Abstract
Setting: Georgia, a country with a high-burden of multi-drug-resistant tuberculosis (MDR-TB).
Objective: To determine the proportion of loss to follow-up (LFU) among MDR-TB patients treated nationwide from 2009 to 2011, and associated risk factors.
Design: Retrospective cohort study involving a review of the National Tuberculosis Programme electronic surveillance database. A Cox proportional hazards model was used to assess risk factors for time to LFU.
Results: Among 1593 patients, 458 (29%) were lost to follow-up. A total of 1240 MDR-TB patients were included in the final analysis (845 treatment success, 395 LFU). Over 40% of LFU occurred during the first 8 months of MDR-TB treatment; 40% of patients had not achieved culture conversion at the time of LFU. In multivariate analysis, the factors associated with LFU included male sex, illicit drug use, tobacco use, history of previous anti-tuberculosis treatment, site of TB disease, and place and year of initiating treatment.
Conclusion: LFU was high among MDR-TB patients in Georgia and posed a significant public health risk, as many were culture-positive at the time of LFU. A multi-pronged approach is needed to address the various patient- and treatment-related characteristics associated with LFU.
Keywords: operational research, SORT IT, Eastern Europe, loss to follow-up, MDR-TB
Multidrug-resistant tuberculosis (MDR-TB), defined as resistance to at least isoniazid and rifampicin, is a major threat to tuberculosis (TB) control and, as mentioned in the 2013 World Health Organization (WHO) global tuberculosis report, the world is ‘off track’ in achieving targets for MDR-TB treatment.1 There were an estimated 450 000 new cases of MDR-TB worldwide in 2012, and the reported treatment success rate was only 48%.1 Among MDR-TB patients with poor treatment outcomes, the majority were due to loss to follow-up (LFU) during treatment (previously termed treatment default).2 According to the WHO, LFU is defined as an interruption of anti-tuberculosis treatment of at least 2 consecutive months. MDR-TB patients who are lost to follow-up have an increased risk of TB-related death and of transmitting drug-resistant TB in the community.3
The highest rates of MDR-TB are observed in Eastern European countries, including Georgia, which is one of the WHO-designated 27 high MDR-TB burden countries.1 In 2012, MDR-TB prevalence was respectively 11% and 32% among new and retreatment TB cases. In 2009, with the assistance of the Green Light Committee (GLC), Georgia became one of the first lower-middle-income countries to provide universal access to diagnosis and treatment of MDR-TB. However, initial MDR-TB treatment outcomes were suboptimal. In an analysis of the first cohort of MDR-TB patients in Georgia who started treatment in 2009, 22% of patients were lost to follow-up.4 LFU rates were also high, at 20%, in a more recent cohort of children receiving treatment for MDR-TB in Georgia.5
LFU rates in Georgia are similar to the high rates reported among MDR-TB patients globally.6 These very high rates highlight the challenges of adherence to treatment and treatment completion among MDR-TB patients for National TB Programmes (NTP) worldwide. Understanding the risk factors associated with LFU would help NTPs to plan effective interventions for addressing this problem. This was the basis for the present study, along with a 2013 directive of the GLC and Global Drug Facility (GDF) Joint Country Mission that recommended an updated strategy to reduce the high LFU among patients with MDR-TB.
The specific objectives of this study were to assess, among MDR-TB patients in Georgia, 1) the proportion of patients lost to follow-up, 2) time of LFU, 3) culture conversion status at LFU, and 4) characteristics associated with LFU. This information will help prioritise future public health interventions aimed at reducing LFU and halting the spread of MDR-TB.
METHODS
Setting
The Ministry of Health oversees TB control in Georgia (population 4.5 million),7 and TB diagnosis and treatment is provided free of charge for the patients through the National Centre for Tuberculosis and Lung Diseases (NCTLD, Tbilisi, Georgia). The Global Fund to Fight AIDS, Tuberculosis and Malaria (Geneva, Switzerland) funds the procurement of quality-assured anti-tuberculosis drugs through the GDF mechanism.
