each tablet was recorded by the teachers. Side effects were not recorded. All 10 iron tablets were taken by 93.4% of children. 67% of children were infected with one or more intestinal worms. Malaria endemicity not reported. Authors provided the ICC (0.1123) and design effect (4.35) to adjust data by the effect of clustering; the estimated effective sample size was used in the analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All 51 schools were assigned to two groups using a random number table. |
| Allocation concealment (selection bias) | Low risk | Not reported. Since the intervention was allocated at school level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: not reported. Personnel: not reported Outcome assessors: not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15.4% of attrition. Losses presumably higher among the control group as two schools were dropped out because they were unable to collect the baseline measurements within the month allotted. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | The second blood sample was withdrawn between 5 and 18 weeks after the end of the iron supplementation. Fourteen of the 49 schools in the study had participated for about 2 months in the fortified rice programme: six in the intervention group and eight in the control group. The mean haemoglobin concentration of children in the 14 schools that had participated in the programme was slightly but significantly higher than that of children in the other 25 schools (126.4 g/L versus 125.0 g/L, P ¼ 0.031). Analysis was not adjusted by the effect of clustering in data. |
Schultink 1995.
| Methods | Randomised clinical trial. 2‐arm design with individual randomisation. | |
| Participants | 87 children, both sexes, aged 2‐5 years, from Subdistrict Kelurahan Tenga of East Jakarta, Indonesia. The initial selection criterion was a haemoglobin concentration < 110 g/L. 96 children were invited to receive anthelmintic treatment before starting iron supplementation; only 87 accepted and were randomised. | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 44): children were supplemented daily with 30 mg elemental iron (as ferrous sulphate dissolved in 5 mL syrup); Group 2 (n = 43): children were supplemented twice a week with 30 mg elemental iron (as ferrous sulphate dissolved in 5 mL syrup) (total 60 mg of iron per week). Length of the intervention: 8 weeks. |
|
| Outcomes | Haemoglobin, ferritin, zinc protoporphyrin, mean changes of haematological variables (anaemia prevalence taken from Beaton 1999). | |
| Notes | Parents and supervising health staff were instructed that each child should take 5 mL from the small bottle on Mondays and Fridays and 5 mL from the large bottle on the remaining days of the week using a standardized spoon for 8 wk. Bottles had similar appearance Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjects were assigned at random to two groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: were not aware of the treatment Personnel: all mothers received two small bottles (each 80 mL) and two large bottles (each 170 mL), each containing a syrup of similar appearance Outcome assessors: supervising staff were not aware of the bottle content. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A complete set of data was obtained for 33 subjects in the group supplemented twice weekly (group 1) (75%) and for 32 subjects in the group supplemented daily (group 2) (74.4%). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Baseline haemoglobin concentrations were different between groups. The results include 25 children with Hb> 110 g/L and the initial description only mentions 16 |
Sen 2009 (C).
| Methods | Cluster‐randomised controlled trial. 4‐arm design with randomisation at school level. | |
| Participants | 240 school age females, aged 9‐13 years, attending four schools in Vadodara area of India. Females were excluded from the analysis if menstruation commenced. None of the Females were involved in athletic sports on a regular basis. Baseline prevalence of anaemia: 68.3%. Socioeconomic status not described in detail but participants were described as "underprivileged". | |
| Interventions | Schools were allocated to one of the following groups: Group 1 (n = 65): females received 100 mg elemental iron (as ferrous gluconate) and 500 μg (0.5 mg) folic acid folic acid oral once weekly; Group 2 (n = 89): females received the same supplement twice weekly (200 mg of elemental iron per week); Group 3 (n = 59): females received 100 mg elemental iron (as ferrous gluconate) daily; Group 4 (n = 41): females received no supplement. Length of the intervention: 1 year. Groups 1 and 2 were combined and compared with groups 3 and 4 as appropriate. |
|
| Outcomes | Physical work capacity, haemoglobin change and adherence. | |
| Notes | Malaria endemicity not reported. Analyses in this review include the estimated effective sample size only, after adjusting the data to account for the clustering effect. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Of 17 schools meeting the inclusion criteria, 4 schools were selected randomly using a random numbers table. Once the four schools were selected, the chit system (chits representing school 1, 2, 3, 4) was used. The order of placing a school in a category was: the first school that is picked up (from the four) goes to daily; the chit is then put back; the next chit picked up goes to twice weekly; the next to once weekly and the one left over, to control so that all schools have an equal probability of being allocated to any of the four groups (information communicated by the author). |
| Allocation concealment (selection bias) | Low risk | Not reported. Since allocation was at school level it is unlikely that there is selection bias at individual level. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants, personnel and outcome assessors: Each school received a different intervention, although it is unclear if the intervention was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 schools. In these schools a random sample of 240 children was selected. 163 had pre and postintervention data for work capacity (68% followed up). Females who started their periods were excluded from the analysis. For cognitive tests results relate to a sub‐sample of 161 females available pre and post‐test. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | The design effect was not taken into account in the analysis. |
Siddiqui 2004.
| Methods | Randomised controlled trial with. 2‐arm design with individual randomisation. | |
| Participants | 60 children, both sexes (30 females (50%)), aged 5‐10 years, attending a private school, blue collar workers, in Karachi Pakistan. Inclusion: anaemia (haemoglobin <110 g/L). Exclusion criteria: acute disease (diarrhoea, fever, cough, running nose) or history of chronic disease (joint pain, bleeding disorders). Socioeconomic status not described. | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 30): Children took supplements containing 60 mg of elemental iron (as 200 mg ferrous sulphate) once a week for 2 months (8 doses total); Group 2 (n = 30): Children took 60 mg of elemental iron supplements (as 200 mg ferrous sulphate) daily for 56 days. Length of the intervention: ˜ 2 months (weekly dosing was 8 weeks but daily dosing only 56 days). |
|
| Outcomes | Haemoglobin, hematocrit, serum iron, total iron binding capacity, serum ferritin. | |
| Notes | Both groups de‐wormed prior to start of study (mebendazole). Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly assigned to one of the groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. Outcome assessors: not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Unclear whether 60 participants reflected all anaemic children in the school or whether (and if so how) the 30 males and 30 females were selected out of all eligible students in the school. Age in weekly group significantly different than those in daily group. Did not assess or adjust/exclude iron status indicators for inflammation |
Sinisterra 1997 (C).
| Methods | Cluster‐randomised trial. 2‐arm design with randomisation at school level (5 schools). | |
| Participants | Children (909 randomised, 842 followed up), both sexes (408 female (48%)), aged 6‐13 years, attending rural schools in the district of Anton, Cocle, Panama. Exclusion criterion: severe anaemia (Hb ≤90 g/L) and clinical conditions that could affect iron status. Baseline prevalence of anaemia: approximately 42.4%. Socioeconomic status not explicitly reported. | |
| Interventions | Schools received one of the following interventions: Group 1 (n = 176 at follow up, number randomised not clear): children received daily 60 mg of elemental iron (as ferrous sulphate) and "nutricrema"; Group 2 (n = 210 at follow up, number randomised not clear): "nutricrema". Group 3 (n = 225 at follow up, number randomised not clear): children received daily 60 mg of elemental iron (as ferrous sulphate) and "nutricrema" once a week; Group 4 (composed by two schools n=195 at follow up, number randomised not clear): Milk plus a fortified cookie plus folic acid Length of the intervention: 6 months Only groups 1 and 3 were randomised and thus included in our analysis. |
|
| Outcomes | Anaemia (Hg < 120 g/L), haemoglobin, attitudes, beliefs, growth. | |
| Notes | Malaria endemicity not reported. We adjusted the results of this study to account for the effect of clustering in data; the estimated effective sample size was used in the analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Groups allocated by drawing of lots (communicated by the author). |
| Allocation concealment (selection bias) | Low risk | Not described. Since the intervention was allocated at school level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported Personnel: Not reported Outcom assessors: Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.3% of losses to follow up. Unclear whether they were balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | The prevalence of anaemia among those receiving weekly supplementation was 20 percentage points higher than those receiving daily supplementation (54.7% vs 34.7%). |
Soemantri 1997.
| Methods | Randomised controlled trial. 2‐arm design with individual randomisation. | |
| Participants | 97 children, both sexes (sex distribution not reported), aged 7‐11 years, attending the primary school Batang, in Indonesia. Inclusion criteria: anaemia (Hb below 120 g/L); not taking iron supplements during the last six months; no evidence of hepatosplenomegaly, haemoglobinopathy, acute or chronic disease, severe anaemia. Baseline prevalence of anaemia: 67.36%. Socioeconomic status not reported. | |
| Interventions | Children were divided into 2 groups and randomly assigned Group 1 (n = 52): children received daily 3 mg of iron per kilogram (as ferrous sulphate); Group 2 (n = 45): children received once a week 3 mg of elemental iron per kilogram (as ferrous sulphate) (approximately 85 mg of iron per week). Length of the intervention: 3 months. |
|
| Outcomes | Anthropometric measurements (weight for age Z‐score, height for age Z‐score) and haemoglobin. | |
| Notes | The solutions were given by the school teachers on school days with careful supervision. All children with intestinal parasites were treated prior to supplementation. Z‐scores used the National Center for Health Statistics data as a reference. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly assigned to the study groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. Outcome assessors: not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two children (3.8%) were excluded from the daily group because of gastrointestinal side effects. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Sungthong 2002.
| Methods | Double‐blind, randomised, placebo‐controlled trial. 3‐arm design with individual randomisation. | |
| Participants | Of 50 government schools located outside the municipality, selected schools had to met the following criteria: 1) high prevalence of underweight according to school‐records (no # or prevalence given to define "high"); 2) a least 150 students in school; 3) not >1 h away by car from research centre; 4) teachers willing to cooperate in study; 5) no previous iron supplementation programme implemented. Subsequently 2 schools selected. 397 school age children in grades 1‐6 (9.7 years of age in average), both sexes (212 females (53%)) only those with written parental consent included. Excluded those with severe Iron deficiency anaemia (Hb equal or lower than 80 g/L and serum ferritin equal or lower than 20 μg/L) severe malnutrition weight‐for‐height <3rd percentile of Thai reference, chronic illness such as thalassaemia, haemolytic disease and physical handicaps. Participants assigned to group stratified by anaemia status. Baseline prevalence of anaemia:˜ 35%. This study took place in a socioeconomically disadvantaged community. |
|
| Interventions | Participants were allocated to one of the following groups: Group1 (n = 134): each child received 2 bottles with tablets, the first was to be taken on Monday only while the second was to be taken for the remaining days of the week (60 mg of elemental iron (as ferrous sulphate) weekly; Group 2 (n = 140): each child received 2 bottles with tablets, the first was to be taken on Monday only while the second was to be taken for the remaining days of the week. Both bottles had 60 mg of elemental iron (as ferrous sulphate) daily); Group 3 (n = 123): Same procedure as groups 1 and 2 but children received placebo. The tablets were similar in colour, shape, size, and taste as the iron tablets. Length of the intervention: 16 weeks |
|
| Outcomes | Haemoglobin, serum ferritin, mean changes of both, height, weight. | |
| Notes | This area is free from malaria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were stratified by anaemic status to balance the proportion of anaemic and non anaemic children across the intervention groups. The children were then assigned by simple random allocation within each stratum using a computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Tablets placed in packages labelled only with participants' name, content not known to any of the project personnel. 2 supplement packages similar in appearance: On Mondays received one packages which contained iron for daily and weekly group, but placebo for control group. Rest of week consumed tablets from other package, which were iron for daily group and placebos for weekly and placebo control group. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: neither parents nor participants knew the content of supplement packages. Personnel: researchers did not know the content of supplement packages. Outcome assessors: not reported but probably blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6 of 397 enrolled lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Baseline prevalence of anaemia was different among study arms (39, 40 & 28%, for daily, weekly and placebo, respectively), but haemoglobin concentrations were not statistically different. |
Tavil 2003.
