Abstract
Setting: Civilian population of the Republic of Azerbaijan.
Objectives: To determine patterns of anti-tuberculosis drug resistance among new and previously treated pulmonary tuberculosis (TB) cases, and explore their association with socio-demographic and clinical characteristics.
Design: National cross-sectional survey conducted in 2012–2013.
Results: Of 789 patients (549 new and 240 previously treated) who met the enrolment criteria, 231 (42%) new and 146 (61%) previously treated patients were resistant to any anti-tuberculosis drug; 72 (13%) new and 66 (28%) previously treated patients had multidrug-resistant TB (MDR-TB). Among MDR-TB cases, 38% of new and 46% of previously treated cases had pre-extensively drug-resistant TB (pre-XDR-TB) or XDR-TB. In previously treated cases, 51% of those who had failed treatment had MDR-TB, which was 15 times higher than in relapse cases (OR 15.2, 95%CI 6–39). The only characteristic significantly associated with MDR-TB was a history of previous treatment (OR 3.1, 95%CI 2.1–4.7); for this group, history of incarceration was an additional risk factor for MDR-TB (OR 2.8, 95%CI 1.1–7.4).
Conclusion: Azerbaijan remains a high MDR-TB burden country. There is a need to implement countrywide control and innovative measures to accelerate early diagnosis of drug resistance in individual patients, improve treatment adherence and strengthen routine surveillance of drug resistance.
Keywords: operational research, SORT IT, drug-resistant tuberculosis, Azerbaijan, surveillance
The Republic of Azerbaijan is one of the 18 high tuberculosis (TB) priority countries of the World Health Organization (WHO) European Region and one of the world's 27 countries with a high burden of multidrug-resistant TB (MDR-TB).1 High MDR-TB burden countries are defined by the WHO as having at least 10% MDR-TB among newly registered TB cases.2 These high MDR-TB burden countries have been identified within the framework of the WHO global project on anti-tuberculosis drug resistance. For this project, countries report on levels and patterns of anti-tuberculosis drug resistance on the basis of either routine surveillance data or surveys.3
The first anti-tuberculosis drug resistance survey (DRS) in the Republic of Azerbaijan, conducted from August 2006 to July 2007, covered the civilian population in Baku city only.4,5 The DRS revealed high levels of drug resistance: 22.3% (95% confidence interval [CI] 19–26) MDR-TB was found among new cases and 55.8% (95%CI 52–60) among previously treated TB cases; extensively drug-resistant TB (XDR-TB) was found in 12.8% of all identified MDR-TB cases. Five years later, in 2012, the country had an estimated 2800 MDR-TB cases, of which 800 were patients with new pulmonary TB.6 This estimate was based on the results of the latest DRS, routine surveillance data and expert opinion. Despite this high level of estimated drug resistance, treatment success rates among new smear-positive pulmonary TB cases in the last few years have increased from 59% in 2005 to 77% in the last 2 years.6 This relatively high level of treatment success suggests that levels of drug resistance in the whole country might be lower than the rate found in the previous 2007 survey in Baku.
The country therefore decided to conduct another survey, this time nationwide, to obtain up-to-date information on anti-tuberculosis drug resistance among pulmonary TB cases. It was anticipated that survey findings would also assist the National TB Programme (NTP) in forecasting and procurement of anti-tuberculosis drugs, optimisation of standard treatment regimens and targeting of specific groups for rapid diagnostic tests, for example Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA). The nationwide anti-tuberculosis DRS was designed in collaboration with the WHO Regional Office for Europe (Copenhagen, Denmark), and a detailed survey protocol was developed in line with WHO guidelines.3 Specific objectives of the current study were to determine, among new and previously treated pulmonary TB cases in the general population (excluding the penitentiary setting): 1) anti-tuberculosis drug resistance patterns, 2) anti-tuberculosis drug resistance patterns between different categories of previously treated cases, and 3) socio-demographic and clinical characteristics associated with anti-tuberculosis drug resistance patterns.
