Abstract
This cohort study assessed drug susceptibility testing (DST) patterns and associated treatment outcomes from Transnistria, Moldova, from 2009 to 2012. Of 1089 newly registered tuberculosis (TB) patients with available DST results, 556 (51%) had some form of drug resistance, while 369 (34%) had multidrug-resistant TB (MDR-TB). There were four cases of extensively drug-resistant TB. MDR-TB patients had poor treatment success (45%); human immunodeficiency virus positivity and a history of incarceration were associated with an unfavourable treatment outcome. This first study from Trans-nistria shows a high level of drug-resistant TB, which constitutes a major public health problem requiring urgent attention.
Keywords: operational research, SORT IT, incarceration, migration
Moldova (including the territory of Transnistria) has the third highest rate of multidrug-resistant tuberculosis (MDR-TB, defined as resistance to at least isoniazid and rifampicin) in the world. In 2012, an estimated 24% of new TB and 62% of retreatment TB cases were MDR-TB.1
In 2009, universal drug susceptibility testing (DST) was introduced for all newly diagnosed TB patients to allow tailoring of treatment to individual resistance profiles. Treatment outcomes for TB may be influenced not only by DST patterns, but also by comorbidities such as HIV/AIDS (human immunodeficiency virus/acquired immune-deficiency syndrome), alcohol use and incarceration. Knowledge of DST patterns in various contexts is essential to guide the tailoring of standardised and individualised TB drug regimens. A better understanding of DST profiles is also vital for the rational procurement of second- and third-line anti-tuberculosis drugs; however, no information has been published in this regard. Furthermore, knowledge on factors associated with unfavourable treatment outcomes can help target interventions. In the light of the above, the 2013 National TB Programme Review published by the World Health Organization (WHO) called for operational research on treatment outcomes among drug-susceptible and drug-resistant TB.2
We thus conducted a 4-year audit (2009–2012) of the pattern of primary drug resistance, including drug-susceptible and drug-resistant TB (mono- and polyresistant, MDR-TB and extensively drug-resistant [XDR-TB]) and treatment outcomes in relation to resistance. For MDR-TB, we determined specific factors associated with unfavourable outcomes for the 2009–2011 cohort for whom treatment outcomes were available.
METHODS
Study design
This was a retrospective cohort study using routine countrywide programme data.
Study sites and population
This descriptive cohort study used data collected by the National TB Control Programme (NTP). Transnistria has eight districts, each with one TB diagnostic and treatment centre, called a TB dispensary, all of which were included in the study. The study population included all TB patients registered between 2009 and 2012 with positive culture and DST. As the duration of treatment for MDR-TB is ⩾24 months, we included the 2009–2011 cohort for whom outcomes were available at the time of writing.
Diagnosis and DST
All TB dispensaries provide sputum smear microscopy. All presumptive TB patients (formerly called ‘TB suspects’) undergo smear tests and TB culture. Specimens for culture and DST were collected and transported to the Transnistria TB Reference Laboratory (TTRL). This is done using a dedicated vehicle once every 7–14 days and on a pre-determined schedule: the 14 days applies to remote dispensaries. Patients are requested to arrive at the dispensary and provide fresh sputum samples to be taken on the day scheduled for transporting specimens. Conventional culture (Löwenstein-Jensen), BACTEC™ (BD, Sparks, MD, USA) liquid culture and molecular diagnostics (polymerase chain reaction test) are available. Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) was introduced in three dispensaries with high TB caseloads in August 2012. All positive cultures are subjected to DST.