| Methods | Randomised clinical trial. 2‐arm design with randomisation at individual level. | |
| Participants | 94 children aged 5 months to 6 years (median age was 18 months of age), both sexes (35 females (37.2%)), attending Dr Sami Ulus Children's Hospital in Ankara, Turkey, from December 1999 to December 2000. Inclusion criteria: iron deficiency anaemia (defined as haemoglobin (Hb) levels below 100 g/L, transferrin saturation levels below 12%, and ferritin levels below 12 ng/mL) and negative supplement intake during the past 3‐4 weeks. Exclusion criteria: chronic, metabolic, and genetic diseases. Socioeconomic status not described. | |
| Interventions | Participants were randomly allocated to one of the following groups: Group 1 (n = 48): children received daily 6 mg/kg of elemental iron as ferrous sulphate; Group 2 (n = 46): children received 6 mg/kg of elemental iron as ferrous sulphate 2 days a week (Tuesday and Friday) (120 mg of iron per week). . Twenty‐three healthy children whose age and gender distribution were compatible with the other groups were included in the study as the control group. This group was not included in the analyses. Length of the intervention: 2 months |
|
| Outcomes | Haemoglobin, hematocrit; red blood cell count, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, iron deficiency anaemia, serum iron, serum iron binding capacity, transferrin saturation, transferrin. | |
| Notes | Malaria endemicity not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into two groups. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. Outcome assessors: not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear the final number of participants per group. The report mentions that 'thirty three patients who had not been regularly conforming to the iron deficiency treatment as recommended or who were intolerant to the medication due to the side effects were excluded from the study. The patients presenting no increase in the Hb levels despite the iron treatment were reevaluated at the end of the first month, and those who were detected as thalassaemia traits were also excluded.' The tables do not present the final numbers. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | It is unclear the failure rate of the intervention and whether the results are biased because of the exclusions. |
Taylor 2001.
| Methods | Double‐blind randomised controlled trial. Factorial design (6 arms) with individual randomisation. | |
| Participants | 425 children, both sexes (50% females), aged 6 ‐15 years (mean age 11.2 years), attending three rural primary schools in Kwa‐Zulu Natal, South Africa.The sample was stratified by school, age and sex. Four children with anaemia were included in the study (Hb < 80 g/L) ‐ all of these children were allocated to receive iron. Females over 12 years of age were excluded as the safety of albendazole in pregnancy has not been established. Socioeconomic status not reported although it was stated that the study was carried out in the third poorest province in South Africa. Anaemia at baseline was 35%. | |
| Interventions | Children were allocated to 1 of the following groups: Group 1 (n = 56): children received 400 mg of albendazole weekly, 40 mg/kg of praziquantel weekly, and 65 mg of elemental iron (as 200 mg ferrous fumarate) plus 100 μg (0.1 mg) of folic acid weekly; Group 2 (n = 60): children received 400 mg of albendazole weekly, 40 mg/kg of praziquantel weekly, and placebo for iron and folic acid weekly; Group 3 (n = 60): children received 400 mg of albendazole for three days, 40 mg/kg of praziquantel weekly, and 65 mg of elemental iron (as 200 mg ferrous fumarate) plus 100 μg (0.1 mg) of folic acid weekly; Group 4 (n = 57): children received 400 mg of albendazole for three days, 40 mg/kg of praziquantel weekly, and placebo for iron and folic acid weekly; Group 5 (n = 101): children received placebo for albendazole, placebo for praziquantel and 65 mg of elemental iron (as 200 mg ferrous fumarate) plus 100 μg (0.1 mg) of folic acid weekly; Group 6 (n = 91): children received only placebos. For the purposes of this review only groups 5 and 6 were analysed. Length of the intervention: 10 weeks, children were followed up for a year with measures at baseline, 6 months and 12 months. |
|
| Outcomes | Height, weight, full blood count, anaemia (could not be extracted), presence of malarial parasites, presence of hookworm infection, urine infection or presence of blood in urine. | |
| Notes | All groups received interventions under supervision by teachers It was reported that the area is endemic for malaria, schistosomiasis and hookworm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial, with individual randomisation. 6‐arm trial (factorial design). Sample stratified by school, age and sex. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Each pupil’s treatment was individually packaged at each phase of the study. Both the field team and pupils were blinded as to the type of drugs used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: all pupils were blinded to the type of supplement used. Personnel: field team were blinded to the type of supplement used. Outcome assessors: not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 428 children entered the study. 4 children who were anaemic at baseline were all allocated to receive iron treatment. It was stated that intention to treat analysis was not carried out as data was missing for children who were absent from school on the day specimens were collected. It was stated that the sample sizes varied at each phase of the study. There was considerable variation in the size of treatment groups ‐ it was not clear why. The numbers available at each assessment point and missing data were not stated. The number with data on Hb at both 6 and 12 months follow up was 275 (64% of the original sample). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | It was stated that groups were similar at baseline for prevalence of anaemia and other variables. Although the figure suggests there was considerable variation in mean Hb levels at baseline ‐ although the differences between groups were not significant. Children with anaemia all received iron. The lack on information on attrition and missing data mean that results are difficult to interpret. |
Thu 1999.
| Methods | Double‐blind, placebo‐controlled trial. 2‐arm design with randomisation at individual level. | |
| Participants | 68 children, both sexes (88 females (54%)) 6–24 months of age, living in the Chi Lang Bac commune, Thanh Mien district, Hai Duong province in Vietnam. Exclusion criteria: infectious disease at the time of enrolment and a birth weight < 2.5 kg according to the birth record. Baseline prevalence of anaemia: ˜50%. Socioeconomic status not reported. | |
| Interventions | Participants were allocated to one of the following groups: Group 1 (n = 55): children received daily 8 mg elemental iron (as ferrous sulphate), 5 mg elemental zinc(as zinc sulphate), 333 mg retinol, and 20 mg vitamin C 5 d/wk for 3 mo; Group 2 (n = 54): children received 20 mg elemental iron (as ferrous sulphate), 17 mg zinc, 1700 mg retinol, and 20 mg vitamin C once a week (Thursdays); the rest of the week were given a placebo; Group 3 (n = 54): children received a placebo Monday to Friday that was similar in colour and appearance to the supplement. Length of the intervention: 12 weeks. |
|
| Outcomes | Haemoglobin, serum retinol, zinc. weight and length (score z, measured 3 mo after the intervention ceased). | |
| Notes | The syrup was put into the children’s mouth by syringe by a research staff member who visited the children daily between 0700 and 1000. Before the start of the study, the acceptability of the syrup was tested in 12 children. Mothers of these children reported good acceptance and no side effects. Acceptability throughout the study remained good, and the children took all of the supplements as intended. Z‐scores used the National Center for Health Statistics data as a reference. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomly divided into 3 groups by using a table with randomly assorted digits. |
| Allocation concealment (selection bias) | Low risk | Blind supplementation was guaranteed by coding the 3 treatment groups as A, B, and C and by putting the syrups to be used for each group in bottles having a corresponding code. Neither the main researcher and his assistants nor the mothers knew which supplement was represented by which code |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blind supplementation was guaranteed by coding the 3 treatment groups as A, B, and C and by putting the syrups to be used for each group in bottles having a corresponding code. Participants: children received a placebo similar in colour and appearance to the supplement Personnel: Neither the main researcher and his assistants nor the mothers knew which supplement was represented by which code. Outcome assessors: Neither the main researcher and his assistants nor the mothers knew which supplement was represented by which code. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 168 children enrolled at baseline, complete data sets were available for 163 children for anthropometric data and for 160 children for biochemical data. Reasons for attrition included families’ moving to other places (n=3), mothers’ refusing further participation because of time limitations (n=2), and fear of blood collection (n=3). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Verhoef 2002.
| Methods | Double‐blind, placebo‐controlled trial. 2x2 factorial design with randomisation at individual level. | |
| Participants | 328 children, both sexes (148 females (45%)), aged 2–36 months. The study was done during rainy seasons in the period 1998–2000 in Mtito Andei Division, Eastern Province, Kenya. Children were randomly sampled. At screening, children were judged eligible for the study when they met the following criteria: the haemoglobin concentration was 60–110 g/L (anaemic); the axillary temperature was below 37·5C; there were no symptoms suggestive of malaria or anaemia, or any systemic illness occurring in combination with a blood dipstick test result indicating current or recent malarial infection; the parents intended to stay in the study area during the intervention period and gave their consent; no allergy to sulfa drugs was reported; and no sulfa drugs had been used in the previous 3 weeks. Children with a positive malaria dipstick test result but without symptoms of systemic illness were included. |
|
| Interventions | Participants were randomly assigned to one of four groups Group 1 (n = 82): children received intermittently sulphadoxine‐pyrimethamine and iron supplement of 6 mg elemental iron (as ferrous fumarate) per kg body weight weekly (approximately 65 mg of elemental iron per week); Group 2 (n = 82): children received intermittently sulphadoxine‐pyrimethamine and iron placebo; Group 3 (n = 82): children received intermittently sulphadoxine‐pyrimethamine placebo and iron supplement of 6 mg elemental iron (as ferrous fumarate) per kg body weight weekly (approximately 65 mg of elemental iron per week); Group 4 (n = 82): children received intermittently sulphadoxine‐pyrimethamine placebo and iron placebo. Iron was administered twice per week as ferrous fumarate in a suspension at a target dose of Length of the intervention: 12 weeks For the purposes of this review, groups 1 and 3 were combined (iron) and compared with the combination of groups 2 and 4 (no iron). |
|
| Outcomes | Malaria attacks, adverse drug reactions, anaemia, iron deficiency, serum ferritin (could not be extracted) and difference in mean haemoglobin change from that of placebo (we calculated final haemoglobin concentrations). | |
| Notes | Sulphadoxine‐pyrimethamine was administered by the clinical officer employed by the project once every 4 weeks at therapeutic doses. Iron was administered by community health‐workers. Children in all groups were under intense health surveillance throughout the intervention period. Malaria transmission is highly seasonal in this area |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced block randomisation (41 blocks). The allocation schedule was generated by one of the researchers for each block, by means of tables with randomised permutations, and only after acceptance of all children making up a block. |
| Allocation concealment (selection bias) | Low risk | The order of the children listed in each block was concealed from the person generating the allocation schedule. Both placebos and active compounds were administered as suspensions that were indistinguishable in taste and appearance. Bottles were colour‐coded, but none of the field investigators was aware of the code until after crude analysis and a plan for further analysis had been prepared. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 328 children undergoing randomisation, 307 (94%) completed the trial and 21 (6%) did not (migrated or moved temporarily from the study area, 13; parents withdrew consent, three; developed severe anaemia, one; died, one; developed malaria but treated elsewhere, one; unknown reasons, two). Balanced losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yang 2004 (C).
