Abstract
Setting: Estonia has a high proportion of multidrug-resistant tuberculosis (MDR-TB). It is important to link molecular and epidemiological data to understand TB transmission patterns.
Objective: To use 24-locus variable numbers of tandem repeat (VNTR) typing and national TB registry data in Estonia from 2009 to 2012 to identify the distribution of drug resistance patterns, Mycobacterium tuberculosis isolate clustering as an index for recent transmission, socio-demographic and clinical characteristics associated with recent transmission, and the distribution of transmission between index and secondary cases.
Design: A retrospective nationwide cross-sectional study.
Results: Of 912 cases with isolate and patient information, 39.1% of isolates were from the Beijing lineage. Cluster analysis identified 87 clusters encompassing 69.1% of isolates. The largest cluster comprised 178 isolates from the Beijing lineage, of which 92.1% were MDR- or extensively drug-resistant TB (XDR-TB). Factors associated with recent transmission were polyresistant TB, MDR- and XDR-TB, human immunodeficiency virus positivity, Russian ethnicity, non-permanent living situation, alcohol abuse and detention. XDR-TB cases had the highest risk of recent transmission. The majority of transmission cases involved individuals aged 30–39 years.
Conclusion: Recent TB transmission in Estonia is high and is particularly associated with MDR- and XDR-TB and the Beijing lineage.
Keywords: Beijing lineage, clustering, MDR-TB, SORT IT, operational research
The smallest Baltic country, Estonia (population 1.3 million) suffers from a relatively high rate of tuberculosis (TB) in the European context, and is among the world's 27 high multidrug-resistant TB (MDR-TB) burden countries.1 The TB incidence rate declined from 36.6 per 100 000 population in 2007 to 21.6/100 000 in 2012. However, a quarter (25.5%) of the 234 new cases reported in 2012 were MDR-TB (defined as resistance to at least isoniazid and rifam-picin [RMP]), of which 6.6% were extensively drug-resistant TB (XDR-TB, defined as MDR-TB plus resistance to a fluoroquinolone and one of the three injectables).2 Recent studies in Estonia showed that human immunodeficiency virus (HIV) infection, homelessness and alcohol abuse significantly increase the risk for XDR-TB.3 However, the extent to which these determinants contribute to TB transmission is unknown.
Molecular epidemiology has contributed significantly to our understanding of TB transmission. Studies using this approach have helped to determine what proportion of active TB is due to reinfection or reactivation and to identify risk factors for TB transmission within communities.4 For such studies, the most commonly used methods for Mycobacterium tuberculosis DNA fingerprinting are insertion sequence (IS) 6110 restriction fragment length polymorphism (RFLP)5 and 24-locus variable numbers of tandem repeat (VNTR) typing,6 which were internationally standardised in 1993 and 2006, respectively. VNTR typing has various advantages over RFLP typing, including its ease of use, short turnaround time and digital output format. In addition, several population-based studies have shown that the predictive value of VNTR typing to study TB transmission is similar to that of RFLP typing in different Western European settings.7,8 The current gold standard in molecular epidemiology of TB is therefore VNTR typing, which produces a numeric pattern enabling the identification of M. tuberculosis strains.9 It is assumed that patients infected with M. tuberculosis isolates with identical DNA fingerprints are 1) infected with the same M. tuberculosis strain and 2) have infected each other, have a common source of infection or were independently infected by a highly prevalent strain circulating in the community. Conversely, it is assumed that patients who are infected with a strain with a unique DNA fingerprint in a population are either suffering from a reactivation of a previous infection or from infection with a strain that was newly introduced into the population by the patient. Investigators have thus used molecular clustering, defined as matching of identical DNA finger-prints, as an index of TB transmission.
The first study to use RFLP DNA fingerprinting results conducted in Estonia showed high clustering rates among MDR-TB isolates, and suggested an association between the Beijing genotype of M. tuberculosis and drug resistance.10 A later study that compared DNA fingerprints from MDR-TB cases reported in Europe during the years 2003–2007 showed that these Beijing strains persisted in Estonia and were part of a large cluster of MDR-TB strains (designated as cluster E0051) detected in 12 different countries.11 In 2009, Estonia started using VNTR typing within the framework of the TB PAN-NET project;12 one of the project's aims was to study TB transmission in European countries.