Regular supervision and external quality assessment are provided through the WHO supranational reference laboratory network. Treatment for drug-susceptible and -resistant TB is individualised and follows Moldova's national guidelines.3
Data and analysis
All information related to study objectives was sourced from a dedicated electronic database (SIME-TB) and validated against individual patient cards. TB patients are registered with a unique registration number—these data were transferred to the SIME-TB electronic database. Treatment outcomes were standardised in accordance with WHO guidelines,4 and stratified into favourable (cured and treatment completed) and unfavourable outcomes (died, loss to follow-up, failure and unknown). For assessing factors associated with unfavourable outcomes in MDR-TB, we used odds ratios (ORs) and adjusted ORs. Adjusted ORs were obtained using multivariate logistic regression and all P values were based on the Walds' test. Variables included in the multivariate model were socio-demographic variables (age, sex, occupation, residence, educational level, travel to a neighbouring country in the last 3 months), clinical characteristics (HIV status, presence of diabetes mellitus [DM]) and history of incarceration and alcohol use. A 95% confidence interval (CI) was set with a 5% error.
Ethics approval
Ethics approval was obtained from the Ethics Advisory Committee of the International Union Against Tuberculosis and Lung Disease, Paris, France. There is no local ethics committee in Transnistria, but the study received formal approval of the NTP.
RESULTS
DST patterns among new TB patients
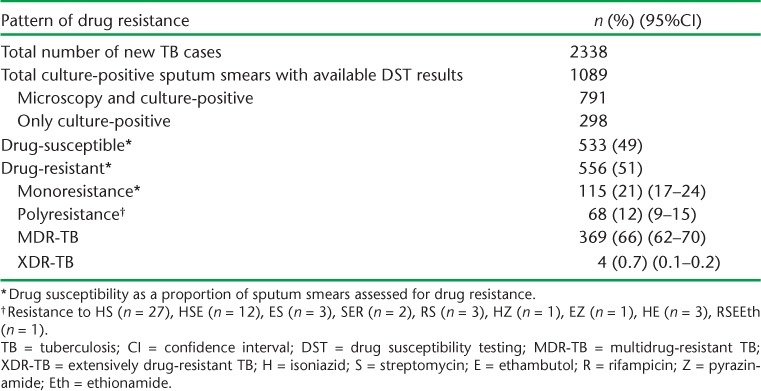
Of the 2338 new TB patients registered during the study period, 1089 (46%) underwent DST following culture and were thus assessed for drug resistance. Table 1 shows the DST patterns. A total of 51% (556) of all new cases had drug resistance, of whom 369 (34%) were MDR-TB. There were four registered cases of XDR-TB.
TABLE 1.
Pattern of primary drug resistance among newly registered TB patients, Transnistria, 2009–2012
Treatment outcomes in relation to drug resistance
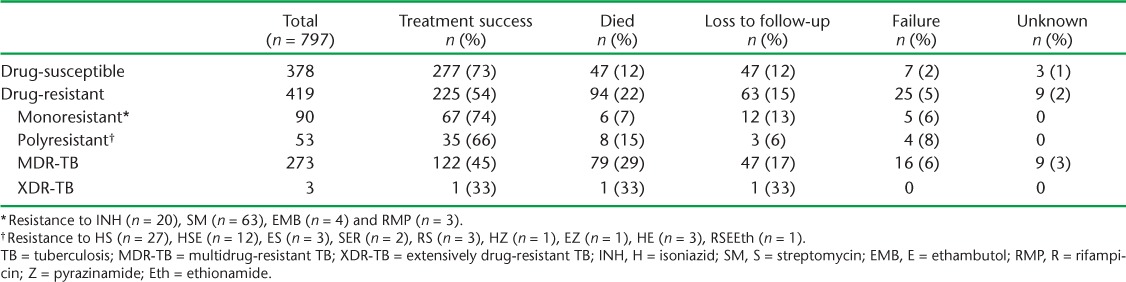
Table 2 shows treatment outcomes by resistance type among 797 patients for whom treatment was complete and outcomes were available. Of 378 patients with drug-susceptible TB, 277 (73%) had achieved treatment success (below the WHO target of 85%). The main problems were attrition (12% deaths and 12% lost to follow-up). Of 273 patients treated for MDR-TB, 122 (45%) had successful treatment outcomes.
TABLE 2.