| Methods | Cluster‐randomised trial. 3‐arm trial with randomisation at classroom level. | |
| Participants | 353 preschool children, both sexes 58 females (44.7%), aged 3–6 years, attending a kindergarten in Baotou City. Inclusion criterion: absence of major diseases, haemoglobin 90‐140g/L. | |
| Interventions | Classrooms were allocated to one of the following groups: Group 1 (n = 120): children received tablets containing 30 mg elemental iron: 5 mg zinc, 300 μg vitamin A, 50 mg vitamin C: 7.5 μg vitamin D3, 150 μg (0.15 mg) folic acid five times every week (Monday to Friday); Group 2 (n = 120): children received the same tablets as group 1 only once a week; Group 3 (n = 113): children received a placebo similar in colour and appearance to the iron supplement tablets. Placebo was given daily Length of the intervention: 14 weeks |
|
| Outcomes | Haemoglobin, serum ferritin, erythrocyte protoporphyrin, iron deficiency (serum ferritin <30 μg/L), height‐for‐age Z‐scores, weight‐for‐age Z‐scores). | |
| Notes | Study translated from Chinese. We attempted to contact the author to validate the extraction Teachers and nurses received iron supplement tablets from kindergarten doctors and helped children to take the tablets with semi‐liquid food. All groups have the same food in the kindergarten except for the tablets. We adjusted the results of this study to account for the effect of clustering in data; the estimated effective sample size was used in the analyses. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly assigned to the treatment or the control according to their classroom. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Every week, teachers and nurses received iron supplement tablets from kindergarten doctors and helped children to take the tablets. Since the intervention was allocated at classroom level, it is unlikely there was a selection bias at the individual level. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: children received a placebo similar in colour and appearance to the supplement Personnel: Every week, teachers and nurses received iron supplement tablets from kindergarten doctors. Outcome assessors: Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow up not reported. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | The data was not adjusted by the effect of clustering. |
Young 2001.
| Methods | Randomised controlled trial. 2‐arm with individual randomisation after stratifying children by anaemia status. | |
| Participants | 577 Malawian children, both sexes (sex distribution not reported), 15 and 60 months of age, were enrolled as they attended the mobile child health clinic in their area. Exclusion criteria:children with severe anaemia (Hb < 70 g/L). Baseline prevalence of anaemia: 83%. Socioeconomic status not reported. | |
| Interventions | Children were allocated to one of the following groups: Group 1 (n = 73 at follow up): children received 60 mg of iron once a week; Group 2 (n = 73 at follow up) children received 60 mg of elemental iron (as ferrous sulphate) plus 7500 IU vitamin A, 45 mg vitamin C, 600 IU vitamin D3, 3 mg vitamin B1, 1.5 mg B2 and 22.5 mg vitamin B3 once a week; Group 3 (n = 85 at follow up): children received 60 mg of elemental iron (as ferrous sulphate) daily. Groups 1 and 2 were combined and reported independently for the relevant subgroup analysis. Length of the intervention: 12 weeks |
|
| Outcomes | Haemoglobin concentration. | |
| Notes | All children received treatment for hookworm at baseline with albendazole. Adherence to the treatment, as reported by guardians along with a monthly tablet count, was similar in each group, approximately 52%. Reported adverse effects ranged from 2.7% to 9.4%, with the weekly iron/vitamin group reporting the least adverse effects and the daily iron group the most. Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The children were stratified according to haemoglobin (Hb) levels (using the HemoCue) and then randomised. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Supplements were administered by guardian but unclear whether he/she was aware of which treatment was being administered |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported. Personnel: Not reported. Outcome assessors: Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition of 60% (n=346). No description of why more than half of the sample was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yurdakok 2004.
| Methods | Randomised control trial. 3 arm design with individual randomisation. | |
| Participants | 79 infants, both sexes (sex distribution not reported), 4 months of age at baseline, identified for potential enrolment at Hacettepe University Ihsan Dogramaci Children's Hospital Well Baby Clinic, Ankara Turkey. Inclusion criteria: 1) gestational age more than 37 weeks, 2) birthweight >2500g, 3) singleton birth, 4) no congential malformation,5) no perinatal disease, 6) breast milk as the only source of food on admission, 7) no history of iron supplementation or therapy, 8) no known hematologic disorder of mother‐infant pairs, 9) mother intended to breasted exclusively until 6 months of age and to continue breastfeeding with introduction of complementary foods no earlier than 7 months of age. Infants or mothers with iron deficiency or iron deficiency anaemia identified at baseline were excluded from the study. Baseline prevalence of anaemia: unknown. Socioeconomic status not reported. | |
| Interventions | Infants were randomised to one of the following groups: Group 1 (n = 27): infants received daily 1 mg/kg/d iron (as ferrous sulphate); Group 2 (n = 27): infants received 7 mg of iron/kg/week (as ferrous sulphate) every Tuesday. The whole dose was divided into three, but all iron was provided only once a week (approximately 45 mg of elemental iron per week); Group 3 (n = 25): infants received no supplementation. The dose was adjusted monthly according to infant's weight. Length of the intervention: 3 months |
|
| Outcomes | Haemoglobin, mean corpuscular volume, red cell distribution width, transferrin saturation, serum ferritin and adverse effects; Iron Deficiency or Iron Deficiency Anaemia are reported together. | |
| Notes | Mothers gave supplement at home in morning one hour before breastfeeding (this seems difficult to control/estimate, as it seems to assume there is no night nursing or on demand feeding). Malaria endemicity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants: Not reported. Personnel: Not reported. Outcome assessors: Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 of 79 (15%) did not finish the study. 3 mothers withdrew from the study. Mothers that introduced complementary foods early or gave other milks (not breast milk) were removed from study (n=5), non‐compliance for iron supplementation were removed from the study (n=2). Infants who contracted infectious disease were removed (n=2). Withdrawns did not significantly differ across three groups. No differences at baseline between those who completed the study and those who did not. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2003 | Prospective study conducted in government schools in Northeast Delhi. 2088 adolescent females participated in the study, with 702 females receiving daily iron‐folate supplementation, 695 females on weekly iron‐folate supplementation and 691 females serving as controls. The authors concluded that though the weekly regimen took longer, it was as effective and practical as daily regimens in raising haemoglobin levels. This study was excluded because the authors looked only at adolescent females, which is out of the scope of this review. |
| Ahmed 2001 | Randomised, double‐blind, placebo‐controlled study in a 2x2 factorial design conducted in urban Bangladesh. Female postmenarchal adolescent subjects were randomised to a placebo (for vitamin A and for iron/folic acid), vitamin A only, iron and folic acid only, and iron, folic acid and vitamin A weekly for 12 weeks. Haemoglobin concentrations were raised significantly more in response to iron and folic acid and iron, folic acid, and vitamin A when compared to vitamin A alone or to placebo. This study was excluded because the study evaluated post‐menarchal females specifically, which is out of the scope of this review. |
| Ahmed 2005 | Randomised double‐blind clinical trial conducted in Bangladesh. Anemic (haemoglobin < 120 g/L) females (n=197) aged 14‐18 y from rural schools in Dhaka District were entered into a randomised double‐blind trial and received twice‐weekly supplements of iron and folic acid or multiple micronutrients (15 micronutrients, including iron and folic acid) for 12 wk. In conclusion, twice‐weekly MMN supplementation for 12 wk significantly improved the status of the micronutrients assessed but was not more efficacious than was supplementation with iron and folic acid alone in improving the hematologic status of anaemic adolescent females. The study was excluded because the authors did not compare intermittent iron supplementation versus daily/placebo and hence the study is out of the scope of this review. |
| Avila‐Jimenez 2011 | Randomised trial conducted in Mexico City, Mexico. The trial included 1,699 healthy, at term, singleton babies during their first year of life and excluded those with low weight at birth, unknown gestational age, bleeding disorder or any other medical conditions that may be associated with anaemia (i.e., malabsorption). Children were randomly assigned to receive a daily, weekly (7 mg/dose) or monthly dose of supplements containing 30 mg elemental iron (as either ferrous sulphate or aminochelate iron), for one year. The study was excluded because the authors compared two intermittent iron supplementation regimens and such comparison is out of the scope of this review. |
| Azeredo 2010 | A prospective population study performed in the city of Viçosa, Southeastern Brazil, in 2007‐8. A total of 103 non‐anaemic children, aged between six and 18 months of age, were included and divided into two supplementation groups: daily dosage (group 1, n=34) and weekly dosage (group 2, n=69). After six months of supplementation, the daily dosage was found to be more effective than the weekly scheme to prevent anaemia in infants. The study was excluded because it was not randomised. |
| Beasley 2000 | Single‐blind randomised trial performed in three rural villages of the Muheza district of Tanzania. Females between the ages of 12‐18 were randomised to a treatment 12 doses of ferrous sulphate or a control of 12 doses of vitamin B12 over the 16 weeks following anti‐helminthic treatment. The use of a strict placebo was not allowed due to ethical reasons. The authors found a significantly greater improvement in serum ferritin in the iron supplemented group but no significant differences in haemoglobin when compared to the control group. This study was excluded because the study evaluated adolescent females which is out of the scope of this review. |
| Briars 2003 | Randomised clinical trial performed in Dunedin, New Zealand. Free‐living healthy adult women of childbearing age (n=138) were randomised to receive a 2,800 μg (2.8 mg) folic acid weekly, a daily 400 μg (0.4 mg) folic acid supplement, or placebo. Authors found that a weekly high‐dose folic acid supplement was as effective as a daily supplement in lowering homocysteine concentrations. The study was excluded because vitamin and minerals were given as foodlets and they are out of the scope of this review. |
| Februhartanty 2002 | Single‐blind experimental community study carried out in Kupang, East Nusa Tenggara, Indonesia. Postmenarchal adolescent females (n=150) were randomised to weekly iron supplementation, placebo, or supplementation during 4 consecutive days of their menstrual cycle. The authors found that weekly iron supplementation for 16 weeks led to a greater improvement in haemoglobin concentration, compared with supplementation during four consecutive days of menstruation. This study was excluded because supplementation was administered to only postmenarchal adolescent females which is out of the scope of this review. |
| Hafeez 1998 | Randomised trial conducted at Combined Military Hospital (CMH), Lahore, Pakistan, from January 1996 to June 1996. 130 children aged 1‐6 years (average 27 months), both sexes, with iron deficiency anaemia (Hb 110 g/L) were divided into 2 subgroups, group A in which children received daily oral dosage of 6 mg/kg of elemental iron as ferrous gluconate and group B, in which children received the same dosage of iron on three consecutive days per week. The intervention lasted 2 months. Haemoglobin and ferritin concentrations increased in both groups with no differences between them. The study was excluded because the intermittent supplements were given on consecutive days. |
| Hop 2005 | Randomised, double‐blind, placebo‐controlled trial conducted in Vietnam. Infants aged 6‐12 mo (n=138) were allocated to one of the following groups: daily multiple micronutrient, daily placebo, weekly multiple micronutrient, or daily iron supplements. All were supplemented for 6 mo, 7 d/wk, under supervision. DMM supplementation had the best overall performance of the micronutrient supplements tested; it reduced the rate of length–growth faltering and had the best hematinic effect. The study was excluded because vitamin and minerals were given as foodLETs and they are out of the scope of this review. |
| Jackson 2003 | The objectives of this study were to ascertain whether, short‐term supplementation with iron and folic acid could reduce anaemia and iron deficiency, and be well tolerated by adolescent females over an 8‐week period. It included 608 postmenarchal adolescent schoolgirls with mild to moderate anaemia. The females were randomly assigned to three groups (iron alone, folic acid alone, and iron with folic acid) and given weekly supplements for eight weeks. Iron and folic acid tablets contained 60 mg elemental iron (as ferrous sulphate) and 3500 μg (3.5 mg) folic acid, respectively. A fourth group of females who had normal Hb concentrations and received no treatment was also included at baseline and after eight weeks.