METHODS
Study design
This was a national cross-sectional survey.
Setting
The Republic of Azerbaijan is the largest country in the Caucasus region, administratively divided into 59 rayons; it has 11 cities and includes the Autonomous Republic of Nakhchivan. In 2012, the population of the Republic of Azerbaijan was estimated to be 9 235 000. In 2012, 8140 TB cases were notified: 4616 new and 3524 previously treated cases.6 The proportion of smear-positive TB cases was low: only 36% of new notified TB cases had a smear-positive microscopy result. The national TB laboratory network includes the National TB Reference Laboratory (NRL) in Baku, five regional culture laboratories and 67 peripheral microscopy laboratories. A sputum transportation system covers the country. In 2012, among the 2800 MDR-TB cases estimated by the WHO, 596 were diagnosed in the civilian population (MDR-TB case detection rate 21%).6
National drug resistance survey
Patients
Patient recruitment for the survey started in October 2012 and was completed in April 2013. All individuals aged ⩾15 presumed to have pulmonary TB submitted at least two sputum specimens. The sputum specimens were transported to the NRL twice a week. Collected samples were subjected to microscopy and culture on Löwenstein-Jensen (LJ) and MGIT™ (BD, Sparks, MD, USA) media.
Laboratory procedures
All culture-positive isolates were subjected to drug susceptibility testing (DST) on LJ medium for first-line anti-tuberculosis drugs (FLDs) (isoniazid [INH], rifampicin [RMP], streptomycin [SM], ethambutol [EMB] and pyrazinamide), and MDR-TB isolates were further subjected to DST for second-line anti-tuberculosis drugs (SLDs) (ofloxacin, capreomycin [CPM], amikacin [AMK], prothionamide, cycloserine and para-amino-salicylic acid). All culture-positive isolates were also subjected to line-probe assay testing (LPA) (Geno-Type® MTBDRplus, Hain Life Sciences, Nehren, Germany) according to the manufacturer's instructions. In case of discordance, DST results on LJ medium were taken as the final determining result. For external quality assurance of DST, all positive cultures were sent to the Supranational Reference Laboratory (SRL) in Borstel, Germany, for retesting; results were sent back to Azerbaijan for comparison.
Questionnaire and data variables
A standard questionnaire was completed for each participant documenting socio-demographic and clinical characteristics. The questionnaire was piloted in two regions for 2 weeks in 2012, and subsequently adapted to obtain more accurate responses. At the sputum collection sites, study participants were registered in a DRS journal and were assigned a unique patient DRS number. Data variables included sex, age, migration, social status, living conditions, financial conditions, smoking status, alcohol use, drug use, location of TB facility, previous incarceration history, human immunodeficiency virus (HIV) status, type and category of TB and DST results against FLDs and SLDs.
Sample size
The 100% sampling method was chosen due to the size of the country and the health care structure in the Republic of Azerbaijan. The sample size for culture-positive patients was calculated according to WHO guidelines,3 and assumptions for simple random sampling included 1) the number of new and retreatment sputum smear- and/or culture-positive pulmonary cases notified in 2011; 2) WHO estimates of the proportions of MDR-TB among notified TB cases; 3) a 95%CI and an absolute precision of respectively 4% and 10% for new and previously treated TB cases, so that in both groups a relative precision of <20% was achieved; and 4) a sample size inflation accounting for 20% expected loss of samples. A total of 544 culture-positive pulmonary TB patients would therefore need to be enrolled in the survey: 435 new and 109 previously treated patients from the civil sector. The survey did not include the following groups of patients: those with chronic TB disease (i.e., failure after any kind of anti-tuberculosis treatment on two or more occasions), those aged <15 years, those who provided sputum specimens 5 days after the onset of anti-tuberculosis treatment, patients with extra-pulmonary TB or culture-negative pulmonary TB, as well as those currently incarcerated.
