Abstract
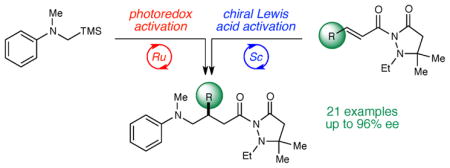
We report the highly enantioselective addition of photogenerated α-amino radicals to Michael acceptors. This method features a dual-catalyst protocol that combines transition metal photoredox catalysis with chiral Lewis acid catalysis. The combination of these two powerful modes of catalysis provides an effective, general strategy to generate and control the reactivity of photogenerated reactive intermediates.
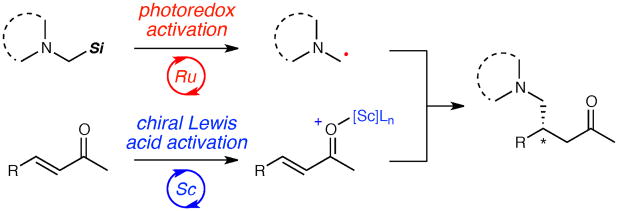
While photochemistry has long been appreciated as a powerful tool in organic synthesis,1 stereocontrol in photo-chemical reactions remains a significant challenge with few general solutions.2 A number of novel dual catalytic systems have recently been developed to address this long-standing problem. The combination of transition metal photoredox catalysts with chiral amine,3 carbene,4 and Brønsted acid organocatalysts5 has enabled a number of highly enantioselective photoinduced reactions. We recently reported the first method combining photoredox and chiral Lewis acid catalysis in the context of an asymmetric [2+2] photocycloaddition.6 Compared to organocatalysts, chiral Lewis acids possess a greater diversity of structures known to provide effective enantiodifferentiating environments for a wide range of mechanistically distinct organic reactions.7 We wondered if the ability to combine organic chemists’ detailed understanding of asymmetric Lewis acid catalysis with the emerging versatility of photoredox activation might provide a robust approach to controlling stereochemistry in photocatalytic reactions. Herein, we report our application of the principle of cooperative Lewis acid–photoredox catalysis to highly enantioselective reactions of α-amino radicals (Scheme 1).
Scheme 1.
Design plan for cooperative Lewis acid–photoredox catalysis of α-amino radical additions.
Our group has an established interest in the chemistry of α-amino radicals.8 Pioneering studies by Mariano9 and Pandey10 demonstrated that the photosensitized oxidation of amines, α-amino acids, and α-silylamines offers the most straightforward method for the production of these highly nucleophilic, functionalized radical intermediates. More recently, several groups have shown that transition metal photoredox sensitizers can be used to produce α-amino radicals under visible light irradiation.11 Although the utility of these amine-functionalized radical species in the synthesis of complex alkaloids has long been appreciated,12 methods to control the enantioselectivity of their addition reactions are extremely rare. To the best of our knowledge, the only prior example of an asymmetric reaction in this class is a single, elegant addition reaction reported by Bach, in which a chiral hydrogen-bonding photosensitizer catalyzes the intramolecular conjugate addition of a photogenerated α-amino radical to a quinolone scaffold.13 A more general method to control the stereochemistry of such additions, particularly in an intermolecular context, is an unrealized goal with great synthetic potential.
We recently reported the photocatalytic functionalization of N-aryl tetrahydroisoquinolines via an α-amino radical intermediate.8 This study provided two important observations that informed the design of our exploratory investigations. First, we found that the conjugate addition of the α-amino radicals to Michael acceptors was catalyzed by Brønsted acids. If chiral Lewis acids could have a similar effect, they might also control the stereochemistry of these additions.14 Indeed, Sibi, Porter, and others have established that chiral Lewis acids can dictate the enantioselectivity of radical conjugate additions,15 although these investigations have been limited to simple, unfunctionalized alkyl radicals. Second, we found that the rate-limiting step was a chain-propagating H-atom abstraction process that was only efficient with N-aryl tetrahydroisoquinoline substrates with especially activated α-amino C–H bonds. We wondered if this narrow restriction on viable substrates could be overcome by an alternative method for generating the α-amino radical. In particular, we were inspired by Mariano’s inisght that α-silyl amines undergo facile oxidative fragmentation and generate α-amino radicals several orders of magnitude more efficiently than their non-silylated analogues.9e