The current study used VNTR patterns generated within the framework of the TB PAN-NET project to construct a database that was linked to the Estonian National TB Registry (NTR) to assist TB control activities. In particular, we performed cluster analysis of the DNA fingerprints with the aim of identifying risk groups that can be targeted by the National TB Programme (NTP) to improve prevention of TB transmission.
METHODS
Study design
This was a retrospective nationwide cross-sectional study.
Setting
Estonia is a small European country neighbouring Latvia, Finland and the Russian Federation, with a population of approximately 1.3 million. About 30% of the population lives in the capital, Tallinn.13 About 70% of the population are of Estonian ethnicity, 25% are Russians and the remaining 5% include various ethnic groups from the former Soviet Union and Eastern Europe.
Since 1998, the institutions managing anti-tuberculosis treatment in Estonia have followed NTP guidelines, which are in line with the World Health Organization TB guidelines.14 TB case finding is mainly passive, and diagnosis is established through sputum smear microscopy, culture and chest radiography for pulmonary TB and other investigations for extra-pulmonary TB. All TB patients are registered with a unique registration number at the NTR, given standardised, mainly hospital-based, treatment and monitored for treatment outcomes according to national and international recommendations.15 All TB services, anti-tuberculosis treatment, and, if necessary, opioid substitute therapy (OST), are free in the country.
Patient sample
All patients with a positive culture and both DST and VNTR typing results available for that culture during the period January 2009–December 2012 were included in the study.
Data sources and collection
There are two TB culture laboratories in Estonia: one in Tallinn and the TB reference laboratory in Tartu. All specimens for TB diagnosis in the country are sent to one of these two laboratories. All positive cultures are subjected to first-line drug susceptibility testing (DST)16 and, in case of resistance to first-line drugs, DST for second-line drugs is performed.17 The TB reference laboratory in Tartu archives all TB isolates that have DST results available and is responsible for DNA genotyping. Within the framework of the TB PAN-NET project, genotyping of the isolate DNA was performed by the Institute Pasteur de Lille (Lille, France) using the standard VNTR typing method6 and mycobacterial interspersed repetitive units (MIRU) VNTR typing kits from Genoscreen (Lille, France). The typing was rigorously quality controlled by the inclusion of positive (M. bovis bacille Calmette-Guérin DNA) and negative controls and internal procedures. When evaluated in independent proficiency studies of VNTR typing, this laboratory systematically obtained 100% intra- and inter-laboratory reproducibility scores.9,18 A database with VNTR patterns of all culture-positive cases is kept at the Tartu Reference Laboratory.
All patient characteristics and demographic data were collected from the Estonian NTR. VNTR patterns were collected from the TB reference laboratory and DST results were collected from the NTR. Data from different sources were matched using unique patient identifiers, names and birth dates. Data from the linked databases were collected into an EpiData database (EpiData Association, Odense, Denmark).
Data analysis and statistics
Cluster analysis of VNTR patterns and lineage assignment was performed using the MIRU-VNTRplus database.19 Cluster analysis was conducted using the unweighted pair group method with arithmetic mean (UPGMA) and the categorical coefficient.20 A cluster was defined as at least two isolates with 100% identical VNTR typing patterns. An index case was defined as chronologically the first case in a cluster. Clustering of VNTR patterns was used as a measure of TB transmission. Lineages were assigned using the similarity search provided in the MIRU-VNTRplus database using a distance maximal genotypic distance of 0.17 and tree-based identification using the neighbour-joining algorithm.21,22
The number and sizes of clusters and the genetic background of these clusters were tabulated. Associations between different patient characteristics and isolate clustering were assessed, and risk ratios (RRs) and corresponding 95% confidence intervals (CIs) were calculated. Differences at the 5% level (P < 0.05), calculated using Pearson's χ2 test, were considered statistically significant. All data were analysed using EpiData version 2.2.2.182 (EpiData Association).