Data validation
Data validation included 1) re-interviewing at least 10% of patients — this percentage was chosen based on the average figure from expert opinion, 2) monitoring visits to TB facilities to compare the data on patients registered for treatment and data on patients whose sputum was sent to the NRL for the study, 3) re-checking DST results at the SRL, and 4) validating patient treatment categories using an electronic database of new TB patients registered in 2009–2012.
Analysis and statistics
Patient data were double-entered into an Epi Info™ 3.5 (Centers for Disease Control and Prevention, Atlanta, GA, USA) database adapted by the WHO and EpiData (version 3.1 for data entry, EpiData Association, Odense, Denmark). To determine the anti-tuberculosis drug resistance patterns among new and previously treated TB cases, frequencies of drug resistance were tabulated. MDR-TB rates (which included those with XDR-TB and pre-XDR-TB, as defined in Table 1) among new and previously treated cases were then calculated. We also compared the proportion of samples of new and previously treated cases of any RMP resistance sent from TB diagnostic facilities to Baku with all other regions in Azerbaijan.
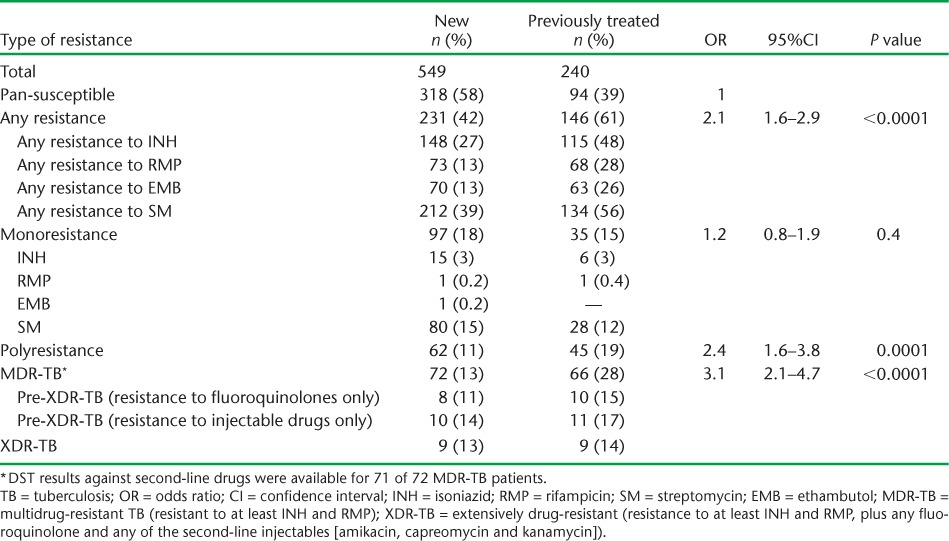
TABLE 1.
Drug resistance patterns among new and previously treated TB patients in Azerbaijan, 2012–2013
To determine factors associated with anti-tuberculosis drug resistance patterns, univariable and multivariable logistic regression was carried out. All data variables collected were included in the regression models. Levels of significance were set at 5%. All analyses were performed using Stata (Version 12; Stata Corp, College Station, TX, USA).
Ethics approval
Permission and approval for the study were obtained from the Ethics Committee of the Ministry of Health of the Republic of Azerbaijan. Ethics approval was also obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
Of 789 bacteriologically confirmed pulmonary TB cases in the survey, 549 were new patients and 240 were previously treated. Anti-tuberculosis drug resistance patterns among new and previously treated cases are shown in Table 1. Among all new and previously treated cases, respectively 231 (42%) and 146 (61%) were resistant to any anti-tuberculosis drug, and 72 (13%) and 66 (28%) had MDR-TB. Among those with MDR-TB, 18 (25%) new and 21 (32%) previously treated cases had pre-XDR-TB, and 13% of new and 14% of previously treated TB cases had XDR-TB. Compared to cases of drug-susceptible TB, those with polyresistance and MDR-TB (including those with XDR-TB and pre-XDR-TB) had significantly increased odds of having been previously treated for TB (P < 0.05). Those with MDR-TB had an odds ratio (OR) of 3.1 (95%CI 2.0–4.7) for being previously treated compared with cases of drug-susceptible TB. Among all MDR-TB cases, 57 (79%) new and 52 (79%) previously treated cases were also resistant to SM and EMB. There was a non-significantly higher proportion of any RMP resistance and MDR-TB among samples of new and previously treated cases sent from diagnostic facilities in Baku (47/239, 20%), compared to all other regions in Azerbaijan (94/550, 17%).
