Abstract
Setting: A tertiary care facility in Ukraine, a high multi- and extensively drug-resistant tuberculosis (MDR/XDR-TB) burden country.
Objectives: To assess the management and treatment outcomes of MDR, pre-XDR-TB and XDR-TB.
Design: Cohort study using programme data, 2006–2011.
Results: Of 484 individuals with drug-resistant TB, 217 (45%) had MDR-, 153 (32%) pre-XDR- and 114 (24%) XDR-TB. Of all resistant types completing the intensive phase of treatment, 322 (67%) were alive and had culture converted. This included 157 (72%) with MDR- and 61 (54%) with XDR-TB. At the end of the continuation phase of treatment, 106 (22%) had treatment success and 378 (78%) had unfavourable outcomes, including 110 (23%) failures, 21 (4%) deaths, 71 (15%) losses to follow-up and 176 (36%) with an unknown outcome. This was associated with more than one lung cavity being affected, a history of treatment with second-line anti-tuberculosis drugs, poor adherence and XDR-TB. A total of 226 (47%) patients reported at least one adverse drug reaction, the most common being gastrointestinal and vestibular toxicity.
Conclusion: Outcomes of MDR- and XDR-TB were satisfactory in the intensive phase; however, this was not sustained during the ambulatory period. If we are to do better, urgent measures are needed to improve ambulatory management, including making safer, shorter and more effective drug regimens available.
Keywords: operational research, SORT IT, decentralisation, MDR-TB drug regimens
With a population of 45 million, Ukraine is Europe's second largest country. Tuberculosis (TB) is a major public health problem, and multidrug-resistant TB (MDR-TB) is common. Rates of MDR-TB, defined as Mycobacterium tuberculosis strains resistant to at least isoniazid and rifampicin, are respectively 16% (range 14–18) and 44% (40–49) in new cases and retreatment cases.1 Extensively drug-resistant TB (XDR-TB), defined as MDR-TB plus resistance to any fluoroquinolone and any of the second-line anti-tuberculosis injectable drugs (amikacin, kanamycin or capreomycin), has also been reported in Ukraine. Pre-XDR-TB is defined as MDR-TB with resistance to either a fluoroquinolone or an injectable drug.
Ukraine is one of the world's 27 high MDR-TB burden countries. Treatment for such patients can take ⩾ 2 years, with second-line drugs that are less effective, more toxic and expensive, costing US$4000–6000 per treatment. A recent meta-analysis of treatment outcome data from 9153 MDR-TB patients at 32 sites worldwide showed poor treatment success rates of 54%.2 In Ukraine, the reported treatment success for MDR-TB for the 2010 cohort was 29%.1 Ways to improve treatment success are thus urgently required.3
The Institute of Phthisiology and Pulmonology, now renamed the F G Yanovsky Academy of Medical Science of Ukraine, Kiev, is a specialised centre for the management of patients with MDR- and XDR-TB. This institute, also known as the National Tuberculosis Institute (NTI), has an internationally accredited national reference laboratory for the diagnosis of drug-resistant TB (DR-TB), as well as a specialised team of doctors, researchers, counsellors and educators who provide education and care. Patients are hospitalised at the centre during the intensive phase of treatment, and receive individualised drug regimens based on drug susceptibility testing (DST). Second-line drugs are readily available.4 During the continuation phase of treatment, which is ambulatory, responsibility for TB management moves to regional dispensaries, whose resources in terms of infrastructure, staffing and supervision are more limited. In particular, counselling and patient support services are weak and this may affect TB management in the continuation phase.4 The decentralisation of the continuation phase was introduced only in 2010.
The World Health Organization (WHO) has called for the successful treatment of 75% of MDR-TB patients in Europe by 2015. Specific targets for death, failure and loss to follow-up (LTFU) were as follows: 10% for death and failure and 5% for LTFU.5 A PubMed search revealed no information on treatment outcomes from Ukraine, and progress toward these targets has thus not been assessed. Operational research to improve MDR-TB treatment in Ukraine is considered a priority.