Ethics approval
Ethics approval was granted both by the Research Ethics Committee of the University of Tartu, Tartu, Estonia, and by the International Union Against Tuberculosis and Lung Disease Ethics Advisory Group, Paris, France. Permission for collecting, handling and analysing patient data was obtained from the Estonian Data Protection Inspectorate and the Ministry of Social Affairs, Talinn, Estonia.
RESULTS
Of the 1371 TB cases identified in Estonia during the 4-year study period, 1286 (93.8%) had pulmonary TB, 1080 (78.8%) were new cases with no previous anti-tuberculosis treatment and 1066 (77.8% of total) were culture-positive TB cases. Of these, 917 (86.0%) had VNTR patterns available for their isolates in the National TB Reference Laboratory database. For 912 cases, including nine patients registered twice as a result of recurrent TB, a link between the patient and isolate information could be established.
Lineages could be assigned to 86.2% of the 912 isolates (cases). The distribution of lineages according to DST pattern is shown in Table 1. The largest overall proportion of isolates (n = 357, 39.2%) was from the Beijing lineage. Drug-susceptible isolates were evenly distributed across the four main lineages (Beijing, Haarlem, Latin-American-Mediterranean [LAM] and Ural). In contrast, most MDR- and XDR-TB isolates were from the Beijing lineage (94.0% and 96.3%, respectively). In addition, five of seven RMP-monoresistant isolates (71.4%) were from the LAM lineage.
TABLE 1.
Anti-tuberculosis drug resistance patterns and the distribution of different lineages of patient isolates in culture-positive TB cases, Estonia, 2009–2012
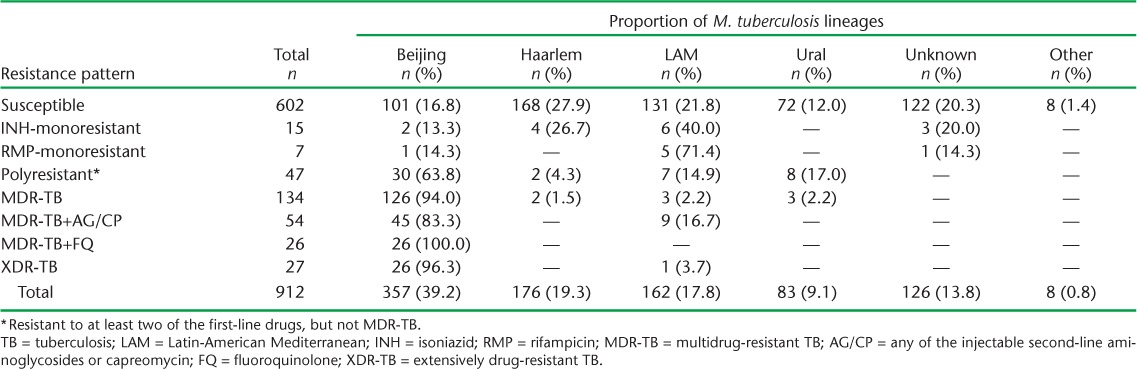
In total, there were 87 clusters comprising 630 (69.1%) isolates (Figure 1). Ten clusters had more than 10 isolates, with four clusters having over 20 isolates. The largest cluster, containing 178 isolates, consisted of the Beijing lineage strain MtbC 15-9 type 100-32, according to the standard VNTR typing nomenclature;21,23 164 (92.1%) of these were MDR- or XDR-TB. In contrast, the second largest cluster comprised 32 isolates from the LAM lineage, of which 30 (94.0%) were drug-susceptible. The third largest cluster comprised 30 isolates from another Beijing lineage strain (MtbC 15-9 type 94-32), of which 9 (30.0%) were MDR- or XDR-TB.
FIGURE 1.
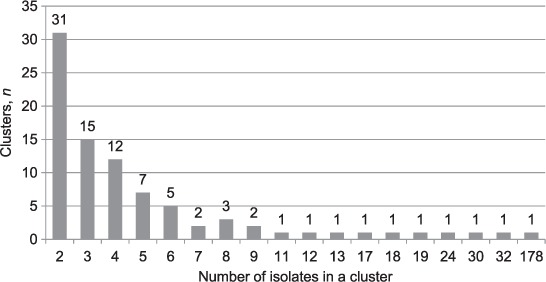
Number and sizes of clusters present in patients with culture-positive tuberculosis, Estonia, 2009–2012.