Second-line drug (SLD) regimens were individualised based on drug susceptibility testing (DST) results, guided by WHO criteria.8 The regimens were designed to include four or more active drugs based on the DST results, and included a fluoroquinolone and an injectable. According to country guidelines, an injectable agent was given for a minimum of 8 months. All treatment is given by directly observed therapy (DOT). In 2008–2012, food vouchers were given to patients with MDR-TB receiving out-patient treatment; however, the voucher programme was discontinued in January 2013.
As standard of care in Georgia, patients with confirmed MDR-TB are admitted to an MDR-TB ward at one of four TB hospitals for the initiation of the intensive phase of treatment. Patients are recommended, but not required, to remain hospitalised at least until their sputum smears convert to negative. After discharge, patients with MDR-TB are referred to out-patient TB units (TB clinics) for the continuation phase of treatment. TB medications are delivered to TB units by NCTLD coordinators. The patient visits the clinic to receive DOT from a nurse.
Study design, population and period
A retrospective cohort study design was utilised. All patients in the country of Georgia with MDR-TB and registered in the civil sector for treatment at the NCT-BLD in Tbilisi, Georgia, and affiliated centres throughout the country from January 2009 to December 2011 were included in the study.
Laboratory
Culture and DST were performed at the Georgian National TB Reference Laboratory. All patients had DST-confirmed MDR-TB. Sputum cultures were performed monthly until three consecutive negative culture results had been obtained, and then every 3 months until treatment completion. For all Mycobacterium tuberculosis-positive sputum cultures, first- and second-line DST were performed using the absolute and proportion concentration methods, respectively, as previously described.9
Data management and analysis
All data were extracted from the electronic National TB Surveillance database. Variables collected included treatment start and completion dates, patient demographics, history of incarceration, drug use, comorbidities, history of anti-tuberculosis treatment, culture conversion dates and treatment outcomes (per WHO definitions2).
Analyses were performed using Stata v12.1 (Stata Corp, College Station, TX, USA) and SAS v9.3 (Statistical Analysis System Institute Inc, Cary, NC, USA). Descriptive statistics were used to determine time to LFU among patients with MDR-TB, and to compare characteristics between patients with LFU and those with a successful outcome (defined as cure or completed treatment). Patients with extensively drug-resistant TB, or those who died, failed treatment or were transferred out, were not included in the analysis. A Cox proportional hazards model was used to assess risk factors for time to LFU. An alternative Cox proportional hazards model comparing LFU vs. all other treatment outcomes (including those who died or failed treatment) was also performed. Model building and selection were based on purposeful selection of covariates, as previously described.10 P < 0.05 was considered significant.
Ethics
The study was approved by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France, and the Institutional Review Boards of the National Center for Disease Control and Public Health (NCDC) in Tbilisi, Georgia, and Emory University in Atlanta, GA, USA.
RESULTS
During 2009–2011, 1593 patients were initiated on MDR-TB treatment in Georgia, of whom 458 (29%) were lost to follow-up. Of the 1593 patients, 353 (22%) were excluded from data analysis for the following reasons: extensively drug-resistant TB (defined as MDR-TB plus resistance to a fluoroquinolone and an injectable), treatment failure, transfer out, death, and either no final outcome available or missing data (Figure 1). A total of 1240 patients were thus included in the analysis of risk factors for LFU. Of these, 845 had a successful outcome and 395 were lost to follow-up.
FIGURE 1.
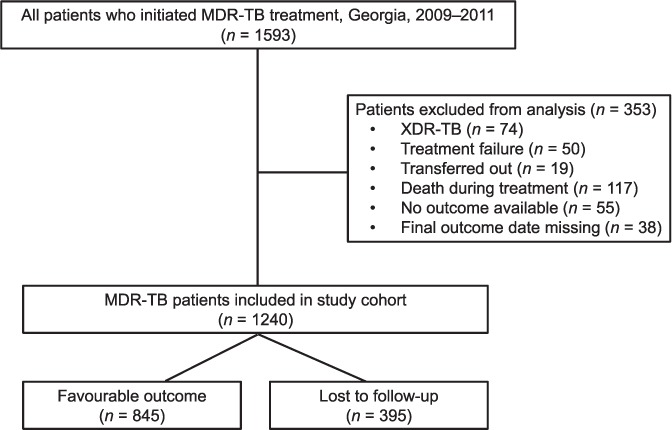
Study cohort diagram. MDR-TB = multidrug-resistant tuberculosis; XDR-TB = extensively drug-resistant TB.
