Abstract
Setting: Latvia, an Eastern European country with a high burden of tuberculosis (TB).
Objective: To describe treatment outcomes among new drug-susceptible TB patients and assess the association of treatment outcomes with selected social determinants and risk factors.
Design: A retrospective cohort study of patients aged ⩾15 years registered during 2006–2010, with a review of records in the National Tuberculosis Registry.
Results: Of 2476 patients, 1704 (69%) were male; the median age was 42 years. About two thirds of patients were unemployed or retired, 7% were human immunodeficiency virus (HIV) positive and 35% had a history of alcohol use. Treatment success was achieved in 2167 (88%) patients. Older age, unemployment, HIV infection and alcohol use were found to be independently associated with unsuccessful treatment (death, loss to follow-up, failure, transfer out and other). For many variables, including HIV infection, diabetes mellitus and tobacco use, it was not possible to distinguish between ‘not recorded’ and ‘not present’ in the registry.
Conclusion: The treatment success rate among new drug-susceptible TB patients exceeded the 85% global target for TB control. Additional attention and support is required for most vulnerable patients, such as those who are unemployed or retired, HIV infected and alcohol users. The National TB Registry should be revised to improve definitions and staff should be trained for proper data collection and recording.
Keywords: SORT IT, risk factors, social determinants, unsuccessful treatment
Latvia is one of the world's 27 countries with the highest burden of multidrug-resistant tuberculosis (MDR-TB),1 and one of the 18 high priority countries for TB control in the World Health Organization (WHO) European Region.2 Latvia has the third highest estimated TB incidence rate among European Union countries.3 The TB notification rate in the country increased dramatically after the collapse of the Soviet Union in the early 1990s, reaching its highest level of 74 new cases per 100 000 population in 1998. After that it progressively decreased until 2011, when it reached 37/100 000. In 2012, the incidence rate increased to 43, probably due to the global economic crisis that also hit Latvia, increasing unemployment and worsening the socio-economic status of large parts of the population.4 The gross domestic product per capita dropped from US$14 858 in 2008 to US$10 723 in 2010,5 and the government had to reform all state-financed institutions, including health institutions, to increase efficiency and reduce public spending; access to medical care thus fell for some population groups.6
The Centre for Disease Prevention and Control (CDC) of the Ministry of Health is responsible for the National TB Registry, where data for each patient diagnosed have been entered since 2000. These data include information on several social and medical conditions, such as anti-tuberculosis drug resistance, human immunodeficiency virus (HIV) status, diabetes mellitus (DM), tobacco smoking, and alcohol and drug use. Several studies have been published using National TB Registry data.7–12 The 2012 report of the joint WHO-European Centre for Disease Prevention and Control mission also provides some information on the prevalence of risk factors among TB patients.13 However, there is limited published information on the association of anti-tuberculosis treatment outcomes with social determinants and risk factors in Latvia. Such information could guide the Ministry of Health in establishing collaboration between TB services and other medical and social services (e.g., drug users, DM patients, etc.) which may contribute to better treatment results. This is especially important, given that Latvia has a considerable number of people with a history of incarceration, among whom the incidence of TB is 17 times higher than in the general population.13
The present article aims to describe anti-tuberculosis treatment outcomes among new drug-susceptible TB patients and to assess the association of treatment outcomes with selected social determinants and risk factors.
METHODS
Study design
This was a retrospective cohort study.
Setting
Latvia is a country of the Baltic region with 2 million inhabitants, 650 000 of whom live in the capital city of Riga (2012). It is one of the former Soviet Union countries that joined the European Union in 2004.
According to the National Plan for Tuberculosis Control 2013–2015,14 TB services are delivered by the Centre of TB and Lung Diseases of the Riga East Clinical University Hospital (Riga, Latvia), six pulmonary wards in regional hospitals and 23 out-patient pulmonary clinics. Diagnostic and treatment services are free of charge and financed by the state. Patients are usually hospitalised for TB diagnosis and treatment, and remain in hospital until sputum smear conversion or longer if clinically indicated. Directly observed treatment (DOT) is provided by family doctors or specialists in pulmonary medicine during the ambulatory phase. Patients are treated with a standardised 6-month daily regimen consisting of four drugs (isoniazid [INH], rifampicin [RMP], pyrazinamide and ethambutol) during the intensive phase, followed by INH and RMP in the continuation phase. All patients undergo drug susceptibility testing; treatment is adjusted according to the results. All TB patients are offered HIV testing; those found to be HIV-infected are offered cotrimoxazole preventive therapy (CPT) and antiretroviral therapy (ART). According to the WHO 2013 global tuberculosis report,1 about 85% of TB patients knew their HIV status, 14% of whom were HIV-positive. Of the HIV-positive TB patients, 57% were placed on ART.
