Abstract
Setting: Drug-resistant tuberculosis (TB) is an important public health problem in Latvia.
Objective: To document trends, characteristics and treatment outcomes of registered patients with multi-drug-resistant (MDR-) and extensively drug-resistant (XDR-) TB in Latvia from 2000 to 2010.
Design: A retrospective national cohort study.
Results: Of 1779 patients, 1646 (92%) had MDR- and 133 (8%) XDR-TB. Over 11 years, the proportion of XDR-TB among MDR-TB patients increased from 2% to 18%. Compared to MDR-TB patients, those with XDR-TB were significantly more likely to have failed MDR-TB treatment (OR 8.4, 95%CI 4.3–16.2), have human immunodeficiency virus infection (OR 3.2, 95%CI 1.8–5.7), be illegal drug users (OR 5.7, 95%CI 2.6–11.6) or have had contact with MDR-TB patients (OR 1.9, 95%CI 1.3–2.8). Cure rates for XDR-TB were 50%. Compared with MDR-TB patients, those with XDR-TB had a higher risk of treatment failure (29% vs. 8%, respectively, P < 0.001). Unfavourable treatment outcomes were significantly associated with being male; having smear-positive disease; pulmonary cavities; failure, default or relapse after previous MDR-TB treatment; and a history of incarceration.
Conclusion: More MDR-TB in Latvia is now also XDR-TB. This study identified several risk factors for XDR-TB and, for unfavourable treatment outcomes, highlighting the importance of early diagnosis and appropriate management of MDR-/XDR-TB.
Keywords: operational research, SORT IT, Europe, Latvia, XDR-TB
Latvia is a Baltic country in Eastern Europe with a population of about 2 million, according to the last census conducted in 2011. Tuberculosis (TB) is a major public health problem, with approximately 1000 cases registered per year and an estimated TB incidence rate of 53 per 100 000 population per year.1 Multidrug-resistant TB (MDR-TB, defined as TB resistant to at least isoniazid [INH] and rifampicin [RMP]) has been a major challenge. MDR-TB rates in the country increased to 14% of new cases and 54% of previously treated cases in 1997.2 Since then, they have decreased in absolute numbers by a factor of about three, and in 2012, 11% of patients registered with new TB and 32% with previously treated TB had MDR-TB.1
Extensively drug-resistant TB (XDR-TB, defined as MDR-TB plus resistance to a fluoroquinolone and one of three second-line injectables)3 was first reported in Latvia in 2000. Since then, according to the TB registry data, there has been a progressive increase in XDR-TB in the country. XDR-TB is difficult and expensive to treat, requiring first-, second- and third-line drugs that are less well tolerated by patients than MDR-TB drugs. The newer anti-tuberculosis drugs, such as bedaquiline and delamanid, which would allow newly designed and potentially effective treatment regimens, are also not available in the country. A study on drug-resistant TB in Latvia from 2000 to 2004 showed poor treatment outcomes, with cure rates of <40% for XDR-TB.4
Since this first report, no further studies on XDR-TB have been published in Latvia. In the meantime, Latvia has implemented various measures to improve treatment outcomes for XDR-TB patients. Drug susceptibility testing (DST) is performed for all culture-positive patients. In 2000, the country introduced BACTEC™ (BD, Sparks, MD, USA) for earlier diagnosis of MDR-TB. Newer generation fluoroquinolones such as moxifloxacin (MFX) and levofloxacin (LFX) and third-line medications such as linezolid, amoxicillin/clavulanic acid and clarithromycin, are included in the treatment regimens as soon as BACTEC results indicating pre-XDR-TB and XDR-TB are obtained. There is also adjuvant surgery for cavitary disease. Since 2008, Latvia has participated in several ICH-GCP (International Conference on Harmonisation/Good Clinical Practice) clinical trials. The impact of these measures has not been evaluated and up-to-date information about the trends, burden of disease and treatment outcomes of XDR-TB in Latvia is needed.
The aim of the present study was to document trends, characteristics and treatment outcomes of patients registered with MDR- and XDR-TB in Latvia over a 10-year period from 2000 to 2010.
METHODS
Study design
This was a retrospective national cohort study of MDR- and XDR-TB patients.
