Abstract
St. Lawrence Island, Alaska is the largest island in the Bering Sea, located 60 km from Siberia. The island is home to approximately 1600 St. Lawrence Island Yupik residents who live a subsistence lifestyle. Two formerly used defense sites (FUDS) exist on the island, one of which, Northeast Cape, has been the subject of a $123 million cleanup effort. Environmental monitoring demonstrates localized soil and watershed contamination with polychlorinated biphenyls (PCBs), organochlorine (OC) pesticides and arsenic. This study examined whether the Northeast Cape FUDS is a source of exposure to OC pesticides. A total of 71 serum samples were collected during site remediation from volunteers that represented three geographic regions of the island. Additionally, ninespine stickleback (Pungitius pungitius) and Alaska blackfish (Dallia pectoralis) were collected from Northeast Cape after remediation to assess continuing presence of OC pesticides. Chlordane compounds, DDT compounds, mirex and hexachlorobenzene (HCB) were the most prevalent and present at the highest concentrations in both fish tissues and human serum samples. After controlling for age and sex, activities near the Northeast Cape FUDS were associated with an increase in serum HCB as compared to residents of the farthest village from the site. Positive but non-significant relationships for sum-chlordane and sum-DDT were also found. Organochlorine concentrations in fish samples did not show clear geographic trends, but appear elevated compared to other sites in Alaska. Taken together, the results suggest that contamination of the local environment at the Northeast Cape FUDS may increase exposure to select persistent OC pesticides.
Introduction
Persistent organic pollutants (POPs) remain an important public health issue for arctic indigenous peoples (Godduhn and Duffy 2003; AMAP 2004; Suk et al. 2004; AMAP 2009; Kitts et al. 2012). POPs primarily arrive in the Arctic via long range transport and global distillation (Burkow and Kallenborn 2000). In some instances locally contaminated sites, such as formerly used defense sites (FUDS), act as point sources of contaminants (Burkow and Kallenborn 2000; Carpenter et al. 2005; Scrudato et al. 2012). Consumption of traditional subsistence foods, especially lipid-rich, high trophic level and long-lived animals such as pinnipeds and bowhead whales (Balaena mysticetus), is of central importance in the exposure of arctic indigenous peoples to POPs (Dewailly et al. 1993; Bjerregaard et al. 2001; Odland et al. 2003). Some Arctic populations have regularly exceeded Tolerable Daily Intakes (TDIs) for such legacy POPs as the chlordane metabolite oxychlordane, toxaphene, and polychlorinated biphenyls (PCBs) (AMAP 2014). Arctic pollution in the United States is poorly studied as compared to Canada and Europe, despite the large size of Alaska and the importance of subsistence foods to Alaska Natives (AMAP 2004).
Low dose exposure to organochlorine (OC) compounds may adversely affect health and normal biological function (Damstra 2002). Many OC pesticides are endocrine disrupting compounds that influence disease states such as cancer, even at low doses (Salehi et al. 2008; Mrema et al. 2013). Organochlorine pesticide exposure during pregnancy is also thought to influence cognitive development in children (Puertas et al. 2010; Torres-Sanchez et al. 2013). Ubiquitous exposure to endocrine disruptors, including OC compounds, also affects reproductive development (Teilmann et al. 2002). Therefore, despite decreasing concentrations for most banned or restricted (legacy) pesticides in the Arctic (AMAP 2002), exposure remains a health concern. Moreover, some legacy pesticides displayed increasing concentrations between 1999-2003 and 2004-2006, such as hexachlorobenzene, β-HCH, trans-nonachlor, and toxaphene among Yupik mothers of western Alaska (AMAP 2014). A review of research in the Canadian Arctic suggests that some indigenous populations are exposed to OC pesticides at levels that present a health risk (Van Oostdam et al. 1999; Elabbas et al. 2011; Laird et al. 2013). Limited research from Alaska suggests that Alaska Natives also maintain relatively high body burdens of OC compounds (Middaugh et al. 2001; Rubin et al. 2001).
The aim of the current study was to investigate the prevalent serum concentrations of OC pesticides in a small population sample of Alaska Natives from St. Lawrence Island. It is the largest island in the Bering Sea and is located approximately 209 km west of Nome, Alaska. St. Lawrence Island has two extant villages (Gambell and Savoonga) as well as the FUDS at the former village of Northeast Cape (NEC; Figure 1). Gambell and Savoonga are home to approximately 1600 full time residents, who are almost exclusively Alaska Natives and primarily St. Lawrence Island Yupik people. Serum concentrations of PCBs in Alaska Natives on St. Lawrence Island tend to be higher than those of the U.S. general population (Carpenter et al. 2005), suggesting that this population may be exposed to additional sources of POPs. The traditional food animals of this population are contaminated by numerous OC compounds (Welfinger-Smith et al. 2011).
Figure 1.
St. Lawrence Island
Two FUDS exist on St. Lawrence Island in Gambell and NEC, and both are associated with environmental contamination (Scrudato et al. 2012). Contamination in Gambell is composed primarily of diesel range organics, while NEC is contaminated with PCBs, diesel range organics, and to a lesser extent OC pesticides. Mirex and DDE have been measured in NEC soil at low μg/kg concentrations (Scrudato et al. 2012). It is unclear to what extent NEC increases exposure to POPs for St. Lawrence Island residents.
The research aims of this study were developed in conjunction with tribal leaders from the Native villages of Gambell and Savoonga and village community members. The details of this ongoing community based participatory research program are described elsewhere (Miller et al. 2013). Community members expressed concern regarding exposure to contaminants through ingestion of traditional foods and from FUDS. The aims of this study were to: 1) document concentrations of persistent chlorinated pesticides in the blood serum of the people of St. Lawrence Island, 2) inform them of results, 3) estimate exposure patterns and concentrations, and 4) assess whether residents who utilize the land in proximity to the NEC FUDS as a hunting and fishing area have higher OC pesticide body burdens.
Current OC pesticide contamination on the island was examined using two resident freshwater fish species as sentinels. Ninespine stickleback (Pungitius pungitius; hereafter ‘stickleback’) and Alaska blackfish (Dallia pectoralis) were collected from the Suqitugheneq (Suqi) River, which drains the Northeast Cape FUDS. This allowed examination of whether the military site remains a point source of OC pesticides after remediation. Additionally, stickleback were collected from a second site at NEC and from the village of Gambell for comparison purposes. Stickleback were used as the principle sentinel species due do their circumpolar artic distribution and presence at contaminated sites. We also compared the pesticide body burdens of St. Lawrence Island residents with the body burdens in stickleback and blackfish from the FUDS in order to determine if patterns in residents reflect exposures of naturally occurring aquatic species.
Methods
Human serum samples
Participants were directly recruited via bilingual community health researchers on the island. Blood samples were obtained from a total of 130 individuals on St. Lawrence Island during August 2001, and between August and October 2003. There were no exclusion criteria, other than being less than 18 years of age. Blood samples were initially collected for analysis of PCBs. For 71 participants, sufficient serum remained to conduct OC pesticide analysis. Participants were recruited from three groups: residents of Gambell, residents of Savoonga who spent little to no time at NEC, and residents of Savoonga with historical familial ties to NEC, including subsistence camps and/or occupational experience at the military site. Gambell is the most distant from NEC and used as the referent group in regression analyses (Figure 1). While time spent at NEC varied, typically several months are spent at a subsistence camp in the summer. Food sharing is common among these communities.