Thus the optimization of the enantioselective α-amino radical addition began with an exploration of the reaction between α-silylmethyl aniline 1 and crotonyl oxazolidinone 2a (Table 1). Irradiation with a household 23 W fluorescent light bulb in the presence of 2 mol% Ru(bpy)3Cl2 resulted in the slow formation of the expected radical conjugate addition product 3a in 28% yield after 18 h (entry 1). In accord with our initial hypotheses, Sc(III)-pybox complexes provided both a significant increase in the rate of the reaction and modest enantioselectivity (entries 2–5); the sBuPybox complex gave the optimal combination of yield and ee in this screen. An examination of reaction concentration showed that the rate and ee were improved somewhat at lower concentrations (entry 6). We next made the surprising observation that the concentration of Ru(bpy)3Cl2 had an effect on ee (entries 6–8). It seemed unlikely that the photocatalyst itself could be involved in the stereochemistry-determining conjugate addition step. Speculating instead that the chloride counteranion might be responsible for the observed effect on ee, we found that the addition of either KCl or Bu4N+Cl− also improved enantioselectivity, while addition of non-coordinating anions did not (entries 9–12). Finally, inspired by the success of Sibi’s chiral relay auxiliary in other enantioselective radical addition processes, we discovered that the use of Michael acceptor 2b provided substantially higher ee (entry 13). A re-screening of chiral ligands at this point revealed that iBuPybox provided the optimal chiral environment with this acceptor, affording γ-aminocarbonyl adduct 3b in 93% ee (entry 14). Control experiments verified the necessity of each reaction component (entries 15–18). Two experiments are particularly notable. First, the electrofugal silyl substituent is critical for successful reaction; N,N-dimethyl aniline (5) produces only a trace of the conjugate addition product (entry 15), consistent with our design strategy. Second, we observed the formation of 52% yield of 3b after 18 h even in the absence of Lewis acid (entry 16). Thus the rate acceleration afforded by the Lewis acid catalyst must be large enough to overcome a significant racemic background addition in the absence of Lewis acid catalyst.
Table 1.
Optimization of the asymmetric α-amino radical additiona
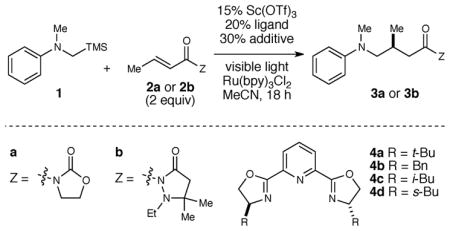
| ||||||
|---|---|---|---|---|---|---|
| entry | acceptor | ligand | [Ru] mol% | additive | yield (%)b | ee (%)c |
| 1d,e | 2a | none | 2% | none | 28 | -- |
| 2d | 2a | tBuPybox (4a) | 2% | none | 18 | 49 |
| 3d | 2a | BnPybox (4b) | 2% | none | 75 | 27 |
| 4d | 2a | iBuPybox (4c) | 2% | none | 15 | 42 |
| 5d | 2a | sBuPybox (4d) | 2% | none | 70 | 43 |
| 6 | 2a | sBuPybox (4d) | 2% | none | 94 | 50 |
| 7 | 2a | sBuPybox (4d) | 5% | none | 93 | 59 |
| 8 | 2a | sBuPybox (4d) | 15% | none | 97 | 66 |
| 9 | 2a | sBuPybox (4d) | 2% | KCl | 74 | 59 |
| 10 | 2a | sBuPybox (4d) | 2% | Bu4N+Cl− | 91 | 67 |
| 11 | 2a | sBuPybox (4d) | 2% | Bu4N+PF6− | 52 | 43 |
| 12 | 2a | sBuPybox (4d) | 2% | Bu4N+ClO4− | 40 | 46 |
| 13 | 2b | sBuPybox (4d) | 2% | Bu4N+Cl− | 80 | 89 |
| 14 | 2b | iBuPybox (4c) | 2% | Bu4N+Cl− | 83 | 93 |
| 15f | 2b | iBuPybox (4c) | 2% | Bu4N+Cl− | <5 | -- |
| 16e | 2b | none | 2% | Bu4N+Cl− | 52 | -- |
| 17 | 2b | iBuPybox (4c) | 0% | Bu4N+Cl− | 0 | -- |
| 18 | 2b | iBuPybox (4c) | 2% | none | 91 | 86 |
Unless otherwise noted, reactions were conducted at 0.05 M in 1 and irradiated at a distance of 30 cm from a 23 W compact fluorescent light bulb.
Yields determined by 1H NMR using an internal standard.
Enantiomeric excess determined by chiral SFC analysis.
Reaction conducted at 0.25 M in 1.
Reaction conducted without Lewis acid.
Reaction conducted with N,N-dimethyl amine in place of silyl amine 1.
