Abstract
Settings: Tuberculosis (TB) health facilities in the Gomel Region, Republic of Belarus—settings with a high burden of multidrug-resistant TB (MDR-TB) and human immunodeficiency virus (HIV) infection.
Objective: To determine treatment outcomes among MDR-TB patients diagnosed in 2009–2010 and factors associated with unsuccessful outcomes (death, failure and loss to follow-up).
Design: Retrospective cohort study involving a review of an electronic patient database maintained under the National Tuberculosis Control Programme.
Results: Of 517 patients diagnosed, 78 (15%) did not start treatment. Among 439 patients who started treatment (84% males, median age 45 years, 15% HIV-infected), 291 (66%) had unsuccessful outcomes (35% deaths, 18% treatment failure and 13% lost to follow-up). Multivariate regression analysis showed that patients aged ⩾45 years (aRR 1.2, 95%CI 1.1–1.3), HIV-infected patients and those not receiving antiretroviral therapy (ART) (aRR 1.5, 95%CI 1.4–1.6) and those with a previous history of anti-tuberculosis treatment (aRR 1.2, 95%CI 1.1–1.4) had significantly higher risk of unsuccessful outcomes.
Conclusion: Treatment outcomes among MDR-TB patients were poor, with high rates of death, failure and loss to follow-up (including pre-treatment loss to follow-up). Urgent measures to increase ART uptake among HIV-infected MDR-TB patients, improved access to second-line anti-tuberculosis drug susceptibility testing and comprehensive patient support measures are required to address this grim situation.
Keywords: operational research, SORT IT, Eastern Europe
According to World Health Organization (WHO) estimates, the Republic of Belarus is one of the world's 27 countries with the highest burden of multidrug-resistant tuberculosis (MDR-TB, defined as resistance to isoniazid [INH] and rifampicin [RMP]).1 In a nationwide survey, it was found that nearly 32% of newly diagnosed TB patients and 76% of previously treated cases had MDR-TB, the highest national MDR-TB rates ever recorded. Among MDR-TB patients, nearly 12% had extensively drug-resistant tuberculosis (XDR-TB, defined as MDR-TB plus resistance to a fluoroquinolone and a second-line injectable).2,3 As per WHO aggregate data, treatment success rates among MDR-TB patients registered in Belarus have been poor, at 40%, with high levels of death, loss to follow-up and treatment failure.1 This is lower than the overall treatment outcomes reported globally.4–6 While two studies have examined the burden of and socio-demographic and clinical factors associated with MDR-TB in Belarus,2,3 no information on whether these factors are associated with treatment outcomes has been published. An understanding of the factors associated with poor treatment outcomes could help in devising strategies to prevent them.
In the present study, we describe the characteristics, management and treatment outcomes of MDR-TB patients diagnosed in the Gomel Region, in south-eastern Belarus. The specific objectives were to assess, among MDR-TB patients diagnosed during 2009–2010, 1) the number (proportion) who initiated treatment and the time from diagnosis of MDR-TB to treatment initiation; 2) the number (proportion) tested for HIV, found to be HIV-positive and initiated on antiretroviral treatment (ART) and cotrimoxazole prophylaxis therapy (CPT); 3) treatment outcomes among patients initiated on treatment; and 4) demographic and clinical factors associated with unsuccessful treatment outcomes.
METHODS
Study design
This was a retrospective cohort study involving a review of routinely maintained records under the Belarus National Tuberculosis Control Programme (NTP).