Setting
General setting
Latvia is a small country in Eastern Europe that shares borders with Lithuania, Estonia, Belarus and Russia, and a sea border with Sweden. It is a middle-income country with a gross domestic product per capita of approximately US$8500 per year; the economy is mainly agricultural. The health system is funded largely through government taxation, with about 66 hospitals serving the population of 2.2 million. The average life expectancy at birth is 74 years, one of the lowest in the European Union. Clinically reported alcoholism is high, with over 20 000 cases registered with health care institutions in 2012.5
TB control
The MDR-TB treatment programme was started in 1997 after DOTS expansion to the whole country, which was started in 1995. Diagnosis, registration, treatment and follow-up of all drug-susceptible and -resistant TB cases follow national guidelines, which are based on World Health Organization (WHO) treatment guidelines.6–8 TB services and treatment are offered free of charge to all patients. There is good surveillance and reporting of data: all patients newly diagnosed with TB and those under retreatment are registered in the National Tuberculosis Register. Patients with laboratory-confirmed MDR-TB are also registered in an MDR-TB patient database, which was used as the data source for the study. All patients are then followed up using treatment cards, with their results updated in the register. Nationwide data from treatment facilities are collated and reported in aggregate form on a quarterly basis.
In 2012, 993 TB cases were notified nationwide (87% case detection rate) and a 73% treatment success rate was reported among new smear-positive pulmonary TB cases. In 2012, there were 110 patients with human im-munodeficiency (HIV) associated TB, a significant increase over the previous year (74 patients).9 Of the 120 estimated MDR-TB cases in 2012, 110 were registered.1
Diagnosis and management of MDR- and XDR-TB
Since 2000, all registered TB cases in the country have undergone culture and DST. All positive cultures undergo DST against first-line drugs; INH- and RMP-resistant cultures are assessed for second-line drug susceptibility. DST is centralised at the Mycobacteriology Laboratory at the Riga Eastern Clinical University Hospital, Riga, Latvia, and supervision is provided by the Supranational Reference Laboratory in Stockholm, Sweden. This includes regular participation in external quality assurance checks, with good results. Second-line DST was implemented for all MDR-TB cases in 1998 using solid Löwenstein-Jensen media and BACTEC™ 460TB (BD), which was then replaced by the Mycobacteria Growth Indicator Tube™ (MGIT) System (BD) in 2000. Since 2004, specimens from MDR-TB suspects have been tested using line-probe assays (LPAs) (INNO-LiPA™ Rif.TB, Innogenetics, Ghent, Belgium, from 2004 to 2006 and GenoType® MTB-DRplus, Hain Lifesciences, Nehren, Germany, since 2006). LPA results are used to modify treatment. Since 2010, Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) has also been used; empirical second-line treatment is started immediately upon receipt of an RMP-resistant result.
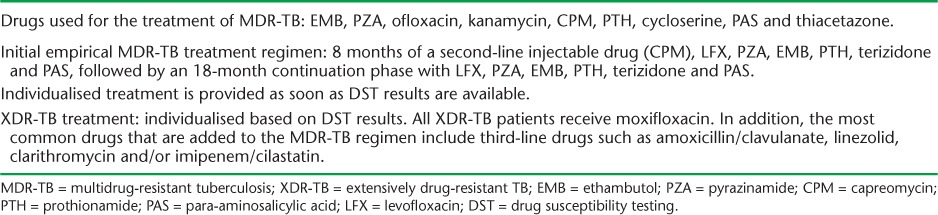
Details of MDR- and XDR-TB treatment are shown in Table 1. Briefly, once patients have been identified with MDR-TB, they are treated empirically with an MDR-TB regimen that may be modified depending on the DST results. Patients diagnosed with XDR-TB will receive regimens that include first-, second- and third-line drugs. Patients are managed under an expert consilium with hospitalisation dependent on smear or culture conversion, tolerance to medications and social factors. All drugs are given daily by direct observation for the entire duration of treatment, which varies from 18 to 36 months. In hospital, drug administration is daily, while out-patients in the community receive their drugs seven, six or five times a week, depending on the distance to the medical facility and the patient's drug tolerance. Boxes of drugs are given every month to medical personnel to disburse. There have been no interruptions of drug supplies to date. Standardised treatment outcomes are reported, with loss to follow-up defined as treatment interruption >2 months and failure as two or more positive cultures during the final 12 months of treatment.8
TABLE 1.