Authors found that the females receiving iron (alone or in combination) had greater mean rise in Hb than females who received folic acid alone. Eight weeks of supplements given on a weekly basis were well tolerated, causing few symptoms and was effective in reducing anaemia by 30‐40%. The study was excluded because the control group was not randomised. Also the control group had normal Hb values while the females receiving the intervention were anaemic. |
| Jaleel 2004 | Prospective study that included 90 apparently healthy individuals. They were divided into 3 groups of 30 subjects each. First group comprised of male subjects of age between 25‐45 years, second group was of postmenopausal women of age between 46‐65 years and third group of reproductive age group that is between 15‐45 years. Each group was further divided into 3 subgroups of 10 male subjects, 10 postmenopausal and 10 women of reproductive age groups. The first subgroup was given iron supplements (as ferrous
sulphate 300 mg) daily. The second subgroup received supplementation (as ferrous sulphate 300 mg) on weekly basis that is 6 times for 36 days and third subgroup received iron supplements in double dose (ferrous sulphate 600 mg) on weekly basis that is 6
times for 36 days. It was concluded, that 600mg of iron given on a weekly gave similar results as that of subjects receiving 300 mg on a daily basis. This study was excluded because the populations assessed are out of the scope of this review. |
| Jayatissa 1999 | In Sri Lanka 36% of all adolescents have inadequate iron intakes. Daily and weekly iron supplementation of 659 adolescent schoolgirls, divided into three groups, was studied in an eight‐week double‐blind trial. One group received 60 mg of elemental iron, 250 μg (0.25 mg) of folic acid, and 100 mg of vitamin C daily. The second group was given the same doses on a weekly basis. The third group was given a placebo. All of the participants were de‐wormed at the beginning of the study. Anaemia was more common among older adolescents. Haemoglobin levels increased significantly at the end of the study. The prevalence of anaemia was reduced from 25% to 9.5% by weekly supplementation and from 18.5% to 8.6% by daily supplementation. The difference in haemoglobin levels between the two groups receiving supplementation was not significant. The study was excluded because it is not clear whether is randomised or not. Based on the methods the selection of participants was at random but not the allocation of the intervention. |
| Kanal 2005 | This was a community trial in which social marketing and community mobilization approaches were applied to introduce weekly iron‐folic acid supplementation to prevent anaemia in Cambodian women of reproductive age. The programme was implemented in three very different environments: secondary schoolgirls, women working in garment factories in the vicinity of Phnom Penh, and women in rural villages. All three groups of women showed substantial improvements in knowledge about the causes, consequences, and prevention of anaemia, and the large majority reported interest in continuing to take the supplements. The study was excluded because it was not randomised. |
| Kapur 2003 | A community‐based trial that compared the effect of nutrition education and/or iron supplementation (weekly) on iron status of 400 children, 9‐36 months, living in an urban slum in Delhi. Children and care takers were selected by using a random number table. Children were assigned to one of the following groups. Group 1, received nutrition education. Group 2, received supplements with 20 mg elemental iron. Group 3, received nutrition education with supplementation with 20 mg elemental iron and Group 4, control given placebo. To ensure objectivity and to avoid spill over effect, specifically with respect to nutrition education, caution was maintained in forming groups and allocation of subjects therein. Subjects from the Anganwadis in Block A (A1, A2, A3 and A4) and Block B (including B1, B2, B3, B4, B5 and B6) (which were more or less adjoining) were allocated to experimental groups 3 (NE+ S) and 4 (NE), where nutrition education was a component. Control and Experimental group 2 (supplementation group), on the other hand, included subjects from Block C (including C1, C2 and C3), Block D (including D1, D2 and D3) and Block E (including E1, E2, E3, E4 and E5) which are situated at a distance of about 1 ‐ 2 Km from the Experimental groups 3 and 4 (information provided by the author). The intervention program was of four months duration, with a treatment phase of 8 wk followed by 8 wk of no treatment.There was no significant effect of any of the intervention at 8 weeks. At 16 wk, there was significant positive effect of nutrition education group (p less than 0.05). This study was excluded because the allocation was not at random. |
| Kianfar 2000 | A randomised trial comparing the effects of daily and intermittent iron supplementation regimens in adolescent schoolgirls in the areas of Zahedan and Rasht, Iran. 1853 subjects were selected by stepwise random sampling and randomised to a daily group, supplemented with 50 mg elemental iron per day, a once weekly group supplemented with 50 mg elemental iron and a twice weekly group, also supplemented with 50 mg elemental iron. The authors concluded that the once and twice weekly regimens were effective in treating anaemia but that the daily schedule was more effective at increasing iron stores than a weekly dose in the short‐term. This study was excluded because it evaluated adolescent females which is out of the scope of this review. |
| Lechtig 2006 | This paper is one of a series of papers that describes the experiences of a multiple micronutrient intervention programme implemented in poor urban mothers and their young children of Chiclayo, Peru. It summarizes the lessons learned for consideration of future programming. The study was excluded because the authors gave foodlets and that intervention is out of the scope of this review. |
| Leenstra 2009 | A double‐blind, randomised controlled study using a factorial design carried out in primary schools in Kisumu, Western Kenya. The study aimed to evaluate the effect of weekly iron and vitamin A supplementation on haemoglobin, iron status,and malaria and non‐malaria morbidity in adolescent schoolgirls. Weekly iron supplementation was found to greatly increase haemoglobin levels in menstruating and iron‐deficient females but not in iron‐replete and non menstruating females. This study was excluded because it looked only at adolescent school females between the ages of 12‐18 years which is out of the scope of this review. |
| Lima 2006 | A controlled, community‐based intervention was carried out with 378 infant to evaluate the impact of weekly treatment with ferrous sulphate on haemoglobin level, morbidity and nutritional status in a sample of anaemic infants from Zona da Mata Meridional in the state of Pernambuco, Brazil. Participating infants were divided into three groups: two received 45 mg of elemental iron weekly, from 12 to 18 months of life (69 children with moderate/severe anaemia, and 111 with mild anaemia); the third group was composed of 65 non‐anaemic children, who received no intervention. The remaining 133 children constituted the control group. Less than half the children receiving ferrous sulphate recovered from anaemia at the end of follow‐up while 40.3% of the children without anaemia at baseline, who did not receive treatment, developed anaemia. The study was excluded because it was not randomised. |
| Lin 2001 | 270 rural preschool children aged 3‐7 years with low levels of vitamin A and iron living in Beijing, China. Participants were divided into four groups based on their determinations: control, lower serum vitamin A, lower iron, and both lower iron and serum vitamin A. Forty‐one subjects who had lower iron and lower serum vitamin A (< 1.12 mumol/L) were divided into two groups: one of them supplemented with 30 mg elemental iron (as ferrous sulphate 0.15 g) once a day for 8 weeks, and the other group supplemented with iron and 12,500 IU vitamin A twice a week for 8 weeks. Authors concluded that supplementation with vitamin A and iron was helpful to improve body iron nutritional status and immunological function obviously in preschool children with iron‐deficiency and sub‐clinical deficiency of vitamin A. The study was excluded because the daily and weekly groups did not receive the same nutrients. |
| López de Romaña 2005 | Randomised, double‐blind, masked, controlled trial conducted in Peru. Infants aged 6 to 12 mo (n=313) were assigned to receive either a daily dose of iron, a daily dose of multiple micronutrients, a weekly dose of multiple micronutrients, or a placebo for 6 mo. None of the supplements tested prevented growth faltering or the morbidities common during infancy. The daily multiple micronutrient intervention was the most efficacious for preventing anaemia, iron, and zinc deficiencies, 15%, 20%, and 50% of this group still remained anaemic, zinc deficient, and iron deficient, respectively, at the end of the study. The study was excluded because vitamin and minerals were given as foodLETs and they are out of the scope of this review. |
| López de Romaña 2006 | Randomised community trial undertook in 26 Peruvian communities with stunting rates above the average. Households were selected if they had at least one child under 5 years of age and at least one woman or adolescent females of childbearing age (12 through 44 years). A total of 866 households (448 in the intervention group and 418 in the comparison group‐unclear) were selected. Women received Nutrivit capsules while children received Foodlets. Authors concluded that weekly supplementation with multi‐micronutrients had a protective effect on the haemoglobin levels of both women and adolescent females of childbearing age and children under 5 years of age. The study was excluded because vitamin and minerals were given as foodlets and they are out of the scope of this review. |
| Menendez 1997 | Randomized clinical trial in Tanzania. Newborns (n=832) were randomly assigned to group DI, receiving daily oral iron (2 mg/kg daily) plus weekly Deltaprim (3.125 mg pyrimethamine plus 25 mg dapsone); group IP, receiving iron plus weekly placebo; group DP, receiving daily placebo plus weekly Deltaprim; or group PP. supplementation was given from 8 to 24 weeks of age, and the weekly chemoprophylaxis from 8 to 48 weeks. The groups that received iron supplementation had a lower frequency of severe anaemia. The study was excluded because only the malaria prophylaxis was given on a weekly basis. |
| Mwanakasale 2009 | Placebo‐controlled intervention trial conducted in Nchelenge district in Luapula province of Zambia. Children between 9 and 15 years of age received once a week either 200 mg of ferrous sulphate or 100 mg of vitamin C. Both study groups received a single dose of praziquantel at baseline and the follow‐up lasted 9 months. The study was excluded because it was not randomised. |
| Perrin 2002 | It is a commentary paper on Shah 2002. |
| Risonar 2008 | 242 Filipino schoolchildren aged 6–12 years with haemoglobin (Hb) concentration <120 g/L and enrolled for school year 2003–2004. UNICEF iron‐folate tablets containing 60 mg elemental iron and 400 μg (0.40 mg) folic acid were given weekly through directly observed supplementation by the teachers for 27 weeks. The intervention reduced anaemia prevalence among anaemic schoolchildren and resulted in high compliance to and coverage of iron supplementation. The study was excluded because it does not have a control group. |
| Rivera 1998 | Report that presents the results of a regional program providing weekly iron supplements to school‐age children attending 30 schools, between 1995 and 1997, in Chiriqui, Panama, This study was excluded because the study design (pre‐post without a control group) is out of the scope of this review. |
| Schümann 2009 | Randomised doubly‐masked, placebo‐controlled trial undertook in Cambodia. Children aged 6‐24 months (n= 250) received twice‐weekly administration of 3 RDAs of iron and folic acid, with and without a complement of 2 RDAs of 11, and 1 RDA of 3 additional essential micronutrients as compared to a placebo control (PlbCON) given as foodLETs. Supplementation of micronutrients along with iron and folic acid mitigates the excess morbidity of iron‐folate alone, without reducing its efficacy in correcting anaemia and building iron stores. The study was excluded because vitamin and minerals were given as foodLETs and they are out of the scope of this review. |
| Shah 2002 | Randomised‐controlled trial of healthy adolescent females in government female schools of Dharan, Nepal. The study aimed to compare the effectiveness of weekly versus daily iron folate supplementation. Females (n=209) were randomised to either a daily iron‐folate group, a weekly iron‐folate group, or a placebo group which did not receive any tablets. The authors concluded that once weekly iron folate supplementation was an effective alternative to daily regimens. The study was excluded because it evaluated only adolescent females which is not in the scope of this review. |
| Sharma 2000 | Randomised experimental trial of adolescent females in poor communities in urban areas of Delhi and rural parts of Rajasthan. Subjects were randomised to either daily iron folate supplementation, weekly iron folate supplementation or weekly iron folate with vitamin C. The authors concluded that the response of haemoglobin levels was greater following daily iron folate supplementation when compared to weekly iron supplementation, but that the addition of vitamin C led to greater increases in haemoglobin than administration of iron and folate alone. This study was excluded because it evaluated only adolescent females which is out of the scope of this review. |