The demographic and disease characteristics of the MDR-TB patients included (n = 1240) are summarised in Table 1. Among the overall cohort, the majority of patients were male (73%); the mean age was 36.2 years. The proportion of previously treated patients (57%) was high, and approximately one fifth had a history of incarceration. Over one third of patients reported use of alcohol (34%) or tobacco (36%), while the rate of illicit drug use (4%) and co-infection with the human immunodeficiency virus (3%) was low. A similar number of patients initiated treatment in 2009 (n = 420) and 2011 (n = 428), with a slight dip in 2010 (n = 382). Approximately half of all patients initiated treatment in Tbilisi, with the rest receiving treatment in other regions of Georgia.
TABLE 1.
Characteristics associated with loss to follow-up among multidrug-resistant tuberculosis patients, Georgia, 2009–2011 (n = 1240)
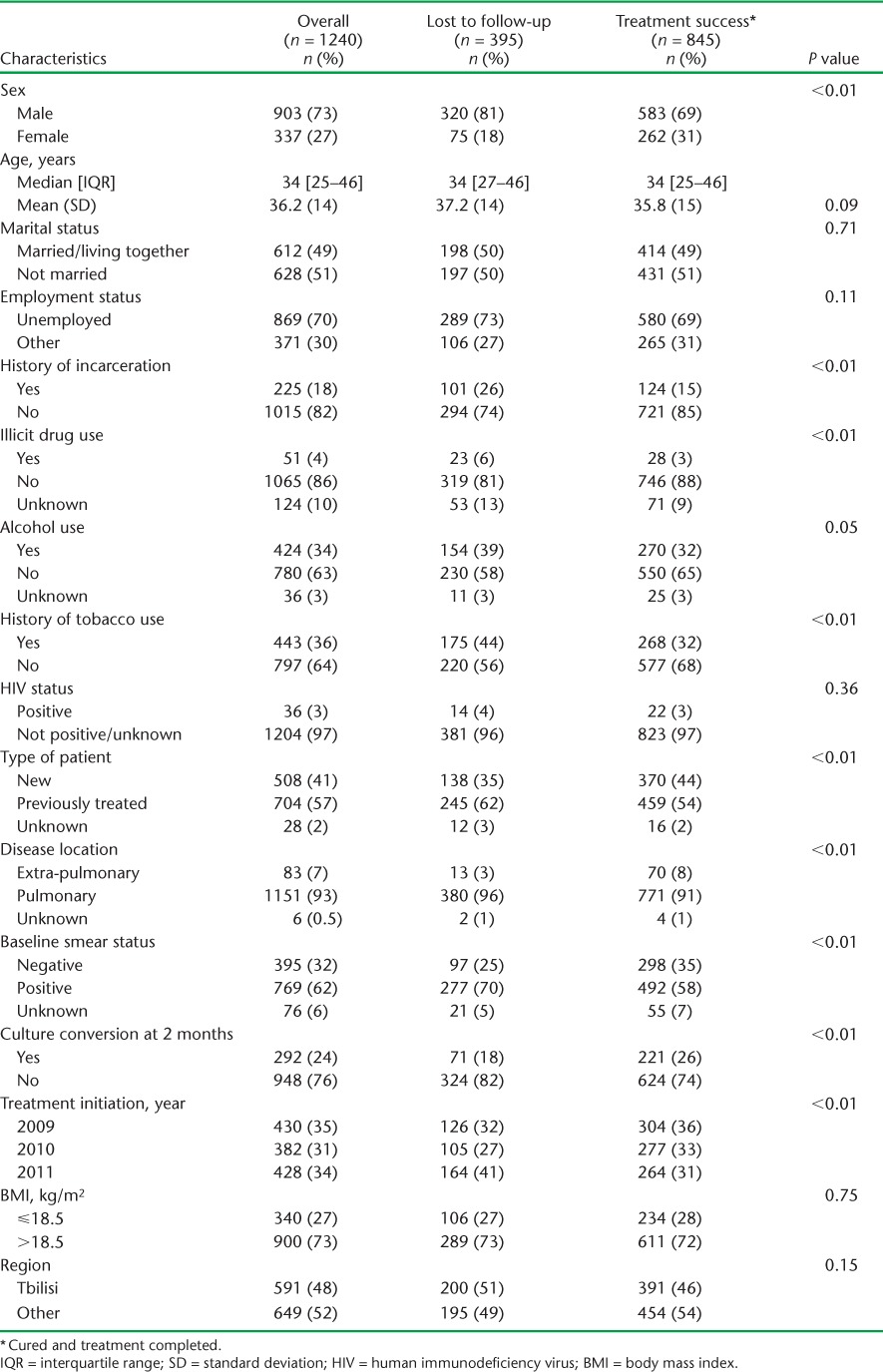
There were numerous differences in characteristics among those lost to follow-up vs. patients with a favourable outcome (Table 1). Patients who were lost to follow-up were significantly more likely to be male (81% vs. 69%), have a history of incarceration (26% vs. 15%), illicit drug use (6% vs. 3%), tobacco use (44% vs. 32%), to have received previous