Anti-tuberculosis drugs used for the treatment of MDR-TB and XDR-TB in Latvia
Patient population
All patients diagnosed and registered in the Latvian national TB registry with MDR- and XDR-TB from 1 January 2000 to 31 December 2010 were included in the study.
Data variables, sources of data and data collection
Data variables included the number of all patients registered in Latvia with TB, MDR-TB and XDR-TB each year. The following information was recorded for all patients over the 11-year period: TB registration number, age, sex, type and category of TB, presence of cavities at the start of treatment, HIV status, history of incarceration, presence/absence of diabetes mellitus, clinically reported alcoholism, drug use and contact with MDR-TB, and standardised treatment outcomes. Sources of data were the Latvia TB and MDR-TB Registers. Data were collected in an MS Excel file (Microsoft, Redmond, WA, USA) between May and December 2013.
Analysis and statistics
Data from the Excel file were exported into EpiData for analysis (version 2.2.2.182, EpiData Association, Odense, Denmark). A descriptive and trend analysis was carried out, and categorical variables were compared against outcomes of interest using the χ2 test, unadjusted odds ratios (ORs) for baseline characteristics, unadjusted relative risks (RRs) for treatment outcomes and 95% confidence intervals (CIs). Multivariable logistic regression was carried out which included all variables from the unadjusted models; as there were no appreciable differences in the significance of the variables between the models, only the unadjusted ORs and RRs are reported. Levels of significance were set at 5%.
Ethics
Approval for the study was received in 2010 from the Riga Stradins University Ethics Committee, Riga, Latvia. Ethics approval was also obtained from the International Union Against Tuberculosis and Lung Disease Ethics Advisory Group, Paris, France.
RESULTS
Of the 1779 patients registered over the 11-year period, 1646 had MDR-TB and 133 XDR-TB. The MDR-TB group had 1247 (76%) males; the mean age was 42 years (± standard deviation [SD] 12.9), while the XDR-TB group had 86 (65%) males and a mean age of 39 years (±12.5). The Figure shows the trend over the 11 years among patients registered each year with MDR- and XDR-TB. MDR-TB peaked in 2001, at 240 cases, and then gradually decreased, reaching 69 cases in 2010. XDR-TB increased from 4 cases in 2000 to peak at 19 cases in 2004; since then, the number of cases per year has varied between 10 and 20. More importantly, the proportion of MDR-TB that was XDR-TB increased from 2% in 2000 to 18% in 2010.
FIGURE.
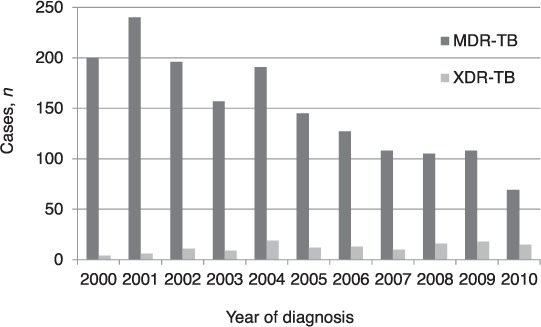
All MDR- and XDR-TB patients registered annually in Latvia, 2000–2010. MDR-TB = multidrug-resistant tuberculosis; XDR-TB = extensively drug-resistant TB.