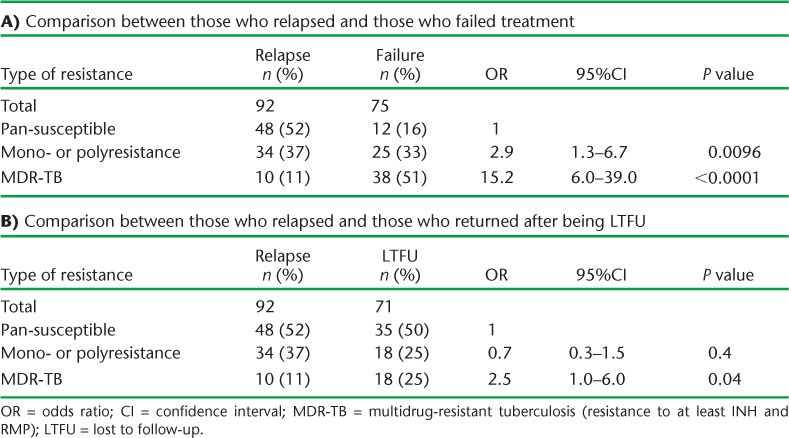
Patterns and comparisons of drug resistance in and between patients with previously treated TB are shown in Table 2A and B. In Table 2A, patients with relapse TB are compared with those who failed treatment; patients who failed treatment had significantly higher odds of mono- and polyresistance, and particularly of MDR-TB, with an OR of 15.2 (95%CI 6.0–39.0). In Table 2B, patients with relapse TB are compared with those who returned after being lost to follow-up (LTFU); patients who were LTFU had significantly higher odds of MDR-TB (OR 2.9, 95%CI 1.3–6.7).
TABLE 2.
Drug resistance patterns among previously treated tuberculosis patients in Azerbaijan, 2012–2013.
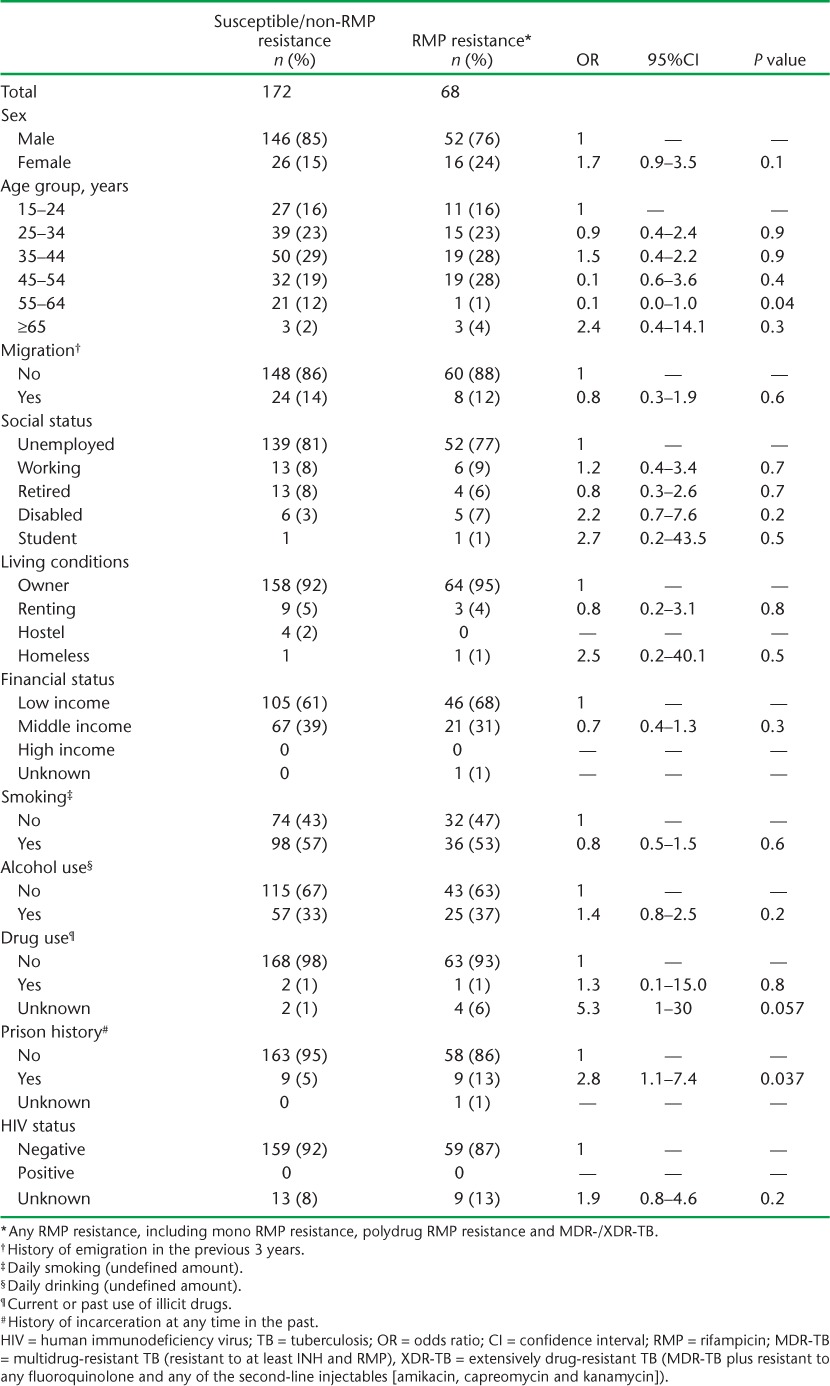