An attempt was made to ensure an approximately equal distribution of age and gender in each location. Brief interviews were conducted to verify age, sex, and usage of NEC as a subsistence hunting or gathering site. Interviews were conducted by the bilingual community health researchers, who were trained and supervised by project personnel as part of the community based research project. Results from this study were reported back to individual community members in written form. In addition, Dr. Carpenter was available to discuss individual results.
Participants provided two separate 8-hr fasting blood samples in 10ml glass vacutainers, 20ml total. Blood samples were collected from participants and placed immediately in a cooler with ice packs. Samples were received by the Institute for Health and the Environment at the University at Albany within 7 days. Blood was allowed to clot at room temperature before centrifugation to collect serum. Serum extractions were performed based on EPA methods 8081A, 3620, and 3630. In brief, 1g serum was placed into 1ml formic acid and 1ml DI water was added for a 1:1:1 ratio; 10μl of a 100 ppb stock surrogate solution was added to each sample and all QA/QC samples. Samples were stirred on a vortex mixer for 1 min and allowed to stand for 30 min.
Samples were exacted on a Baker spe™-12g Column Processing System connected to a vacuum pump at a rate of 3ml/min with pre-rinsed C18 cartridges. After 40 min the C18 cartridges were eluted with 20ml methanol/DCM (1:9) in a clean culture tube. The eluant was transferred to a RapidVap concentrator tube and concentrated to approximately 1.5ml. A solvent swap to hexane was performed by adding 10ml hexane and allowing the sample to concentrate to 1ml.
Florisil SPE cartridges were employed to clean up and remove polar lipids and other interferents from the serum extracts. The Florisil SPE cartridge was rinsed with 4ml hexane, and the sample was quantitatively applied and eluted with 10ml hexane; this fraction was labeled Fr-1. The cartridge was then eluted with 10ml acetone/hexane (1:9) and labeled Fr-2. Both fractions were transferred to concentrator tubes and concentrated to 1ml. Fr-2 was solvent swapped with 10ml hexane twice. The final extract was transferred to a 2ml GC vial and capped with a PTFE lined septa cap for analysis.
Pesticides were analyzed using high resolution GC on an Agilent 6890 gas chromatograph equipped with dual electronic pressure controlled injection ports run in splitless mode and dual Ni 63 electron capture detectors. Four μl sample were injected on dual capillary columns, a JT Baker 5% phenylmethyloctadecylsilyl bonded (DB-5) fused silica (25m, 0.33μm film, 0.25mm id) capillary column and a fused silica Apiezon-L (30m, 0.33μm film, 0.25mm id) column. Total serum lipid concentration was determined by drying and weighing the residual of a hexane extract. PCB-85 was partially co-eluted with p,p’-DDE. Pesticide recoveries from spiked serum were typically within 20%, except for alpha-HCH (75.5%) gamma-HCH (55%), delta-HCH (55.7%), heptachlor epoxide (68.9%) cis-nonachlor (68.2%), gamma-chlordane (78.1%), o,p’-DDE (78.8%), o,p’-DDT (69.9%), and aldrin (73%). The limit of quantification varied by compound, but was in the range of 3-12ng/ml wet weight. All samples were blank corrected.
Fish Samples
Stickleback were collected from three locations on the island. The primary study site was the Suqi River (June 2012, June-July 2013), which runs through the contaminated NEC military site. Fish sampling locations included both the region downstream of the military complex, as well as the portion up gradient of the site (upstream Suqi). Alaska blackfish were also collected from the Suqi River (June 2012). Stickleback were also collected from the Tapisaggak (Tapi) River (June 2012), located approximately 5 km east of the NEC military site, and from Troutman Lake (June 2012), a coastal lake situated adjacent to the village of Gambell.
All fish were collected with unbaited minnow traps, euthanized with an overdose of pH-neutral MS222 fish anesthetic, held on ice at the collection site, and then transferred to −80°C in the lab. AXYS Analytical Services Ltd. conducted analysis of pesticide concentrations in fish samples. Percent recovery from matrix spikes was typically within 10%, and never outside of 20% difference. Limit of quantification varied by compound, but was in the range of 0.01-0.1 ng/g wet weight. All samples were blank corrected. Composite samples of multiple whole stickleback were used to generate average tissue concentrations. Composite samples were made up of approximately 10 individual stickleback totaling approximately10g from the same study site. A total of 15 composite stickleback samples were analyzed, representing approximately 150 individual fish (3 composite samples from Troutman Lake, 2 composite samples from the Tapi River, and 10 composite samples from the Suqi River). In addition, 5 individual Alaska blackfish from the Suqi River, ranging from 8.1-10.4g, were analyzed. Neither stickleback nor Alaska blackfish are regularly consumed, but rather act as useful indicator species due to their common presence in contaminated sites of the Bering Sea region.
Statistical analysis
Statistical analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, North Carolina), and some descriptive statistics were calculated in Excel (Microsoft Corp, Redmond, Washington). Data from the National Health and Nutrition Examination Survey (NHANES): Third National Report on Human Exposure to Environmental Chemicals were used as a qualitative comparison group (CDC 2005). Data from the 2001-2002 NHANES research cycle were used because they are temporally most comparable to the current study.
Multiple linear regression was used to estimate the influence of residence area/subsistence camp location on serum pesticide concentrations. Normality of continuous variables was assessed visually with quantile-quantile plots and with the Kolmogorav-Smirnov test. Non-normally distributed variables were natural log transformed and reassessed for normality. Both sex and age appeared to be confounders based on the distribution of these factors in the sample and were controlled for in all multivariate analyses. NEC was considered the main exposure location; Gambell was considered the referent location. This was done because individuals residing in Savoonga may be exposed through food sharing, while those in Gambell are less likely to receive food from NEC. The criterion for significance was set at p<0.05.
Compounds that were absent from ≥50% of human sera samples were not included in regression analyses, unless these could be summed as part of a group (e.g., Σ-DDT). Even after summing individual hexachlorocyclohexane (HCH) isomers, only 21% of participants displayed any detectable HCH isomer in their serum sample, and therefore HCH isomers were not included. For the final analysis, 4 OC pesticides (or summed groups) were found to be present in a sufficient number of samples to warrant multivariate analysis. These compounds were Σ-DDT, Σ-chlordanes, mirex, and HCB.
Σ-DDT was defined as the sum of o,p-DDE, op’-DDT, and p,p’-DDE. Σ-chlordane was defined as the sum of heptachlor, heptachlor epoxide, alpha-chlordane, gamma-chlordane, oxychlordane, trans-nonachlor, and cis-nonachlor. These compounds were considered together because they are present in technical chlordane mixtures or are environmental and biological degradation products of technical chlordane compounds; often both are the case (Sovocool et al. 1977; Tashiro and Matsumura 1978). Data points below the level of detection were imputed as one half of the minimum observed concentration. This is likely to bias associations to the null, but was necessary to maintain a normal distribution. Imputation was only used for mirex, because HCB, Σ-DDT, and Σ-chlordanes had no missing data points.