| Shobha 2003 | Randomised trial of 244 adolescent females at an Andhra Pradesh residential social welfare school in the Ranga Reddy district of India. Females were stratified by anaemia status and then randomly assigned to either a daily or twice weekly supplementation regimen. Supervised administration of iron twice weekly was found to be similarly advantageous as daily supplementation in this population. This study was excluded because it evaluated only adolescent females which is out of the scope of this review. |
| Smuts 2005 | Randomised trial undertook in South Africa. Infants aged 6‐12 mo (n=265) were individually randomised to 1 of 4 intervention groups a daily multiple micronutrient supplement, a daily placebo supplement; a multiple micronutrient supplement 1 d of the week and placebo supplement on the other days of the week, and a daily iron supplement . For 6 mo, the blinded supplements were provided to mothers at monthly health clinic sessions, and consumption was verified during weekly household visits by community health workers, when morbidity was also checked. The DMM was the most effective intervention tested, not only for improving anaemia but also for improving iron, zinc, riboflavin, and tocopherol status. The study was excluded because vitamin and minerals were given as foodlets and they are out of the scope of this review. |
| Soekarjo 2004 | A school‐based grade‐randomised intervention was conducted in rural and urban, East Java, Indonesia among adolescents. 1757 females and 1859 males were randomised to weekly supplementation (650 mg iron, 250 μg (0.25 mg) folic acid), weekly vitamin A supplementation (10,000 IU), or both, were compared to a group not receiving any supplements. Weekly iron supplementation was not effective at raising haemoglobin levels, likely due to poor compliance and side effects. This study was excluded because it evaluated an exclusively adolescent population, which is out of the scope of this review. |
| Sotelo‐Cruz 2002 | 20 anaemic children aged 2 to 5 years living in Hermosillo, Mexico. Group A received oral ferrous sulphate twice a day whereas group B received the some dose per kilogram of weight like that of group A once a week for three months. Haemoglobin concentrations improved in both groups. The study was excluded because it is not randomised. |
| Tee 1999 | Study that investigated whether long‐term, weekly iron folate supplements administered at school would improve haemoglobin and ferritin concentrations in adolescent females, including those with mild‐to‐moderate anaemia and haemoglobin concentrations indicating borderline anaemia. 266 females with haemoglobin concentrations of 80–119.9 g/L (group A) and 358 females with haemoglobin concentrations of 120–130 g/L (group B) who were otherwise healthy. Two hundred sixty‐six females in group A and 268 females in group B were randomly assigned to receive either 60 or 120 mg iron plus 3500 μg (3.5 mg) folic acid weekly for 22 wk. Ninety of the females in group B were randomly assigned to receive only 5000 μg (5 mg) folic acid weekly. Authors concluded that long‐term, weekly iron‐folate supplementation was found to be a practical, safe, effective, and inexpensive method for improving iron nutrition in adolescent schoolgirls. Study was excluded because children in both arms were given iron on a weekly basis. Group C did not receive supplements but was not followed up. |
| Tomashek 2001 | Randomised double‐blind study, in which 215 anaemic children, initially treated for malaria and helminth infection, received 12 weeks of thrice‐weekly oral iron and folic acid. Group I received placebo and chloroquine treatment for symptomatic malaria infection (i.e., no presumptive anti‐malarial treatment given). Group II received placebo and monthly presumptive treatment with sulphamethoxazole‐pyrimethamine (SP). Group III also received
monthly SP and thrice‐weekly vitamins A and C (VAC). Mean haemoglobin concentration increased from 66 to 102 g/L, with no significant differences among groups. Study was excluded because all the participants were given iron and folic acid on a weekly basis. |
| UNICEF 2006 | Report that presents the results of a National program providing weekly iron supplements (ferrous fumarate) to infants and pregnant women living in priority districts in the Republic of Panama. This study was excluded because the study design (pre‐post without a control group) is out of the scope of this review. |
| Vir 2008 | Study performed in school and non‐school females aged 11 to 18 years that aimed to assess the effectiveness of weekly iron‐folic acid supplementation in reducing the prevalence of anaemia in adolescent females. The project provided weekly iron‐folic acid tablets, family life education, and deworming tablets every 6 months to 150,700 adolescent school females and non‐school females of a total district population of 3,647,834. Groups were not evaluated simultaneously. In 4 years, the overall prevalence of anaemia was reduced from 73.3% to 25.4%. Hemoglobin levels and anaemia prevalence were influenced significantly at 6 months. No difference in the impact on haemoglobin or anaemia prevalence was observed between supervised and unsupervised females. The study was excluded because it was not randomised. |
| Wijaya‐Erhardt 2007 | A double‐blind, randomised, placebo‐controlled trial. Indonesian infants.
aged 6–12 mo were randomly allocated to 1 of 4 groups: daily multiple‐micronutrients food like tablets (foodlets), weekly multiple‐micronutrient foodlets, daily iron foodlets, or daily placebo Data were obtained at baseline and 23 wk. DI and daily multiple‐micronutrients foodlets are efficacious in improving and weekly multiple‐micronutrient is efficacious in maintaining iron stores. The study was excluded because vitamin and minerals were given as foodlets and they are out of the scope of this review. |
| Zavaleta 2000 | A randomised, double‐blind, placebo‐controlled study conducted among adolescent school females in Lima, Peru. 312 adolescents females were randomly assigned to either 60mg ferrous sulphate Mon‐Friday (daily), 60 mg of ferrous sulphate twice weekly with placebo 3 times per week, or a placebo five days per week. Both iron supplementation regimens were found to be effective at reducing iron deficiency and the daily supplementation schedule was found to be more effective at raising haemoglobin concentration and reducing anaemia. This study was excluded because it evaluated adolescent females which is out of the scope of this review. |
Characteristics of studies awaiting assessment [ordered by study ID]
Husseini 1999.
| Methods | Cluster‐randomised trial. 2‐arm design with randomisation at village level. |
| Participants | 822 children, both sexes, 5 to 26 months (average age 17.5 months). Inclusion criterion: haemoglobin <90 g/L |
| Interventions | Villages were allocated to one of the following groups> Group 1: children received 20 mg of iron daily; Group 2: children received 25 mg of iron once a week; Length of the intervention: 18 months with visits every 4 weeks. Intervention supervised and unsupervised. |
| Outcomes | Anaemia, haemoglobin, ferritin |
| Notes | Information obtained from Beaton 1999 |
Kargarnovin 2010.
| Methods | Randomised trial. 2‐arm design with individual randomisation. |
| Participants | 160 anaemic infants, both sexes, 6‐24 months living in the South of Tehrna, Iran. |
| Interventions | Children were allocated to one of the following groups: Group 1 (n=80): children were given daily containing 40 mg of elemental iron (as ferrous sulphate); Group 1 (n=80): children were given 40 mg of elemental iron (as ferrous sulphate) once a week on Friday mornings. Length of the intervention: 6 months. |
| Outcomes | Haemoglobin, erythrocyte volume, total iron binding capacity, transferrin saturation, serum transferrin |
| Notes | Article written in Farsi. The author was contacted to obtain the information. Figure 2 is not available in PDF and the author has been contacted to obtain this information. The article concludes that weekly administration of iron compared with daily consumption seems superior due to similar effects, better compliance of mothers and lower costs for the treatment of anaemia in infants between 6 and 24 months |
Reid 2001.
| Methods | Double‐blind randomised trial. 3 arm study with randomisation at individual level. |
| Participants | 125 Mexican preschoolers with low Hb, age 12‐40 months of age |
| Interventions | Participants were allocated to one of the following treatments: Group 1: placebo Group 2: iron (Fe), Group 3: iron + B‐12 (Fe+B‐12), or Group 4: multiple micronutrients (MM = iron, vitamin B12, vitamin B2, vitamin B6, vitamin A, vitamin E, folic acid zinc, cooper). Doses were 2 x RDA, 3 times/wk, under supervision. Length of the intervention: 3 months |
| Outcomes | Anaemia, haematocrit, ferritin, retinol, serum B12 |
| Notes | This study has not been published. If the data is made available to us, we will include it in future updates of the review. |
Characteristics of ongoing studies [ordered by study ID]
Zeeba Zaka‐ur‐Rab 2010.
| Trial name or title | A clinical trial to compare the effects of daily versus intermittent iron supplementation on markers of oxidative stress and anti‐oxidant status in children with iron deficiency anaemia |
| Methods | Randomised, controlled trial. Method of generating randomisation sequence: random number table. Method of allocation concealment: sequentially numbered, sealed, opaque envelopes Blinding and masking:Outcome Assessor Blinded |
| Participants | 150 children between 1‐15 years of age with iron deficiency anaemia. Exclusion criteria: children with history of fever within last 4 weeks, acute or chronic medical disorders, hemolytic anaemia, haemoglobin <6gm%, patients receiving iron/vitamin/mineral supplements (including herbal drugs), blood transfusion within 8 weeks. |
| Interventions | Intervention: sodium feredetate: 6 mg/kg daily; Control: sodium feredetate: 6 mg/kg on day 1 and day 4. Length of the intervention: 8 weeks. |
| Outcomes | Changes in malonyl dialdehyde, oxidized glutathione, superoxide dismutase, glutathione peroxidase, catalase, changes in hemo globin, serum ferritin, total iron binding capacity and serum iron. Timepoint: 8 weeks |
| Starting date | Date of first enrolment: 02‐03‐2009 |
| Contact information | Dr. Zeeba Zaka‐ur‐Rab Deptt. of Pediatrics, J.N. Med. College, A.M.U. 2, Wazir Manzil, Luxmibai Marg, 202 001 Aligarh, UTTAR PRADESH India Tel: 0571 ‐2402928 Email: zzrab@yahoo.co.in |
| Notes |
Differences between protocol and review
We added a description of the methodology followed to produce the 'Summary of findings' tables in the 'Assessment of risk of bias in included studies' section.
We made the following changes to the outcomes section:
we modified the order of the primary outcomes so that the effects of the same indicator, presented either as a continuous or as a dichotomous variable, could be assessed together (for example, anaemia and haemoglobin concentrations);
since there are no official cut‐offs for children younger than 6 months, we changed the definition of anaemia from "haemoglobin < 110 g/L or < 115 g/L for children 6 to 59 months or 5 to 11 years old, respectively, adjusted by altitude where appropriate" to "haemoglobin below a cut‐off defined by trialists, taking into account the age group and altitude";
for our secondary outcome 'all‐cause morbidity', we replaced 'at least one event' with 'at least one reported illness' to make it clearer;
we renamed our secondary outcome 'folic acid status' as 'folate status' and replaced the units with 'as measured by trialists'. Folate may be measured in serum, plasma or red blood cells and the most frequently used units may vary.
as the duration of the trials was mostly short, we changed the definition of our secondary outcome 'growth impairment (stunting and wasting)' to 'height‐for‐age and weight‐for‐age Z‐scores' and moved this to the end of our list of outcomes.
Contributions of authors
All four review authors contributed to drafting the text of the review, commented on the drafts and approved the final version.
Sources of support
Internal sources
Centers for Disease Control and Prevention (CDC), Division of Nutrition, Physical Activity, and Obesity, USA.
Micronutrients Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
External sources
-
Micronutrients Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Dr Therese Dowswell received partial financial support for her work on this review.
-
Government of Luxembourg, Luxembourg.
WHO acknowledges the Government of Luxembourg for their financial support to the Micronutrients Unit for conducting systematic reviews on micronutrient interventions
Declarations of interest
Luz Maria De‐Regil ‐ none known. Maria Elena D Jefferds ‐ none known. Allison C Sylvetsky ‐ none known. Therese Dowswell ‐ none known.
Disclaimer: Luz Maria De‐Regil is a full‐time staff member of the World Health Organization (WHO), Allison C Sylvetsky did a 6‐week internship at WHO (summer 2010), and Therese Dowswell has received financial support from the WHO for her work on this review. Maria Elena Jefferds is a full‐time staff member of the US Centers for Disease Control and Prevention. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the official position, decisions, policy or views of these Organisations.