The CDC is responsible for the National TB Registry, which follows case definitions and treatment outcome categories as recommended by the WHO.15,16
Study population and study period
The study was conducted between June 2013 and January 2014, and included all new drug-susceptible TB patients aged ⩾15 years registered for treatment during 2006–2010. We could not include data from recent (post-2010) patient cohorts, due to the partial restructuring of the TB database during the period, with the changes in definitions making it inappropriate for combining with 2006–2010 data.
Data variables and data source
Data variables related to the study objectives were extracted from the National TB Registry. These included age, sex, residence, employment status, year of TB registration, type of TB, treatment outcome, HIV infection, DM status, smoking tobacco, use of alcohol and drugs, and history of incarceration.
Data analysis
The data were exported into EpiData, version 2.2.2.182 (EpiData Association, Odense, Denmark) for analysis. The treatment outcome categories ‘cured’ and ‘treatment completed’ were combined as ‘successful outcome’, while ‘died’, ‘lost to follow-up’, ‘failure’, ‘transferred out’ and ‘other’ were grouped under ‘unsuccessful outcome’ for the purpose of analysis and were examined for association with the other variables recorded. Relative risks (RRs) with 95% confidence intervals (CIs) were calculated as measures of the association. The level of significance was set at 5%. Variables found to be statistically significant during bivariate analysis were included in a multivariate model (log-binomial regression); adjusted RRs were calculated to assess the independent effects of each variable. STATA version 12.1 (Stata Corp, College Station, TX, USA) was used for multivariate analysis.
Ethics
Ethics approval was received from the Committee of Ethics of Riga Stradins University, Riga, Latvia, and from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
The sociodemographic and clinical profiles of the study population are shown in Table 1. Of the 2476 patients studied, 1704 (69%) were male; the median age was 42 years (interquartile range [IQR] 32–53); the majority of the patients (67%) were aged between 25 and 54 years. They were almost equally distributed between urban and rural areas. More than two thirds of the patients were unemployed or retired. For many of the patient characteristics, such as history of incarceration, HIV infection, DM status, tobacco smoking, and use of alcohol and drugs, it was not possible to distinguish between ‘not recorded’ and ‘not present’. Nevertheless, every tenth patient had a history of incarceration, and a small proportion (3%) were in prison at the time of diagnosis.
TABLE 1.
Sociodemographic and clinical characteristics of new drug-susceptible TB patients aged ⩾15 years registered for treatment, Latvia, 2006–2010

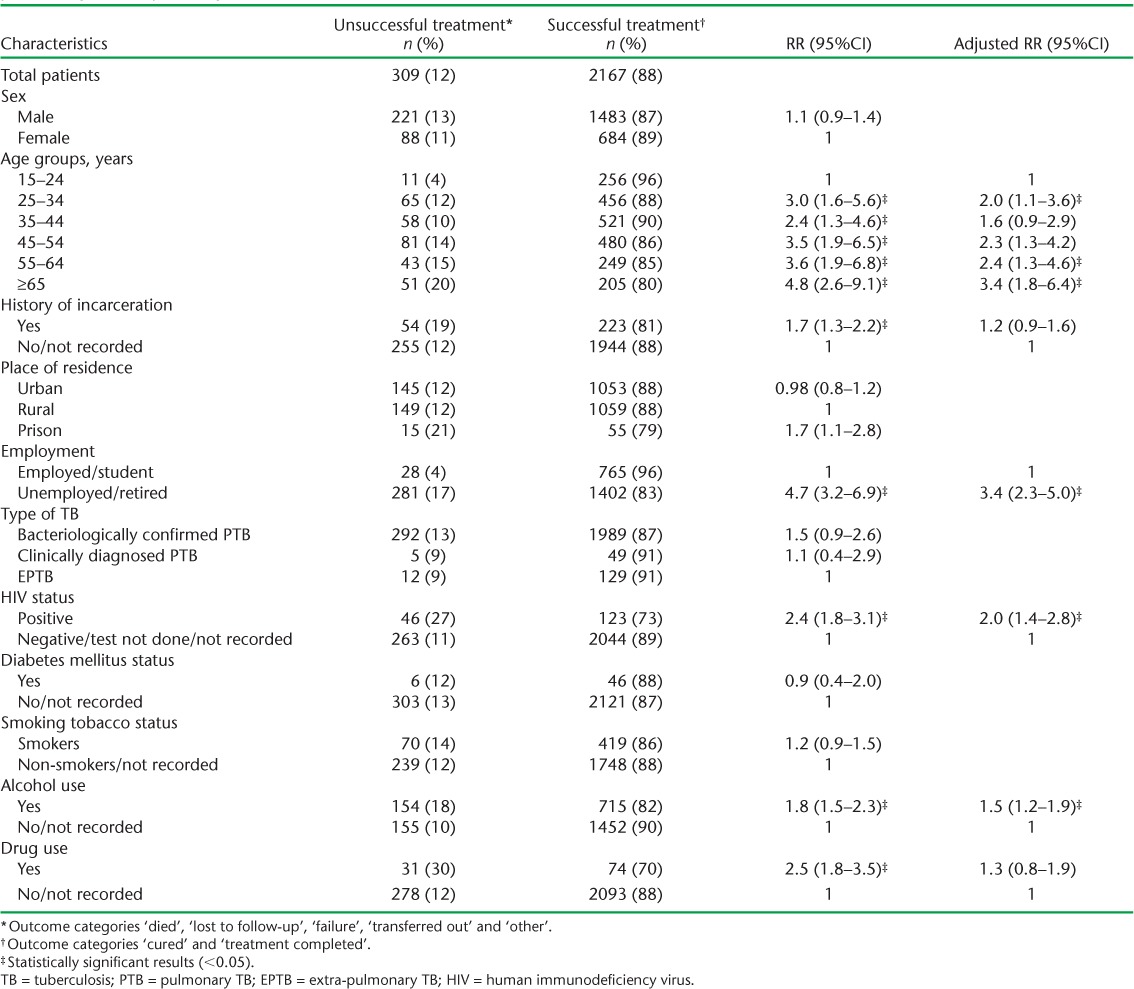