Setting
The Republic of Belarus, one of the 15 former Republics of the Soviet Union, has a population of 9.5 million; the Gomel Region, where this study was conducted, had a population of 1.5 million in 2012. The Ministry of Health has the overall responsibility for TB control in the country. It discharges this function through the Republican Scientific and Practical Centre for Pulmonology and Tuberculosis (RSPCPT) in the capital city (Minsk) and the health departments of the regional executive committees. The Department of Monitoring and Evaluation of the RSPCPT is the central unit of the NTP, with functions of guidance, monitoring and supervision of TB services both directly and through the oblast (regional) TB coordinators. The regional health authorities are responsible for the delivery of TB services in the same way as any other health service. The Ministry of Internal Affairs runs a parallel system of health care, including TB services, for the penitentiary system. Belarus adopted the DOTS strategy in 2001 and expanded its implementation countrywide by 2005, including the penitentiary system. Anti-tuberculosis control interventions are delivered through a network of dedicated TB facilities and primary health care services.
Management of MDR-TB
‘Presumptive TB’ patients are evaluated at the primary health polyclinics from where sputum specimens are transported to regional reference laboratories for direct smear microscopy and other tests.7 During the study period, all patients underwent culture and drug susceptibility testing (DST) against first-line drugs (INH, RMP, streptomycin and ethambutol) using phenotypic methods (liquid culture using BACTEC™ MGIT™, BD, Sparks, MD, USA). All drug-susceptible, new and previously treated TB patients received WHO-recommended standardised first-line drug regimens. Treatment was changed to an individualised second-line regimen, comprising an 8-month intensive phase with at least six drugs and a 12-month continuation phase with four drugs, after receipt of DST results.8 Access to second-line DST was limited during the study period. MDR-TB patients were admitted to hospital during the intensive phase of treatment, or for longer, until their bacteriological sputum culture became negative. For continuation of treatment, patients were referred to TB dispensaries, or to primary health care facilities for those living in rural areas. All treatment was delivered under direct observation (DOT) by a health worker. Clinical follow-up was supervised by a TB specialist. All TB patients were offered HIV testing; those found to be HIV-infected were offered CPT and ART using a first-line regimen consisting of zidovudine/stavudine, lamivudine and efavirenz. Diagnosis and treatment services were provided free of charge. Case definitions, outcome categories, recording and reporting followed WHO guidelines, and all patient information was captured in an electronic National TB Register.
Study participants and study period
All MDR-TB patients (culture-confirmed and INH- and RMP-resistant) diagnosed in the Gomel Region during the period 2009–2010 were included. The study was conducted between June 2013 and March 2014.
Data variables and data source
We extracted information on the following variables: age, sex, date of MDR-TB diagnosis (defined as the date when the laboratory results were available), date of treatment initiation, history of previous anti-tuberculosis treatment (particularly second-line anti-tuberculosis drugs), HIV status, ART initiation, CPT initiation and treatment outcome. The source of data was an electronic patient database maintained at the Gomel Regional Tuberculosis Hospital, Gomel, Belarus.
Data analysis
Data were extracted from the electronic database and imported into EpiData (version 2.2.2.182, EpiData Association, Odense, Denmark) for analysis. Univariate analysis was performed to describe the demographic and clinical characteristics of MDR-TB patients. The treatment outcome categories ‘cured’ and ‘treatment completed’ were combined under ‘successful outcome’; ‘death,’ ‘lost to follow-up’ (LFU), ‘failures’ and ‘transfer out’ were combined under ‘unsuccessful treatment outcome’. Pre-treatment LFU was defined as failure to initiate treatment after MDR-TB diagnosis. Bivariate analysis was performed to examine the factors associated with unsuccessful treatment outcomes. Relative risks (RRs) with 95% confidence intervals (CIs) were calculated as measures of association. We conducted a multivariate analysis (log-binomial regression) using STATA version 12.1 (Stata Corp, College Station, TX, USA) to assess adjusted relative risks (aRRs) and independent effects of each factor on treatment outcomes. Variables found to be significantly associated in bivariate analysis (P < 0.1), in addition to age and sex, were included in the model.