Use of log transformed dependent variables in linear regression changes the interpretation of the coefficients from an absolute additive change to a relative change. That is, the model becomes multiplicative. Regression coefficients are expressed here as risk ratios (RR), or as % change where % change = (eβ – 1)* 100. The fit of regression was assessed using moments of the raw, studentized and jackknife residuals, as well as through visual assessment of residual* plots and residual*leverage plots. High leverage observations were defined a priori as those with leverage values of >2(). No high leverage observations existed in the dataset. Sensitivity analysis was conducted to determine the influence of imputation on the model of mirex. Complete case analysis was conducted for mirex to determine the effect of imputation. Statistical tests for confounding were not relied upon because low sample sizes may mask significant confounder associations. The covariates of age and sex were included in all final models.
The concentrations of OC pesticides in composite fish samples were assessed with descriptive statistics. Analysis of composite samples and low sample sizes in some water bodies precluded the use of statistical tests. Visual comparisons of fish data were used for those compounds which appeared to be related to military contamination based on human sera samples.
Sample size was determined predominantly on economic terms and therefore the statistical tests tended to be underpowered. Post hoc power and sample size calculations were conducted with OpenEpi (Sullivan et al. 2009).
Results
The study sample includes 40 women and 31 men ranging in age from 18 to 53 (Table 1). Total serum lipid concentrations had a mean of 4.78 g/L and a range from 2.2 to 8.6 g/L. Of the 25 OC pesticides analyzed in the serum samples, 16 individual compounds were detected: aldrin, heptachlor, heptachlor epoxide, alpha-chlordane, gamma-chlordane, oxychlordane, trans-nonachlor, cis-nonachlor, o,p-DDE, o,p’-DDT, p,p’-DDE, alpha-HCH, delta-HCH, gamma-HCH (lindane), HCB, and mirex (Table 2). After summing compounds there were no missing values for Σ-chlordanes or Σ-DDT. PCB levels for the entire study population were previously reported (Carpenter et al. 2005).
Table 1.
Participant Characteristics
| Age mean (range) | Male | Female | |
|---|---|---|---|
| Total (n=71) | 33.6 (18-53) | 31 | 40 |
| Savoonga (n=26) | 32.5 (18-45) | 10 | 16 |
| Gambell (n=21) | 31.9 (18-45) | 8 | 13 |
| NEC (n=24) | 36.5 (20-53) | 13 | 11 |
NEC=Northeast Cape Formerly Used Defense Site
Table 2.
Distribution of organochlorine pesticides in participant serum samples (μg/kg lw)
| Mean | St. Dev. | Median | 95th % | % Detect | |
|---|---|---|---|---|---|
| HCH, alpha | * | * | nd | 1.5 | 12.7 |
| HCH, gamma | * | * | nd | 15.1 | 8.5 |
| HCH, delta | * | * | nd | nd | 1.4 |
| Aldrin | * | * | nd | 1.4 | 7.0 |
| Heptachlor | * | * | nd | 3.2 | 22.5 |
| Heptachlor epoxide | * | * | nd | 27.3 | 35.2 |
| Oxychlordane | 85.9 | 70.6 | 75.3 | 248.9 | 94.4 |
| Gamma-chlordane | * | * | nd | 2.8 | 7.0 |
| Alpha-chlordane | * | * | nd | 6.1 | 38.0 |
| Trans-nonachlor | 52.1 | 36.3 | 50.3 | 120.3 | 90.1 |
| Cis-nonachlor | * | * | nd | nd | 1.4 |
| HCB | 85.9 | 52.9 | 72.0 | 163.5 | 100 |
| o,p’-DDT | * | * | nd | 5.6 | 22.5 |
| o,p’-DDE | * | * | nd | 10.4 | 18.3 |
| p,p’-DDE/PCB85 | 386.4 | 272.6 | 316.5 | 967.3 | 100 |
| Mirex | 22.1 | 19.5 | 19.4 | 69.7 | 84.5 |
Not enough data for accurate calculation, proportion <LOD greater than 50%; nd=non-detectable
All samples contained p,p’-DDE/PCB-85, which was present at the highest mean concentration of 386.4 μg/kg lw. The mean for the sum of DDT compounds was 389.2 μg/kg lw. While HCB was also present in all samples, its concentration was notably lower with a mean of 85.9 μg/kg lw. Chlordane components and degradation products were also prevalent compounds. Oxychlordane and trans-nonachlor were present in 94% and 90% of samples, respectively, and together comprise the majority of Σ-chlordane compounds. Σ-chlordane compounds were present at an average concentration of 148.1 μg/kg lw, while the mean for the sum of only oxychlordane and trans-nonachlor was 137.9 μg/kg lw. Mirex was present at an average concentration of 22.1 μg/kg lw.
Those utilizing NEC as a hunting or fishing area were both older and more likely to be male, based on this sample. This necessitated control of these factors in multivariate models because being older and being male are both likely to be associated with increased OC body burden. Results from log-linear models are presented in Table 4. Age was a significant predictor of OC body burden in all models. A 10 year increase in age predicted a significant elevation in serum pesticide concentrations of 27.1% for Σ-DDT compounds, 52% for Σ-chlordane compounds, 115.9% for mirex, and 36.8% for HCB. Female sex was negatively associated with OC body burden in all models, but significantly associated only with serum mirex concentration, with a 129.3% decrease. Female sex was non-significantly associated with a 20.6% reduction in serum HCB concentrations.
Table 4.
Associations between predictors (age, sex and location) on serum pesticide concentrations from log-linear regression.
| Dependent Variable |
Predictor | Risk Ratio |
95% CI | % change | P value |
|---|---|---|---|---|---|
| Σ-DDT | |||||
| Age(10 years) | 1.27 | (1.06, 1.51) | 27.1 | 0.009 | |
| Female sex | 0.89 | (0.67, 1.19) | −10.5 | 0.45 | |
| NEC | 1.36 | (0.94, 1.96) | 36.2 | 0.09 | |
| Savoonga | 1.23 | (0.87, 1.76) | 23.9 | 0.23 | |
| Σ-Chlordane | |||||
| Age(10 years) | 1.52 | (1.26, 1.83) | 52.0 | <0.001 | |
| Female sex | 0.83 | (0.61, 1.14) | −16.5 | 0.25 | |
| NEC | 1.32 | (0.89, 1.95) | 32.4 | 0.15 | |
| Savoonga | 1.38 | (0.94, 1.99) | 37.5 | 0.09 | |
| Mirex | |||||
| Age(10 years) | 2.16 | (1.80, 2.58) | 115.9 | <0.0001 | |
| Female sex | 0.43 | (0.32, 0.59) | −129.3 | <0.0001 | |
| NEC | 1.03 | (0.71, 1.50) | 2.9 | 0.88 | |
| Savoonga | 1.04 | (0.72, 1.48) | 3.5 | 0.85 | |
| HCB | |||||
| Age(10 years) | 1.37 | (1.15, 1.62) | 36.8 | 0.0004 | |
| Female sex | 0.79 | (0.60, 1.05) | −20.6 | 0.10 | |
| NEC | 1.60 | (1.13, 2.27) | 60.5 | 0.009 | |
| Savoonga | 1.36 | (0.97. 1.90) | 35.9 | 0.07 |
Gambell was the referent group for both NEC and Savoonga. Female compared to male.