The final treatment outcomes are shown in the Figure: ‘successful treatment’ was achieved in 2167 (88%) patients. The associations of sociodemographic and clinical characteristics with ‘unsuccessful treatment’ are shown in Table 2. Older age, unemployment, HIV infection and alcohol use were found to be independently associated with unsuccessful treatment.
FIGURE.
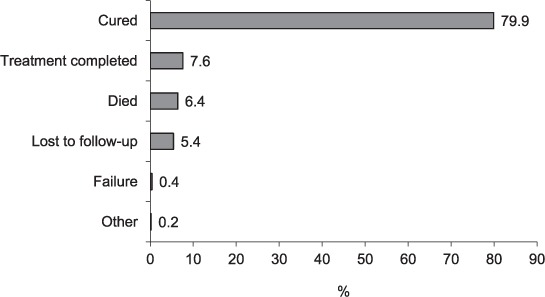
Treatment outcomes among new drug-susceptible tuberculosis patients aged ⩾15 years registered for treatment, Latvia, 2006–2010 (n = 2476).
TABLE 2.
Associations between socio-demographic and clinical characteristics with unsuccessful treatment among new drug-susceptible TB patients aged ⩾15 years registered for treatment, Latvia, 2006–2010
DISCUSSION
Overall, we found a high treatment success rate (88%) among new drug-susceptible TB patients, which is higher than the WHO target of 85%. However, taking into account the long-term goal to control and ultimately eliminate TB in the European region, additional efforts are needed to improve treatment results and reduce the transmission of Mycobacterium tuberculosis.17 We identified several factors associated with unsuccessful treatment outcomes that merit detailed discussion.
First, we found that age was independently associated with unsuccessful treatment outcome; this is similar to reports from other parts of the globe.18 The highest risk, among those aged ⩾55 years, may be related to the presence of DM, the prevalence of which was twice as high (~4%) as in the other age groups. This may be an underestimate of the true picture due to suboptimal documentation, given the national policy of routine screening of TB patients for DM in Latvia. According to the 2013 estimates of the International Diabetes Federation (Brussels, Belgium),19 about 6% of the adult population in Latvia had DM. Given the well-known link between DM and TB,20 we would expect a higher prevalence of DM among TB patients; we therefore believe the 2% DM prevalence among TB patients to be an underestimation, and that documentation needs to be strengthened.
Second, patients with a history of alcohol use had a 50% higher risk of an unsuccessful outcome. Previous studies have shown that alcohol is likely to impair the immune response against M. tuberculosis and delay treatment response.21–23 Alcohol use is also likely to predispose patients to adverse drug effects, which may lead to treatment interruption and loss to follow-up.24,25 Despite the well-recognised implications of alcohol use, few programmes have tried to address TB and alcoholism in an integrated manner. To our knowledge, there have been only two such examples from Russia26 and Estonia.27 The project in Russia led to an increase in the proportion of successful anti-tuberculosis treatment outcomes and alcohol abstinence by 18%. TB treatment outcome was also improved in Estonia by integrating TB, psychiatric and social services. This model could be tried in Latvia, given the similarity in the settings.
