Abstract
Chemists have long aspired to synthesize molecules the way that plants do — using sunlight to facilitate the construction of complex molecular architectures. Nevertheless, the use of visible light in photochemical synthesis is fundamentally challenging because organic molecules tend not to interact with the wavelengths of visible light that are most strongly emitted in the solar spectrum. Recent research has begun to leverage the ability of visible light absorbing transition metal complexes to catalyze a broad range of synthetically valuable reactions. In this review, we highlight how an understanding of the mechanisms of photocatalytic activation available to these transition metal complexes, and of the general reactivity patterns of the intermediates accessible via visible light photocatalysis, has accelerated the development of this diverse suite of reactions.
The year 2012 marked the centennial anniversary of a now-famous article entitled "The Photochemistry of the Future," in which the pioneering chemist Giacomo Ciamician challenged the scientists of his day to imagine a chemical industry that could synthesize chemicals in the same manner that plants do — using sunlight as a safe, inexpensive, abundant, and renewable source of chemical potential (1). In the past several decades, chemists have made remarkable strides towards increasingly efficient conversion of solar energy into electricity and chemical fuels (2, 3). The use of solar energy in the synthesis of value-added, structurally complex organic compounds has, however, been considerably less well investigated, and Ciamician's grand vision has yet to be fully realized (4, 5).
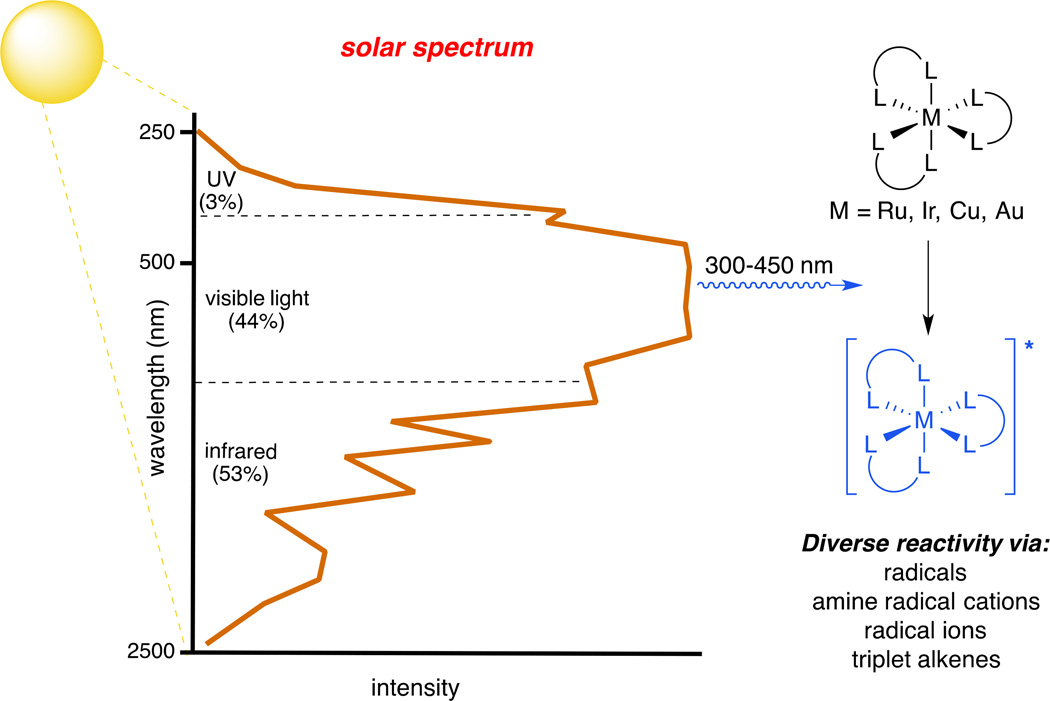
The photochemical synthesis of complex organic molecules is challenging for several reasons. First, organic molecules tend to absorb only photons in the ultraviolet (UV) region that are not abundant in the solar radiation that penetrates the atmosphere (Fig 1). This has constrained the rate of development in large-scale industrial photochemical synthesis, as energy-efficient sources of UV light have only recently become available. Moreover, UV photons are quite high in energy — on the order of a carbon–carbon bond — and can cause significant unproductive decomposition reactions to occur, particularly when relatively weak bonds are present or when the target compounds possess significant structural complexity. There are many notable examples of classic syntheses of complex targets that utilize photochemical key steps, but photochemical synthesis as a whole has long been considered to be the purview of a small community of specialists, rather than as a core component of the standard synthetic repertoire (6, 7).
Fig. 1. Converting solar energy into chemical potential.
Certain transition metal complexes are strong absorbers of visible light and can thereby harness solar energy for chemical synthesis, particularly by driving radical-mediated transformations from their photoexcited states (10).
Recently, there has been a dramatic renaissance of interest in photochemistry among synthetic organic chemists. This development has been inspired in large part by the recognition that the same transition metal complexes that have been so productively utilized to convert sunlight to electrochemical potential can also be used to catalyze useful and unique organic reactions that are initiated by visible light irradiation (8, 9).
The objective of this review is to explain the features of visible light photocatalysis that have captured the attention of synthetic chemists. First, we will briefly outline the diverse mechanisms by which transition metal photocatalysts can be used to activate organic compounds towards subsequent transformations. Then, we will show that transition metal photocatalysts have been used to generate a wide range of highly reactive intermediates (radicals, amine radical cations, radical ions, and triplet alkenes), each of which display distinctive patterns of reactivity that can be used to access different classes of complex organic structures.
Mechanisms of visible light photocatalysis
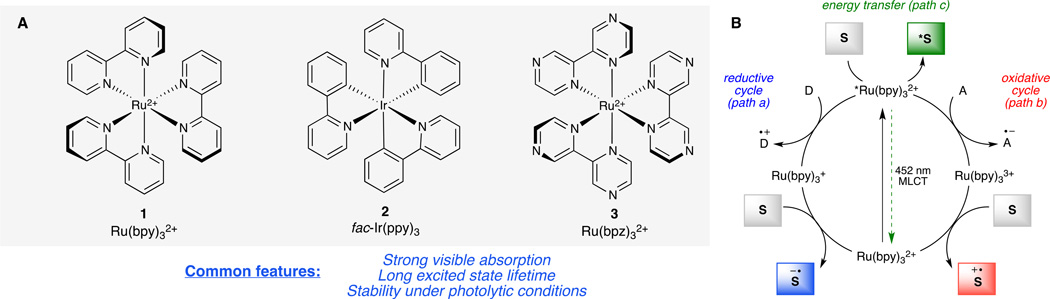
A wide variety of transition metal complexes with varying photochemical properties have been examined as photocatalysts for synthetic applications. The majority of these have been ruthenium and iridium complexes, although copper and gold species have also recently been investigated in this context (11, 12). Figure 2 summarizes the key photochemical properties of Ru(bpy)32+ [bpy is 2,2’-bipyridine] complex (1), the most well studied transition metal complex in the context of both synthetic photocatalysis as well as solar energy conversion. This complex exhibits a strong, broad absorbance in the visible range (λmax = 452 nm) that results in the production of a long-lived excited state (τ ~ 0.9 μs) (13, 14).