Once sex and age were accounted for, participants maintaining a subsistence camp at NEC tended to have higher serum pesticide concentrations. The strength and significance of this association varied for specific compounds. NEC, as compared to Gambell, was not significantly associated with a 36.2% rise in serum concentrations of Σ-DDT compounds, but significantly associated with a 60.5% increase in serum HCB concentrations. Living in Savoonga was not significantly associated with a 37.5% elevation in Σ-chlordane compounds as compared to Gambell. There was a similar 32.4% elevation in Σ-chlordane for those with camps at NEC, compared to Gambell. Mirex concentrations did not appear to be related to residence or subsistence camp location.
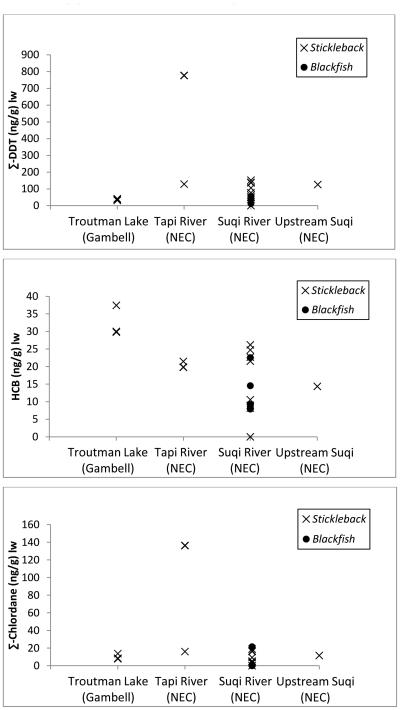
Fish samples were analyzed for 28 individual OC pesticide compounds, and of these 22 individual compounds were detected in any sample (Supplemental Material). These 22 compounds represent approximately 9 technical grade pesticide formulations: DDT, HCH, chlordane, HCB, aldrin, dieldrin, endrin, endosulfan, and mirex. Chlordane compounds, DDT compounds and HCB were present in more than 75% of samples; these compounds appear prevalent in fish samples regardless of geographic location. Of the chlordane compounds, trans-nonachlor was the most prevalent and present at highest concentrations. Median concentrations of trans-nonachlor and oxychlordane were 0.16 ng/g lw and non-detectable, but maximum concentrations were 98.26 ng/g lw and 23.63 ng/g lw, respectively (Figure 2). Of the DDT compounds, p,p’-DDE was the most prevalent and present at highest concentrations of any pesticide with a median of 5.16 ng/g lw and a maximum of 769.89 ng/g lw. Other notable compounds included dieldrin (present in approximately 60% of samples) and mirex (present in approximately 50% of samples). HCB was present at a median of 4.65 ng/g lw and a maximum of 37.44 ng/g lw.
Figure 2.
Major organochlorine pesticides in sentinel fish separated by geographic location (ng/g lw). NEC = Northeast Cape.
The highest detected concentration of p,p’-DDE was present in a Tapi River composite sample. Concentrations of trans-nonachlor and oxychlordane were also highest in this Tapi River sample. The highest HCB concentrations were in the three Troutman Lake composite samples. The highest concentrations of dieldrin were present in Suqi River and Tapi River composite samples. Mirex concentrations were highest in Suqi River composite samples. Visual comparisons of pesticide concentrations between geographic locations did not reveal obvious trends (Figure 2).
Discussion
Population comparisons
The sample of participants from St. Lawrence Island residents contained more women, and was younger than the U.S. general population. Total serum lipid concentrations are similar to those seen in other Arctic indigenous groups (Butler Walker et al. 2003). The median concentration of OC pesticides tended to be higher among St. Lawrence Island residents than the NHANES 2001-2002 U.S. population sample (Table 3). The 95th percentiles tended to be comparable between St. Lawrence Island residents and NHANES participants, probably because most St. Lawrence Island participants were under 50 years of age. A sample of older St. Lawrence Island residents may include individuals with higher body burdens accumulated over time.
Table 3.
Comparison of current study sample to nationally representative sample of National Health and Nutrition Examination Study (NHANES) data from 2001-2002, stratified by sex (μg/kg lw)
| Current median |
Current 95th percentile |
NHANES median |
NHANES 95th percentile |
||
|---|---|---|---|---|---|
| Men | Lindane | nd | 8.7 | nd | nd |
| Oxychlordane | 75.3 | 279.1 | 11.1 | 48.1 | |
| Trans-nonachlor | 54.3 | 124.7 | 18.3 | 77.2 | |
| HCB | 94.3 | 160.5 | nd | nd | |
| o,p’-DDT | nd | 4.0 | nd | nd | |
| p,p’-DDE/PCB85 | 358.5 | 706.4 | 245 | 1900 | |
| Mirex | 28.7 | 75.8 | nd | 50.8 | |
| Women | Lindane | nd | 34.2 | nd | nd |
| Oxychlordane | 72.6 | 215.9 | 11.0 | 52.5 | |
| Trans-nonachlor | 40.2 | 104.3 | 17.5 | 76.8 | |
| HCB | 54.2 | 202.3 | nd | nd | |
| o,p’-DDT | nd | 6.5 | nd | nd | |
| p,p’-DDE/PCB85 | 264.9 | 1159.5 | 256 | 2630 | |
| Mirex | 11.6 | 33.3 | nd | 63.0 |
nd=non-detectable
Some compounds were detected in the St. Lawrence Island samples that were not detected in the NHANES samples. Most notably, HCB was present in all St. Lawrence Island serum samples, and completely absent from NHANES samples. Both o,p’-DDT and γ-HCH (lindane) were present in a small number of St. Lawrence Island samples, but absent from NHANES samples. In general, median concentrations for the current study tended to be higher than medians from NHANES samples, with the exception that females in NHANES have a higher median p,p’-DDE concentration. The 95th percentile for p,p’-DDE in the current study was lower than corresponding NHANES estimates for both men and women. The 95th percentile for mirex in the current study was lower than corresponding NHANES data for women only. As mentioned previously, the current study included primarily people under the age of 50, which likely censored the upper end of the distribution of serum pesticide concentrations. It is likely that the predominant difference in exposure to OC pesticides between St. Lawrence Island residents and the U.S general population is dietary.
DDT and related compounds were present at the highest concentration of the OC pesticides detected in this study. This may have been influenced in part by the co-elution of PCB-85 with p,p’-DDE in the analytical method. Residents of St. Lawrence Island are also exposed to PCBs at a higher rate than the general population, and PCBs were present in these serum samples (Carpenter et al. 2005). Nonetheless, DDT related compounds are also prevalent in the blood of other Alaska Native groups (Middaugh et al. 2001; Rubin et al. 2006) and residents of St. Lawrence Island are also exposed to DDT compounds (Tables 2 & 3).