Socio-demographic factors associated with an increased risk of clustering were Russian ethnicity, non-permanent living conditions, alcohol abuse and history of incarceration, while factors associated with a reduced risk of clustering were older age, being born in Ukraine, being of Belarusian ethnicity, living in Tartu and having a lower education level (Table 2). The clinical factors associated with an increased risk of clustering were polyresistant, MDR- and XDR-TB, and being HIV-positive (Table 3). XDR-TB cases had the highest risk of clustering (RR 1.63, P < 0.001). Extra-pulmonary TB was associated with a reduced risk of clustering.
TABLE 2.
Socio-demographic characteristics of patients with culture-positive tuberculosis in relation to clusters, Estonia, 2009–2012
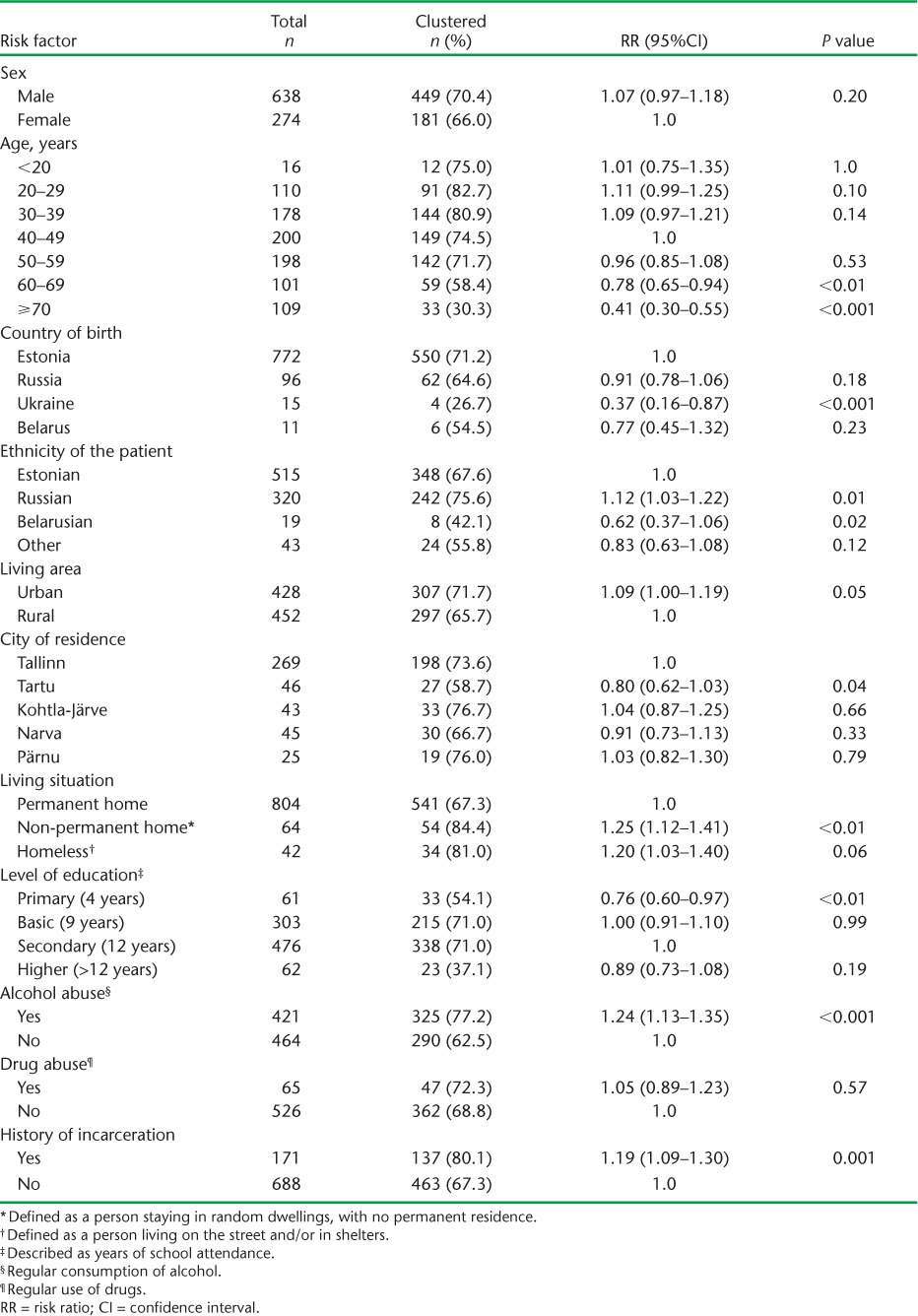
TABLE 3.