Edited (no change to conclusions)
References
References to studies included in this review
Aguayo 2000 {published data only}
- Aguayo VM. School‐administered weekly iron supplementation ‐ effect on the growth and hemoglobin status of non‐anemic Bolivian school‐age children. A randomized placebo‐controlled trial. European Journal of Nutrition 2000;39(6):263‐9. [DOI] [PubMed] [Google Scholar]
Arcanjo 2011 (C) {published data only}
Awasthi 2005 (C) {published data only}
- Awasthi S, Verma T, Vir S. Effectiveness of biweekly versus daily iron‐folic acid administration on anaemia status in preschool children. Journal of Tropical Pediatrics 2005;51(2):67‐71. [DOI] [PubMed] [Google Scholar]
Baqui 2003 {published data only}
- Baqui AH, Walker CL, Zaman K, Arifeen S, Chowdhury HR, Wahed MA, et al. Weekly iron supplementation does not block increases in serum zinc due to weekly zinc supplementation in Bangladeshi infants. Journal of Nutrition 2005;135(9):2187‐91. [DOI] [PubMed] [Google Scholar]
- Baqui AH, Zaman K, Persson LA, Arifeen S, Yunus M, Begum N, et al. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhea and acute lower respiratory infection in Bangladeshi infants. Journal of Nutrition 2003;133(12):4150‐7. [DOI] [PubMed] [Google Scholar]
- Black MM, Baqui AH, Zaman K, Ake Persson L, Arifeen S, Le K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. American Journal of Clinical Nutrition 2004;80(4):903‐10. [DOI] [PubMed] [Google Scholar]
- Fischer Walker CL, Baqui AH, Ahmed S, Zaman K, Arifeen S, Begum N, et al. Low‐dose weekly supplementation of iron and/or zinc does not affect growth among Bangladeshi infants. European Journal of Clinical Nutrition 2009;63(1):87‐92. [DOI] [PubMed] [Google Scholar]
Berger 1997 {published data only}
- Berger J, Aguayo VM, Téllez W, Luján C, Traissac P, San Miguel JL. Weekly iron supplementation is as effective as 5 day per week iron supplementation in Bolivian school children living at high altitude. European Journal of Clinical Nutrition 1997;51(6):381‐6. [DOI] [PubMed] [Google Scholar]
Da Silva 2008 {published and unpublished data}
- Silva DG, Franceschini Sdo C, Sigulem DM. Growth in non‐anemic infants supplemented with different prophylactic iron doses. Jornal de Pediatria 2008;84(4):365‐72. [DOI] [PubMed] [Google Scholar]
Desai 2004 (C) {published data only}
- Desai MR, Dhar R, Rosen DH, Kariuki SK, Shi YP, Kager PA, et al. Daily iron supplementation is more efficacious than twice weekly iron supplementation for the treatment of childhood anemia in western Kenya. Journal of Nutrition 2004;134(5):1167‐74. [DOI] [PubMed] [Google Scholar]
Ekvall 2000 {published data only}
- Ekvall H, Premji Z, Bjorkman A. Micronutrient and iron supplementation and effective anti‐malarial treatment synergistically improve childhood anaemia. Tropical Medicine and International Health 2000;5(10):696‐705. [DOI] [PubMed] [Google Scholar]
Engstrom 2008 (C) {published data only}
- Engstrom EM, Castro IR, Portela M, Cardoso LO, Monteiro CA. Effectiveness of daily and weekly iron supplementation in the prevention of anemia in infants. Revista de Saúde Pública 2008;42(5):786‐95. [DOI] [PubMed] [Google Scholar]
Ermis 2002 {published data only}
- Ermis B, Demirel F, Demircan N, Gurel A. Effects of three different iron supplementations in term healthy infants after 5 months of life. Journal of Tropical Pediatrics 2002;48(5):280‐4. [DOI] [PubMed] [Google Scholar]
Evangelista‐Salazar 2004 {published data only}
- Evangelista‐Salazar E. Evaluation of the preventive effect of the intermittent provision of iron and vitamin C on the reduction of the iron and neurodevelopment in infants [Evaluacion del efecto preventivo de la administracion intermitente de hierro y vitamina C sobre la disminucion de la reserva de hierro y el neurodesarrollo en lactantes]. Doctoral Thesis. Universidad de Colima, Mexico 2004.
- Martínez H, Evangelista Salazar JJ, Avila Jiménez L. Iron supplementation for preventing anaemia in infants [Suplementación con hierro para prevenir anemia en la primera infancia]. In: Luis Durán Arenas, Onofre Muñoz Hernández editor(s). La traducción del conocimiento. del resultado de la investigación a la aplicación de los servicios de salud. México, D.F: CAMS, 2006:71‐86. [Google Scholar]
Faqih 2006 {published data only}
- Faqih AM, Kakish SB, Izzat M. Effectiveness of intermittent iron treatment of two‐ to six‐year‐old Jordanian children with iron‐deficiency anemia. Food and Nutrition Bulletin 2006;27(3):220‐7. [DOI] [PubMed] [Google Scholar]
Hall 2002 (C) {published and unpublished data}
- Hall A, Roschnik N, Ouattara F, Toure I, Maiga F, Sacko M, et al. A randomised trial in Mali of the effectiveness of weekly iron supplements given by teachers on the haemoglobin concentrations of schoolchildren. Public Health Nutrition 2002;5(3):413‐8. [DOI] [PubMed] [Google Scholar]
Khademloo 2009 {published data only}
- Khademloo M, Karami H, Ajami A, Yasari M. Comparison of the effectiveness of weekly and daily iron supplementation in 6‐ to 24‐months‐old babies in urban health centers of Sari, Iran. Pakistan Journal of Biological Sciences 2009;12(2):195‐7. [DOI] [PubMed] [Google Scholar]
Liu 1995 (C) {published data only}
- Liu X‐N, Kang J, Zhao L, Viteri FE. Intermittent iron supplementation in Chinese pre‐schoolchildren is efficient and safe. Food and Nutrition Bulletin 1995;16(2):139‐46. [Google Scholar]
- Liu XNA, Liu PY. The effectiveness of weekly iron supplementation regimen in improving the iron status of Chinese children and pregnant women. Biomedical and Environmental Sciences 1996;9(2‐3):341‐7. [PubMed] [Google Scholar]
Nguyen 2002 {published data only}
- Nguyen XN, Berger J, Dao TQ, Nguyen CK, Traissac P, Ha HK. Efficacy of daily and weekly iron supplementation for the control of iron deficiency anaemia in infants in rural Vietnam [Efficacite de la supplementation en fer quotidienne et hebdomadaire pour le controle de l'anemie chez le nourrisson en milieu rural au Vietnam]. Santé (Montrouge, France) 2002;12(1):31‐7. [PubMed] [Google Scholar]
Olsen 2000 {published data only}
- Olsen A, Nawiri J, Friis H. The impact of iron supplementation on reinfection with intestinal helminths and Schistosoma mansoni in western Kenya. Transactions of the Royal Society of Tropical Medicine Hygene 2000;94(5):493‐9. [DOI] [PubMed] [Google Scholar]
- Olsen A, Nawiri J, Magnussen P, Krarup H, Friis H. Failure of twice‐weekly iron supplementation to increase blood haemoglobin and serum ferritin concentrations: results of a randomized controlled trial. Annals of Tropical Medicine and Parasitology 2006;100(3):251‐63. [DOI] [PubMed] [Google Scholar]
Palupi 1997 {published data only}
- Palupi L, Schultink W, Achadi E, Gross R. Effective community intervention to improve hemoglobin status in preschoolers receiving once‐weekly iron supplementation. American Journal of Clinical Nutrition 1997;65(4):1057‐61. [DOI] [PubMed] [Google Scholar]
Roschnik 2003 (C) {unpublished data only}
- Roschnik N, Phiri V, Mukaka M. The impact of weekly school‐based iron supplementation. Mangochi District, Malawi (Report). Save the Children, USA February 2003.
Roschnik 2004 (C) {published and unpublished data}
- Roschnik N, Parawan A, Baylon MA, Chua T, Hall A. Weekly iron supplements given by teachers sustain the haemoglobin concentration of schoolchildren in the Philippines. Tropical Medicine and International Health 2004;9(8):904‐9. [DOI] [PubMed] [Google Scholar]
Schultink 1995 {published data only}
- Schultink W, Gross R, Gliwitzki M, Karyadi D, Matulessi P. Effect of daily vs twice weekly iron supplementation in Indonesian preschool children with low iron status. American Journal of Clinical Nutrition 1995;61(1):111‐5. [DOI] [PubMed] [Google Scholar]
Sen 2009 (C) {published and unpublished data}
- Sen A, Kanani SJ. Impact of iron‐folic acid supplementation on cognitive abilities of school girls in Vadodara. Indian Pediatrics 2009;46(2):137‐43. [PubMed] [Google Scholar]
- Sen A, Kanani SJ. Physical work capacity of young underprivileged school girls impact of daily vs intermittent iron folic acid supplementation: a randomized controlled trial. Indian Pediatrics 2009;46(10):849‐54. [PubMed] [Google Scholar]
Siddiqui 2004 {published data only}
- Siddiqui IA, Jaleel A, Rahman MA. Preventive strategy to control iron deficiency anemia in children and adults. Journal of the Pakistan Medical Association 2003;53(4):131‐3. [PubMed] [Google Scholar]
- Siddiqui IA, Rahman MA, Jaleel A. Efficacy of daily vs. weekly supplementation of iron in schoolchildren with low iron status. Journal of Tropical Pediatrics 2004;5:276‐8. [DOI] [PubMed] [Google Scholar]
Sinisterra 1997 (C) {published data only}
- Sinisterra OT, Valdes VE, Valverde C. Effect of supplementation with iron salts and knowledge, attitudes and practices in relation to anaemia among schoolchildren in the province of Cocle, Ministry of Health, Republic of Panama (report). [Efecto de la suplementacion con sales de hierro y de conocimientos, actitudes y practicas en relacion a la anemia en escolares de la provincia de Cocle, Republica de Panama: reporte]. Vol. DCE/18, Panama: INCAP, Ministry of Health, Republic of Panama, 1997. [Google Scholar]
Soemantri 1997 {published data only}
- Soemantri AG, Hapsari DE, Susanto JC, Rohadi W, Tamam M, Irawan PW, et al. Daily and weekly iron supplementation and physical growth of school age Indonesian children. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28 Suppl 2:69‐74. [PubMed] [Google Scholar]
Sungthong 2002 {published data only}
- Sungthong R, Mo‐Suwan L, Chongsuvivatwong V, Geater AF. Once weekly is superior to daily iron supplementation on height gain but not on hematological improvement among schoolchildren in Thailand. Journal of Nutrition 2002;132(3):418‐22. [DOI] [PubMed] [Google Scholar]
- Sungthong R, Mo‐suwan L, Chongsuvivatwong V, Geater AF. Once‐weekly and 5‐days a week iron supplementation differentially affect cognitive function but not school performance in Thai children. Journal of Nutrition 2004;134(9):2349‐54. [DOI] [PubMed] [Google Scholar]
Tavil 2003 {published data only}
- Tavil B, Sipahi T, Gökçe H, Akar N. Effect of twice weekly versus daily iron treatment in Turkish children with iron deficiency anemia. Pediatric Hematology and Oncology 2003;20(4):319‐26. [PubMed] [Google Scholar]
Taylor 2001 {published data only}
- Taylor M, Jinabhai CC, Couper I, Kleinschmidt I, Jogessar VB. The effect of different anthelmintic treatment regimens combined with iron supplementation on the nutritional status of schoolchildren in KwaZulu‐Natal, South Africa: a randomized controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(2):211‐6. [DOI] [PubMed] [Google Scholar]
Thu 1999 {published data only}
- Thu BD, Schultink W, Dillon D, Gross R, Leswara ND, Khoi HH. Effect of daily and weekly micronutrient supplementation on micronutrient deficiencies and growth in young Vietnamese children. American Journal of Clinical Nutrition 1999;69(1):80‐6. [DOI] [PubMed] [Google Scholar]
Verhoef 2002 {published data only}
- Verhoef H, West CE, Kraaijenhagen R, Nzyuko SM, King R, Mbandi MM. Malarial anemia leads to adequately increased erythropoiesis in asymptomatic Kenyan children. Blood 2002;15(00(10)):489‐94. [DOI] [PubMed] [Google Scholar]
- Verhoef H, West CE, Nzyuko SM, Vogel S, Valk R, Wanga MA, et al. Intermittent administration of iron and sulfadoxine‐pyrimethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet 2002;21(360(9337)):908‐14. [DOI] [PubMed] [Google Scholar]
Yang 2004 (C) {published data only}
- Yang Q, Yin S, Zhao X, An J. Effect of daily or once weekly iron supplementation on growth and iron status of preschool children. Wei sheng yan jiu = Journal of Hygiene Research 2004;33(2):205‐7. [PubMed] [Google Scholar]
Young 2001 {published data only}
- Young MW. The effectiveness of weekly iron and vitamin supplementation of Malawian preschool children. South African Medical Journal = Suid‐Afrikaanse tydskrif vir geneeskunde 2001;91(1):49‐50. [PubMed] [Google Scholar]
Yurdakok 2004 {published data only}
- Yurdakök K, Temiz F, Yalçin SS, Gümrük F. Efficacy of daily and weekly iron supplementation on iron status in exclusively breast‐fed infants. Journal of Pediatric Hematology/Oncology 2004;26(5):284‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2003 {published data only}
- Agarwal KN, Gomber S, Bisht H, Som M. Anemia prophylaxis in adolescent school girls by weekly or daily iron‐folate supplementation. Indian Pediatrics 2003;40(4):296‐301. [PubMed] [Google Scholar]
Ahmed 2001 {published data only}
- Ahmed F, Khan MR, Jackson AA. Concomitant supplemental vitamin A enhances the response to weekly supplemental iron and folic acid in anemic teenagers in urban Bangladesh. American Journal of Clinical Nutrition 2001;74(1):108‐15. [DOI] [PubMed] [Google Scholar]
Ahmed 2005 {published data only}
- Ahmed F, Khan MR, Akhtaruzzaman M, Karim R, Marks GC, Banu CP, et al. Efficacy of twice‐weekly multiple micronutrient supplementation for improving the hemoglobin and micronutrient status of anemic adolescent schoolgirls in Bangladesh. American Journal of Clinical Nutrition 2005;82(4):829‐35. [DOI] [PubMed] [Google Scholar]
Avila‐Jimenez 2011 {unpublished data only}
- Ávila Jiménez L, Méndez, Villalpando‐Hernández S, Martínez‐Salgado H. Preventive iron supplementation and nutritional status of infants measured by anthropometry assigned to IMSS obligatory regimen [Suplementación preventiva con hierro y estado nutricio medido por antropometría de lactantes del régimen obligatorio del IMSS]. Unpublished article sent by Dr Homero Martinez.