There are few data on serum OC pesticide concentrations in Alaska Natives. The results from the current study have both similarities and dissimilarities to those reported in a study of Alaska Natives from the Aleutian and Pribolof Islands (API) (Middaugh et al. 2001). These Alaska Natives are an ideal comparison group because they also reside on remote Bering Sea islands and rely heavily on traditional food sources. Overall, concentrations tended to be similar, although the upper ranges were higher for Aleutian and Pribilof villages. Mean concentrations of oxychlordane are almost identical when comparing the current study (85.9 μg/kg lw) with API villagers (84.4 μg/kg lw). Interestingly, trans-nonachlor concentrations were not as similar, with the current study reporting a mean of 52.07 μg/kg lw while API villagers had a mean of 207 μg/kg lw. API villagers had a mean mirex concentration of 12.5 μg/kg lw and a maximum of 165 μg/kg lw, in comparison to the current study which had a mean mirex concentration of 22.1 μg/kg lw and a maximum of 80.9 μg/kg lw. The mean concentration of p,p’-DDE in the current study (386.4 μg/kg lw) was notably lower than in API villagers (1740 μg/kg lw ). Unfortunately, Middaugh et al. (2001) did not report concentrations of HCB. The differences in concentrations between API and St. Lawrence Island samples may be due in part to the low overall age range in the current study.
A case control study of breast cancer using banked serum samples from Alaska Natives collected between 1983-1987 detected HCB in 96.8% of cases and 100% of controls (Rubin et al. 2006). This indicates that HCB exposure is likely widespread among Alaska Natives. Median concentrations from women in the case control study were 1.49 μg/L ww for cases and 2.63 μg/L ww for controls, higher than the median of 0.27 μg/L ww for women in the current study. Overall mean wet weight HCB concentrations in the current study were 0.39 μg/L ww. Serum HCB concentrations of 28 μg/kg lw were reported from a study of 20 pregnant Inuit women from the North Slope of Alaska (Van Oostdam et al. 2004). Among women on St. Lawrence Island, HCB concentrations appear to be slightly higher with a median of 54.2 μg/kg lw.
A review of POP concentrations in plasma of arctic indigenous groups in Canada reported similar concentrations of p,p’-DDE, oxychlordane and trans-nonachlor (Donaldson et al. 2010). Concentrations in the current study tended to be on the lower end of the distribution seen in Canada. Therefore, despite our relatively small sample of St. Lawrence Island residents, our results suggest that organochlorine pesticide exposure on St. Lawrence Island is similar to other arctic indigenous groups in the U.S. and Canada. Further research with a larger and more representative sample could confirm this assessment.
Sources of exposure
A history of activities near NEC was positively associated with select OC pesticide concentrations (Table 4). A significant association was found between having subsistence camps at NEC and serum concentration of HCB. The association between NEC and Σ-DDT was positive but not significant. The serum concentration of Σ-chlordane was positively but non-significantly associated with NEC, and there was a quantitatively stronger association for Savoonga. Food sharing practices within Savoonga may explain higher serum pesticide concentrations as compared to Gambell. Mirex concentration did not appear to be related to NEC. Overall the data are suggestive of a relationship between subsistence hunting and gathering activities at NEC and an increased exposure to OC compounds, although individuals may also be exposed through inhalation of volatile contaminants in air or dust and ingestion of drinking water. Consumption of locally contaminated plants and animals may reasonably be expected to produce an increase in exposure. Plants and animals harvested in proximity of the NEC FUDS are contaminated with POPs, specifically PCBs, mirex and DDE (Scrudato et al. 2012). There is also evidence that plants gathered from the military site are contaminated due to residual soil and dust particles which contain POPs (Scrudato et al. 2012). Lack of detailed dietary information precludes further investigation into specific sources of pesticide residues. However, many of the traditional food animals harvested on St. Lawrence Island contain appreciable concentrations of organochlorine pesticides (Welfinger-Smith et al. 2011).
Despite non-significance, the modeled effect estimates for mirex are larger than for other compounds, likely due to the lower concentration and reduced range of the distribution. For example, being female is associated with a 129.3% reduction in mirex concentration. This represents the difference between mean concentrations of approximately 34 μg/kg lw for men and 13 μg/kg lw for women after controlling for age and location.
Sentinel fish
Although stickleback and Alaska blackfish are not consumed as traditional foods by the population of St. Lawrence Island, these are useful sentinel species for assessment of contaminant levels. Organochlorine pesticide concentrations in stickleback and Alaska blackfish from St. Lawrence Island appear to be similar to those found in higher trophic level species elsewhere in Alaska. Unfortunately, data are lacking on OC concentrations in low trophic level fish species in Alaska, which would provide a better comparison for stickleback and blackfish.
Concentrations of HCB and chlordanes in Alaskan fish tend to be higher than those from the contiguous United States (Flanagan Pritz et al. 2014). HCB and mirex concentrations in the current study are similar to those reported in several fish species from the Aleutian Islands. For example in Aleutian Dolly Varden (Salvelinus malma), mean HCB concentrations were 0.77 ng/g ww and mean mirex concentrations were 0.19 ng/g ww (Hardell et al. 2010). Concentrations of chlordanes in St. Lawrence Island fish appear similar to those in lake trout (Salvelinus namaycush) and grayling (Thymallus arcticus) from Schrader Lake in northeast Alaska, where the mean concentrations of Σ-chlordane were 0.7 ng/g ww and 0.2 ng/g ww, respectively (Wilson et al. 1995).
Median concentrations of DDE compounds in St. Lawrence Island fish samples were similar to those reported in Aleutian Island fish; in Aleutian Dolly Varden mean DDE concentrations were 1.1 ng/g ww. However, one Tapi River composite sample contained p,p’-DDE concentrations (26.48 ng/g ww) higher than would be expected based on the trophic level of stickleback. This is within the range seen in adipose tissue from seals (Erignathus barbatus), walrus (Odobenus rosmarus) and polar bear (Ursus maritimus) taken as food on the island (Welfinger-Smith et al. 2011); however, seals and bears from other areas of Alaska contain higher concentrations than those on the island (Kucklick et al. 2002). The p,p’-DDE concentration in the Tapi River stickleback sample was within the range (0-56 ng/g ww) observed in sockeye salmon (Oncorhycus nerka) from the Aleutian Islands (Hardell et al. 2010), and similar to the mean (S.D.) concentration of 34.9 (96.6) ng/g ww in lake trout from Peter Lake in NWT, Canada (Kidd et al. 1998). Based on these comparisons, it appears that resident St. Lawrence Island stickleback and blackfish accumulate OC pesticides at a rate higher than predicted by trophic level alone. This is suggestive of local sources of contamination.
Overall, the tissue concentrations in stickleback suggest OC pesticide contamination is geographically widespread on St. Lawrence Island. Concentrations of Σ-DDT, Σ-chlordanes and HCB in the downstream section of the Suqi River were not consistently above other sites (Figure 2). There was no evidence that fish in the downstream section of the Suqi River have higher concentrations of any detected OC pesticide (supplemental material). Atmospheric transport and deposition are likely to be the major source of exposure for most of the island, but this does not preclude military contamination as a source of exposure for some individuals or populations. Significant remediation of the military site has occurred, and this may be reflected in tissue concentration of the resident fish. In addition, previously undocumented military contamination of the Tapi River and Troutman Lake are possible, as both are in proximity to FUDS.