Fig. 2. Visible light photocatalysis.
(A) Ruthenium and iridium complexes, such as 1–3, readily absorb visible light and can mediate numerous photochemical transformations (13, 14). (B) Photoexcited Ru*(bpy)32+ can act as an electron shuttle, interacting with sacrificial electron donors D (path i) or acceptors A (path ii) to yield either a strongly reducing or oxidizing catalyst toward organic substrates S. Ru*(bpy)32+ can also directly transfer energy to an organic substrate to yield electronically excited species (path iii). Abbreviations: bpy, 2,2'-bipyridine; bpz, 2,2’-bipyrazine; ppy, 2-phenylpyridine.
This photoexcited complex is both a stronger oxidant and reductant than its corresponding ground state; remarkably, both electrochemical potentials lie within a range that can perform useful chemical work. Thus, photoactivation of Ru(bpy)32+ has most commonly been used to drive processes initiated by single-electron transfer. This property of Ru(bpy)32+ has been instrumental in the development of many strategies for the conversion of solar energy into electricity and into electrochemically generated chemical fuels (15–17). In the context of organic synthesis, Ru*(bpy)32+ can initiate the one-electron reduction of a variety of electron-deficient substrates, or it can effect the one-electron oxidation of electron-rich substrates. In other words, the photoinduced electron transfer properties of Ru(bpy)32+ can easily be coupled to electrochemically induced transformations of organic molecules (Fig. 2B, paths i and ii).
Alternatively, Ru*(bpy)32+ can directly transfer energy to a suitable organic substrate (Fig. 2B, path iii) (13). The resulting electronically excited organic compound reacts quite differently than it would in the ground state. These high-energy intermediates are often useful for reactions that construct strained or otherwise structurally unusual molecular scaffolds that are difficult to assemble by non-photochemical means (18, 19).
Many terms have been used to describe these various modes of photochemical activation. The photoactivation of organic molecules by electron-transfer processes has been variously referred to as photoinduced electron transfer (PET) sensitization or, more recently, as photoredox catalysis. Similarly, the indirect generation of electronically excited states of organic substrates has been called photosensitization or energy transfer photocatalysis. We prefer to use the blanket term "photocatalysis" to describe both modes of activation, as the unambiguous determination of the mechanism of a photochemical reaction can be challenging and the use of a single term highlights the similarities between these two approaches to photochemical synthesis (20).
Photogeneration of organic radicals
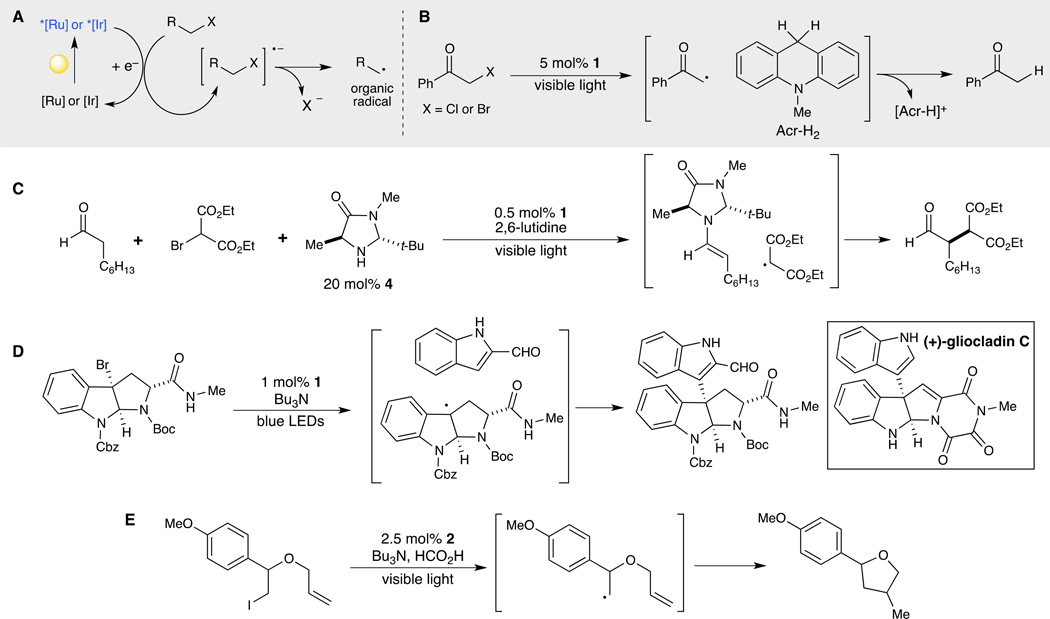
Much of the activity in visible light photocatalysis over the past five years has concerned reactions of photogenerated organic radicals (Fig. 3A). The photocatalytic reductive dehalogenation of α-haloketones with dihydroacridine in the presence of Ru(bpy)32+ (1) was first described by Fukuzumi in 1990; the key intermediate in this transformation is a highly electrophilic α-ketoradical generated by reduction of the bromide by photoexcited Ru*(bpy)32+ (Fig. 3B) (21). This strategy for the photoreductive generation of highly reactive radical intermediates from alkyl halides is an intriguing complement to more established methods for the production of carbon-centered radical species; in particular, many of the most common methods for the thermal formation of organic radicals involve highly toxic tin or pyrophoric boron compounds (22). The ability to access the diverse reactivity of electrophilic radicals via visible light activation, therefore, is highly attractive.
Fig. 3. Photoreduction of alkyl halides for radical reactions.
(A) Photoexcited ruthenium or iridium complexes can reduce electron deficient alkyl halides to radical ions that readily undergo fragmentation to an electrophilic organic radical. (B) This reactivity was first explored by Fukuzumi in the dehalogenation of α-halocarbonyl compounds (21) and later revisited by MacMillan (C) in the context of an asymmetric α-alkylation of aldehydes through the merging of photo and organocatalysis (23). (D) The generation of radicals through visible light photoreduction of alkyl halides is mild and selective, as demonstrated in the synthesis of (+)-gliocladin C (30). (E) The range of alkyl an aryl halides susceptible to photoreduction can be extended by tuning the photoelectrochemical properties of the catalyst (31). Abbreviations: Acr-H2, 9,10-dihydro-10-methylacridine; Boc, tert-butyloxycarbonyl; Bu, butyl; Cbz, carbobenzyloxy; LED, light-emitting diode; Ph, phenyl; t-Bu, tertiary-butyl.