Clinical characteristics of patients with culture-positive tuberculosis in relation to clusters, Estonia, 2009–2012
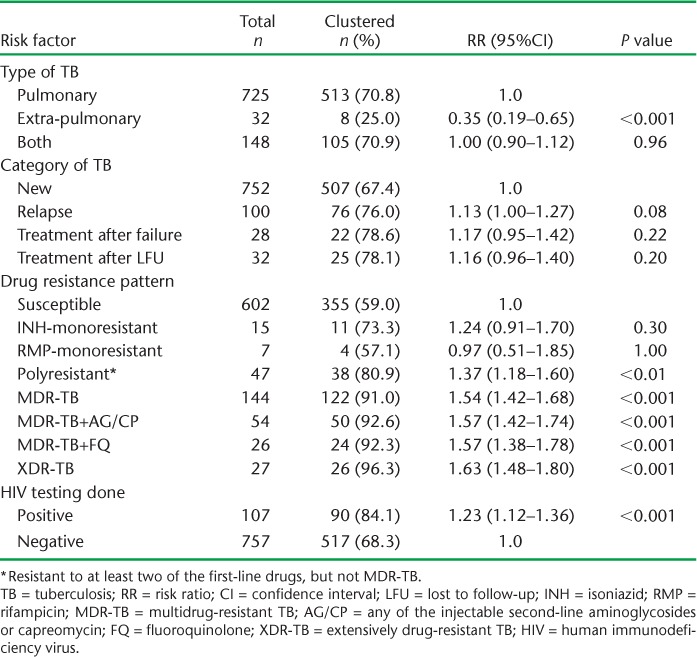
The distribution of transmission between index cases and secondary cases in relation to different age groups is shown in Figure 2. Transmission occurred most frequently from the 30–39 year age group. The majority of secondary cases were distributed among those aged 30–59 years.
FIGURE 2.
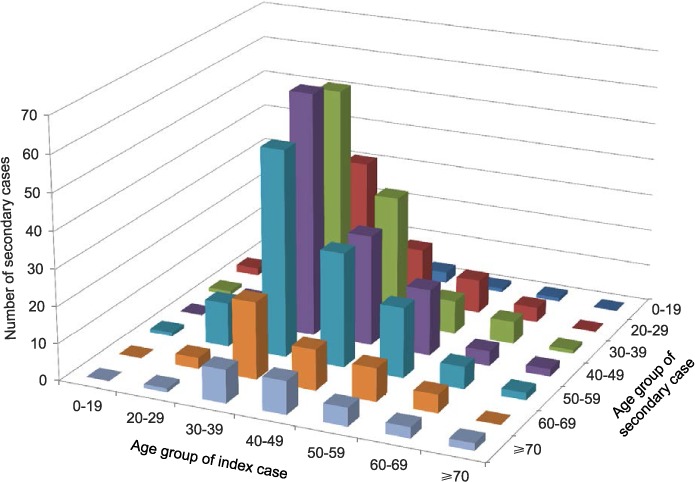
Distribution of tuberculosis transmission between age groups of index cases and secondary cases, Estonia, 2009–2012.
DISCUSSION
This is the first molecular epidemiological study in Estonia using the VNTR methodology to examine associations between patients, their characteristics, M. tuberculosis strains and clustering to determine where recent TB transmission has occurred. There were several important findings.