- Ávila‐Jiménez L, Méndez I, Villalpando‐Hernández S, Martínez‐Salgado H. Effect of iron supplementation on growth velocity in infants [Efecto de una suplementación con hierro sobre la velocidad de crecimiento en lactantes]. Unpublished article sent by Dr Homero Martinez.
Azeredo 2010 {published data only}
- Azeredo CM, Cotta RM, Sant'Ana LF, Franceschini Sdo C, Ribeiro Rde C, Lamounier JA, et al. Greater effectiveness of daily iron supplementation scheme in infants [Efectividad superior del esquema diario de suplementación de hierro en lactantes]. Revista de Saúde Pública 2010;44(2):230‐9. [DOI] [PubMed] [Google Scholar]
Beasley 2000 {published data only}
- Beasley NM, Tomkins AM, Hall A, Lorri W, Kihamia CM, Bundy DA. The impact of weekly iron supplementation on the iron status and growth of adolescent girls in Tanzania. Tropical Medicine and International Health 2000;5(11):794‐9. [DOI] [PubMed] [Google Scholar]
Briars 2003 {published data only}
- Adank C, Green TJ, Skeaff CM, Briars B. Weekly high‐dose folic acid supplementation is effective in lowering serum homocysteine concentrations in women. Annals of Nutrition and Metabolism 2003;47(2):55‐9. [DOI] [PubMed] [Google Scholar]
Februhartanty 2002 {published data only}
- Februhartanty J, Dillon D, Khusun H. Will iron supplementation given during menstruation improve iron status better than weekly supplementation?. Asia Pacific Journal of Clinical Nutrition 2002;11(1):36‐41. [DOI] [PubMed] [Google Scholar]
Hafeez 1998 {published data only}
- Hafeez A, Ahmad P. Iron deficiency anaemia: continuous versus intermittent treatment in anaemic children. Journal of the Pakistan Medical Association 1998;48(9):269‐72. [PubMed] [Google Scholar]
Hop 2005 {published data only}
- Hop LT, Berger J. Multiple micronutrient supplementation improves anemia, micronutrient nutrient status, and growth of Vietnamese infants: double‐blind, randomized, placebo‐controlled trial. Journal of Nutrition 2005;135(3):660s‐5s. [DOI] [PubMed] [Google Scholar]
Jackson 2003 {published data only}
- Jackson RT, Al‐Mousa Z, Al‐Raqua M, Prakash P. Effect of short‐term weekly supplementation on anemia and symptoms in adolescent Kuwaiti girls. Kuwait Medical Journal 2003;35(4):275‐80. [Google Scholar]
Jaleel 2004 {published data only}
- Jaleel A, UrRahman MA. Daily iron supplementation versus intermittent dose. Saudi Medical Journal 2004;25(8):1124‐5. [PubMed] [Google Scholar]
Jayatissa 1999 {published data only}
- Jayatissa R, Piyasena P. Adolescent schoolgirls: daily or weekly iron supplementation. Food and Nutrition Bulletin 1999;20(4):429‐34. [Google Scholar]
Kanal 2005 {published data only}
- Kanal K, Busch‐Hallen J, Cavalli‐Sforza T, Crape B, Smitasiri S, Cambodian Weekly Iron‐Folic Acid Program Team. Weekly iron‐folic acid supplements to prevent anemia among Cambodian women in three settings: process and outcomes of social marketing and community mobilization. Nutrition Reviews 2005;63(12 Pt 2):S126‐33. [DOI] [PubMed] [Google Scholar]
Kapur 2003 {published data only}
- Kapur D, Sharma S, Agarwal KN. Effectiveness of nutrition education, iron supplementation or both on iron status in children. Indian Pediatrics 2003;40(12):1131‐44. [PubMed] [Google Scholar]
Kianfar 2000 {published data only}
- Kianfar H, Kimiagar M, Ghaffarpour M. Effect of daily and intermittent iron supplementation on iron status of high school girls. International Journal for Vitamin and Nutrition Research 2000;70(4):172‐7. [DOI] [PubMed] [Google Scholar]
Lechtig 2006 {published data only}
- Lechtig A, Gross R, Vivanco OA, Gross U, López de Romaña D. Lessons learned from the scaling‐up of a weekly multimicronutrient supplementation program in the integrated food security program (PISA). Food and Nutrition Bulletin 2006;27 Suppl Peru(4):160‐5. [DOI] [PubMed] [Google Scholar]
Leenstra 2009 {published data only}
- Leenstra T, Kariuki SK, Kurtis JD, Oloo AJ, Kager PA, Ter Kuile FO. The effect of weekly iron and vitamin A supplementation on hemoglobin levels and iron status in adolescent schoolgirls in western Kenya. European Journal of Clinical Nutrition 2009;63(2):173‐82. [DOI] [PubMed] [Google Scholar]
Lima 2006 {published data only}
- Lima AC, Lima MC, Guerra MQF, Romani SAM, Eickmann SH, Lira PIC. Impact of weekly treatment with ferrous sulfate on hemoglobin level, morbidity and nutritional status of anemic infants. Jornal de Pediatria 2006;82(6):452‐7. [DOI] [PubMed] [Google Scholar]
Lin 2001 {published data only}
- Lin X, Tang Y, Long Z. Effects of vitamin A and iron supplementation on the improvement of iron status and immunological function in preschool children. Chinese Journal of Preventive Medicine ‐ Zhonghua Yu Fang Yi Xue Za Zhi 2001;35(6):374‐7. [PubMed] [Google Scholar]
López de Romaña 2005 {published data only}
- López de Romaña G, Cusirramos S, López de Romaña D, Gross R. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, growth, and morbidity of Peruvian infants. Journal of Nutrition 2005;135(3):646s‐52s. [DOI] [PubMed] [Google Scholar]
López de Romaña 2006 {published data only}
- López de Romaña D, Verona S, Vivanco OA, Gross R. Protective effect of multimicronutrient supplementation against anemia among children, women, and adolescent girls in lower‐income areas of Chiclayo, Peru. Food and Nutrition Bulletin 2006;27 Suppl Peru(4):S143‐50. [DOI] [PubMed] [Google Scholar]
Menendez 1997 {published data only}
- Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, et al. Randomised placebo‐controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 1997;350(9081):844‐50. [DOI] [PubMed] [Google Scholar]
Mwanakasale 2009 {published data only}
- Mwanakasale V, Siziya S, Mwansa J, Koukounari A, Fenwick A. Impact of iron supplementation on schistosomiasis control in Zambian school children in a highly endemic area. Malawi Medical Journal 2009;21(1):12‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perrin 2002 {published data only}
- Perrin E, Rothman R, Coyne‐Beasley T, Ford C, Bordley WC. Is weekly iron and folic acid supplementation as effective as daily supplementation for decreasing incidence of anemia in adolescent girls?. Archives of Pediatrics and Adolescent Medicine 2002;156(2):128‐30. [DOI] [PubMed] [Google Scholar]
Risonar 2008 {published data only}
- Risonar MG, Tengco LW, Rayco‐Solon P, Solon FS. The effect of a school‐based weekly iron supplementation delivery system among anemic schoolchildren in the Philippines. European Journal of Clinical Nutrition 2008;62(8):991‐6. [DOI] [PubMed] [Google Scholar]
Rivera 1998 {published data only}
- Rivera G, Thomson M. Effect of ferrous salts supplementation on iron status of schoolchildren, Chiriqui, Panama, 1995‐1997 [Efecto de la suplementacion con sales ferrosas en el estado nutricional de hierro de escolares, Chiriqui, Panama, 1995‐1997]. Panama: Ministry of Health, Republic of Panama, 1998. [Google Scholar]
Schümann 2009 {published data only}
- Schümann K, Longfils, P, Monchy D, Xylander S, Weinheimer H, Solomons NW. Efficacy and safety of twice‐weekly administration of three RDAs of iron and folic acid with and without complement of 14 essential micronutrients at one or two RDAs: a placebo‐controlled intervention trial in anemic Cambodian infants 6 to 24 months of age. European Journal of Clinical Nutrition 2009;63(3):355‐68. [DOI] [PubMed] [Google Scholar]
Shah 2002 {published data only}
- Shah BK, Gupta P. Weekly vs daily iron and folic acid supplementation in adolescent Nepalese girls. Archives of Pediatrics and Adolescent Medicine 2002;156(2):131‐5. [DOI] [PubMed] [Google Scholar]
Sharma 2000 {published data only}
- Sharma A, Prasad K, Rao KV. Identification of an appropriate strategy to control anemia in adolescent girls of poor communities. Indian Pediatrics 2000;37(3):261‐7. [PubMed] [Google Scholar]
Shobha 2003 {published data only}
- Shobha S, Sharada D. Efficacy of twice weekly iron supplementation in anemic adolescent girls. Indian Pediatrics 2003;40(12):1186‐90. [PubMed] [Google Scholar]
Smuts 2005 {published data only}
- Smuts CM, Dhansay MA, Faber M, Stuijvenberg ME, Swanevelder S, Gross R, et al. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, and growth in South African infants. Journal of Nutrition 2005;135(3):653s‐9s. [DOI] [PubMed] [Google Scholar]
Soekarjo 2004 {published data only}
- Soekarjo DD, Pee S de, Kusin JA, Schreurs WH. Schultink W, Muhilal, et al. Effectiveness of weekly vitamin A (10,000 IU) and iron (60 mg) supplementation for adolescent boys and girls through schools in rural and urban East Java, Indonesia. European Journal of Clinical Nutrition 2004;58(6):927‐37. [DOI] [PubMed] [Google Scholar]
Sotelo‐Cruz 2002 {published data only}
- Sotelo‐Cruz N, Gómez‐Rivera N, Ferrá‐Fragoso S, Pereyda‐Galaz DE. Ferrous sulfate weekly dose for iron deficiency in preschool children. Gaceta Médica de México 2002;138(3):225‐30. [PubMed] [Google Scholar]
Tee 1999 {published data only}
- Tee ES, Kandiah M, Awin N, Chong SM, Satgunasingam N, Kamarudin L, et al. School‐administered weekly iron‐folate supplements improve hemoglobin and ferritin concentrations in Malaysian adolescent girls. American Journal of Clinical Nutrition 1999;69(6):1249‐56. [DOI] [PubMed] [Google Scholar]
Tomashek 2001 {published data only}
- Tomashek KM, Woodruff BA, Gotway CA, Bloland P, Mbaruku G. Randomized intervention study comparing several regimens for the treatment of moderate anemia among refugee children in Kigoma Region,Tanzania. American Journal Tropical Medicine and Hygiene 2001;64(3‐4):164‐71. [DOI] [PubMed] [Google Scholar]
UNICEF 2006 {published data only}
- Ministry of Health Panama, UNICEF, PAHO. [Situacion de deficiencia de hierro y anemia]. Status of iron deficiency and anaemia. Panama: UNICEF, 2006. [Google Scholar]
Vir 2008 {published data only}
- Vir SC, Singh N, Nigam AK, Jain R. Weekly iron and folic acid supplementation with counselling reduces anemia in adolescent girls: a large‐scale effectiveness study in Uttar Pradesh, India. Food and Nutrition Bulletin 2008;29(3):186‐94. [DOI] [PubMed] [Google Scholar]
Wijaya‐Erhardt 2007 {published data only}
- Wijaya‐Erhardt M, Erhardt JG, Untoro J, Karyadi E, Wibowo L, Gross R. Effect of daily or weekly multiple‐micronutrient and iron foodlike tablets on body iron stores of Indonesian infants aged 6‐12 mo: a double‐blind, randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2007;86(6):1680‐6. [DOI] [PubMed] [Google Scholar]
Zavaleta 2000 {published data only}
- Zavaleta N, Respicio G, Garcia T. Efficacy and acceptability of two iron supplementation schedules in adolescent school girls in Lima, Peru. Journal of Nutrition 2000;130(2):462s‐4s. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Husseini 1999 {unpublished data only}
- Husseini T. Bagor, Indonesia Trial. Information included in Beaton 1999.