We aimed to assess the management of DR-TB among individuals who started treatment. Among patients with MDR-, pre-XDR- and XDR-TB, our specific objectives were to report on 1) the baseline demographic, clinical and radiological characteristics; 2) sputum culture conversion at the end of the intensive phase; 3) treatment outcomes at the end of the intensive phase at the NTI and at the end of the continuation phase at the regional dispensaries; 4) factors associated with unfavourable treatment outcomes at the end of the intensive phase; and 5) adverse drug reactions.
METHODS
Study design and setting
This was a retrospective cohort study using routine programme data. The study was conducted at the NTI in Kiev, the capital of Ukraine, the second largest country (by surface area and population) in Europe. The study population included all adult (aged ⩾18 years) MDR-, pre-XDR- and XDR-TB patients, both new and those with a previous history of anti-tuberculosis treatment, who started treatment between 1 January 2006 and 31 December 2011.
The NTI treats patients from across the country and accepts all types of TB cases, including new, retreatment and MDR-TB cases; both self-presenting patients and referrals are accepted. The institute has specialised departments, isolation wards, radiology and a national reference laboratory, which performs DST against both first- and second-line anti-tuberculosis drugs, as well as specialised services such as bronchoscopy, thoracoscopy, ultrasound and surgical interventions for severe TB. Drug-susceptible and -resistant TB is managed according to national guidelines.6,7 In brief, the intensive phase involves hospitalisation for 6–8 months, followed by a 12–18-month continuation phase on an ambulatory basis in regional TB dispensaries. Patients may also attend the NTI during the ambulatory phase if they wish. Culture and DST are performed on all patients. Conventional culture (Löwenstein-Jensen) has been available since 2006; from 2008 onwards, BD BACTEC™ liquid culture (BD, Franklin Lakes, NJ, USA) also became available. Laboratory quality control procedures are in place and external quality assessment of DST is done on a regular basis by the Supranational Laboratory in Latvia (Novads, Latvia), one of the laboratories accredited by the WHO/International Union against Tuberculosis and Lung Disease (The Union).8 Sputum smear and culture are performed monthly during the intensive phase and then every 3 months during the continuation phase. All services are offered free of charge.
Treatment outcomes are standardised and shown in Table 1. All health workers who manage TB have been trained in the recognition, management and reporting of adverse drug reactions. All patients were routinely monitored for adverse drug reactions using audiogram, complete blood count, urinalysis, blood chemistry, blood electrolytes and self-reporting, following counselling sessions with a neurologist, an otolaryngologist and an ophthalmologist. Adverse drug reactions were reported according to a standardised format.6
TABLE 1.
Standardised definitions for treatment outcomes for drug-resistant tuberculosis, Kiev, Ukraine

Data collection and statistical analysis
Data on patient characteristics, management and treatment outcomes were entered into a dedicated Excel database (Microsoft, Redmond, WA, USA) that was regularly validated and cross-checked using patient master and ambulatory cards. Treatment outcomes were stratified by type of drug resistance. To examine factors associated with unfavourable outcomes at the end of the intensive phase of anti-tuberculosis treatment, outcomes were stratified into ‘favourable’ (alive and culture converted to negative) and ‘unfavourable’ (died, failure, LTFU and not evaluated). The measures of risk were determined using crude odds ratios (ORs) and adjusted odds ratios (aORs). ORs were adjusted using multi-variant logistic regression, and all related P values were based on the Wald test. Data were analysed using EpiData (version V2.2.2.180, EpiData Association, Odense, Denmark) and Stata version 12 (Stata Corp, College Station, TX, USA). The χ2 test was used to compare differences between groups and the χ2 for trend to assess linear relationships; 95% confidence intervals (CIs) were used throughout.
Ethical clearance was received from the ethics review committee of the NTI, Kiev, Ukraine, and the Ethics Advisory Group of The Union, Paris, France.