The synthetic potential of this method for photochemical radical generation, however, was not fully recognized until 2008, when it was exploited by MacMillan to develop an enantioselective α-alkylation of aldehydes with organocatalyst 4 (Fig. 3C) (23). This transformation is built upon MacMillan's long-standing interest in enantioselective reactions of achiral aldehydes involving the in situ formation of chiral enamines by condensation with secondary amine organocatalysts (24–26). MacMillan showed that a range of α-ketoradicals, generated from Ru(bpy)32+-catalyzed photoreduction of α-halocarbonyl compounds, react efficiently and with excellent stereocontrol with chiral enamines. While this initial work focused upon the formation of radicals from α-haloketones and α-haloesters, MacMillan has subsequently reported conditions for the formation of highly electrophilic benzyl and trifluoromethyl radicals from the corresponding alkyl halides as well (27, 28).
The direct enantioselective catalytic α-alkylation of carbonyl compounds is synthetically highly useful. However, an arguably broader impact of MacMillan’s initial report was the demonstration that photocatalytic reduction of alkyl halides can be used to access the general complexity-building reactivity of electrophilic organic radicals. This strategy has subsequently been exploited by many research groups and has resulted in the development of a diverse range of new chemical transformations. For example, Stephenson has used the reductive dehalogenation of activated alkyl and aryl halides to effect photocatalytic alkylation of heteroarenes (29). This is an exceptionally mild and convenient method to construct an important bond type, and Stephenson has demonstrated its utility in the context of the total synthesis of the cytotoxic indole alkaloid (+)-gliocladin C (Fig. 3D) (30).
The generation of a wide variety of alkyl and aryl radicals by photoreduction of the corresponding halides has been a consistent theme of Stephenson's research program (31). An important general feature of this work, and indeed, of many of the recent reactions involving photoredox catalysis, is the ready availability of a large number of known photoactive transition metal complexes with well characterized photochemical and electrochemical properties. Thus, more strongly reducing photocatalysts can be used to compensate for organohalide substrates that are more difficult to reduce than α-haloketones. Stephenson has applied iridium complex 2, which is a significantly more strongly reducing photocatalyst than Ru(bpy)32+, in the reduction of simple alkyl and aryl iodides and has shown that they participate in synthetically powerful radical cyclization and dehalogenation processes (Fig. 3E) (32).
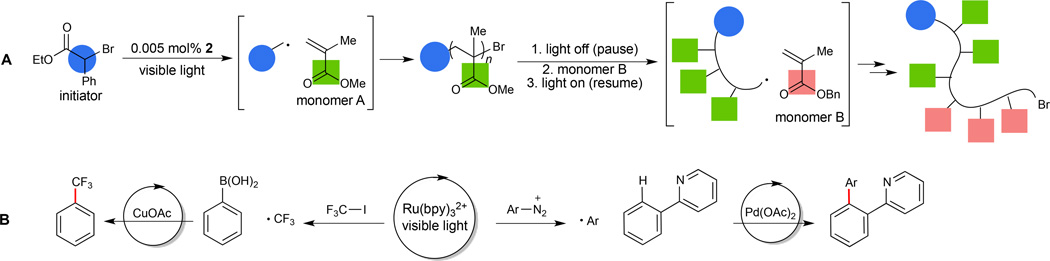
The utility of photocatalytic radical reactions has also been recognized in applications beyond small molecule synthesis. Recently, Hawker reported an efficient method for photocatalytic atom-transfer radical polymerization (ATRP) that enables the light-controlled synthesis of poly(methylmethacrylate) polymers with iridium photocatalyst 2 (33) (Fig. 4A). This reaction shows the same high practical convenience, functional group tolerance, and living nature as other conventional methods for ATRP. However, in contrast to standard copper-initiated reactions, Hawker's method is subject to a high degree of temporal control; polymerization occurs only under constant irradiation with visible light, but chain growth can be reinitiated after several hours in the dark without loss of the living nature of the polymerization. Moreover, this approach provides a route for block copolymer synthesis, as turning off the light source allows a new monomer to be introduced and incorporated into the growing chain upon reapplication of the light source.
Fig. 4. Applications of photocatalysis in polymerization and organometallic chemistry.
(A) The ability to temporally control radical formation under photocatalytic conditions has important benefits in atom transfer radical polymerization reactions (33). Turning off the light source halts polymerization to allow a new monomer to be added (monomer B) before polymerization is reinitialized. (B) Likewise, photogenerated radical species can be intercepted with either copper or palladium organometallic complexes in co-catalytic transformations (34, 35). Abbreviations: Ar, aryl; Bn, benzyl; OAc, acetate; Ph, phenyl.
Photogenerated electrophilic radicals have also proven to be important in the development of reactions involving organometallic species. Sanford has recently reported new methods for Pd-catalyzed C–H functionalization of arenes and Cu-catalyzed trifluoromethylation of organoboronic acids (Fig. 4B) (34, 35), both of which rely on Ru(bpy)32+ to generate the key electrophilic radical, from photoreduction of an aryl diazonium salt or from CF3I, respectively. These radicals are proposed to interact with an organometallic intermediate to promote the formation of new carbon–carbon bonds.
The ability of photocatalysts to generate organic radicals by selective reduction of carbon-halogen bonds has thus already had a substantial impact in the area of radical chemistry. Many known, synthetically valuable radical reactions can be conducted under exceptionally mild, tin-free conditions by exploiting the ability of transition metal photocatalysts to generate radical species by photoinduced electron transfer.
Photocatalytic activation of amines
Amines are common additives in visible light photocatalysis and are generally used as reductants. They assist catalysis either by directly reducing the photoexcited Ru*(bpy)32+ catalyst to generate an even more strongly reducing Ru(bpy)3+ complex or by turning over the oxidized Ru(bpy)33+ photocatalyst after a direct substrate reduction step (Fig. 2). Several recent reviews have described both mechanisms in detail; however, in either scenario, the amines have generally been sacrificial or catalytic co-reductants in photoredox processes that are not themselves incorporated into the synthetic target (8, 36).