First, the most frequent isolates were from the Beijing lineage, which were particularly associated with drug-resistant TB. Over 90% of patients with MDR-TB, MDR-TB with additional resistance to second-line drugs and XDR-TB, were infected with isolates from the Beijing lineage. The association between severe drug resistance and the Beijing lineage is well established worldwide;24,25 in Estonia, this association was already demonstrated in 2001 with isolates that originated from 1994.10 Our data thus demonstrate a continuous, longitudinal persistence of this association in Estonia. Another interesting but unexpected finding was that the small number of isolates with RMP monoresistance largely came from the LAM lineage (5/7). Of note, 4/5 isolates were distributed into two clusters and one had a unique VNTR pattern. This indicates both recent transmission of RMP-monoresistant TB and independent acquisition of this resistance pattern. Nevertheless, patients with RMP monoresistance require MDR-TB treatment rather than first-line anti-tuberculosis treatment,26 indicating that this particular pattern of drug resistance needs to be carefully monitored in the future.
Second, almost 70% of the isolates were clustered, indicating a high degree of recent TB transmission in the community. This is significantly higher than shown previously for Estonia from cases examined 20 years ago (49%).10 Importantly, there were several clusters suggesting transmission chains involving large groups of ⩾10 persons. The largest cluster, of 178 isolates, consisted of the Beijing lineage strain, which was recently reported to be the major source of MDR-/XDR-TB in many European Union countries.27 In this largest cluster, the prevalence of MDR-/XDR-TB exceeded 90%. This evidence for active, extensive spread of MDR-/XDR-TB clones of M. tuberculosis Beijing isolates in Estonia is of particular concern, and calls for the need for new measures to better prevent transmission in the populations involved. However, the prominence of this Beijing 100-32 cluster, and of Beijing isolates overall in this setting, means that we must interpret the molecular clustering results in terms of recent transmission with some caution. Although highly discriminatory, 24-locus VNTR typing lacks resolution power for accurately discriminating closely related clones composing Beijing strain populations in comparison with IS6110-RFLP or with the use of additional hypervariable VNTR loci.8,28 There may thus be some minimal overestimation in our assessment of recent transmission.
Third, we found certain socio-demographic and clinical factors that were significantly associated with clustering, indicating that the clustering detected reflects recent transmission to a good extent. These factors included an absence of a permanent home, alcohol abuse, previous incarceration and having polyresistant TB, MDR- or XDR-TB, or HIV infection. While the associations of clustering with incarceration, alcohol abuse, MDR-/XDR-TB and HIV are well established,29,30 the other socio-demographic associations are novel. However, it makes intuitive sense that those without a permanent home are at increased risk of TB,31 and are likely to congregate and spread the infection. We are unaware of previous reports that polyresistant TB is significantly associated with recent transmission. Most of the polyresistant isolates were clustered and were also from the Beijing lineage. This is important because polyresistant Beijing strains are associated with a risk of developing amplified resistance leading to MDR-TB.32 Transmission patterns of polyresistant isolates therefore also need to be carefully monitored.
Fourth, we found that certain factors were associated with a reduced risk of recent transmission. Patients with extra-pulmonary TB had a 65% reduced risk of recent transmission compared with pulmonary TB patients, and this reflects the predominantly non-infectious nature of extra-pulmonary disease. In patients born in Ukraine, there was a significantly lower risk of clustering, possibly because they acquired latent tuberculous infection in their home country which reactivated after coming to Estonia. Old age was significantly associated with a reduced risk of recent transmission, which may reflect the increasing isolation of elderly people from today's modern society.
The strengths of this study were the large number of patients studied, the countrywide research and the integration of molecular epidemiological data into the TB programme risk factor analysis. The conduct and reporting of the research also followed internationally agreed recommendations for reporting on observational studies.33,34
There were two limitations. First, a detailed epidemiological contact investigation was not performed; it therefore remains unclear how person-to-person transmission occurred within each cluster. Second, we used only VNTR typing data for clustering analysis and lineage assignment. Spoligotyping, which is available but not currently in use in Estonia, and additional use of VNTR hypervariable loci on Beijing isolates would have allowed a degree of independent confirmation of lineages and more precise discrimination of cases within clusters, respectively.28,35 However, as indicated above, our results from the analysis of risk factors for clustering allows a good degree of confidence in the epidemiological relevance of the clusters identified.
The most important implication of this study is to show the value of molecular epidemiology in understanding the transmission of the TB epidemic in Estonia and, in particular, of the most dangerous MDR-/XDR-TB forms. It will be important to continue to support this field, invest in new molecular epidemiology technology and improve the already good linkages with the NTP.