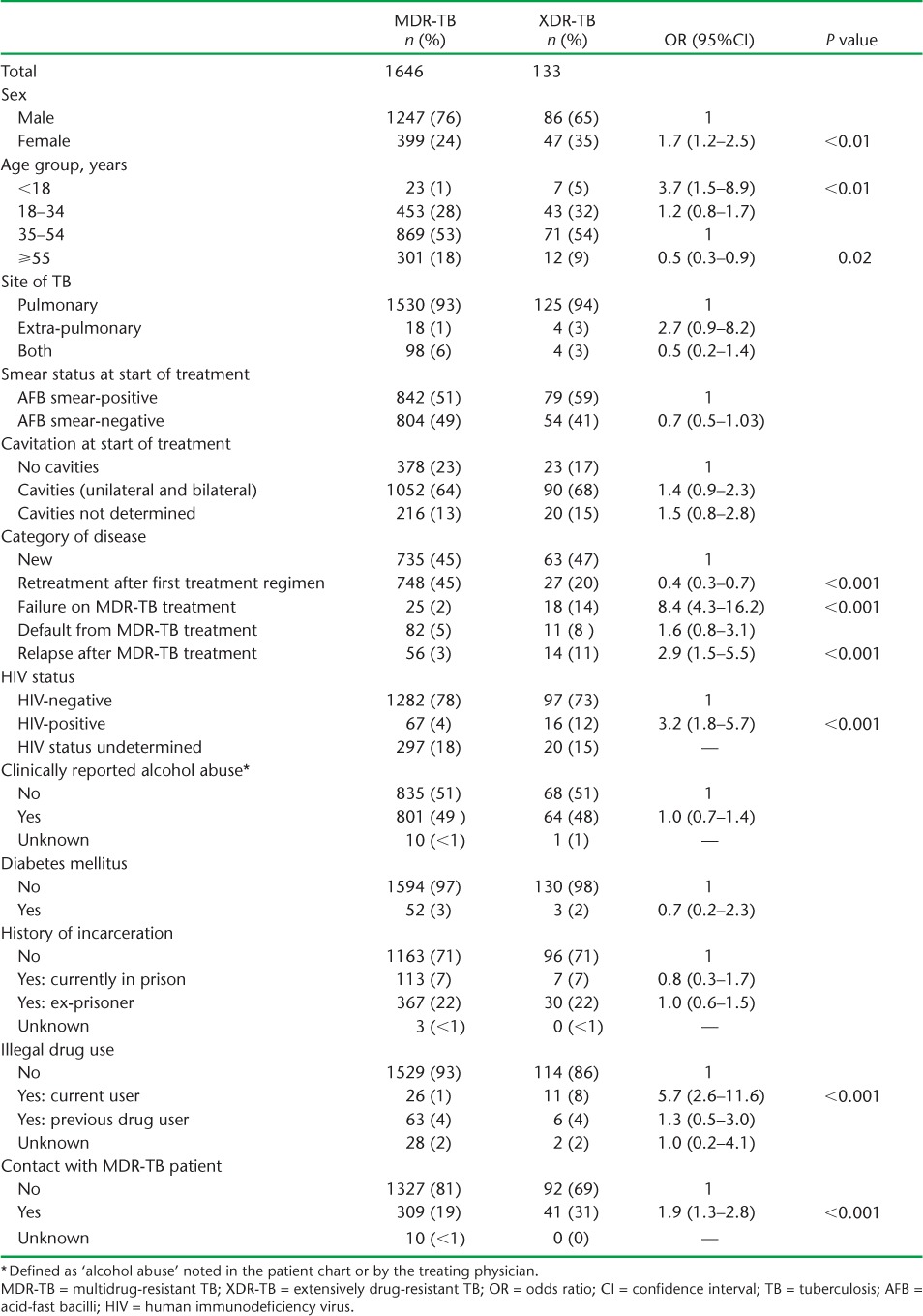
The demographic, clinical and social characteristics of patients diagnosed with MDR- and XDR-TB over the 11-year period are shown in Table 2. Among patients with XDR-TB, and compared with MDR-TB patients, there were significantly more females, patients aged <18 years, patients who had failed or relapsed after MDR-TB treatment, HIV-infected patients, those who currently used illegal drugs, and individuals who had contact with index MDR-TB patients.
TABLE 2.