Demographic, social and HIV characteristics of new and previously treated patients in relation to whether they had drug-susceptible/non-RMP resistance or any kind of RMP resistance, including MDR-TB, are shown in Tables 3 and 4, respectively. For previously treated patients, those with any kind of RMP resistance were significantly more likely to have been in prison than those who had drug-susceptible/non-RMP resistance. There were no other differences between the two groups.
TABLE 3.
Demographic, social and HIV characteristics of new pulmonary TB patients in relation to patterns of drug resistance in Azerbaijan, 2012–2013
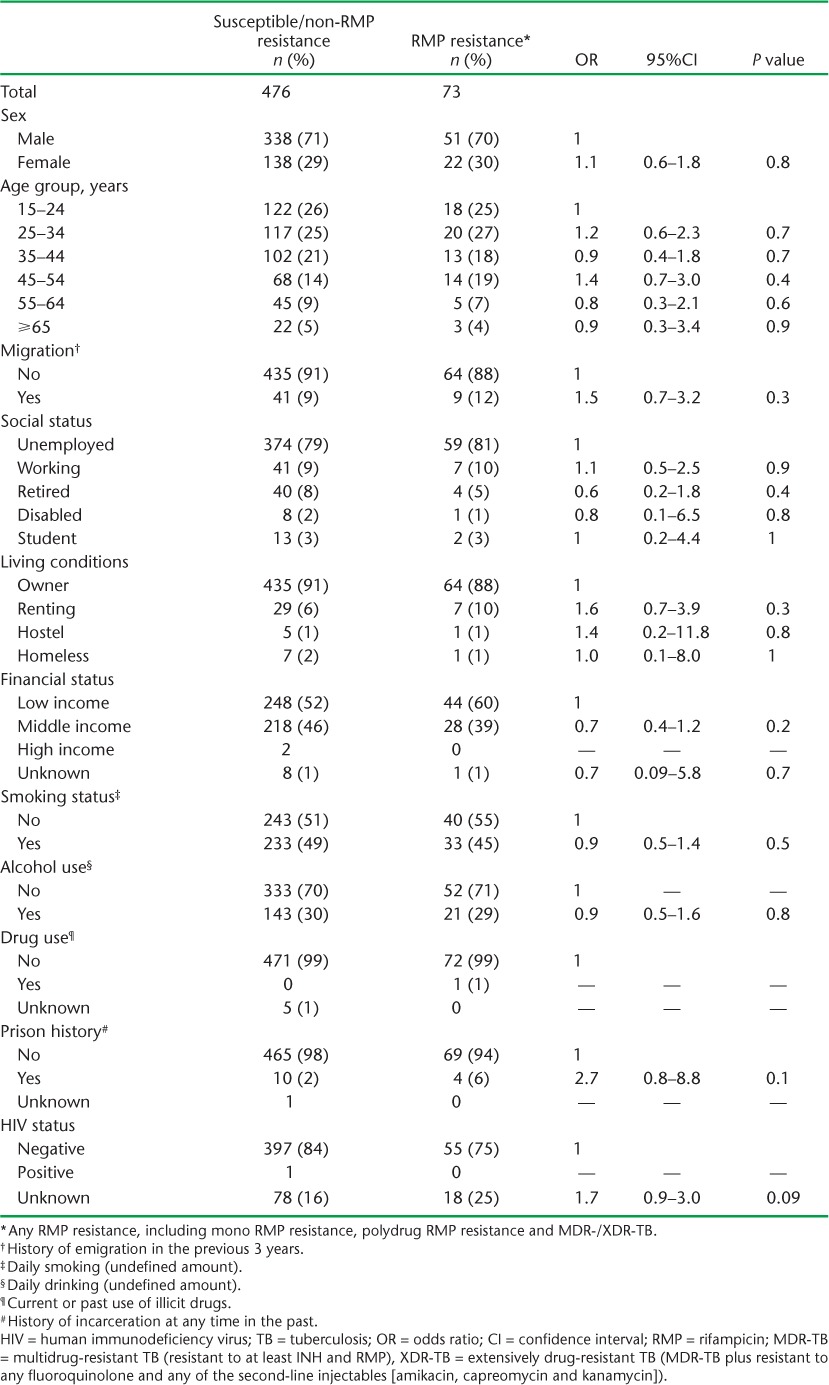
TABLE 4.
Demographic, social and HIV characteristics of previously treated pulmonary TB patients in relation to patterns of drug resistance, Azerbaijan, 2012–2013
DISCUSSION
This was the first nationwide DRS in Azerbaijan. Among new and previously treated TB cases, the prevalence of MDR-TB was respectively 13% and 28%. In addition, the rate of resistance to any anti-tuberculosis drug, including MDR- and XDR-TB, remained high, and removes any grounds for complacency. A history of previous treatment was a significant risk factor for all types of drug resistance. Among those who had been previously treated, treatment failure was the highest risk factor for MDR-TB (including XDR-TB). A previous prison history was also a significant risk factor for any RMP resistance, including MDR-TB, among previously treated cases.