The patterns of pesticide body burdens in sentinel fish species tended to reflect human body burdens. The predominant compounds in both fish tissues and human blood samples were chlordane compounds, DDT compounds, and HCB. This is true both in terms of concentration and % samples above the detection limit. The Suqi River is sometimes used as a potable water source by people who use NEC, however none of the other water bodies sampled are routinely used as potable water sources, and significant human exposure from water contamination is unlikely.
Median lipid adjusted total chlordane concentrations were approximately 16-fold higher in human serum than in sentinel fish samples. The single largest contributor to total chlordane concentration in fish samples was trans-nonachlor, whereas oxychlordane was the predominant compound in human samples. In human serum samples, median lipid adjusted p,p’-DDE concentrations were approximately ten-fold higher than the concentration in fish. However, the range was similar due an outlier in the fish data. Median lipid adjusted HCB concentrations were approximately four-fold higher in human serum than in fish samples.
Potential health effects
Many organochlorine pesticides appear to be capable of altering endocrine homeostasis, predominantly by their action as estrogen receptor agonists (Lemaire et al. 2006), but also through androgen receptor antagonism (Kelce et al. 1995; Lemaire et al. 2004). Serum concentrations of p,p’-DDE in the same range as the current study are associated with reductions in dihydrotestosterone concentrations among otherwise healthy adult men (Emeville et al. 2013). Among adults who lived in proximity to a former organochlorine production site, elevated concentrations of HCB, p,p’-DDT, p,p’-DDD and mirex were associated with decreases in luteinizing hormone among perimenopausal and postmenopausal women, but not premenopausal women (Freire et al. 2013). Many organochlorine pesticides are cancer promoting agents, and may also promote more malignant forms of cancer (Mrema et al. 2013). Additionally, exposure to relatively low concentrations of DDE are associated with cognitive deficits in a number of studies (Ribas-Fito et al. 2003; Eskenazi et al. 2006; Torres-Sanchez et al. 2013).
Little information is available on the potential health effects of cumulative and simultaneous exposure to multiple POPs. Residents of St. Lawrence Island are exposed not only to organochlorine pesticides, but also to PCBs and bioaccumulative metals such as mercury (Carpenter et al. 2005; Welfinger-Smith et al. 2011). Furthermore, there are marked health disparities between Alaska Natives/American Indians and the U.S. general population. Alaska Natives and American Indians have comparatively poor access to, and utilization of, health care resources, and tend to be of lower socio-economic status and have higher rates of alcohol use disorders and nicotine dependence (Castor et al. 2006; Falk et al. 2006). These factors may influence susceptibility to OC exposures.
Traditional foods
An in depth discussion of the merits of the traditional diet are outside the scope of this research. Briefly, despite contamination by POPs, the traditional diet is widely accepted to be the healthiest diet available to indigenous peoples of the Arctic. The transition to nutrient-poor store bought foods is associated with inadequate intake of micronutrients, while a traditional diet tends to increase consumption of micronutrients and decrease intake of sugar carbohydrates and fat (Kuhnlein et al. 2004; Bersamin et al. 2007; Erber et al. 2010; Hopping et al. 2010). Further, high intake of omega-3 fatty acids in subsistence diets may be protective against metabolic syndrome and obesity related diseases, although not protective against cancer (Ebbesson et al. 2005; Makhoul et al. 2011).
Limitations
This study represents a non-random convenience sample of a poorly studied population; additionally, the sample was small leading to low statistical power. Nevertheless, the results of this study provide valuable information on general exposure trends in this remote population. This study did not include older members of the population, which likely led to an underestimation of the true population parameters for serum OC concentrations.
The compounds DDE and PCB-85 were co-eluted in our analytical method. This could bias the results toward a positive association between NEC and Σ-DDT if PCB-85 was present in higher concentrations among individuals using NEC as a hunting and fishing site. Previous research suggests this may be the case (Carpenter et al. 2005).
In the models, the main exposure variable was a categorical variable meant to capture exposure to pesticides via both direct contact with the military site as well as site specific food contamination. A lack of detailed exposure information, such as amount of time spent in close proximity to NEC and the type and amount of food consumed from the area present the possibility of exposure misclassification, which would likely bias the results toward the null. Residual confounding is possible if those with subsistence camps at NEC were more likely to eat traditional foods compared to participants in Gambell or Savoonga. While traditional food consumption is widespread throughout the island, this confounding bias cannot be ruled out.
One limitation of composite fish samples is the lack of knowledge of individual fish data; it is possible for outliers to influence the overall mean of a composite sample without the investigators’ knowledge. However, composite samples also reflect a larger number of individuals, allowing relatively few analyzed samples to better represent population trends. Soil samples were not collected from any sites, and therefore OC compounds which partitioned into the organic fraction of soils were not assessed. The geographic distribution of compounds in soils and sediments might be different than those in water and sentinel fish species.
Post hoc power and sample size
Based on the observed differences in mean serum pesticide concentrations it was possible to calculate the actual statistical power and the sample size needed to detect this effect with 80% power. This analysis indicated that several statistical tests used were underpowered, meaning that a significant effect might easily be missed. Power to detect the observed mean difference in Σ-DDT was 61.4%, for HCB 77.2%, for Σ-chlordanes 11.1%, and for mirex 15%. Sample size calculations indicate that attainable increases in sample size would provide acceptable power to detect effect sizes of the magnitude seen in this study. For example, to detect the observed differences between mean serum pesticide concentrations between NEC and Gambell the group size would need to be 36 (72 total) for Σ-DDT, 331 (662 total) for Σ-chlordane, and 203 (406 total) for mirex. For HCB the necessary sample size is 25 per group, which was nearly met, explaining the significant result.
Conclusions
The residents of St. Lawrence Island appear to be exposed to OC pesticides at a comparatively higher rate than the general U.S. population. The body burdens of OC pesticides tend to be similar to those of other Alaska Native groups and First Nations of Canada. Data suggest that exposure is predominately mediated through the subsistence way of life via consumption of traditional food animals that accumulate pesticides that have undergone long range transport. Data from sentinel fish species suggest current OC pesticide contamination is widespread without clear relationships to FUDS. Associations exist between local military contamination at NEC and select OC pesticides in human serum. This may be due to an increase in exposure due to the military site, which may have occurred before site remediation.