RESULTS
Baseline characteristics of the study population
Table 2 shows the baseline demographic, clinical and radiological characteristics of patients with DR-TB. Of 484 DR-TB patients included in the study, 217 (45%) had MDR-, 153 (32%) pre-XDR and 114 (24%) XDR-TB. Those with a previous history of second-line anti-tuberculosis drug use were more likely to have pre-XDR- and XDR TB (χ2 for trend, P < 0.001).
TABLE 2.
Baseline demographic, clinical and radiological characteristics of patients with drug-resistant TB starting treatment at the National Tuberculosis Institute, Kiev, Ukraine, 2006–2011
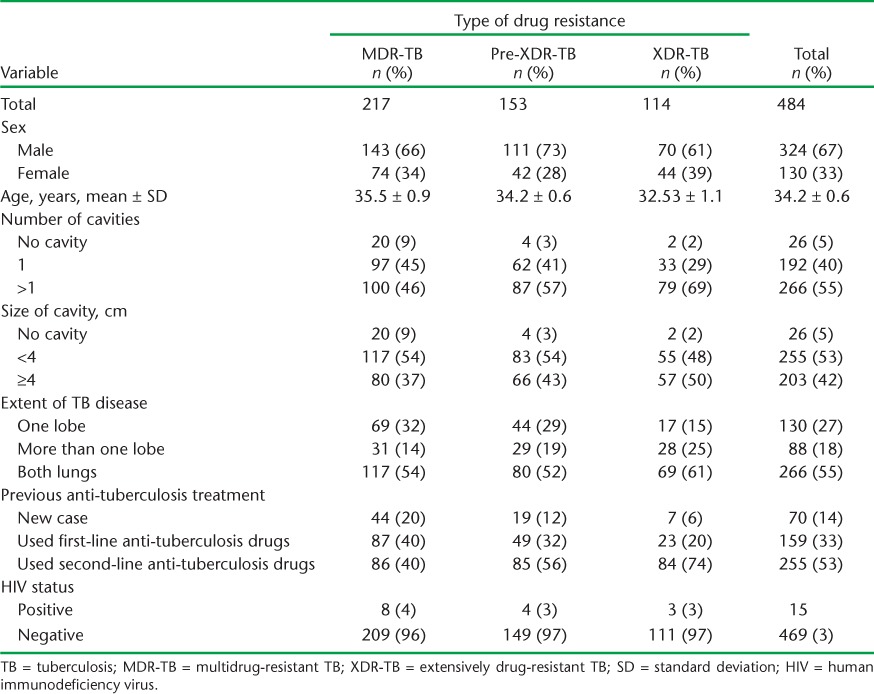
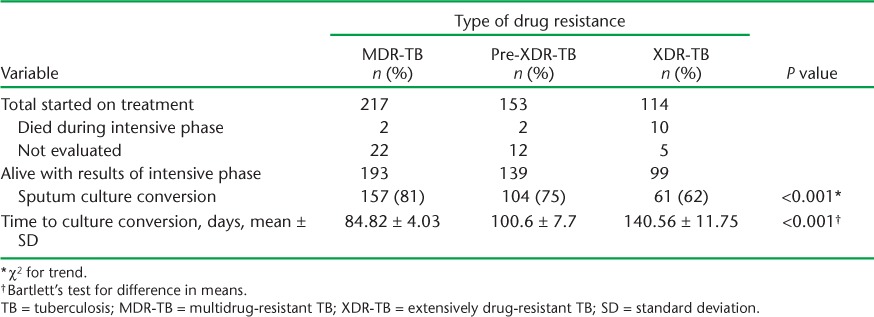
Data on sputum culture conversion at the end of the intensive phase are summarised in Table 3, which shows lower culture conversion in pre-XDR- and XDR-TB patients than in MDR-TB patients; this relationship was linear and significant. The mean number of days to culture conversion also showed a similar picture.
TABLE 3.