anti-tuberculosis treatment (62% vs. 54%), have pulmonary disease (96% vs. 91%) and have baseline smear-positive results (70% vs. 58%) than patients with a favourable outcome. The culture conversion rate at 2 months was also significantly lower in LFU patients vs. those with a favourable outcome (18% vs. 26%, respectively).
With regard to timing of LFU, the median time of follow-up among patients lost to follow-up was 10 months (interquartile range 5–17); 161 patients (41%) were lost to follow-up during the first 8 months of MDR-TB treatment, 81 (20%) during the next 9–12 months and 152 (39%) after 12 months of initiating MDR-TB treatment (Figure 2). At the time of LFU, 61% of patients (241/395) had achieved culture conversion. The culture conversion rate at the time of LFU was much lower in patients interrupting treatment during the early period (37%) than compared to those lost to follow-up from 9 to 12 months (73%) or after 12 months (80%) of starting MDR-TB treatment (Figure 3).
FIGURE 2.
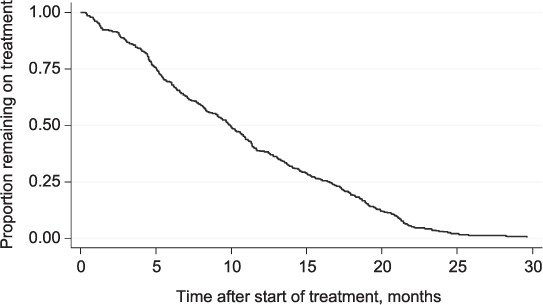
Time to loss to follow-up among MDR-TB patients, Georgia, 2009–2011. MDR-TB = multidrug-resistant tuberculosis.
FIGURE 3.
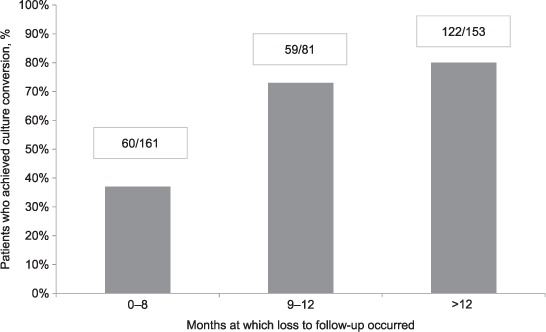
Rates of culture conversion among multidrug-resistant tuberculosis patients, Georgia, 2009–2011 (n = 395).