Bibliography
- AMAP . Artic Pollution 2002: Persistent organic pollutants, heavy metals, radioactivity, human health, changing pathways. Arctic Monitoring and Assessment Programme (AMAP; Oslo, Norway: 2002. [Google Scholar]
- AMAP . Assessment 2002: Persistent organic pollutants in the Arctic. Arctic Monitoring and Assessment Programme (AMAP; Oslo, Norway: 2004. [Google Scholar]
- AMAP . Assessment 2009: Human health in the Arctic. Arctic Monitoring and Asessment Programme (AMAP; Oslo, Norway: 2009. [Google Scholar]
- AMAP . Trends in stockholm convention persistent organic pollutants (POPs in arctic air, human media and biota. Arctic Monitoring and Asessment Programme (AMAP; Oslo, Norway: 2014. [Google Scholar]
- Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska Native communities: the CANHR Study. Int. J. Circumpolar Health. 2007;66(1):62–70. doi: 10.3402/ijch.v66i1.18228. [DOI] [PubMed] [Google Scholar]
- Bjerregaard P, Dewailly E, Ayotte P, Pars T, Ferron L, Mulvad G. Exposure of Inuit in Greenland to organochlorines through the marine diet. J Toxicol. Environ. Health. A. 2001;62(2):69–81. doi: 10.1080/009841001455490. [DOI] [PubMed] [Google Scholar]
- Burkow IC, Kallenborn R. Sources and transport of persistent pollutants to the Arctic. Toxicol. Lett. 2000;112–113:87–92. doi: 10.1016/s0378-4274(99)00254-4. [DOI] [PubMed] [Google Scholar]
- Butler Walker J, Seddon L, McMullen E, Houseman J, Tofflemire K, Corriveau A, Weber J-P, Mills C, Smith S, Van Oostdam J. Organochlorine levels in maternal and umbilical cord blood plasma in Arctic Canada. Sci. Total Environ. 2003;302(1–3):27–52. doi: 10.1016/s0048-9697(02)00319-4. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, DeCaprio AP, O’Hehir D, Akhtar F, Johnson G, Scrudato RJ, Apatiki L, Kava J, Gologergen J, Miller PK. Polychlorinated biphenyls in serum of the Siberian Yupik people from St. Lawrence Island, Alaska. Int. J. Circumpolar Health. 2005;64(4):322–335. doi: 10.3402/ijch.v64i4.18010. [DOI] [PubMed] [Google Scholar]
- Castor ML, Smyser MS, Taualii MM, Park AN, Lawson SA, Forquera RA. A nationwide population-based study identifying health disparities between American Indians/Alaska Natives and the general populations living in select urban counties. Am. J. Public Health. 2006;96(8):1478–1484. doi: 10.2105/AJPH.2004.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Third report on human exposure to environmental chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2005. [Google Scholar]
- Damstra T. Potential effects of certain persistent organic pollutants and endocrine disrupting chemicals on the health of children. J Toxicol Clin Toxicol. 2002;40(4):457–65. doi: 10.1081/clt-120006748. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Ayotte P, Bruneau S, Laliberté C, Muir D, Norstrom RJ. Inuit exposure to organochlorines through the aquatic food chain in arctic quebec. Environ. Health Perspect. 1993;101(7):618–620. doi: 10.1289/ehp.93101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SG, Van Oostdam J, Tikhonov C, Feeley M, Armstrong B, Ayotte P, Boucher O, Bowers W, Chan L, Dallaire F, Dallaire R, Dewailly É, Edwards J, Egeland GM, Fontaine J, Furgal C, Leech T, Loring E, Muckle G, Nancarrow T, Pereg D, Plusquellec P, Potyrala M, Receveur O, Shearer RG. Environmental contaminants and human health in the Canadian Arctic. Sci. Total Environ. 2010;408(22):5165–5234. doi: 10.1016/j.scitotenv.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Ebbesson SO, Risica PM, Ebbesson LO, Kennish JM, Tejero ME. Omega-3 fatty acids improve glucose tolerance and components of the metabolic syndrome in Alaskan Eskimos: the Alaska Siberia project. Int. J. Circumpolar Health. 2005;64(4):396–408. doi: 10.3402/ijch.v64i4.18016. [DOI] [PubMed] [Google Scholar]
- Elabbas LE, Finnilä MA, Herlin M, Stern N, Trossvik C, Bowers WJ, Nakai J, Tuukkanen J, Heimeier RA, Åkesson A, Håkansson H. Perinatal exposure to environmental contaminants detected in Canadian Arctic human populations changes bone geometry and biomechanical properties in rat offspring. J Toxicol. Environ. Health. A. 2011;74(19):1304–1318. doi: 10.1080/15287394.2011.590103. [DOI] [PubMed] [Google Scholar]
- Emeville E, Giton F, Giusti A, Oliva A, Fiet J, Thome JP, Blanchet P, Multigner L. Persistent organochlorine pollutants with endocrine activity and blood steroid hormone levels in middle-aged men. PLoS ONE. 2013;8(6):e66460. doi: 10.1371/journal.pone.0066460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber E, Hopping BN, Beck L, Sheehy T, De Roose E, Sharma S. Assessment of dietary adequacy in a remote Inuvialuit population. J. Hum. Nutr. Diet. 2010;23:35–42. doi: 10.1111/j.1365-277X.2010.01098.x. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, Jewell NP. In utero exposure to dichlorodiphenyltrichloroethane (DDT and dichlorodiphenyldichloroethylene (DDE and neurodevelopment among young Mexican American children. Pediatrics. 2006;118(1):233–41. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders. Alcohol Res Health. 2006;29(3):162–171. [PMC free article] [PubMed] [Google Scholar]
- Flanagan Pritz CM, Schrlau JE, Massey Simonich SL, Blett TF. Contaminants of emerging concern in fish from western U.S. and Alaskan national parks — spatial distribution and health thresholds. J. Am. Water Resour. Assoc. 2014;50(2):309–323. [Google Scholar]
- Freire C, Koifman RJ, Sarcinelli PN, Rosa ACS, Clapauch R, Koifman S. Association between serum levels of organochlorine pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. Int. J. Hyg. Environ. Health. 2013;217(2):370–378. doi: 10.1016/j.ijheh.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Godduhn A, Duffy LK. Multi-generation health risks of persistent organic pollution in the far north: use of the precautionary approach in the Stockholm Convention. Environ. Sci. Policy. 2003;6(4):341–353. [Google Scholar]
- Hardell S, Tilander H, Welfinger-Smith G, Burger J, Carpenter DO. Levels of polychlorinated biphenyls (PCBs and three organochlorine pesticides in fish from the Aleutian Islands of Alaska. PLoS ONE. 2010;5(8):e12396. doi: 10.1371/journal.pone.0012396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopping BN, Mead E, Erber E, Sheehy C, Roache C, Sharma S. Dietary adequacy of Inuit in the Canadian Arctic. J. Hum. Nutr. Diet. 2010;23:27–34. doi: 10.1111/j.1365-277X.2010.01099.x. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995;375(6532):581–5. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kidd KA, Hesslein RH, Ross BJ, Koczanski K, Stephens GR, Muir DCG. Bioaccumulation of organochlorines through a remote freshwater food web in the Canadian Arctic. Environ. Pollut. 1998;102(1):91–103. [Google Scholar]