Treatment outcomes in relation to drug resistance patterns, Transnistria, 2009–2012
Factors associated with unfavourable treatment outcomes in MDR-TB
Among 273 individuals started on MDR-TB treatment, HIV status (OR 6.7, 95%CI 2.7–16.5) and history of incarceration (OR 2.5, 95%CI 1.4–4.47) were significantly associated with an unfavourable outcome, while age, sex, occupation, residence, educational level, migration for a period of 3 months during the previous year, presence of a household TB contact, DM and alcohol use were not (data not shown). Of the 273 patients treated for MDR-TB, 39 (16%) were HIV-positive.
DISCUSSION
This is the first study of its kind from Transnistria showing that one in three new culture-positive TB patients had MDR-TB and low treatment success. Factors associated with an unfavourable outcome included HIV positivity and a history of incarceration. Half of all new culture-positive cases had some type of drug resistance; this is of major public health concern.
A number of findings merit discussion. First, even in drug-susceptible TB, one in four patients either died or were lost to follow-up. Economic migration to neighbouring high HIV and MDR-TB prevalence countries such as Ukraine, Moldova and Russia is common, and may be contributing to drug resistance and the spread of HIV. There has as yet been no national study on HIV prevalence among TB patients in Transnistria, and we also do not know the extent to which migration might have influenced treatment adherence. These aspects merit further research.
Second, although treatment success for MDR-TB was low, at 45%, and this is known to be a global problem,5,6 intermittent shortages of and ruptures in MDR-TB drug supplies occurred during the study period and may have aggravated this problem.2 Between 2009 and 2011, only 30% of MDR-TB patients received drugs through The Global Fund to Fight AIDS, Tuberculosis and Malaria (Geneva, Switzerland) — although this has now reached about 50% of the current need. This operational gap needs to be covered by introducing an effective mechanism for procurement and sustainable supply of good quality drugs for all patients.
Third, HIV positivity and incarceration were significant risk factors for unfavourable outcomes. HIV is known to be associated with higher mortality, while individuals with a history of incarceration may have social backgrounds that negatively influence treatment adherence, such as poor socio-economic status.7,8 These groups merit specific attention.
In conclusion, this first study from Transnistria shows a high level of drug-resistant TB; this constitutes a major public health problem requiring urgent attention.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by WHO-TDR, the WHO Regional Office for Europe (Copenhagen, Denmark), the Operational Research Unit (LUXOR), Brussels Operational Center, Médecins Sans Frontières (MSF Luxembourg), the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union; Paris, France), The Union South-East Asia Regional Office, New Delhi, India. The programme was funded by the United States Agency for International Development (Washington DC, USA) through a grant managed by WHO-TDR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
The authors alone are responsible for the content of this paper which may not necessarily represent the policies, decisions or views of the WHO. In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution Intergovernmental organisation license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. www.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf Accessed August 2014. [Google Scholar]
- 2.World Health Organization. Review of the National Tuberculosis Programme in the Republic of Moldova, 4–15 February 2013. Geneva, Switzerland: WHO; 2013. http://www.euro.who.int/en/health-topics/communicable-diseases/tuberculosis/publications/2013/review-of-the-national-tuberculosis-programme-in-the-republic-of-moldova Accessed August 2014. [Google Scholar]
- 3.Ministry of Health. National tuberculosis management guidelines 2012. Chisnau, Moldova: Ministry of Health; 2012. [Google Scholar]
- 4.World Health Organization. Definitions and reporting framework for tuberculosis: 2013 revision. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.2. http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf Accessed August 2014. [Google Scholar]
- 5.World Health Organization. Resolution EUR/RC61/R7. Roadmap to prevent and combat drug-resistant tuberculosis: the consolidated action plan to prevent and combat multidrug- and extensively drug-resistant tuberculosis in the WHO European Region, 2011–2015. Copenhagen, Denmark: WHO; 2011. [Google Scholar]
- 6.Ahuja S D, Ashkin D, Avendano M et al. Multidrug-resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLOS MED. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi N R, Nunn P, Dheda K et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 8.Jakubowiak W M, Bogorodskaya E M, Borisov S E, Danilova I D, Kourbatova E V. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11:46–53. [PubMed] [Google Scholar]