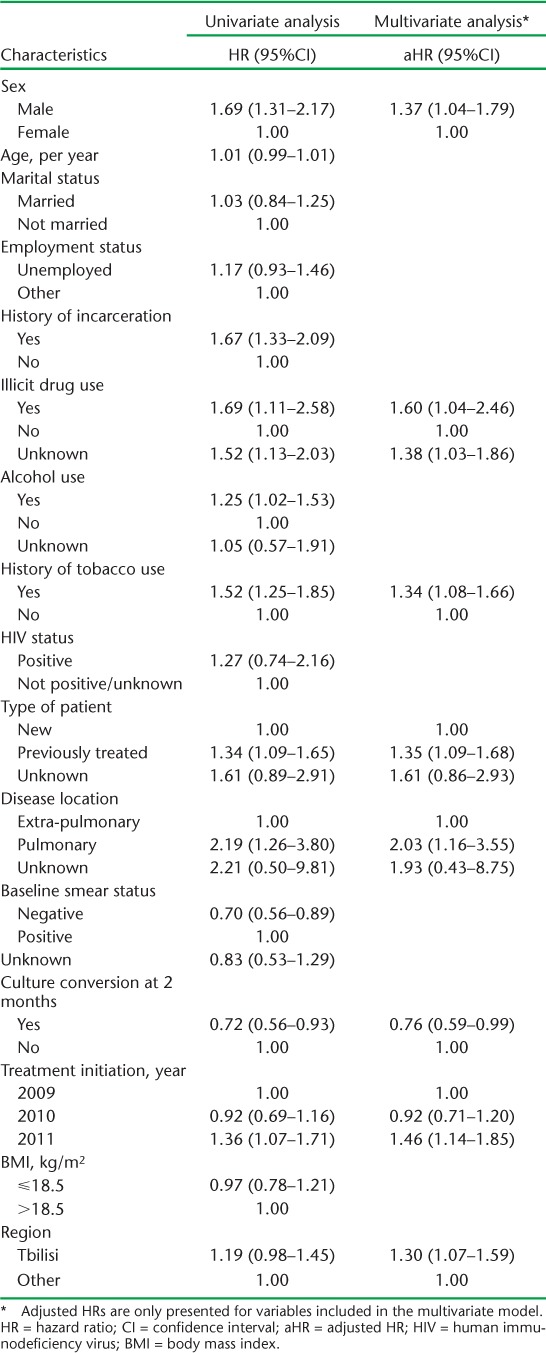
The results of the univariate and multivariate survival analyses for risk factors for time to LFU are shown in Table 2. In multivariate analysis, factors associated with LFU included male sex, illicit drug use, tobacco use, history of previous anti-tuberculosis treatment, site of TB disease, and place and year of initiating treatment. Achieving culture conversion at 2 months (hazard ratio 0.76, 95%CI 0.59–0.99) had a protective effect against LFU. The alternative model comparing LFU vs. all other treatment outcomes found similar results (results not shown); however, unknown illicit drug use status and culture conversion at month 2 were no longer significant in multivariate analysis.
TABLE 2.
Univariate and multivariate analysis evaluating risk factors for time to loss to follow-up among multidrug-resistant tuberculosis patients, Georgia, 2009–2011 (n = 1240)
DISCUSSION
In this study, we found that 29% of all patients initiating treatment for MDR-TB during 2009–2011 were lost to follow-up during treatment, and that 40% had not achieved culture conversion at the time of LFU. A similarly high rate of LFU among MDR-TB patients has been demonstrated in other studies,6 and these data together illustrate the immense challenges in achieving completion of currently recommended SLD regimens for MDR-TB. Our LFU rate was also substantially higher than the WHO recommended target of 5%.11 We identified a combination of various patient and treatment characteristics that were associated with LFU; reducing LFU will take a multipronged approach targeting multiple elements.
About 40% of LFU occurred early during the intensive phase (first 8 months), and two thirds of these patients were culture-positive at the time of LFU. While a previous study from Peru found the same rate of culture positivity (40%) at the time of LFU,3 no other studies have reported on the rate of culture conversion at the time of LFU. Given the higher rates of culture positivity, patients with early LFU are at a higher risk of spreading MDR-TB disease in the community and of having a poor long-term outcome.3 Furthermore, the reasons for LFU are likely to be different. Adverse events may be more likely to be responsible for LFU during the intensive phase of treatment, when patients are receiving an injectable agent. Previous studies evaluating severe adverse events during MDR-TB treatment found that approximately 65% occur in the first 6 months of treatment.12,13 Unfortunately, there is no standard system for reporting drug-related adverse events or management in Georgia. The only insight available comes from a United States Agency for International Development TB Prevention Project in Georgia, in which MDR-TB patients who were lost to follow-up were contacted and interviewed. The study found that 50% of patients with MDR-TB reported treatment-related side effects as the most important reason for LFU.14 As injectable agents and current SLDs will likely continue to be used for at least the next few years, proper reporting and management of adverse events is an essential component of MDR-TB treatment.8,15 To ensure proper treatment of adverse events, continual training of TB health care workers (HCWs) on the management of SLD-related adverse events will be needed.