- Kitts DD, Chen X-M, Broda P. Polyaromatic hydrocarbons of smoked cured muscle foods prepared by Canadian Tl'azt'en and Llheidli T'enneh First Nation Communities. J Toxicol. Environ. Health. A. 2012;75(21):1249–1252. doi: 10.1080/15287394.2012.709410. [DOI] [PubMed] [Google Scholar]
- Kucklick JR, Struntz WD, Becker PR, York GW, O'Hara TM, Bohonowych JE. Persistent organochlorine pollutants in ringed seals and polar bears collected from northern Alaska. Sci Total Environ. 2002;287(1-2):45–59. doi: 10.1016/s0048-9697(01)00997-4. [DOI] [PubMed] [Google Scholar]
- Kuhnlein HV, Receveur O, Soueida R, Egeland GM. Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J. Nutr. 2004;134(6):1447–1453. doi: 10.1093/jn/134.6.1447. [DOI] [PubMed] [Google Scholar]
- Laird BD, Goncharov AB, Chan HM. Body burden of metals and persistent organic pollutants among Inuit in the Canadian Arctic. Environ. Int. 2013;59:33–40. doi: 10.1016/j.envint.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006;79(12):1160–9. doi: 10.1016/j.lfs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Terouanne B, Mauvais P, Michel S, Rahmani R. Effect of organochlorine pesticides on human androgen receptor activation in vitro. Toxicol. Appl. Pharmacol. 2004;196(2):235–246. doi: 10.1016/j.taap.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Makhoul Z, Kristal AR, Gulati R, Luick B, Bersamin A, O'Brien D, Hopkins SE, Stephensen CB, Stanhope KL, Havel PJ, Boyer B. Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. Eur J Clin Nutr. 2011;65(7):808–817. doi: 10.1038/ejcn.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh J, Verbrugge L, Haars M, Schloss M, Yett G. Assessment of exposure to persistent organic pollutants (POPs in 5 Aleutian and Pribilof villages. State of Alaska Epidemiology Bulletin. 2001;5(5):1–18. [Google Scholar]
- Miller PK, Waghiyi V, Welfinger-Smith G, Byrne SC, Kava J, Gologergen J, Eckstein L, Scrudato R, Chiarenzelli J, Carpenter DO, Seguinot-Medina S. Community-based participatory research projects and policy engagement to protect environmental health on St Lawrence Island, Alaska. Int. J. Circumpolar Health. 2013;72(supplement):e21656. doi: 10.3402/ijch.v72i0.21656. http://dx.doi.org/10.3402/ijch.v72i0.21656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307(0):74–88. doi: 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Odland JO, Deutch B, Hansen JC, Burkow IC. The importance of diet on exposure to and effects of persistent organic pollutants on human health in the Arctic. Acta Paediatr. 2003;92(11):1255–1266. [PubMed] [Google Scholar]
- Puertas R, Lopez-Espinosa M-J, Cruz F, Ramos R, Freire C, Pérez-García M, Abril A, Julvez J, Salvatierra M, Campoy C, Olea N. Prenatal exposure to mirex impairs neurodevelopment at age of 4 years. NeuroToxicology. 2010;31(1):154–160. doi: 10.1016/j.neuro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N, Cardo E, Sala M, Eulalia de Muga M, Mazon C, Verdu A, Kogevinas M, Grimalt JO, Sunyer J. Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics. 2003;111:e580–5. doi: 10.1542/peds.111.5.e580. 5 Pt 1. [DOI] [PubMed] [Google Scholar]
- Rubin CH, Lanier A, Kieszak S, Brock JW, Koller KR, Strosnider H, Needham L, Zahm S, Harpster A. Breast cancer among Alaska Native women potentially exposed to environmental organochlorine chemicals. Int J Circumpolar Health. 2006;65(1):18–27. doi: 10.3402/ijch.v65i1.17885. [DOI] [PubMed] [Google Scholar]
- Rubin CH, Lanier A, Socha M, Brock JW, Kieszak S, Zahm S. Exposure to persistent organochlorines among Alaska Native women. Int. J. Circumpolar Health. 2001;60(2):157–69. [PubMed] [Google Scholar]
- Salehi F, Turner MC, Phillips KP, Wigle DT, Krewski D, Aronson KJ. Review of the etiology of breast cancer with special attention to organochlorines as potential endocrine disruptors. J. Toxicol. Environ. Health B. 2008;11(3-4):276–300. doi: 10.1080/10937400701875923. [DOI] [PubMed] [Google Scholar]
- Scrudato R, Chiarenzelli J, Miller P, Alexander C, Arnason J, Zamzow K, Zweifel K, Gologergen J, Kava J, Waghiyi V. Contaminants at arctic formerly used defense sites. J Local Glob. Health Sci. 2012;1(2):1–15. [Google Scholar]
- Sovocool GW, Lewis RG, Harless RL, Wilson NK, Zehr RD. Analysis of technical chlordane by gas chromatography/mass spectrometry. Anal. Chem. 1977;49(6):734–740. doi: 10.1021/ac50014a018. [DOI] [PubMed] [Google Scholar]
- Suk WA, Avakian MD, Carpenter D, Groopman JD, Scammell M, Wild CP. Human exposure monitoring and evaluation in the Arctic: the importance of understanding exposures to the development of public health policy. Environ. Health Perspect. 2004;112(2):113–20. doi: 10.1289/ehp.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Dean A, Soe MM. OpenEpi: A web-based epidemiologic and statistical calculator for public health. Public Health Reports. 2009;124(3):471–474. doi: 10.1177/003335490912400320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro S, Matsumura F. Metabolism of trans-nonachlor and related chlordane components in rat and man. Arch Environ Contam Toxicol. 1978;7(1):113–27. doi: 10.1007/BF02332042. [DOI] [PubMed] [Google Scholar]
- Teilmann G, Juul A, Skakkebæk NE, Toppari J. Putative effects of endocrine disrupters on pubertal development in the human. Best Pract. Res. Clin. Endocrinol. Metab. 2002;16(1):105–121. doi: 10.1053/beem.2002.0184. [DOI] [PubMed] [Google Scholar]
- Torres-Sanchez L, Schnaas L, Rothenberg SJ, Cebrian ME, Osorio-Valencia E, Hernandez Mdel C, Garcia-Hernandez RM, Lopez-Carrillo L. Prenatal p,p -DDE exposure and neurodevelopment among children 3.5-5 years of age. Environ. Health Perspect. 2013;121(2):263–8. doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oostdam J, Gilman A, Dewailly E, Usher P, Wheatley B, Kuhnlein H, Neve S, Walker J, Tracy B, Feeley M, Jerome V, Kwavnick B. Human health implications of environmental contaminants in Arctic Canada: a review. Sci. Total Environ. 1999;230(1–3):1–82. doi: 10.1016/s0048-9697(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Van Oostdam JC, Dewailly E, Gilman A, Hansen JC, Odland JO, Chashchin V, Berner J, Butler-Walker J, Lagerkvist BJ, Olafsdottir K, Soininen L, Bjerregard P, Klopov V, Weber JP. Circumpolar maternal blood contaminant survey, 1994–1997 organochlorine compounds. Sci. Total Environ. 2004;330(1–3):55–70. doi: 10.1016/j.scitotenv.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Welfinger-Smith G, Minholz JL, Byrne S, Waghiyi V, Gologergen J, Kava J, Apatiki M, Ungott E, Miller PK, Arnason JG, Carpenter DO. Organochlorine and metal contaminants in traditional foods from St. Lawrence Island, Alaska. J Toxicol. Environ. Health. A. 2011;74(18):1195–214. doi: 10.1080/15287394.2011.590099. [DOI] [PubMed] [Google Scholar]
- Wilson R, Allen-Gil S, Griffin D, Landers D. Organochlorine contaminants in fish from an Arctic lake in Alaska, USA. Sci. Total Environ. 1995;160–161:511–519. doi: 10.1016/0048-9697(95)04385-e. [DOI] [PubMed] [Google Scholar]