Sputum conversion at the end of the intensive phase by type of drug resistance and time to culture conversion, National Tuberculosis Institute, Kiev, Ukraine, 2006–2011
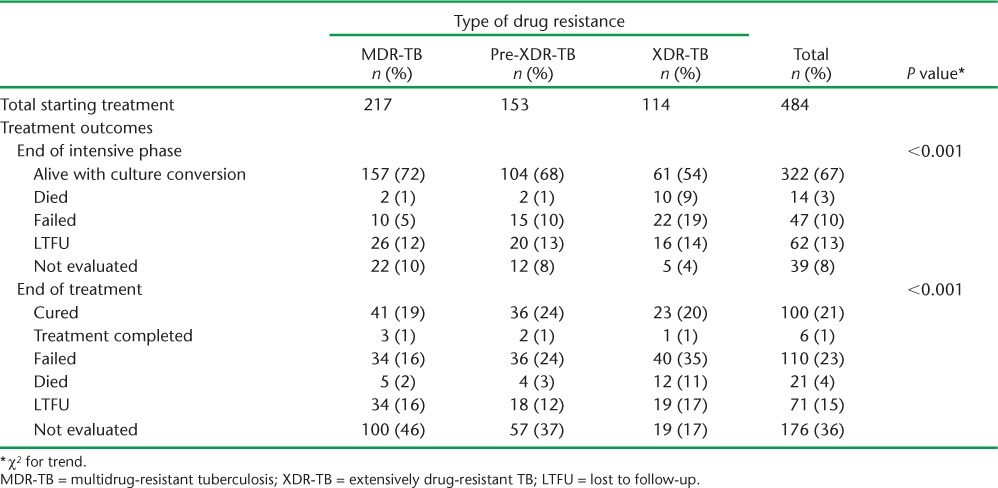
Treatment outcomes at the end of the intensive and continuation phases are shown in Table 4. Of all DR-TB patients started on treatment, 322 (67%) were alive and had culture-converted by the end of the intensive phase; this included 157/217 (72%) MDR patients and 61/114 (54%) with XDR-TB. At the end of the continuation phase of treatment, 378 (78%) patients were recorded as having an unfavourable outcome and only 106 (22%) had overall treatment success; 176 (36%) could not be evaluated due to ‘unknown outcomes’. Even after excluding the ‘unknown outcome’ group, treatment success was 34%. The numbers of failures, LTFU and deaths were high at the end of treatment.
TABLE 4.
Treatment outcomes at the end of the intensive phase and at the end of treatment, National Tuberculosis Institute, Kiev, Ukraine, 2006–2001
Factors associated with an unfavourable treatment outcome at the end of the intensive phase are shown in Table 5. Significant variables associated with unfavourable outcomes included having more than one lung cavity affected (compared to a single cavity), a previous history of second-line anti-tuberculosis drug use, poor treatment adherence and XDR-TB (Table 5).
TABLE 5.
Determinants associated with unfavourable treatment outcomes at the end of the initial phase of treatment for drug-resistant TB, National Tuberculosis Institute, Kiev, Ukraine, 2006–2001

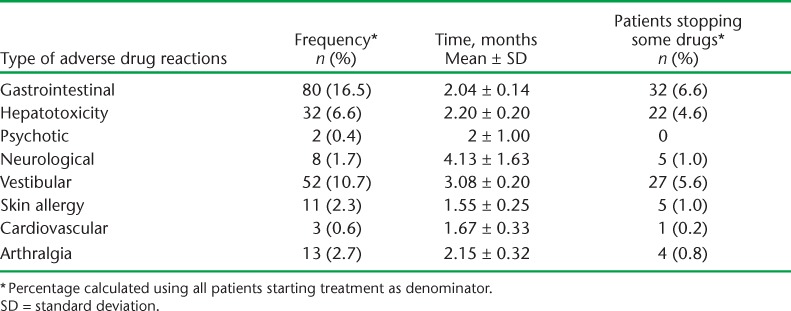
Type and frequency of adverse drug reactions are shown in Table 6. Of 484 patients, 175 (36%) reported at least one adverse drug reaction. The most common adverse drug reactions were gastrointestinal and vestibular toxicity, which occurred in the first 3 months of starting treatment.