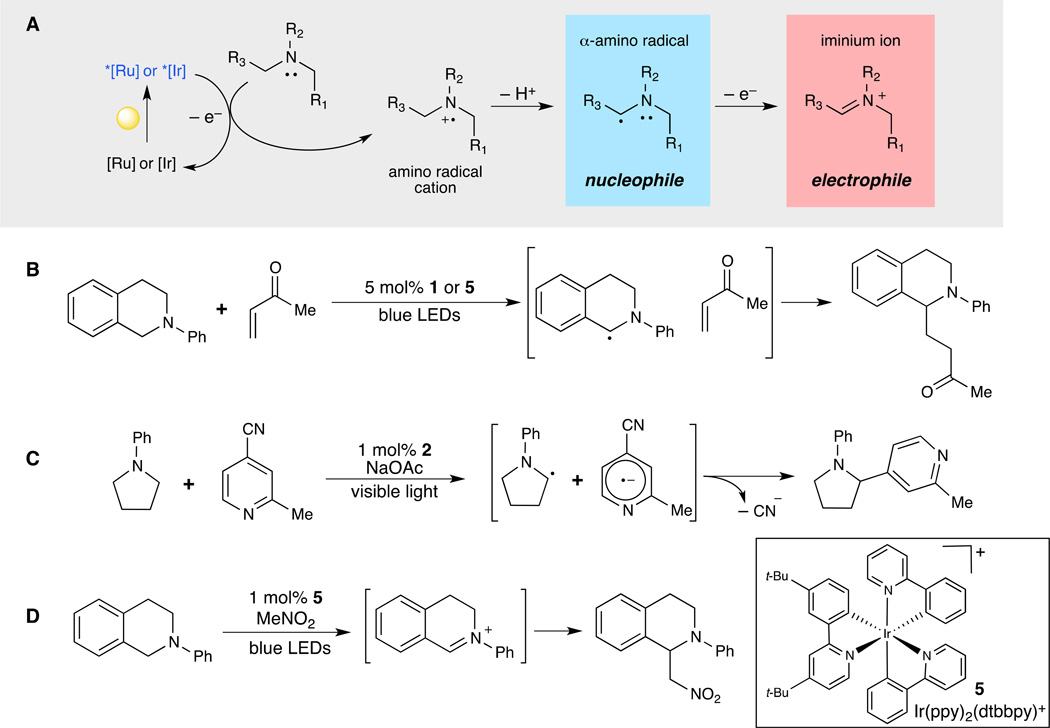
Motivated by the important role that nitrogen-containing compounds play in the chemistry of bioactive small molecules, researchers have recently explored the amine radical cations generated by photocatalytic one-electron oxidation of tertiary amines as substrates for synthetic modification (37). One of the most characteristic features of amine radical cations is an observed decrease in the pKa of the C–H bond adjacent to the nitrogen, deprotonation of which results in the formation of nucleophilic α-amino radicals that are capable of reacting with a diverse range of electrophilic reaction partners (38) (Fig. 5A). The net result of this type of transformation can be viewed as a photochemically induced C–H functionalization of the α-position of amines, an important reaction that has been a long-standing synthetic challenge (39, 40).
Fig. 5. Diverse reactivity of α-amino radical cations.
(A) Tertiary amines readily undergo photooxidation to yield a highly versatile amine radical cation intermediate, which can be transformed into a nucleophilic (α-amino radical) or electrophilic (iminium ion) species. (B) Pandey and Reiser were able to functionalize tetrahydroisoquinolines through the formation of an α-amino radical that readily intercepted various electrophiles (41). (C) Through the utilization of a more strongly reducing iridium photocatalyst (2) MacMillan and coworkers were able to intercept α-amino radicals with cyanoarenes, overall providing a route for α-acylating amines (43). (D) Depending upon the reaction conditions, α-amino radicals can undergo further oxidation to electrophilic iminium ions that can subsequently be trapped with nucleophilic reagents (44). Abbreviations: dtbbpy, 4,4’-di-tert-butyl-2,2’-bipyridyl; LED, light-emitting diode; Me, methyl; Ph, phenyl; ppy, 2-phenylpyridine.
For example, Pandey and Reiser have described the radical functionalization of nitrogen-containing heterocycles using this approach (41). A variety of tetrahydroisoquinolines undergo facile photooxidation by either Ru(bpy)32+ (1) or Ir(ppy)2(dtbbpy)+ [ppy is 2-phenylpyridine; dtbbpy is 4,4’-di-tert-butyl-2,2’-bipyridyl] (5); the resulting amine radical cation undergoes facile deprotonation to produce the corresponding α-amino radical, which was shown to participate in carbon–carbon bond-forming additions to a variety of enone electrophiles in good yields (Fig. 5B). Our laboratory has also studied this transformation, and we have reported a mechanistic analysis of this reaction that verifies the nucleophilic nature of the intermediate radical and shows that this reaction can be accelerated by Brønsted acid co-catalysts (42).
MacMillan reported a more complex application of this reactivity to enable the photocatalytic α-arylation of tertiary amines using electron-deficient cyanoarenes (Fig. 5C) (43). The proposed mechanism involves initial photoreduction of the cyanoarene component by the photoexcited iridium complex 2, coupled with a subsequent oxidation of the amine. Deprotonation of the amine radical cation affords an α-amino radical, which combines with the arene radical anion to expel cyanide and produce the α–arylated amine. The scope of this reaction with respect to both the tertiary amine and the cyanoarene proved to be quite broad, enabling the synthesis of an important class of structures commonly found in bioactive compounds.
The photooxidation of amines has also been used to produce iminium cations, which result formally from a second one-electron oxidation of the α-amino radicals discussed above. These iminium cation intermediates are strong electrophiles that provide reactivity complementary to the nucleophilic reactions of α-amino radicals. Stephenson reported a method for aerobic photocatalytic oxidation of tetrahydroisoquinolines in nitromethane solvent utilizing iridium photocatalyst 5; under these conditions, the iminium cation is trapped by the solvent to afford the product of formal C–H functionalization of the benzylic position adjacent to the amine (44) (Fig. 5D). Subsequently, Stephenson found that a broader range of nucleophiles could be added to these iminium cations in a two-step process involving BrCCl3 as a terminal oxidant (45). Under these conditions, the tetrahydroisioquinolines could be substituted using a variety of carbon-based nucleophiles including copper acetylenes, silyl enol ethers, allyl silanes, and electron-rich heteroarenes.
Depending on reaction conditions, the same family of transition metal photocatalysts has thus been used to generate both nucleophilic α-amino radicals and highly electrophilic iminium cations, and the variety of reactions accessible via photochemical formation of these intermediates is consequently quite broad (46, 47). Moreover, the ability to rapidly modify the environment around an amine holds particular promise in the discovery of structurally complex new bioactive compounds, as nitrogen-containing functional groups are very often important in the binding of a small molecule to a biological target (48).
Photogenerated radical ions
Compared to the chemistry of neutral organic radicals and amine radical cations, reactions that exploit the reactivity of alkene radical ions have enjoyed substantially fewer applications in organic synthesis (49–51). The most common methods for the generation of these reactive intermediates have involved either stoichiometric heavy metal oxidants (52–54) or electrochemical activation (55, 56), which may explain some of the reluctance of chemists to fully explore the synthetic potential of these reactions. Nevertheless, radical ion intermediates exhibit reactivity profiles that are quite distinctive; reactions involving these charged odd-electron species can result in the formation of products that are difficult to assemble by any other means.