Third, as shown in numerous previous studies, treatment outcomes were worse among HIV-infected TB patients than in HIV-negative patients or those with unknown HIV status.28,29 While we did not have any information from the database on links between HIV-infected TB patients and ART, we know from the WHO global tuberculosis report that only 57% of TB-HIV patients received ART in 2012.1 Access to ART needs to be improved urgently.
Fourth, we found a higher risk of unsuccessful treatment outcomes in unemployed or retired TB patients than in the employed. About 68% of all TB cases were detected among unemployed or retired persons, which was higher than that in the general population.30 This result provides further evidence indicating that TB is a disease of the poor and needs special patient support measures.
The strengths of the study are related to the large sample size and national representativeness of the data. It is the first study among drug-susceptible TB patients using countrywide data. The study adhered to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.31
Given its retrospective nature and reliance on routine data collection, the study had some limitations. A major limitation was that it was not possible to distinguish between ‘not present’ and ‘not recorded’ for many variables. Because of this limitation, we may have missed associations between other variables with unsuccessful treatment outcomes. This is a result of the flawed design of the National TB Registry, which records a characteristic only when present. Another limitation was the failure to adopt standardised definitions for some of the variables collected (such as tobacco smoking, alcohol and drug use), which leads to subjective interpretations by health staff recording patient history. This problem urgently needs to be addressed. The electronic database of the National TB Registry was partly revised in 2013, but its structure needs further review; standardised definitions should be adopted for all variables, written guidelines developed and training provided to all staff working with patient records.
In conclusion, we found a treatment success rate among new drug-susceptible TB patients of 88%, exceeding the WHO 85% global target set for TB control. However, more efforts should be made to support more vulnerable TB patients —especially unemployed or retired people, those with HIV infection and alcohol/drug users—to prevent unsuccessful treatment outcomes. To generate accurate information for policy makers, the National TB Registry should be revised and staff should be trained in proper data collection and recording.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by WHO-TDR, the WHO Regional Office for Europe (Copenhagen, Denmark), the Operational Research Unit (LUXOR), Brussels Operational Center, Médecins Sans Frontières (MSF Luxembourg), the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union; Paris, France), and The Union South-East Asia Regional Office, New Delhi, India. We are grateful for the support of the WHO Country Office in Talinn, Estonia, and the Estonia National Institute for Health and Development (Talinn, Estonia) in hosting the training workshops. We also appreciate the active involvement of the WHO Country Office and the Ministry of Health (Talinn, Estonia) in the selection of candidates for training in operational research and identification of research projects.
The SORT IT programme was funded by the United States Agency for International Development (Washington DC, USA) through a grant managed by WHO-TDR. Additional support was provided by the WHO Regional Office for Europe, the Department for International Development (London, UK), and the MSF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
The authors alone are responsible for the content of this paper which may not necessarily represent the policies, decisions or views of the WHO.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution intergovernmental organisation licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.World Health Organization, Regional Office for Europe. Plan to Stop TB in 18 high-priority countries in the WHO European Region, 2007–2015. Copenhagen, Denmark: WHO; 2007. [Google Scholar]
- 3.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2013. Stockholm, Sweden: ECDC; 2013. [Google Scholar]
- 4.Suhrcke M, Stuckler D, Suk J E et al. The impact of economic crises on communicable disease transmission and control: a systematic review of the evidence. PLOS ONE. 2011;6(6):e20724. doi: 10.1371/journal.pone.0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Bank. The World Bank data. GDP per capita (current US$) in Latvia, 2004–2012. Washington DC, USA: World Bank; 2014. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD/countries/LV--XR?display=graph Accessed August 2014. [Google Scholar]
- 6.Eurofund. Impact of the crisis on access to healthcare services in the EU. Dublin, Ireland: Eurofund; 2013. http://www.eurofound.europa.eu/publications/htmlfiles/ef1385.htm Accessed August 2014. [Google Scholar]