Ethics
Administrative approval to conduct the study was obtained from the NTP authorities in Belarus. Ethics approval was obtained from the Ethics Committee of the RSPCPT, Minsk, Belarus, and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
Of 517 MDR-TB patients diagnosed, 439 (85%) were initiated on treatment and the rest were LFU before starting treatment (pre-treatment LFU) (Figure). Pre-treatment LFU was significantly higher among MDR-TB patients with a previous history of anti-tuberculosis treatment than in new MDR-TB patients (18% vs. 8%, P = 0.005) and marginally higher among males than in females (16% vs. 10%, P = 0.25). Among those initiated on treatment, the median duration between date of diagnosis and date of treatment initiation was 1 day (interquartile range [IQR] 1–1); nearly 90% started treatment within 5 days of diagnosis.
FIGURE.
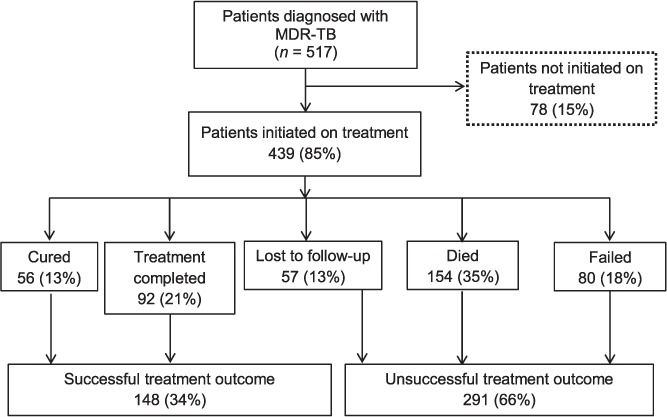
Treatment outcomes of patients diagnosed with MDR-TB, Gomel Region, Republic of Belarus, 2009–2010. MDR-TB = multidrug-resistant tuberculosis.
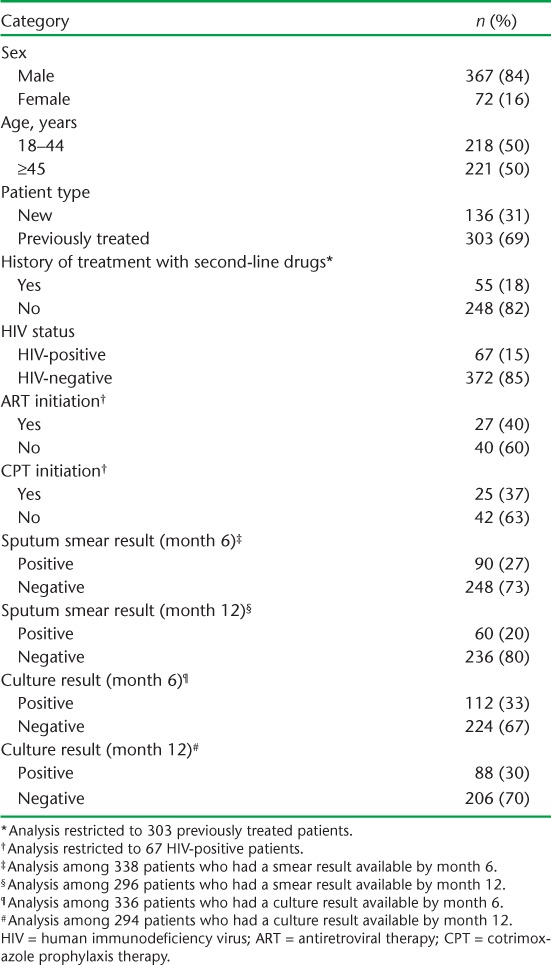
The demographic and clinical characteristics of the MDR-TB patients are shown in Table 1. Nearly 85% were males, and the median age was 45 years (IQR 36–53). Of these, 303 (69%) had a previous history of anti-tuberculosis treatment, among whom about 20% had taken second-line anti-tuberculosis drugs. All had their HIV status ascertained; 15% were HIV-infected. Among the HIV-infected MDR-TB patients, 27 (40%) received ART and 25 (37%) received CPT. Almost all patients who were alive and on treatment had a follow-up smear and culture at 6 and 12 months; about one third of these were culture-positive.