Demographic, clinical and social characteristics of patients registered with MDR- and XDR-TB, Latvia, 2000–2010
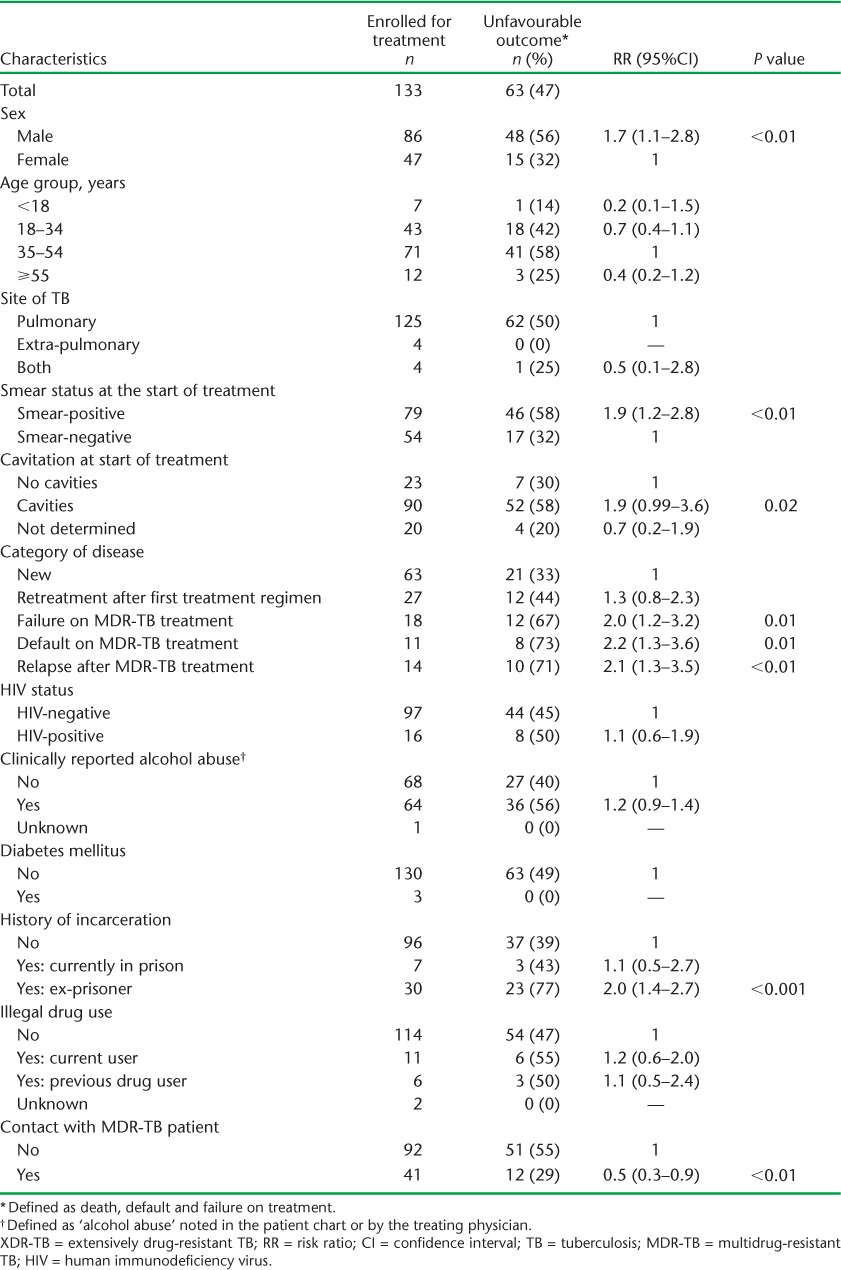
Treatment outcomes are shown in Table 3. Significantly more MDR-TB than XDR-TB patients were cured, the principal reason being a higher rate of treatment failure in XDR-TB patients. Other outcomes were not significantly different between the two groups of patients. The proportions of XDR-TB patients with an unfavourable outcome in relation to demographic, clinical and social characteristics are shown in Table 4. Among patients with unfavourable outcomes, there were significantly more males, patients with smear-positive disease and cavities, patients who had failed, defaulted or relapsed after MDR-TB treatment, and patients with a history of incarceration. In addition, patients who had been contacts of an MDR-TB patient had a significantly lower risk of an unfavourable outcome.
TABLE 3.
Treatment outcomes in patients with MDR- and XDR-TB in Latvia, 2000–2010
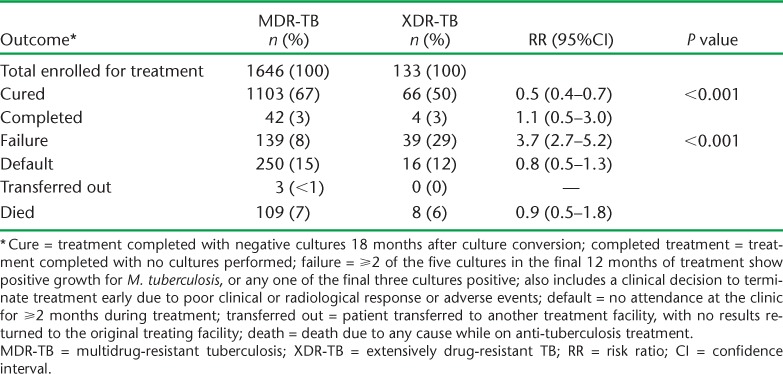
TABLE 4.