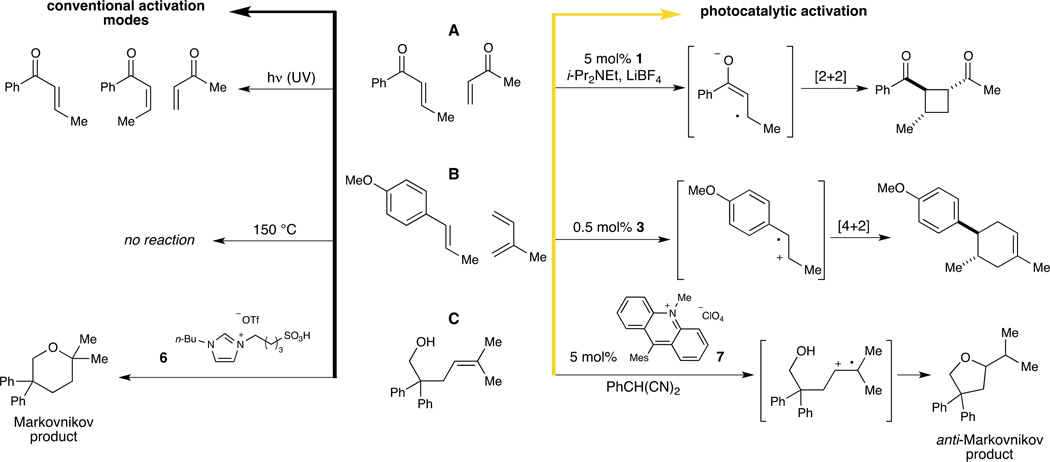
Our group has been particularly interested in exploring cycloaddition reactions of alkene radical ion intermediates, given the efficiency with which this class of reactions can construct stereochemically and structurally complex carbocyclic structures from readily available starting materials (57, 58). In 2008, we demonstrated that aryl enones are readily reduced to the corresponding radical anions upon irradiation in the presence of Ru(bpy)32+ (1); these photogenerated intermediates can then participate in efficient [2+2] intramolecular cycloaddition reactions (59). The extension of this method to intermolecular cycloadditions took advantage of the ability to selectively reduce aryl enones in the presence of less-conjugated Michael acceptors; thus, high selectivity for crossed [2+2] cycloadducts could be achieved for reactions with unsaturated ketones, esters, and thioesters, providing an approach to the synthesis of a broad range of unsymmetrically substituted cyclobutanes (Fig. 6A) (60). The same crossed [2+2] cycloadditions could not be effected by direct photoexcitation with UV light, because the electronically excited triplet enones preferentially undergo alkene isomerization processes rather than productive cycloaddition.
Fig. 6. Unique reactivity of photogenerated radical ions.
Radical ions are underutilized reactive intermediates that can participate in otherwise inaccessible bond formations. (A) Yoon has demonstrated that the photocatalytic activation of enones produces radical anions that readily participate in [2+2] cycloadditions to afford cyclobutane products that are not generated upon UV irradiation (60). (B) Likewise, the photooxidation of electron-rich styrenes yields an electron-deficient radical cation that undergoes facile [4+2] cycloaddition with an electron rich diene, a reaction that is disfavored under thermal conditions (63). (C) Radical ion intermediates also afford products with atypical atom connectivities, such as the exclusive formation of the less common anti-Markovnikov product under photochemical conditions (64). Abbreviations: i-Pr, iso-propyl; Me, methyl; Mes, mesityl; n-Bu, normal-butyl; OTf, trifluoromethanesulfonate; Ph, phenyl; UV, ultraviolet.
Inspired by the ease with which radical anions can be generated by visible light photocatalysis, we have also explored other new reactions involving these reactive intermediates. For example, we recently reported that aryl cyclopropyl ketones can engage in [3+2] cycloadditions via formation of radical anion intermediates (61). This reaction involves the photoinduced reduction of the aryl cyclopropyl ketone moiety followed by fragmentation to afford a distonic radical anion. This intermediate undergoes a series of subsequent intramolecular bond-forming reactions with a pendant alkene to afford cyclopentane products in good yield.
The ability of photoactive transition metal complexes to promote both oxidation and reduction processes has also enabled the development of photocatalytic cycloadditions involving alkene radical cations. The scope of these reactions is complementary to that of radical anion reactions; one-electron oxidations are most facile with electron-rich alkenes. For example, electron-rich styrenes undergo facile one-electron photooxidation in the presence of ruthenium photocatalysts. We showed that the resulting alkene radical cations undergo efficient [2+2] cycloaddition with a variety of alkene partners to afford cyclobutane products with high diastereoselectivity (62).
[4+2] Cycloadditions of electron-rich alkenes can also be promoted by one-electron photooxidation (Fig. 6B) (63). These reactions provide a valuable alternative to thermal Diels–Alder cycloaddition reactions, which have been applied to the synthesis of complex carbocyclic organic structures for decades. In particular, thermal [4+2] cycloadditions generally proceed efficiently only when one component of the reaction is electron-rich and the other is electron-deficient such that the frontier molecular orbitals are close in energy; cycloadditions between two electron-rich partners are generally quite slow. One-electron oxidation of electron-rich styrenes utilizing Ru(bpz)32+ [bpz is 2,2’-bipyrazine] (3), however, reverses their electronic character and results in the generation of intrinsically electron-deficient radical cations. These can subsequently undergo rapid [4+2] cycloaddition with simple dienes to afford the formal products of electronically mismatched Diels–Alder cycloadditions. Predictably, under forcing thermal conditions no [4+2] cycloaddition is observed, further highlighting the unique reactivity accessible through photogenerated radical ion intermediates.
Nicewicz has also investigated new reactions of photogenerated alkene radical cations, focusing on the addition of alcohols to olefins to form heterocyclic products (Fig. 6C) (64). This type of bond construction commonly relies on acid catalysts, such as 6, that direct bond formation to the most substituted carbon of the alkene to yield the Markovnikov product. However, in Nicewicz's work, the cyclization process is promoted via photooxidation by a mesityl-substituted acridinium salt 7 originally reported by Fukuzumi (65, 66). This organic photocatalyst has many of the same properties as ruthenium and iridium complexes, including strong visible light absorption and a long excited-state lifetime, but its photoexcited state is sufficiently oxidizing to react with trisubstituted aliphatic alkenes (66, 67). The resulting alkene radical cations can undergo nucleophilic attack by a tethered alcohol; however, due to the polarization of the radical cation, the regioselectivity complements that of acid-catalyzed reactions and results in the exclusive formation of the anti-Markovnikov addition product. Nicewicz has also reported the same regioselectivity in additions of nitrogen nucleophiles to these radical cations (68). Although many methods for the Markovnikov functionalization of alkenes are known and widely exploited in synthesis, few methods for the direct anti-Markovnikov addition of nucleophiles to olefins have been reported (69). Thus the chemistry of photogenerated radical cations offers a solution to a long-standing problem of great synthetic significance.