Our study identified several patient characteristics that were associated with a higher risk of LFU. The use of illicit drugs was probably underreported in our study, although it showed a statistically significant association with LFU. Excessive alcohol consumption was associated with LFU in univariate analysis; however, the definition of excessive alcohol consumption was not standardised and was evaluated by physicians subjectively. This was one of the main risk factors for interruption of and non-adherence to treatment in the study conducted in Tomsk oblast, Russia.16
We recommend that all MDR-TB patients be screened routinely for the presence of the risk factors identified in this study; those who have these risk factors should be prioritised for intensive follow-up care. They should also be linked to other support services such as opioid substitution therapy, treatment of alcohol addiction and tobacco cessation services. While results on the effectiveness of patient incentives vary, cash incentives have been seen to perform better than non-cash incentives in improving patient adherence and retention.17 The country plans to re-launch its comprehensive package of adherence interventions (cash incentives) in 2014.
Two treatment-related factors associated with LFU were year and location of treatment initiation. Later year of treatment enrolment has been found to be associated with higher LFU rates in many studies and is thought to be secondary to a decreasing ability to provide individualised patient-centred care as the total MDR-TB cohort size increases.6,18 The HCW-patient ratio may decrease, and limited NTP financial resources are spread over an increasing number of patients. In Georgia, the total number of MDR-TB patients on treatment steadily increased from 2009 to 2011 and may have been responsible for higher LFU rates for the reasons given above. The NCTLD hospital and affiliated clinics in Tbilisi care for the majority of MDR-TB patients in the country, and for the reasons described, this may lead to a higher rate of LFU than in other regions, where more patient-centred, decentralised care is possible. An additional factor that is likely responsible in part for the higher LFU seen in 2011 is a recent trend of patients from former Soviet Union countries, including Georgia, seeking care in other countries. A 2013 report describes the high rate of Georgian MDR-TB patients seeking care in France, including 26 patients in 2012.19 These findings indicate an urgent need to evaluate patient perception of care in Georgia and find ways to improve MDR-TB management.
The main strengths of our study include a large population-based study cohort, the inclusion of culture conversion status at time of LFU, and adherence to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting on observational studies.20 Limitations include use of retrospective, routine programme data, which may be subject to unrecognised errors, lack of information on adverse drug events and comorbidities, including diabetes and mental health disorders, which were not systematically documented. The absence of hospitalisation dates also prevented us from assessing the effect of duration of hospitalisation on LFU.
In conclusion, our study found an alarmingly high rate of LFU among MDR-TB patients and a high rate of culture positivity at the time of LFU. Patients who are lost to follow-up are a threat to the spread of disease in the community, and based on our results a multipronged strategy is needed to address this urgent problem.
The following country-specific recommendations could be made:
To improve adverse drug reaction management by setting up a pharmacovigilance system linked to the electronic TB database
To train HCWs in the management of side effects of anti-tuberculosis medicines
Screening on risk factors identified by the study and intensive follow-up
Incentives for patients and service providers
Decentralisation of treatment facilities within Tbilisi to improve the HCW-patient ratio per treatment centre.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by WHO-TDR, the WHO Regional Office for Europe (Copenhagen, Denmark), the Operational Research Unit (LUXOR), Brussels Operational Center, Médecins Sans Frontières (MSF Luxembourg), the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union; Paris, France), The Union South-East Asia Regional Office, New Delhi, India. We are grateful for the support of the WHO Country Office in Talinn, Estonia, and the Estonia National Institute for Health and Development (Talinn, Estonia) in hosting the training workshops. We also appreciate the active involvement of the WHO Country Office and the Ministry of Health (Talinn, Estonia) in the selection of candidates for training in operational research and identification of research projects.