TABLE 1.
Demographic and clinical characteristics of multidrug-resistant tuberculosis patients enrolled for treatment, Gomel Region, Republic of Belarus, 2009–2010 (n = 439)
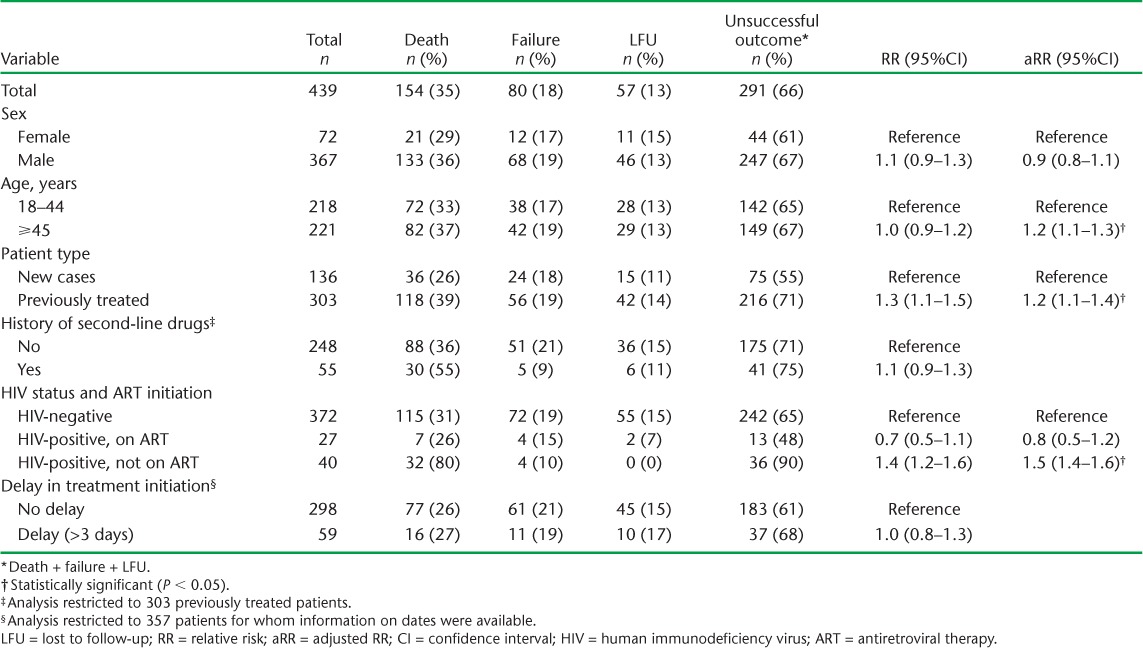
Of the MDR-TB patients initiated on treatment, 291 (66%) had an unsuccessful outcome: 13% LFU, 35% deaths and 18% treatment failures (Figure). Multivariate regression analysis showed that unsuccessful outcome was significantly more likely to occur among patients aged ⩾45 years, those with a history of anti-tuberculosis treatment and HIV-infected patients not receiving ART (Table 2).
TABLE 2.
Factors associated with unsuccessful treatment outcome in multidrug-resistant tuberculosis patients, Gomel Region, Republic of Belarus, 2009–2010
DISCUSSION
Overall, treatment outcomes among MDR-TB patients in the Gomel Region of Belarus were poor, with about two thirds of those started on treatment having an unsuccessful outcome, much higher than reported in other settings.4,5 Even this is an underestimation, given that 15% of the patients were lost before starting treatment: adding these to ‘unsuccessful’ outcomes would increase the proportion of unsuccessful outcomes to 71% (369/517 patients). Significant risk factors for unsuccessful outcomes were age >44 years, previous anti-tuberculosis treatment and untreated HIV infection.