The strengths of this study were the nationwide coverage, the large number of patients included that exceeded the sample size calculation and the 100% sampling of all diagnostic facilities in the civilian sector. The study also incorporated rigorous sample validation measures to ensure the accuracy of the reported data. The questionnaire included socio-demographic characteristics and the HIV status of patients, information that was not available in previous surveys. We were thus able to assess risk factors associated with any RMP resistance and MDR-TB. Due attention was also paid to following internationally agreed recommendations for the conduct and reporting of observational studies.7,8
There were some limitations. First, the survey did not include patients with long-standing chronic TB disease. The previous 2007 DRS in Baku,4 however, did include these patients, making a direct, head-to-head comparison difficult. Second, the survey did not include data from the prison sector, where high MDR-TB rates have been found in detainees.9–11
The current prevalence of drug resistance places the country among the world's high MDR-TB burden countries, and highlights the need to continue with robust TB control efforts. The finding that nearly a third of new and previously treated cases had polyresistance emphasises the need for DST as soon as possible after each TB diagnosis, given the high risk of polyresistant conversion to MDR-TB.12 The high proportion of MDR-TB patients who were also resistant to EMB is of concern, although the reliability of DST for EMB is poor. This does mean, however, that there is a questionmark over the use of EMB in the treatment regimens for these patients, and especially those with pre-XDR- and XDR-TB. This study confirms previous published findings reporting that treatment failure is strongly associated with drug resistance.13–18 In Azerbaijan, many new and previously treated patients are placed on first-line treatment before DST results are available. Furthermore, the coverage of first-line DST in Azerbaijan in 2010 was only 19% and 48%, respectively, among new and previously treated cases.6 Given that patients who had MDR-TB had nearly 15 times the odds of previous treatment failure and 2.8 times the odds of previous treatment returning after loss to follow-up, any patient from these subgroups should be urgently prioritised for rapid DST that includes rapid molecular techniques such as Xpert, which potentially allows a diagnosis of TB and RMP resistance within 2 h.19
Interestingly, of all the socio-demographic risk factors assessed, only one factor, namely having a history of incarceration, was significantly associated with MDR-TB. It is known that the rate of MDR-TB in the prison setting of Azerbaijan was high.9 However, recent reforms in TB infection control in prisons in Azerbaijan have led to a successful decline in the rate of new infections and an improvement in treatment success even for patients with MDR-TB in this setting.20 The association between a history of incarceration and MDR-TB found in this study needs further investigation.
This study has several important implications. First, early detection of drug resistance is vital in all new and previously treated patients using rapid or conventional DST, including Xpert; there is therefore a need for complete coverage of these diagnostic services in the country. Second, ongoing, frequent monitoring of TB drug resistance is necessary throughout the country, including the penitentiary sector, using routine surveillance. Third, the NTP needs to strengthen basic TB control measures, including addressing the incorrect registration of patients' disease categories, which anecdotally occurs in peripheral facilities. Fourth, as we were not able to measure the prevalence of MDR-TB among chronic TB cases or prisoners, future studies should include these high-risk populations and should also assess reasons for the high risk of MDR-TB among former prisoners in the civilian population. Fifth, the study showed a clear need for third-line anti-tuberculosis medicines for the treatment of patients with pre-XDR- and XDR-TB.
In conclusion, Azerbaijan remains a high MDR-TB burden country, with several important risk factors contributing to this epidemic of drug resistance. There is an urgent need to implement several countrywide activities in order to reverse this tide.
Acknowledgments
This research was conducted within the framework of the consolidated programme of Round 5 and 7 of the Global Fund Project in Azerbaijan (AZE-708-G03-T), and implemented by the Ministry of Health of Azerbaijan. The World Health Organization (WHO) supported the survey design, implementation and data management and analysis. Data interpretation was conducted at the Workshop on Anti-TB Drug Resistance Surveillance in Copenhagen (WHO Regional Office for Europe), as well as the Structured Operational Research and Training Initiative (SORT IT). SORT IT is a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the WHO (WHO-TDR, Geneva, Switzerland). The specific SORT IT programme which contributed to this publication was jointly developed and implemented by: WHO-TDR; WHO Regional Office for Europe; the Operational Research Unit (LUXOR), Médecins Sans Frontières, Brussels Operational Center, Luxembourg; the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France; and The Union South-East Asia Regional Office, Delhi, India. We are grateful for the support of the WHO Country Office in Talinn, Estonia and the Estonia National Institute for Health and Development in hosting the SORT IT workshops. We also appreciate the active involvement of the WHO Country Office and the Ministry of Health in the selection of candidates for training in operational research and identification of research projects.