Demographic, clinical and social characteristics of patients with XDR-TB by unfavourable treatment outcome, Latvia, 2000–2010
DISCUSSION
This study highlights the importance of XDR-TB in Latvia. During the 11-year study period, the absolute number of MDR-TB cases decreased in the country, while the number of XDR-TB cases peaked and then remained stable at a high level. As a result, the proportion of patients with XDR-TB increased by a factor of 10; to date nearly 20% of MDR-TB cases have extensive drug resistance.
Some key factors were associated with the development of XDR-TB. Patients who failed, defaulted or relapsed after MDR-TB treatment were at high risk of acquiring XDR-TB, particularly those who failed. This finding emphasises the need for more effective treatment regimens to avoid amplification of resistance; this includes shortening the treatment duration and using drugs with the least adverse effects to ensure better tolerability for patients and better adherence to treatment. Those with HIV infection (there are currently 4863 people living with HIV in the country) and who currently use illegal drugs were at significant risk of developing XDR-TB. These two factors are interlinked in Latvia, with HIV infection being a concentrated epidemic focused around intravenous drug use.10 A recent systematic review suggested that HIV infection in countries such as Latvia is associated with primary MDR-TB,11 and where there is a growing burden of XDR-TB the same association might be expected. Finally, XDR-TB was associated with contact with an MDR-TB case. A systematic review of risk factors for XDR-TB highlights some of the same variables found in our study, but there is no mention of contact with index patients.12 We are not sure why this might be a risk factor, but index MDR-TB patients are on treatment for long periods, during which they might fail treatment or not comply with drug regimens and are therefore at risk of developing and transmitting XDR-TB.
The cure rate of patients with XDR-TB in our study was 50%, which was lower than that observed in MDR-TB patients, mainly due to high failure rates. An unfavourable outcome was more common in males and prisoners, as has been reported in previous studies,13,14 and this presumably reflects poor adherence to treatment. An unfavourable outcome was also associated with more severe disease, defined as more cavities on chest radiography and smear positivity. These treatment results, however, are similar to, if not better than, those reported from other countries such as Peru,15,16 and they are a definite improvement on those reported in Latvia between 2000 and 2004, when cure rates were 38% and failure was at nearly 50%.4 It might be possible to improve treatment outcomes with newer drugs and better combinations of drugs.
The strengths of this study are the long, 11-year study period and the full nationwide sample, which make the results representative for the country. Attention was also paid to following internationally agreed recommendations for reporting on observational studies.17,18 However, the study also had some limitations. First, this is a report of patients registered for treatment and misses those who were diagnosed with MDR- and XDR-TB and who did not start treatment due to death, refusal or loss to follow-up. It therefore underreports the scale of the national problem. Second, some of the data for associated HIV infection were missing, as many patients, particularly those in peripheral health facilities and in prison hospitals, were not tested.
This study has important implications. First, the treatment of MDR-TB is too long and is associated with many adverse effects. Shorter, less toxic regimens are needed19 to improve adherence with less risk of creating XDR-TB. This could be further helped by the introduction of newer drugs such as bedaquiline and delamanid.20,21 Second, more severe disease is associated with poorer outcomes, thus emphasising the need for earlier diagnosis of both MDR-TB and XDR-TB, and intensified case finding among MDR- and XDR-TB high-risk groups such as the HIV-infected and illegal drug users. New diagnostic technology, such as Xpert, which has been in use in Latvia since 2010, may allow more rapid diagnosis of drug-resistant TB.22 Third, it is vitally important to try and prevent the spread of airborne infections and the spread of drug-resistant TB, particularly in the health facility setting, and this requires more attention to acceptable and effective infection control measures.22 Finally, close attention to ensuring good cure rates in patients with drug-susceptible TB is needed to prevent the emergence of acquired drug-resistant disease.