Triplet alkenes
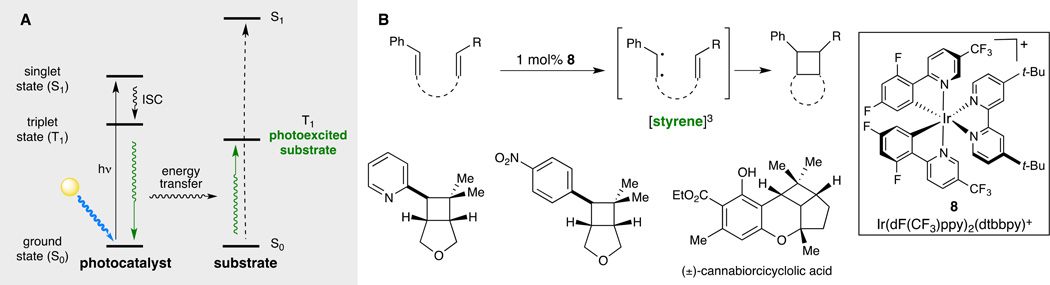
Almost all of the recent investigations in visible light photocatalysis have involved photoredox processes that activate a variety of organic substrates by single-electron oxidation or reduction. Thus the success of these reactions depends upon the relative electrochemical properties of the substrates and photoexcited catalysts; reactions involving thermodynamically unfavorable electron-transfer processes are unlikely to occur at efficient rates. However, the transition metal complexes that have proven to be so powerful in the development of photoredox reactions can also be involved in energy transfer processes that generate highly reactive organic molecules in electronically excited states. In these cases, successful activation depends upon the relative triplet state energies of the catalyst and substrate and not on redox potentials (Fig. 7A).
Fig. 7. Visible light photocatalyzed triplet sensitization.
(A) Visible light induced energy transfer sensitization involves coupling the generation of an electronically excited organic substrate to the relaxation of a photoexcited transition metal chromophore (13). (B) Iridium catalyst 8 mediates the facile [2+2] cycloaddition of styrenes (70). Triplet sensitization provides a means to access interesting and highly strained cyclobutane frameworks that are prevalent in natural products and would be difficult to construct through other methods. Abbreviations: dF(CF3)ppy, 2-(2,4-difluorophenyl)-5-trifluoromethylpyridine, dtbbpy, 4,4’-di-tert-butyl-2,2’-dipyridyl; ISC, intersystem crossing; Me, methyl; Ph, phenyl; t-Bu, tertiary-butyl.
For instance, our group recently reported that styrenes undergo efficient [2+2] cycloaddition via energy transfer when irradiated in the presence of the iridium complex 8 (Fig. 7B) (70). This method involves the formation of electronically excited styrenes in their triplet state, which undergo [2+2] cycloaddition reactions that are superficially similar to the radical cation reactions discussed in the previous section. However, because the mode of substrate activation involves energy transfer rather than electron transfer, the scope of styrenes that participate in this reaction is considerably broader. Highly electron-deficient styrenes and heterostyrenes that cannot be easily photooxidized nevertheless undergo facile cycloaddition.
This alternative approach to visible light photocatalysis is intriguing because it offers convenient access to the reactivity of organic compounds in electronically excited states using photons of much lower energy than those required for direct photoexcitation. The short-wavelength UV photons required to excite simple styrenes to their triplet excited states are more energetic (ca. 115 kcal/mol) than many common bonds found in organic molecules, including carbon–carbon bonds. Thus reactions requiring irradiation with these high-energy UV photons often suffer from competitive homolytic photodecomposition processes. Triplet sensitization using transition metal photocatalysts that are photoexcited with lower-energy visible light, therefore, provides access to the reactivity of electronically excited alkenes that are less susceptible to photoinduced degradation. For example, the cyclobutane cannabanoid cannabiorcicyclolic acid can be synthesized using a clean, high-yielding [2+2] cycloaddition under photocatalytic conditions. Direct irradiation with UV light, however, results in significant photodecomposition of the product after 5 h, and only 19% yield of the cycloadduct is formed along with 9% of unreacted starting material.
Conclusions and outlook
Synthetic organic chemistry benefits greatly when it can incorporate developments from intellectually adjacent disciplines to advance. For instance, organotransition metal chemistry, biocatalysis, and computational chemistry have all been rapidly adopted and have now become tools that are used by synthetic chemists on a routine basis. Photochemistry has the potential to have an equally substantial impact on the field of chemical synthesis by providing a complementary strategy for the activation of organic substrates; the prospect of doing so with renewable solar radiation has motivated photochemists for over a century and seems increasingly relevant as the chemistry community becomes more cognizant of its environmental responsibilities. The growing recognition that operationally facile visible light induced photochemical reactions can be conducted using transition metal photocatalysts is bringing us closer to this long-standing goal.
The pace of development in this area has been remarkable. Recent research in visible light photocatalysis has yielded a generalizable rubric for how organic substrates can undergo photoactivation by transition metal complexes. Both electron-transfer and energy-transfer photocatalysis have been used to generate classes of reactive intermediates whose general reactivity patterns are well-understood, inspiring the design of a wide range of new chemical reactions.
The immediate goals within this area, therefore, are to continue advancing the utility of photocatalytic synthesis by exploiting the reactivity of photogenerated intermediates in the construction of increasingly complex organic targets. Current research is also expanding the range of reactive intermediates that are available using photocatalytic activation, which should increase the diversity of transformations accessible to synthetic chemists interested in photochemical activation strategies (71). Finally, as has been the case with many other advances in synthetic chemistry, the advantages of visible light photocatalysis are being recognized by researchers with interests in areas as diverse as materials science, chemical biology, and drug discovery, suggesting that research in photochemical synthesis will continue to grow in synergistic relationship with intellectually adjacent fields (33, 72, 73). It seems clear that the future of photochemical synthesis remains bright.
Supplementary Material
Acknowledgments
We gratefully acknowledge support from the NIH in the form of a research grant (GM095666) and postdoctoral fellowship to DMS.