In accordance with WHO recommendations, NTPs create cohorts of TB patients from those who were ‘initiated on treatment’ for the purposes of recording and reporting. This means that pre-treatment LFU is usually not accounted for. Several studies from across the globe have highlighted the high pre-treatment attrition among MDR-TB patients and have called for more careful cohort analysis starting from all diagnosed patients rather than only those who are started on treatment.9,10 This need is confirmed by our study findings, and is probably more important from a public health perspective, as untreated patients may have an increased risk of death and of transmitting drug-resistant organisms. Acknowledging the true magnitude of a problem is the first step towards addressing it, and we strongly recommend that the NTP in Belarus take the lead in this direction to revise its recording and reporting system and include an indicator to capture pre-treatment LFU. While this is not explicitly mentioned in the WHO guidelines, there is an indirect indication to this effect by the changed definition of LFU in 2013.11 As per the latest guidance on recording and reporting, the WHO now recommends the inclusion of patients lost before treatment initiation within the ambit of LFU.11 However, this change is currently restricted to TB patients receiving first-line drugs. This indirectly indicates that cohorts should be created out of those ‘diagnosed’ instead of ‘treated’ patients. We recommend that the WHO make this recommendation explicit, extend it to MDR-TB patients and include it in their operational guidance to NTPs worldwide.
Nearly half of all unsuccessful outcomes were accounted for by deaths, which were mainly related to being HIV-infected and not receiving ART and a history of previous anti-tuberculosis treatment. While the WHO 2010 ART guidelines recommend that all HIV-infected TB patients should be initiated on ART, irrespective of CD4 count, and as early as possible in the course of anti-tuberculosis treatment, this was practised in less than half of the patients in the Gomel Region.8,12 This is unacceptable given that ART is the single most important life-saving intervention among HIV-infected patients. While the exact reasons are not known, we speculate, on the basis of anecdotal evidence, that patients are reluctant to start ART due to the high pill burden and fear of adverse drug reactions resulting from the simultaneous consumption of anti-tuberculosis and ART drugs. Further research using qualitative methods is required to ascertain the exact reasons for not receiving ART and devising appropriate interventions. While this was not an issue in our study, recent programme reviews have drawn attention to low levels of HIV testing among TB patients and have recommended increasing the HIV test uptake by using rapid tests and resolving the operational issues of confirming HIV diagnosis.13 Management of HIV-infected MDR-TB patients is a medical and social challenge that requires specialised expertise.14,15 Considering the relatively high HIV prevalence among TB patients in the Gomel Region, which is higher than the national average and increased from 1.9% in 2004 to 13.4% in 2011, developing and strengthening TB-HIV collaboration should be one of the most important future priorities for the regional TB programme.
Patients aged ⩾45 years had a higher risk of unsuccessful outcomes, which may be related to the presence of other comorbidities, such as diabetes mellitus, and to the risk of death, which increases with age. Unfortunately, our patient database did not capture this important information; we were thus unable to assess if diabetes was related to poor treatment outcomes. As per WHO recommendations, all TB patients are routinely screened for diabetes mellitus in Belarus.16 However, implementation of this policy and its recording and reporting needs to be strengthened further and monitored.
The second important reason for unsuccessful outcomes in Gomel was the high rate of treatment failure. This could be related to the history of previous anti-tuberculosis treatment using second-line drugs among study participants, a high prevalence of resistance to second-line anti-tuberculosis drugs and limited access to second-line DST during the study period. The high proportion of previous treatment is related to the frequent use of first-line treatment in the face of widespread and undiagnosed primary MDR-TB, leading to very high levels of failure.1 A nationwide survey published in 2013 showed that 12% of all MDR-TB patients had XDR-TB, and about 40% had resistance to either fluoroquinolones or second-line injectables (pre-XDR-TB).3 The Belarus NTP has already responded to this challenge, and it is now national policy to test all MDR-TB patients for resistance to second-line drugs at baseline and to initiate appropriate treatment.