In conclusion, we have shown that in Estonia, there is a high and perhaps increasing degree of recent TB transmission, especially in certain high-risk groups, and this is particularly associated with MDR- and XDR-TB and the Beijing lineage.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by WHO-TDR, the WHO Regional Office for Europe (Copenhagen, Denmark), the Operational Research Unit (LUXOR), Brussels Operational Center, Médecins Sans Frontières (MSF Luxembourg), the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union; Paris, France), The Union South-East Asia Regional Office, New Delhi, India.
We are grateful for the support of the WHO Country Office in Talinn, Estonia, and the Estonia National Institute for Health and Development (Talinn, Estonia) in hosting the training workshops. We also appreciate the active involvement of the WHO Country Office and the Ministry of Health (Talinn, Estonia) in the selection of candidates for training in operational research and identification of research projects.
The research leading to these results received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement FP7-223681. The SORT IT programme was funded by the United States Agency for International Development (Washington DC, USA) through a grant managed by WHO-TDR. Additional support was provided by the WHO Regional Office for Europe, the Department for International Development (London, UK) and MSF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: PS is a consultant for Genoscreen. The other authors declare no conflict of interest.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited. The authors alone are responsible for the content of this paper which may not necessarily represent the policies, decisions or views of the WHO.
References
- 1.World Health Organization. The global plan to stop TB 2011–2015: transforming the fight towards elimination of tuberculosis. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 2.European Centre for Disease Prevention and Control. Tuberculosis surveillance and monitoring in Europe 2014. Stockholm, Sweden: ECDC; 2014. [Google Scholar]
- 3.Kliiman K, Altraja A. Predictors of extensively drug-resistant pulmonary tuberculosis. Ann Intern Med. 2009;150:766–775. doi: 10.7326/0003-4819-150-11-200906020-00004. [DOI] [PubMed] [Google Scholar]
- 4.van der Zanden A G, Rahim Z, Fedder G et al. Multiple Mycobacterium tuberculosis infections in an HIV-infected patient. Southeast Asian J Trop Med Public Health. 2007;38:704–705. [PubMed] [Google Scholar]
- 5.van Embden J D, Cave M D, Crawford J T et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supply P, Allix C, Lesjean S et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:4498–4510. doi: 10.1128/JCM.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allix-Beguec C, Fauville-Dufaux M, Supply P. Three-year population-based evaluation of standardized mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2008;46:1398–1406. doi: 10.1128/JCM.02089-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Beer J L, van Ingen J, de Vries G et al. Comparative study of IS6110 restriction fragment length polymorphism and variable-number tandem-repeat typing of Mycobacterium tuberculosis isolates in the Netherlands, based on a 5-year nationwide survey. J Clin Microbiol. 2013;51:1193–1198. doi: 10.1128/JCM.03061-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Beer J L, Kodmon C, van Ingen J, Supply P, van Soolingen D. Second worldwide proficiency study on variable number of tandem repeats typing of Mycobacterium tuberculosis complex. Int J Tuberc Lung Dis. 2014;18:594–600. doi: 10.5588/ijtld.13.0531. [DOI] [PubMed] [Google Scholar]
- 10.Kruuner A, Hoffner S E, Sillastu H et al. Spread of drug-resistant pulmonary tuberculosis in Estonia. J Clin Microbiol. 2001;39:3339–3345. doi: 10.1128/JCM.39.9.3339-3345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devaux I, Kremer K, Heersma H, Van Soolingen D. Clusters of multidrug-resistant Mycobacterium tuberculosis cases, Europe. Emerg Infect Dis. 2009;15:1052–1060. doi: 10.3201/eid1507.080994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirillo D. TB PAN-NET: Pan-European network for study and clinical management of drug resistant tuberculosis. Milan, Italy: TB PAN-NET; 2009–2013. [Google Scholar]
- 13.Tiit Eesti Matemaatika Selts. The census of people and housing. Overview of the population in Estonian counties. Tallinn, Estonia: Data Protection Agency; 2013. [Google Scholar]
- 14.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2010.3. [Google Scholar]