- 7.Dalton T, Cegielski P, Akksilp P et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug- resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380:1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurbatova E V, Taylor A, Gammino V M et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis. 2012;92:397–403. doi: 10.1016/j.tube.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leimane V, Dravniece G, Riekstina V et al. Treatment outcome of multidrug/extensively drug-resistant tuberculosis in Latvia, 2000–2004. Eur Respir J. 2010;11:584–593. doi: 10.1183/09031936.00003710. [DOI] [PubMed] [Google Scholar]
- 10.Riekstina V, Leimane V, Holtz T H et al. Treatment outcome cohort analysis in an integrated DOTS and DOTS-Plus TB program in Latvia. Int J Tuberc Lung Dis. 2007;11:585–587. [PubMed] [Google Scholar]
- 11.Podewils L J, Holtz T, Riekstina V et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect. 2011;139:113–120. doi: 10.1017/S0950268810000907. [DOI] [PubMed] [Google Scholar]
- 12.Arinaminpathy N, Dye C. Health in financial crises: economic recession and tuberculosis in Central and Eastern Europe. J R Soc Interface. 2010;7:1559–1569. doi: 10.1098/rsif.2010.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control. Tuberculosis in Latvia. Stockholm, Sweden: ECDC; 2013. [Google Scholar]
- 14.National plan for control of the spread of tuberculosis in 2013–2015. Riga, Latvia: Latvian Ministry of Health; 2013. http://www.vm.gov.lv/images/userfiles/vmrikp_050313_tbpl.pdf. Accessed August 2014. [Latvian] [Google Scholar]
- 15.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneva, Switzerland: WHO; 2009. WHO/HTM/TB/2009.420. [Google Scholar]
- 16.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. Geneva, Switzerland: WHO; 2008. WHO/HTM/TB/2008.402. [Google Scholar]
- 17.European Centre for Disease Prevention and Control. Progressing towards TB elimination. Stockholm, Sweden: ECDC; 2010. [Google Scholar]
- 18.Ananthakrishnan R, Kumar K, Ganesh M et al. The profile and treatment outcomes of the older (aged 60 years and above) tuberculosis patients in Tamilnadu, South India. PLoS ONE. 2013;8:e67288. doi: 10.1371/journal.pone.0067288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. Diabetes atlas. 6th ed. Brussels, Belgium: IDF; 2013. http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf Accessed August 2014. [Google Scholar]
- 20.Harries A D, Satyanarayana S, Kumar A M V et al. Epidemiology and interaction of diabetes mellitus and tuberculosis and challenges for care: a review. Public Health Action. 2013;3(Suppl 1):S3–S9. doi: 10.5588/pha.13.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermudez L E, Wu M, Martinelli J, Young L S. Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res. 1991;10:413–419. [PubMed] [Google Scholar]
- 22.Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T-cell proliferation and monocyte IL-1β TNF and IL-6 production by acute ethanol. J Leukoc Biol. 1995;58:342–350. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- 23.Crews F T, Bechara R, Brown L A et al. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekaran V, Gopi P G, Subramani R, Thomas A, Jaggarajamma K, Narayanan P R. Default during the intensive phase of treatment under DOTS programme. Indian J Tuberc. 2005;52:197–202. [Google Scholar]
- 25.Vijay S, Kumar P, Chauhan L S, Vollepore B H, Kizhakkethil U P, Rao S G. Risk factors associated with default among new smear positive TB patients treated under DOTS in India. PLOS ONE. 2010;5:e10043. doi: 10.1371/journal.pone.0010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller A C, Gelmanova I Y, Keshavjee S. Alcohol use and the management of multidrug-resistant tuberculosis in Tomsk, Russian Federation. Int J Tuberc Lung Dis. 2012;16:891–896. doi: 10.5588/ijtld.11.0795. et.al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Regional Office for Europe. Collaborative action on tuberculosis and alcohol abuse in Estonia First report of a demonstration project. Copenhagen, Denmmark: WHO; 2013. http://www.euro.who.int/__data/assets/pdf_file/0006/237516/WHO-AUD-TB-project-report_10-final-edited-with-PCO_5Dec-2013_NS_kujundatud_koos_TjaK_2.pdf Accessed August 2014. [Google Scholar]
- 28.Nahid P, Jarlsberg L G, Rudoy I et al. Factors associated with mortality in patients with drug-susceptible pulmonary tuberculosis. BMC Infect Dis. 2011;11:1. doi: 10.1186/1471-2334-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van't Hoog A H, Williamson J, Sewe M. Risk factors for excess mortality and death in adults with tuberculosis in western Kenya. Int J Tuberc Lung Dis. 2012;16:1649–1656. doi: 10.5588/ijtld.12.0135. et.al. [DOI] [PubMed] [Google Scholar]
- 30.Central Statistical Bureau of Latvia. Table NBG04: activity rate, employment rate and unemployment rate by statistical region (%) Riga, Latvia: Central Statistical Bureau; 2014. [Google Scholar]
- 31.von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]