This research was conducted within the framework of the consolidated programme of Round 5 and 7 of the Global Fund project in Azerbaijan (AZE-708-G03-T) and the SORT IT programme was funded by the United States Agency for International Development (USAID, Washington DC, USA) and managed by WHO-TDR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
The authors alone are responsible for the content of this paper which may not necessarily represent the policies, decisions or views of the WHO.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. The Global MDR-TB and XDR-TB Response Plan 2007–2008. Geneva, Switzerland: WHO; 2007. WHO/HTM/TB/2007.387. [Google Scholar]
- 2.World Health Organization. Roadmap to prevent and combat drug-resistant tuberculosis. The Consolidated Action Plan to Prevent and Combat Multidrug- and Extensively Drug-Resistant Tuberculosis in the WHO European Region, 2011–2015. Copenhagen, Denmark: WHO; 2011. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Tuberculosis prevalence surveys: a handbook, 2011. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB.2010.17. [Google Scholar]
- 4.World Health Organization. Anti-tuberculosis drug resistance in the world. 4th ed. Geneva, Switzerland: WHO; 2008. The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. WHO/HTM/TB/2008.394. [Google Scholar]
- 5.Zignol M, Dara M, Dean A S et al. Drug-resistant tuberculosis in the WHO European Region: Meta-analysis of surveillance data. Drug Resistance Updates. 2013;16:108–115. doi: 10.1016/j.drup.2014.02.003. http://dx.doi.org/10.1016/j.drup.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 7.von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Edginton M, Enarson D, Zachariah R et al. Why ethics is indispensible for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfyffer G E, Strässle A, van Gorkum T et al. Multidrug-resistant tuberculosis in prison inmates, Azerbaijan. Emerging Infect Dis. 2001;7:855–861. doi: 10.3201/eid0705.017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruddy M, Balabanova Y, Graham C et al. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in Samara Region, Russia. Thorax. 2005;60:130–135. doi: 10.1136/thx.2004.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin S S, Pasechnikov A D, Gelmanova I Y et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–408. [PubMed] [Google Scholar]
- 12.Temple B, Ayakaka I, Ogwang S et al. Rate and amplification of drug resistance among previously treated patients with tuberculosis in Kampala, Uganda. Clin Infect Dis. 2008;47:1126–1134. doi: 10.1086/592252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Werf M J, Langendam M W, Huitric E, Manissero D. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511–1519. doi: 10.1183/09031936.00125711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews J R, Shah N S, Weissman D, Moll A P, Friedland G, Gandhi N R. Predictors of multidrug- and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLOS ONE. 2010;5:e15735. doi: 10.1371/journal.pone.0015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diande S, Sangare L, Kouanda S et al. Risk factors for multidrug-resistant tuberculosis in four centers in Burkina Faso, West Africa. Mikrob Drug Resist. 2009;15:217–221. doi: 10.1089/mdr.2009.0906. [DOI] [PubMed] [Google Scholar]
- 16.Faustini A, Hall A J, Perucci C A. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61:158–163. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casal M, Vaquero M, Rinder H et al. A case-control study for multidrug-resistant tuberculosis: risk factors in four European countries. Microb Drug Resist. 2005;11:62–67. doi: 10.1089/mdr.2005.11.62. [DOI] [PubMed] [Google Scholar]
- 18.Weyer K, Brand J, Lancaster J, Levin J, van der Walt M. Determinants of multidrug-resistant tuberculosis in South Africa: results from a national survey. S Afr Med J. 2007;97:1120–1128. [PubMed] [Google Scholar]
- 19.Boehme C C, Nicol M P, Nabeta P et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Regional Office for Europe. Best practices in prevention control and care for drug-resistant tuberculosis, 2013. Copenhagen, Denmark: WHO; 2013. [Google Scholar]