In conclusion, this study highlights the substantial burden of XDR-TB in Latvia, and underscores the need for shorter, better treatment regimens for MDR-TB, earlier diagnosis, intensified case finding among high-risk groups and implementation of effective hospital infection control measures in TB facilities and other high-risk settings such as HIV facilities.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR, Geneva, Switzerland). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by WHO-TDR; the WHO Regional Office for Europe (Copenhagen, Denmark); the Operational Research Unit (LUXOR), Médecins Sans Frontières (MSF), Brussels Operational Center, Luxembourg; the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France; The Union South-East Asia Regional Office, New Delhi, India. We are grateful for the support of the WHO Country Office in Talinn, Estonia and the Estonia National Institute for Health and Development (Talinn, Estonia) in hosting the training workshops. We also appreciate the active involvement of the WHO Country Office and the Ministry of Health in the selection of candidates for training in operational research and identification of research projects.
The programme was funded by the United States Agency for International Development (USAID) through a grant managed by WHO-TDR. Additional support was provided by the WHO Regional Office for Europe, the Department for International Development, London, UK; and MSF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
The authors alone are responsible for the content of this paper which may not necessarily represent the policies, decisions or views of the WHO.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report, 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.The World Health Organization/International Union Against Tuberculosis and Lung Disease. Global project on anti-tuberculosis drug resistance surveillance. anti-tuberculosis drug resistance in the world. Geneva, Switzerland: WHO; 1997. WHO/TB/97.229. [Google Scholar]
- 3.Extensively drug-resistant tuberculosis (XDR-TB): recommendations for prevention and control. Wkly Epidemiol Rec. 2006;81:430–432. [PubMed] [Google Scholar]
- 4.Leimane V, Dravniece G, Riekstina V et al. Treatment outcome of multidrug/extensively drug-resistant tuberculosis in Latvia, 2000–2004. Eur Respir J. 2010;36:584–593. doi: 10.1183/09031936.00003710. [DOI] [PubMed] [Google Scholar]
- 5.Government of Latvia. Latvia Statistics. Health care and sport: key indicators. Riga, Latvia: Government of Latvia; 2012. www.csb.gov.lv/en/statistikas-temas/health-care-and-sport-key-indicators-30689.html Accessed July 2014. [Google Scholar]
- 6.Ministry of Health. National TB treatment guidelines, Latvia 1997. Riga, Latvia: National TB Programme, Ministry of Health; 1997. [Google Scholar]
- 7.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneva, Switzerland: WHO; 2009. WHO/HTM/TB/2009.420. [Google Scholar]
- 8.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: WHO; 2008. WHO/HTM/TB/2008.402. [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2014. Stockholm, Sweden: ECDC; 2013. www.ecdc.europa.eu/en/publications/Publications/tuberculosis-surveillance-monitoring-Europe-2014.pdf Accessed July 2014. [Google Scholar]
- 10.Balode D, Ferdats A, Dievberna I et al. Rapid epidemic spread of HIV type 1 subtype A1 among intravenous drugs users in Latvia and slower spread of subtype B among other risk groups. AIDS Res Hum Retroviruses. 2004;20:245–249. doi: 10.1089/088922204773004978. [DOI] [PubMed] [Google Scholar]
- 11.Suchindran S, Brouwer E S, van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLOS ONE. 2009;4:e5561. doi: 10.1371/journal.pone.0005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lima B F, Tavares M. Risk factors for extensively drug-resistant tuberculosis: a review. Clin Respir J. 2014;8:11–23. doi: 10.1111/crj.12044. [DOI] [PubMed] [Google Scholar]
- 13.Keshavjee S, Gelmanova I Y, Farmer P E et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–1409. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 14.Miller A C, Gelmanova I Y, Keshavjee S et al. Alcohol use and the management of multidrug-resistant tuberculosis in Tomsk, Russian Federation. Int J Tuberc Lung Dis. 2012;16:891–896. doi: 10.5588/ijtld.11.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitnick C D, Shin S S, Seung K J et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–574. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonilla C A, Crossa A, Jave H O et al. Management of extensively drug-resistant tuberculosis in Peru: cure is possible. PLOS ONE. 2008;13:e2957. doi: 10.1371/journal.pone.0002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P, Vandenbroucke J P, for the STROBE initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 18.Edginton M, Enarson D, Zachariah R et al. Why ethics is indispensible for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Deun A, Maug A K J, Salim M A H et al. Short, highly effective, and inexpensive standardised treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 20.Diacon A H, Donald P R, Pym A et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother. 2012;56:3271–3276. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skripconoka V, Danilovits M, Pehme L et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41:1393–1400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehme C C, Nicol M P, Nabeta P et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of Xpert® MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