- 15.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneva, Switzerland: WHO; 2009. WHO/HTM/TB/2009.420. [Google Scholar]
- 16.Rüsch-Gerdes S, Pfyffer G E, Casal M, Chadwick M, Siddiqi S. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44:688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. Geneva, Switzerland: WHO; 2008. WHO/HTM/TB/2008.392. [PubMed] [Google Scholar]
- 18.Cowan L S, Hooks D P, Christianson S et al. Evaluation of mycobacterial interspersed repetitive-unit-variable-number tandem-repeat genotyping as performed in laboratories in Canada, France, and the United States. J Clin Microbiol. 2012;50:1830–1831. doi: 10.1128/JCM.00168-12. author reply 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weniger T, Krawczyk J, Supply P, Niemann S, Harmsen D. MIRU-VNTRplus: a web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res. 2010;38:W326–331. doi: 10.1093/nar/gkq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokal R R, Michener C D. A statistical method for evaluating systematic relationships. Univ Kansas Sci Bull. 1958;38:1409–1438. [Google Scholar]
- 21.Allix-Beguec C, Niemann S, Weniger T, Supply P, Harmsen D. Automated web based identification of Mycobacterium tuberculosis genotypes: Evaluation and user-strategy of the MIRU-VNTRplus database. J Clin Microbiol. 2008;298:93. doi: 10.1128/JCM.00540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou N, Nei M. The neighbor-joining method - a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Allix-Beguec C, Harmsen D, Weniger T, Supply P, Niemann S. Evaluation and strategy for use of MIRU-VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification of Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2008;46:2692–2699. doi: 10.1128/JCM.00540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokrousov I, Jiao W W, Sun G Z et al. Evolution of drug resistance in different sublineages of Mycobacterium tuberculosis Beijing genotype. Antimicrob Agents Chemother. 2006;50:2820–2823. doi: 10.1128/AAC.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynn J R, Kremer K, Borgdorff M W, Rodriguez M P, van Soolingen D, European concerted action N. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg Infect Dis. 2006;12:736–743. [Google Scholar]
- 26.World Health Organization. Multidrug-resistant TB (MDR-TB): 2013 update. Geneva, Switzerland: WHO; 2013. http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf?ua=1 Accessed August 2014. [Google Scholar]
- 27.De Beer J L, Kodmon C, van der Werf M J, van Ingen J, van Soolingen D, the ECDC MDR-TB molecular surveillance project participants Molecular surveillance of multi-and extensively drug-resistant tuberculosis transmission in the European Union from 2003 to 2011. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.11.20742. pii: 20742. [DOI] [PubMed] [Google Scholar]
- 28.Allix-Beguec C, Wahl C, Hanekom M et al. Proposal of a consensus set of hypervariable mycobacterial interspersed repetitive-unit-variable-number tandem-repeat loci for subtyping of Mycobacterium tuberculosis Beijing isolates. J Clin Microbiol. 2014;52:164–172. doi: 10.1128/JCM.02519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alland D, Kalkut G E, Moss A R et al. Transmission of tuberculosis in New York City: an analysis by DNA-fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 30.Mokrousov I, Valcheva V, Sovhozova N, Aldashev A, Rastogi N, Isakova J. Penitentiary population of Mycobacterium tuberculosis in Kyrgyzstan: Exceptionally high prevalence of the Beijing genotype and its Russia-specific subtype. Infect Genet Evol. 2009;9:1400–1405. doi: 10.1016/j.meegid.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Lönnroth K, Jaramillo E, Williams B G, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 32.Cox H S, Niemann S, Ismailov G et al. Risk of acquired drug resistance during short-course directly observed treatment of tuberculosis in an area with high levels of drug resistance. Clin Infect Dis. 2007;44:1421–1427. doi: 10.1086/517536. [DOI] [PubMed] [Google Scholar]
- 33.von Elm E, Altman D G, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;370:1453–1457. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 34.Edginton M, Enarson D, Zachariah R. Why ethics is indispensible for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oelemann M C, Diel R, Vatin V et al. Assessment of an optimized mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J Clin Microbiol. 2007;45:691–697. doi: 10.1128/JCM.01393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