TABLE 6.
Type of adverse drug reactions and timing during treatment of 484 patients for drug-resistant TB, Kiev, Ukraine, 2006–2001
DISCUSSION
This is the first large cohort study from Ukraine reporting on treatment outcomes of DR-TB. MDR- and XDR-TB treatment outcomes were satisfactory in the intensive phase; however, this was not sustained during the decentralised ambulatory period. Failures, deaths and LTFU during the intensive phase (including for XDR-TB) were relatively low. Satisfactory outcomes for XDR-TB during the intensive phase despite extensive lung involvement may reflect low prevalence of human immunodeficiency virus infection in this cohort. However, by the end of the continuation phase of treatment, only one in four patients had attained treatment success. Furthermore, attrition (deaths and LTFU) and failure rates were high. This study thus highlights the urgent need to improve the ambulatory phase of care for patients with DR-TB.
The study strengths are that it was conducted in a tertiary TB care facility with high clinical and laboratory standards, treatment protocols and outcomes were standardised, and we were able to report on the extent of lung pathology. We also adhered to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines9 for the reporting of observational studies, including ethics.10 An important study limitation was that end of treatment outcomes were unknown in over one third of all patients, and this may have biased our interpretation of treatment success. However, unknown outcomes reflect weaknesses in programme monitoring that need improvement. In addition, the NTI is a reference centre for complicated and severe cases, and as such, treatment outcomes may be worse than in other settings in Ukraine due to the sick-cohort effect. On the other hand, the NTI is well-resourced, with specialised staff, equipment and resources, and this may have had a beneficial effect on outcomes. Finally, we do not know the exact reasons for the poorer treatment outcomes during the ambulatory phase, and this merits specific investigation.
Despite these limitations, Ukraine is a country that is embarking on scaling up MDR- and XDR-TB treatment, and several findings from this study merit discussion. First, treatment outcomes could not be ascertained for over one third of all patients in the continuation phase. This was related to difficulties in obtaining information on treatment outcomes from decentralised sites by the NTI. Ukraine's national TB control programme has made laudable efforts to address this problem by introducing a national electronic TB register system in 2013. This allows universal access to treatment outcomes from all decentralised sites. This problem is thus resolved for future cohorts.
Second, the high rates of LTFU, death and failures, particularly during the continuation phase, may be due to a number of factors, including reduced adherence associated with longer periods of treatment, as well as adverse drug reactions. The latter have a negative effect on adherence, as do weaknesses in patient education and social support systems during the continuation phase, all of which may be to blame. In particular, regions of the former Soviet Union are known to have high rates of alcohol use, unemployment, homelessness and a history of incarceration, all of which accentuate the need for adapted patient support systems.11,12 The high failure rates may also be related to other factors, such as drug shortages, that may have occurred during ambulatory treatment. A recent study from Eastern Europe showed a treatment success rate of 21.8%, which compares well with that in our study, underlining the need to find ways forward in reducing unfavourable outcomes.13
Third, adverse drug reactions were very common, with almost half of all patients having reported at least one adverse drug reaction, and the pattern was diverse. Self-reported gastrointestinal and vestibular toxicity were the most common in our setting, unlike Africa and Asia, where hypothyroidism and psychosis are highly prevalent.14,15
Finally, Van Deun et al. have shown that adapted drug regimens were highly effective for MDR-TB and that it is possible to achieve a relapse-free success rate of 88%.16 Despite the caveat that only patients without extensive drug resistance were included in that study, these results suggest that we can improve overall treatment adherence and outcomes. Importantly, shorter, safer and more effective regimens will facilitate the urgent scale-up of MDR-TB treatment in countries such as Ukraine. This is supported by the finding by Ahuja et al. that treatment success rates at a global level are only 54% and close to pre-chemotherapy levels.2
In conclusion, we have identified a number of operational challenges in the management of MDR- and XDR-TB, particularly a high proportion of unfavourable outcomes in the continuation phase, which negatively affects treatment success. If we are to do better, urgent action is needed to improve treatment adherence during the continuation phase and to make shorter and more effective drug regimens available.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR, Geneva, Switzerland). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by WHO-TDR; the WHO Regional Office for Europe, Copenhagen, Denmark; the Operational Research Unit (LUXOR), Médecins Sans Frontières, Brussels Operational Center, Luxembourg; the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France; and The Union South-East Asia Regional Office, New Delhi, India. We are grateful for the support of the WHO Country Office in Talinn, Estonia, and the Estonia National Institute for Health and Development (Talinn, Estonia) in hosting training workshops. We also appreciate the active involvement of the WHO Country Office and the Ministry of Health in the selection of candidates for training in operational research and identification of research projects.