References and Notes
- 1.Ciamician G. The photochemistry of the future. Science. 1912;36:385–394. doi: 10.1126/science.36.926.385. [DOI] [PubMed] [Google Scholar]
- 2.Balzani V, Credi A, Venturi M. Photochemical conversion of solar energy. ChemSusChem. 2008;1:26–58. doi: 10.1002/cssc.200700087. [DOI] [PubMed] [Google Scholar]
- 3.Kalyanasundaram K. Photochemistry of polypyridine and porphyrin complexes. London ; San Diego: Academic Press; 1992. p. 626. [Google Scholar]
- 4.Protti S, Manzini S, Fagnoni M, Albini A. The contribution of photochemistry to green chemistry. Eco-Friendly Synthesis of Fine Chemicals. 2009:80–111. [Google Scholar]
- 5.Esser P, Pohlmann B, Scharf HD. The photochemical-synthesis of fine chemicals with sunlight. Angew. Chem. Int. Ed. 1994;33:2009–2023. [Google Scholar]
- 6.Bach T, Hehn JP. Photochemical reactions as key steps in natural product synthesis. Angew. Chem. Int. Ed. 2011;50:1000–1045. doi: 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008;108:1052–1103. doi: 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]
- 8.Prier C, Rankic D, MacMillan D. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon TP, Ischay MA, Du J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010;2:527–532. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]
- 10.Solar spectrum adapted from: M. P. Thekaekara, Solar-radiation measurement - techniques and instrumentation. Solar Energy. 1976;18:309–325. [Google Scholar]
- 11.Pirtsch M, Paria S, Matsuno T, Isobe H, Reiser O. [Cu(dap)2Cl] As an efficient visible-light-driven photoredox catalyst in carbon-carbon bond-forming reactions. Chem. Eur. J. 2012;18:7336–7340. doi: 10.1002/chem.201200967. [DOI] [PubMed] [Google Scholar]
- 12.Revol G, McCallum T, Morin M, Gagosz F, Barriault L. Photoredox transformations with dimeric gold complexes. Angew. Chem. Int. Ed. 2013;52:13342–13345. doi: 10.1002/anie.201306727. [DOI] [PubMed] [Google Scholar]
- 13.Juris A, et al. Ru(II) polypyridine complexes: photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988;84 [Google Scholar]
- 14.Kalyanasundaram K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Coord. Chem. Rev. 1982;46 [Google Scholar]
- 15.Takeda H, Ishitani O. Development of efficient photocatalytic systems for CO2 reduction using mononuclear and multinuclear metal complexes based on mechanistic studies. Coord. Chem. Rev. 2010;254:346–354. [Google Scholar]
- 16.Wenger OS. Proton-coupled electron transfer with photoexcited metal complexes. Acc. Chem. Res. 2013;46:1517–1526. doi: 10.1021/ar300289x. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg DR, et al. Proton-coupled electron transfer. Chem. Rev. 2012;112:4016–4093. doi: 10.1021/cr200177j. [DOI] [PubMed] [Google Scholar]
- 18.Bach T. Stereoselective intermolecular [2+2]-photocycloaddition reactions and their application in synthesis. Synthesis. 1998:683–703. [Google Scholar]
- 19.Albini A. Photosensitization in organic-synthesis. Synthesis. 1981:249–264. [Google Scholar]
- 20.Fagnoni M, Dondi D, Ravelli D, Albini A. Photocatalysis for the formation of the C-C bond. Chem. Rev. 2007;107:2725–2756. doi: 10.1021/cr068352x. [DOI] [PubMed] [Google Scholar]
- 21.Fukuzumi S, Mochizuki S, Tanaka T. Photocatalytic reduction of phenacyl halides by 9,10-dihydro-10-methylacridine - control between the reductive and oxidative quenching pathways of tris(bipyridine)ruthenium complex utilizing an acid catalysis. J. Phys. Chem. 1990;94:722–726. [Google Scholar]
- 22.Alonso F, Beletskaya IP, Yus M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 2002;102:4009–4091. doi: 10.1021/cr0102967. [DOI] [PubMed] [Google Scholar]
- 23.Nicewicz D, MacMillan D. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science. 2008;322:77–80. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee S, Yang JW, Hoffmann S, List B. Asymmetric enamine catalysis. Chem. Rev. 2007;107:5471–5569. doi: 10.1021/cr0684016. [DOI] [PubMed] [Google Scholar]
- 25.Beeson TD, Mastracchio A, Hong JB, Ashton K, MacMillan DWC. Enantioselective organocatalysis using SOMO activation. Science. 2007;316:582–585. [PubMed] [Google Scholar]
- 26.Beeson TD, MacMillan DWC. Enantioselective organocatalytic α-fluorination of aldehydes. J. Am. Chem. Soc. 2005;127:8826–8828. doi: 10.1021/ja051805f. [DOI] [PubMed] [Google Scholar]
- 27.Shih HW, Vander Wal MN, Grange RL, MacMillan DW. Enantioselective α-benzylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 2010;132:13600–13603. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham PV, Nagib DA, MacMillan DW. Photoredox catalysis: a mild, operationally simple approach to the synthesis of α-trifluoromethyl carbonyl compounds. Angew. Chem. Int. Ed. 2011;50:6119–6122. doi: 10.1002/anie.201101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furst L, Matsuura BS, Narayanam JM, Tucker JW, Stephenson CR. Visible light-mediated intermolecular C-H functionalization of electron-rich heterocycles with malonates. Org. Lett. 2010;12:3104–3107. doi: 10.1021/ol101146f. [DOI] [PubMed] [Google Scholar]
- 30.Furst L, Narayanam JM, Stephenson CR. Total synthesis of (+)-gliocladin C enabled by visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2011;50:9655–9659. doi: 10.1002/anie.201103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallentin CJ, Nguyen JD, Finkbeiner P, Stephenson CR. Visible light-mediated atom transfer radical addition via oxidative and reductive quenching of photocatalysts. J. Am. Chem. Soc. 2012;134:8875–8884. doi: 10.1021/ja300798k. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen JD, D'Amato EM, Narayanam JM, Stephenson CR. Engaging unactivated alkyl, alkenyl and aryl iodides in visible-light-mediated free radical reactions. Nat. Chem. 2012;4:854–859. doi: 10.1038/nchem.1452. [DOI] [PubMed] [Google Scholar]
- 33.Fors BP, Hawker CJ. Control of a living radical polymerization of methacrylates by light. Angew. Chem. Int. Ed. 2012;51:8850–8853. doi: 10.1002/anie.201203639. [DOI] [PubMed] [Google Scholar]
- 34.Ye Y, Künzi S, Sanford M. Practical method for the Cu-mediated trifluoromethylation of arylboronic acids with CF3 radicals derived from NaSO2CF3 and tert-butyl hydroperoxide (TBHP) Org. Lett. 2012;14:4979–4981. doi: 10.1021/ol3022726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalyani D, McMurtrey K, Neufeldt S, Sanford M. Room-temperature C-H arylation: merger of Pd-catalyzed C-H functionalization and visible-light photocatalysis. J. Am. Chem. Soc. 2011;133:18566–18569. doi: 10.1021/ja208068w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucker J, Stephenson C. Shining light on photoredox catalysis: theory and synthetic applications. J. Org. Chem. 2012;77:1617–1622. doi: 10.1021/jo202538x. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Xia WJ. Photoredox functionalization of C-H bonds adjacent to a nitrogen atom. Chem. Soc. Rev. 2012;41:7687–7697. doi: 10.1039/c2cs35203f. [DOI] [PubMed] [Google Scholar]
- 38.Yoon UC, Mariano PS. Mechanistic and synthetic aspects of amine enone single electron-transfer photochemistry. Acc. Chem. Res. 1992;25:233–240. [Google Scholar]