The SORT IT programme was funded by the United States Agency for International Development (Washington DC, USA) through a grant managed by WHO-TDR. Additional support was provided by the WHO Regional Office for Europe, the Department for International Development (London, UK), and the MSF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The study was supported in part by grants from the US National Institutes of Health (NIH) Fogarty International Center (D43TW007124 and D43TW007124-06S1), and National Institutes for Allergy and Infectious Diseases (K23AI103044), Bethesda, MD, USA.
Footnotes
Conflict of interest: none declared.
The authors alone are responsible for the content of this paper which may not necessarily represent the policies, decisions or views of the World Health Organization.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution intergovernmental organisation license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.World Health Organization. Definitions and reporting framework for tuberculosis: 2013 revision. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.2. [Google Scholar]
- 3.Franke M F, Appleton S C, Bayona J et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis. 2008;46:1844–1851. doi: 10.1086/588292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gegia M, Kalandadze I, Kempker R R, Magee M J, Blumberg H M. Adjunctive surgery improves treatment outcomes among patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis. 2012;16:e391–396. doi: 10.1016/j.ijid.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gegia M, Jenkins H E, Kalandadze I, Furin J. Outcomes of children treated for tuberculosis with second-line medications in Georgia, 2009–2011. Int J Tuberc Lung Dis. 2013;17:624–629. doi: 10.5588/ijtld.12.0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toczek A, Cox H, du Cros P, Cooke G, Ford N. Strategies for reducing treatment default in drug-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2013;17:299–307. doi: 10.5588/ijtld.12.0537. [DOI] [PubMed] [Google Scholar]
- 7.National Statistics Office of Georgia. GeoStat. Tbilisi, Georgia: NSO; 2013. [Google Scholar]
- 8.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.6. [PubMed] [Google Scholar]
- 9.Tukvadze N, Kempker R R, Kalandadze I et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLOS ONE. 2012;7:e31563. doi: 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosmer D W, Lemeshow S, May S. Applied survival analysis: regression modeling of time to event data. Hoboken, NJ, USA: John Wiley & Sons; 2008. [Google Scholar]
- 11.World Health Organization. Roadmap to prevent and combat drug-resistant tuberculosis: the Consolidated Action Plan to Prevent and Combat Multi-drug- and Extensively Drug-Resistant Tuberculosis in the WHO European Region 2011–15. Copenhagen, Denmark: WHO; 2011. Resolution EUR/RC61/R7. [Google Scholar]
- 12.Shin S S, Pasechnikov A D, Gelmanova I Y et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11:1314–1320. [PubMed] [Google Scholar]
- 13.Isaakidis P, Cox H S, Varghese B et al. Ambulatory multi-drug resistant tuberculosis treatment outcomes in a cohort of HIV-infected patients in a slum setting in Mumbai, India. PLOS ONE. 2011;6:e28066. doi: 10.1371/journal.pone.0028066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Social Studies and Analysis. Survey of risk factors associated with default from treatment and long term outcomes of default in patients with multidrug-resistant tuberculosis. Tbilisi, Georgia: IoSSA; 2013. [Google Scholar]
- 15.World Health Organization. A practical handbook on the pharmacovigilance of medicines used in the treatment of tuberculosis. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 16.Gelmanova I Y, Keshavjee S, Golubchikova V T et al. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ. 2007;85:703–711. doi: 10.2471/BLT.06.038331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutge E E, Wiysonge C S, Knight S E, Volmink J. Material incentives and enablers in the management of tuberculosis. Cochrane Database Syst Rev. 2012;1:CD007952. doi: 10.1002/14651858.CD007952.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Lalor M K, Greig J, Allamuratova S et al. Risk factors associated with default from multi- and extensively drug-resistant tuberculosis treatment, Uzbekistan: a retrospective cohort analysis. PLOS ONE. 2013;8:e78364. doi: 10.1371/journal.pone.0078364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard C, Brossier F, Sougakoff W et al. A surge of MDR and XDR tuberculosis in France among patients born in the Former Soviet Union. Euro Surveill. 2013;18:20555. doi: 10.2807/1560-7917.es2013.18.33.20555. [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman D G, Egger M, Pocock S J, Cøtzsche P C, Vandenbroucke J P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]