The programme was funded by the United States Agency for International Development (USAID) through a grant managed by WHO-TDR. Additional support was provided by the WHO Regional Office for Europe, the Department for International Development, London, UK; and MSF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
The authors alone are responsible for the content of this paper which may not necessarily represent the policies, decisions or views of the WHO.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed July 2014. [Google Scholar]
- 2.Ahuja S D, Ashkin D, Avendano M et al. Multidrug-resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLOS MED. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abubakar I, Zignol M, Falzon D et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13:529–539. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Review of the National Tuberculosis Programme in Ukraine: 10–22 October 2010. Copenhagen, Denmark: WHO; 2011. [Google Scholar]
- 5.World Health Organization. Resolution EUR/RC61/R7. Roadmap to prevent and combat drug-resistant tuberculosis: the Consolidated Action Plan to Prevent and Combat Multidrug- and Extensively Drug-Resistant Tuberculosis in the WHO European Region, 2011–2015. Copenhagen, Denmark: WHO; 2011. http://www.euro.who.int/en/publications/abstracts/roadmap-to-prevent-and-combat-drug-resistant-tuberculosis Accessed July 2014. [DOI] [PubMed] [Google Scholar]
- 6.Ukraine Ministry of Health. National treatment protocol No. 1091. Kiev: Ukraine Ministry of Health; 2012. [Google Scholar]
- 7.Ukraine Ministry of Health. National tuberculosis management guidelines. Kiev: Ukraine Ministry of Health; 2012. [Google Scholar]
- 8.World Health Organization. Global laboratory initiative: SRL network. Geneva, Switwerland: WHO; 2014. http://www.stoptb.org/wg/gli/srln.asp Accessed July 2014. [Google Scholar]
- 9.von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P C, Vandenbroucke J P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Edginton M, Enarson D, Zachariah R et al. Why ethics is indispensable for good quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowiak W M, Bogorodskaya E M, Borisov S E, Danilova I D, Kourbatova E V. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11:46–53. [PubMed] [Google Scholar]
- 12.Jakubowiak W M, Bogorodskaya E M, Borisov S E, Danilova I D, Lomakina O B, Kourbatova E V. Impact of socio-psychological factors on treatment adherence of TB patients in Russia. Tuberculosis (Edinb) 2008;88:495–502. doi: 10.1016/j.tube.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Post F A, Grint D, Werlinrud A M et al. Multidrug-resistant tuberculosis in HIV positive patients in Eastern Europe. J Infect. 2014;68:259–263. doi: 10.1016/j.jinf.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Brust J C, Shah N S, Scott M et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012;16:998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaakidis P, Varghese B, Mansoor H et al. Adverse events among HIV/MDR-TB co-infected patients receiving antiretroviral and second line anti-TB treatment in Mumbai, India. PLOS ONE. 2012;7:e40781. doi: 10.1371/journal.pone.0040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Deun A, Maug A K, Salim M A et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]