- 39.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BU. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 2012;18:10092–10142. doi: 10.1002/chem.201201539. [DOI] [PubMed] [Google Scholar]
- 40.Campos KR. Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 2007;36:1069–1084. doi: 10.1039/b607547a. [DOI] [PubMed] [Google Scholar]
- 41.Kohls P, Jadhav D, Pandey G, Reiser O. Visible light photoredox catalysis: generation and addition of N-aryltetrahydroisoquinoline-derived α-amino radicals to Michael acceptors. Org. Lett. 2012;14:672–675. doi: 10.1021/ol202857t. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz Espelt L, Wiensch EM, Yoon TP. Bronsted acid cocatalysts in photocatalytic radical addition of α-amino C-H bonds across Michael acceptors. J. Org. Chem. 2013;78:4107–4114. doi: 10.1021/jo400428m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNally A, Prier C, MacMillan D. Discovery of an α-amino C-H arylation reaction using the strategy of accelerated serendipity. Science. 2011;334:1114–1117. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Condie AG, Gonzalez-Gomez JC, Stephenson CR. Visible-light photoredox catalysis: aza-Henry reactions via C-H functionalization. J. Am. Chem. Soc. 2010;132:1464–1465. doi: 10.1021/ja909145y. [DOI] [PubMed] [Google Scholar]
- 45.Freeman D, Furst L, Condie A, Stephenson C. Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light. Org. Lett. 2012;14:94–97. doi: 10.1021/ol202883v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou YQ, et al. Visible-light-induced oxidation/[3+2] cycloaddition/oxidative aromatization sequence: a photocatalytic strategy to construct pyrrolo[2,1-a]isoquinolines. Angew. Chem. Int. Ed. 2011;50:7171–7175. doi: 10.1002/anie.201102306. [DOI] [PubMed] [Google Scholar]
- 47.Zhu S, et al. Oxygen switch in visible-light photoredox catalysis: radical additions and cyclizations and unexpected C-C-bond cleavage reactions. J. Am. Chem. Soc. 2013;135:1823–1829. doi: 10.1021/ja309580a. [DOI] [PubMed] [Google Scholar]
- 48.Horton DA, Bourne GT, Smythe ML. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 49.Muller F, Mattay J. Photocycloadditions: control by energy and electron-transfer. Chem. Rev. 1993;93:99–117. [Google Scholar]
- 50.Schmittel M, Burghart A. Understanding reactivity patterns of radical cations. Angew. Chem. Int. Ed. 1997;36:2550–2589. [Google Scholar]
- 51.Bauld NL. Cation radical cyclo-additions and related sigmatropic reactions. Tetrahedron. 1989;45:5307–5363. [Google Scholar]
- 52.Floreancig PE. Development and applications of electron-transfer-initiated cyclization reactions. Synlett. 2007:191–203. [Google Scholar]
- 53.Edmonds DJ, Johnston D, Procter DJ. Samarium(II)-iodide-mediated cyclizations in natural product synthesis. Chem. Rev. 2004;104:3371–3404. doi: 10.1021/cr030017a. [DOI] [PubMed] [Google Scholar]
- 54.Guo F, Clift MD, Thomson RJ. Oxidative coupling of enolates, enol silanes and enamines: methods and natural product synthesis. Eur. J. Org. Chem. 2012;2012:4881–4896. doi: 10.1002/ejoc.201200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sperry JB, Wright DL. The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules. Chem. Soc. Rev. 2006;35:605–621. doi: 10.1039/b512308a. [DOI] [PubMed] [Google Scholar]
- 56.Dalko PI. Redox-induced radical and radical ionic carbon-carbon bond-forming reactions. Tetrahedron. 1995;51:7579–7653. [Google Scholar]
- 57.Ischay MA, Yoon TP. Accessing the synthetic chemistry of radical ions. Eur. J. Org. Chem. 2012:3359–3372. [Google Scholar]
- 58.Yoon TP. Visible light photocatalysis: the development of photocatalytic radical ion cycloadditions. ACS Catal. 2013;3:895–902. doi: 10.1021/cs400088e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ischay M, Anzovino M, Du J, Yoon T. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J. Am. Chem. Soc. 2008;130:12886–12887. doi: 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]
- 60.Du J, Yoon T. Crossed intermolecular [2+2] cycloadditions of acyclic enones via visible light photocatalysis. J. Am. Chem. Soc. 2009;131:14604–14605. doi: 10.1021/ja903732v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Z, Shen M, Yoon T. [3+2] Cycloadditions of aryl cyclopropyl ketones by visible light photocatalysis. J. Am. Chem. Soc. 2011;133:1162–1164. doi: 10.1021/ja107849y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ischay MA, Ament MS, Yoon TP. Crossed intermolecular [2+2] cycloaddition of styrenes by visible light photocatalysis. Chem. Sci. 2012;3:2807–2811. doi: 10.1039/C2SC20658G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin S, Ischay M, Fry C, Yoon T. Radical cation Diels-Alder cycloadditions by visible light photocatalysis. J. Am. Chem. Soc. 2011;133:19350–19353. doi: 10.1021/ja2093579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamilton D, Nicewicz D. Direct catalytic anti-markovnikov hydroetherification of alkenols. J. Am. Chem. Soc. 2012;134:18577–18580. doi: 10.1021/ja309635w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuzumi S, et al. Electron-transfer state of 9-mesityl-10-methylacridinium ion with a much longer lifetime and higher energy than that of the natural photosynthetic reaction center. J. Am. Chem. Soc. 2004;126:1600–1601. doi: 10.1021/ja038656q. [DOI] [PubMed] [Google Scholar]
- 66.Ohkubo K, et al. Simultaneous production of p-tolualdehyde and hydrogen peroxide in photocatalytic oxygenation of p-xylene and reduction of oxygen with 9-mesityl-10-methylacridinium ion derivatives. Chem. Commun. 2010;46:601–603. doi: 10.1039/b920606j. [DOI] [PubMed] [Google Scholar]
- 67.Kotani H, Ohkubo K, Fukuzumi S. Photocatalytic oxygenation of anthracenes and olefins with dioxygen via selective radical coupling using 9-mesityl-10-methylacridinium ion as an effective electron-transfer photocatalyst. J. Am. Chem. Soc. 2004;126:15999–16006. doi: 10.1021/ja048353b. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen T, Nicewicz D. Anti-Markovnikov hydroamination of alkenes catalyzed by an organic photoredox system. J. Am. Chem. Soc. 2013;135:9588–9591. doi: 10.1021/ja4031616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moeller KD. Intramolecular anodic olefin coupling reactions: using radical cation intermediates to trigger new umpolung reactions. Synlett. 2009:1208–1218. [Google Scholar]
- 70.Lu Z, Yoon T. Visible light photocatalysis of [2+2] styrene cycloadditions by energy transfer. Angew. Chem. Int. Ed. 2012;51:10329–10332. doi: 10.1002/anie.201204835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farney EP, Yoon TP. Visible-light sensitization of vinyl azides by transition-metal photocatalysis. Angew. Chem. Int. Ed. 2014;53:793–797. doi: 10.1002/anie.201308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leibfarth FA, Mattson KM, Fors BP, Collins HA, Hawker CJ. External regulation of controlled polymerizations. Angew. Chem. Int. Ed. 2013;52:199–210. doi: 10.1002/anie.201206476. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Kamlet AS, Steinman JB, Liu DR. A biomolecule-compatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system. Nat. Chem. 2011;3:146–153. doi: 10.1038/nchem.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.