The other reason for unsuccessful outcome was related to loss to follow-up during treatment. Considering the long duration of treatment with drugs that often have adverse effects, patients need to be counselled, motivated and supported to complete treatment. A recent systematic review among MDR-TB patients mentions several patient support measures aimed at reducing loss to follow-up, including provision of DOT throughout the course of treatment by community health workers and provision of patient education.6 Several other patient support measures, such as incentives and enablers (cash, food vouchers, hospital travel reimbursements), have been used in the past with varied success.17 These measures were introduced in the recent past in Belarus and their impact is yet to be evaluated. Considering the widespread use of mobile telephone services in Belarus, it may be opportune to use this technology for sending patient reminders to improve adherence and retention.18
There were several strengths to our study. First, this is the first report from Belarus to examine treatment outcomes among MDR-TB patients. Second, we had a large sample size and covered all the patients in the Gomel Region. Third, we followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting the study, including the ethics dimension.19,20 Given the retrospective, operational nature of the study and reliance on existing patient databases as data source, the study had several limitations. First, we did not have information on the date of outcome and we could not therefore infer the timing of deaths and LFUs. This information would have been very useful in prioritising NTP efforts. Second, we did not have any information on variables such as diabetes mellitus, smoking, alcoholism, drug use, CD4 count, etc., which have been shown to be associated with poor outcomes in previous studies.21,22 Third, we could not interview patients to ascertain the exact reasons for LFU and non-uptake of ART. Future research should address these aspects.
In conclusion, this study from Gomel showed that treatment outcomes were poor among MDR-TB patients, with very high rates of death, failure and LFU (including pre-treatment LFU). HIV-infected patients not on ART, older age and those with a history of previous anti-tuberculosis treatment had a higher risk of unsuccessful outcomes. Urgent measures to increase ART uptake among HIV-infected MDR-TB patients, improved access to second-line DST and comprehensive patient support measures to improve treatment adherence are required to address this grim situation.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR, Geneva, Switzerland). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by WHO-TDR; the WHO Regional Office for Europe, Copenhagen, Denmark; the Médecins Sans Frontières (MSF), Operational Research Unit (LUXOR), Brussels Operational Center, Luxembourg; the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), France; The Union South-East Asia Regional Office, New Delhi, India. We are grateful for the support of the WHO Country Office in Talinn, Estonia and the Estonia National Institute for Health and Development (Talinn, Estonia) in hosting the training workshops. We also appreciate the active involvement of the WHO Country Office and the Ministry of Health in the selection of candidates for training in operational research and identification of research projects. WHO/HQ (Global TB Programme) was involved in the ideation of this study and the writing of this manuscript.
The programme was funded by the United States Agency for International Development (USAID) through a grant managed by WHO-TDR. Additional support was provided by the WHO Regional Office for Europe, the Department for International Development, London, UK; and MSF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: none declared.
The authors alone are responsible for the content of this paper, which may not necessarily represent the policies, decisions or views of the WHO.