Kargarnovin 2010 {published data only}
- Kargarnovin Z, Tamkins A, Jalaly M, Salavan K, Pam L. Effects of daily versus weekly iron therapy in infants between 6‐24 months for iron deficiency anemia. Journal of Nursing and Midwifery 2010;20(69):48. [Google Scholar]
Reid 2001 {published data only}
- Reid ED, Lopez P, Galaviz IA, Isoard F, Rosado JL, Allen LH. Hematological and biochemical responses of rural Mexican preschoolers to iron alone or iron plus micronutrients. FASEB Journal. 2001; Vol. 15:A731.
References to ongoing studies
Zeeba Zaka‐ur‐Rab 2010 {published data only}
- Dr. Zeeba Zaka‐ur‐Rab. A clinical trial to compare the effects of daily versus intermittent iron supplementation on markers of oxidative stress and anti‐oxidant status in children with iron deficiency anemia . The Clinical Trials Registry‐ India (CTRI).
Additional references
ACC/SCN 1991
- Administrative Committee on Coordination/Subcommittee on Nutrition (United Nations). Controlling iron deficiency. ACC/SCN State of the Art Series. Vol. Nutrition Policy Discussion Paper No 9, Geneva: United Nations Administrative Committee on Coordination: Subcommittee on Nutrition, 1991. [Google Scholar]
Adetifa 2009
- Adetifa I, Okomo U. Iron supplementation for reducing morbidity and mortality in children with HIV. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006736.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2010
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Baqui 2005
- Baqui AH, Fischer Walker CL, Zaman K, Arifeen SE, Chowdhury HR, Wahed MA, et al. Weekly iron supplementation does not block increases in serum zinc due to weekly zinc supplementation in Bangladeshi infants. Journal of Nutrition 2005;135(9):2187‐91. [DOI] [PubMed] [Google Scholar]
Beard 2008
- Beard JL. Why iron deficiency is important in infant development. Journal of Nutrition 2008;138(12):2534‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beaton 1999
- Beaton GH, McCabe GP. Efficacy of intermittent iron supplementation in the control of iron deficiency anaemia in developing countries. An analysis of experience (final report). Ottawa: The Micronutrient Initiative, 1999. [Google Scholar]
Bhutta 2009
- Bhutta Z, Klemm R, Shahid F, Rizvi A, Rah JH, Christian P. Treatment response to iron and folic alone is the same as with multivitamins and/or anthelminthics in severely anemic 6 to 24‐month‐old children. Journal of Nutrition 2009;139(8):1568. [DOI] [PubMed] [Google Scholar]
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Bothwell 2000
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. American Journal of Clinical Nutrition 2000;72(1):257S‐64S. [DOI] [PubMed] [Google Scholar]
Casanueva 2003
- Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. Journal of Nutrition 2003;133(5):1700S‐8S. [DOI] [PubMed] [Google Scholar]
Cole 2010
- Cole CR, Grant FK, Swaby‐Ellis ED, Smith JL, Jacques A, Northrop‐Clewes CA, et al. Zinc and iron deficiency and their interrelations in low‐income African American and Hispanic children in Atlanta. American Journal of Clinical Nutrition 2010;91(4):1027‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidsson 2003
- Davidsson L. Approaches to improve iron bioavailability from complementary foods. Journal of Nutrition 2003;133(5):1560S‐2S. [DOI] [PubMed] [Google Scholar]
De‐Regil 2011
- De‐Regil LM, Pena‐Rosas JP, Flores‐Ayala R, Jefferds MED. WHO/CDC logic model for micronutrient interventions in public health. FASEB Journal March 17, 2011;25:108.1. [Google Scholar]
Fernández‐Gaxiola 2011
- Fernández‐Gaxiola AC, De‐Regil LM, Nasser M. Intermittent iron supplementation for reducing anaemia and its associated impairments in women of reproductive age. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009218] [DOI] [PubMed] [Google Scholar]
Gera 2007
- Gera T, Sachdev HP, Nestel P, Sachdev SS. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. Journal of Pediatric Gastroenterology and Nutrition 2007;44(4):468‐86. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro Version 3.2 for Windows. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group, 2008.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Iannoti 2006
- Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. American Journal of Clinical Nutrition 2006;84(6):1261‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozoff 2000
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 2000;105(4):e51. [DOI] [PubMed] [Google Scholar]
Lozoff 2007
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(4):S560‐71. [DOI] [PubMed] [Google Scholar]
Mills 2009
- Mills RJ, Davies MW, McGuire W. Iron supplementation in enterally fed preterm infants. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005095] [DOI] [Google Scholar]
Moy 2006
- Moy RJD. Prevalence, consequences, and prevention of childhood nutritional iron deficiency: a child public health perspective. Clinical and Laboratory Haematology 2006;28(5):291‐8. [DOI] [PubMed] [Google Scholar]
NIH 2011
- NIH Iron and Malaria Technical Working Group. Chapter 2: Mechanisms. In: Raiten D, Namaste S, Brabin B editor(s). Considerations for the Safe and Effective use of Iron Interventions. Bethesda: Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), 2011 (in press):16‐51. [Google Scholar]
Okebe 2011
- Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria‐endemic areas. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006589.pub3] [DOI] [PubMed] [Google Scholar]
Olsen 2006
- Olsen A, Nawiri J, Magnussen P, Krarup H, Friis H. Failure of twice weekly iron supplementation to increase blood haemoglobin and serum ferritin concentrations: results of a randomized controlled trial. Annals of Tropical Medicine and Parasitology 2006;100(3):251‐63. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2009
- Peña‐Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004736.pub3] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Simmons 1993
- Simmons. Evaluation of a gastric delivery system for iron supplementation in pregnancy. American Journal of Clinical Nutrition 1993;58(5):622‐6. [DOI] [PubMed] [Google Scholar]
Stoltzfus 2011
- Stoltzfus RJ. Iron interventions for women and children in low‐income countries. Journal of Nutrition 2011;141(4):756S–62S. [DOI] [PubMed] [Google Scholar]
Thu 1999
- Thu BD, Schultink W, Dillon D, Gross R, Dhevita Leswara N, Khoi HH. Effect of daily and weekly micronutrient supplementation on micronutrient deficiencies and growth in young Vietnamese children. American Journal of Clinical Nutrition 1999;69(1):80‐6. [DOI] [PubMed] [Google Scholar]
Viteri 1997
- Viteri FE. Iron supplementation for the control of iron deficiency in populations at risk. Nutrition Reviews 1997;55(6):195‐209. [DOI] [PubMed] [Google Scholar]
Viteri 2005
- Viteri FE, Berger J. Importance of pre‐pregnancy and pregnancy iron status: can long‐term weekly preventive iron and folic acid supplementation achieve desirable and safe status?. Nutrition Reviews 2005;63(12 Pt 2):S65‐76. [DOI] [PubMed] [Google Scholar]
WHO 2001
- WHO, UNICEF, UNU. Iron Deficiency Anaemia Assessment, Prevention and Control: A Guide for Programme Managers. 1st Edition. Geneva: World Health Organization, 2001:1‐114. [Google Scholar]
WHO 2009
- World Health Organization. Weekly iron and folic acid supplementation programmes for women of reproductive age: An analysis of best programme practices (WHO Position Statement). Manila: World Health Organization Regional Office for the Western Pacific, 2009. [Google Scholar]
WHO 2009a
- World Health Organization. Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. Geneva: World Health Organization (http://whqlibdoc.who.int/publications/2009/9789241597494_eng.pdf). Geneva: World Health Organization, 2009. [PubMed]
WHO 2009b
- World Health Organization. Weekly iron folic acid supplementation (WIFS) in women of reproductive age: its role in promoting optimal maternal and child health. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
WHO 2011a
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011. [Google Scholar]
WHO 2011b
- WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011. [Google Scholar]
WHO/CDC 2008
- World Health Organization/Centers for Disease Control and Prevention (USA). Worldwide prevalence of anaemia 1993‐2005. In: Benoist B, McLean E, Egli I, Cogswell M editor(s). WHO Global Database on Anaemia. Geneva: World Health Organization, 2008. [Google Scholar]
WHO/CDC 2011
- World Health Organization, Centers for Disease Control and Prevention. Logic model for micronutrient interventions in public health. Vitamin and Mineral Nutrition Information System. (WHO/NMH/NHD/MNM/11.5) (http://www.who.int/vmnis/toolkit/WHO‐CDC‐english_colour.pdf, accessed 7 July 2011).. Geneva: World Health Organization, 2011.
Wright 1990
- Wright AJ, Southon S. The effectiveness of various iron supplementation regimens in improving the Fe status of anemic rats. British Journal of Nutrition 1990;63(3):579–85. [DOI] [PubMed] [Google Scholar]