In accordance with the WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution Intergovernmental Organizations licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report, 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.Skrahina A, Hurevich H, Zalutskaya A et al. Alarming levels of drug-resistant tuberculosis in Belarus: results of a survey in Minsk. Eur Respir J. 2012;39:1425–1431. doi: 10.1183/09031936.00145411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skrahina A, Hurevich H, Zalutskaya A et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull World Health Organ. 2013;91:36–45. doi: 10.2471/BLT.12.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahuja S D, Ashkin D, Avendano M et al. Multidrug-resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLOS MED. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettehad D, Schaaf H S, Seddon J A, Cooke G S, Ford N. Treatment outcome for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:449–456. doi: 10.1016/S1473-3099(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 6.Toczek A, Cox H, du Cros P, Cooke G, Ford N. Strategies for reducing treatment default in drug-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2013;17:299–307. doi: 10.5588/ijtld.12.0537. [DOI] [PubMed] [Google Scholar]
- 7.Ministry of Health of The Republic of Belarus. Clinical guidance for the organization and conduct of TB control in ambulatory out-patient health care organizations. Minsk, Belarus: Ministry of Health, Republic of Belarus; 2012. http://www.levonevski.net/pravo/norm2013/num04/d04866.html. Accessed July 2014. [Google Scholar]
- 8.Ministry of Health, Republic of Belarus. Clinical guidelines for the treatment of TB and drug-resistants forms. Minsk, Belarus: Ministry of Health, Republic of Belarus; 2012. http://www.google.by/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=0CDcQFjAC&url=http%3A%2F%2Fwww.rnpcpf.by%2Fen%2Forganizational-methodical-work%2Flegal-documents.html%3Fdownload%3D34%253A22.08.2012.-939&ei=D99gU6vhO8XfygOyqIC4Cw&usg=AFQjCNHPN2OZdkhFs-BQMyJegTsmSb3vuA&bvm=bv.65636070,d.bGQ. Accessed July 2014. [Google Scholar]
- 9.Khann S, Eang M T, Rajendra Y P, Satyanarayana S, Nagaraja S B, Kumar A M V. Linkage of presumptive multidrug resistant tuberculosis (MDR-TB) patients to diagnostic and treatment services in Cambodia. PLoS ONE. 2013;8:e59903. doi: 10.1371/journal.pone.0059903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chadha S S, Sharath B N, Reddy K et al. Operational challenges in diagnosing multi-drug resistant TB and initiating treatment in Andhra Pradesh, India. PLOS ONE. 2011;6:e26659. doi: 10.1371/journal.pone.0026659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Definitions and reporting framework for tuberculosis. 2013 revision. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.2. [Google Scholar]
- 12.World Health Organization. Global update on HIV treatment 2013: results, impact and opportunities. Geneva, Switzerland: WHO, UNICEF, UNAIDS; 2013. [Google Scholar]
- 13.World Health Organization Regional Office for Europe. Review of the National Tuberculosis Programme in Belarus 10–21 October 2011. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2012. [Google Scholar]
- 14.Isaakidis P, Cox H S, Varghese B et al. Ambulatory multidrug-resistant tuberculosis treatment outcomes in a cohort of HIV-infected patients in a slum setting in Mumbai, India. PLOS ONE. 2011;6:e28066. doi: 10.1371/journal.pone.0028066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaakidis P, Paryani R, Khan S et al. Poor outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrug-resistant tuberculosis in Mumbai, India. PLOS ONE. 2013;8:e68869. doi: 10.1371/journal.pone.0068869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Union Against Tuberculosis and Lung Disease, World Health Organization. Collaborative framework for care and control of tuberculosis and diabetes. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.15. [PubMed] [Google Scholar]
- 17.Lutge E E, Wiysonge C S, Knight S E, Volmink J. Material incentives and enablers in the management of tuberculosis. Cochrane Database Syst Rev. 2012;1:CD007952. doi: 10.1002/14651858.CD007952.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Nglazi M D, Bekker L G, Wood R, Hussey G D, Wiysonge C S. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review. BMC Infect Dis. 2013;13:566. doi: 10.1186/1471-2334-13-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenbroucke J P, von Elm E, Altman D G et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLOS MED. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edginton M, Enarson D, Zachariah R, Reid T, Satyanarayana S, Bissell K. Why ethics is indespensible for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podewils L J, Gler M T S, Qualapio M I, Chen M P. Patterns of treatment interruption among patients with multidrug-resistant TB (MDR-TB) and association with interim and final treatment outcomes. PLOS ONE. 2013;8:e70064. doi: 10.1371/journal.pone.0070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driver C R, Matus S P, Bayuga S, Winters A I, Munsiff S S. Factors associated with tuberculosis treatment interruption in New York City. J Public Health Management Practice. 2005;11:361–368. doi: 10.1097/00124784-200507000-00017. [DOI] [PubMed] [Google Scholar]