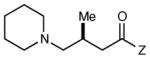
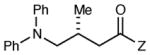
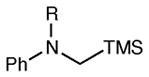
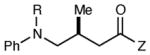
Table 2 summarizes the effects of structurally varied α-silylamines under the optimized conditions for enantioselective conjugate addition. A variety of electron-withdrawing para substituents are easily accommodated on the N-aryl moiety (entries 2–4). Modestly electron-donating substituents slow the rate of the reaction without negatively impacting ee (entry 5). Strongly electron-donating para substituents inhibit reactivity altogether (entry 6), which is consistent with Mariano’s observation that electron-releasing p-methoxy groups retard the desilylation of α-silylmethyl anilines by an order of magnitude compared to their unsubstituted analogs.16 A meta methoxy substituent, on the other hand, is well tolerated and provides the desired product in good yield and with excellent enantioselectivity (entry 7). Gratifyingly, substitution in the ortho position is also tolerated (entries 8 and 9). We found that successful Michael reaction required the presence of one N-aryl substituent; aliphatic amines undergo protodesilylation without adding to the Michael addition (entry 10). On the other hand, both N,N-diaryl and mixed N-alkyl-N-aryl α-silylamines participate in this reaction (entries 11 and 12) although sterically bulky α-amino radicals react sluggishly. Unfortunately, substrates bearing N-acyl groups were recovered unchanged, consistent with the greater difficulty with which they are oxidized (entry 14).
Table 2.
Reactions of Structurally Varied α-Silylamines with Michael acceptor 2b.a
| entry | amine | product | time | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
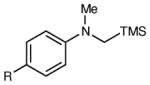
|
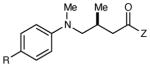
|
||||
| 1 | R = H | 6 h | 87 | 93 | |
| 2 | R = F | 6 h | 94 | 94 | |
| 3 | R = Cl | 6 h | 96 | 93 | |
| 4 | R = Br | 6 h | 90 | 92 | |
| 5 | R = Me | 12 h | 79 | 91 | |
| 6 | R = OMe | 12 h | <5 | -- | |
| 7 |
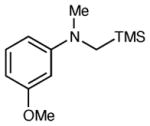
|
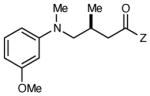
|
6 h | 85 | 92 |
| 8 |
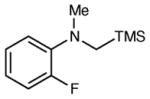
|
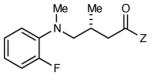
|
6 h | 80 | 96d,e |
| 9 |
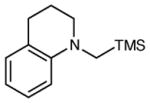
|
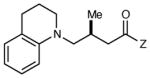
|
12 h | 80 | 90 |
| 10 |
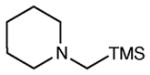
|
|
12 h | <5 | -- |
| 11 |
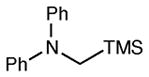
|
|
12 h | 93 | 91d,e |
|
|
||||
| 12 | R = Bn | 6 h | 60 | 94 | |
| 13 | R = i-Pr | 12 h | 33 | 95 | |
| 14 | R = Boc | 12 h | <5 | -- |
Unless otherwise noted, reactions were conducted using 1.5 equiv of 2, 2 mol% Ru(bpy)3Cl2, 15 mol% Sc(OTf)3, 20 mol% (S,S)-4c and 30 mol% Bu4N+Cl− in degassed MeCN (0.05 M) and were irradiated using a 23 W compact fluorescent light bulb.
Values represent the averaged isolated yields of two reproducible experiments.
Enantiomeric excess determined by chiral SFC analysis.
Enantiomeric excess of the corresponding alcohol.
Reaction conducted using (R,R)-4c to facilitate measurement of ee.
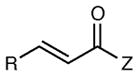
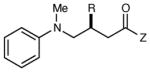
The generality of the reaction with respect to the Michael acceptor is outlined in Table 3. A variety of aliphatic acceptors react smoothly and deliver the expected conjugate addition products with high ee (entries 1–4). Aromatic acceptors are also excellent partners for this transformation, and both electron-poor and electron-rich substrates undergo facile Michael addition (entries 5–7). Substitution at the ortho position is well tolerated, with only marginally diminished enantioselectivity (entry 8). A heterocyclic group was also compatible with the reaction conditions (entry 9).
Table 3.
Reactions of Structurally Varied Michael Acceptors with α-Silylamine 1.a
| entry | amine | product | time | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
|
|
||||
| 1 | R = n-Pr | 6 h | 76 | 93 | |
| 2 | R = i-Pr | 6 h | 71 | 93 | |
| 3 | R = CH2OBn | 6 h | 75 | 91 | |
| 4 | R = t-Bu | 12 h | 35 | 96 | |
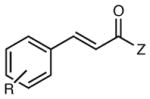
|
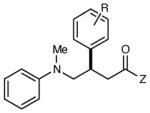
|
||||
| 5 | R = H | 12 h | 74 | 93 | |
| 6 | R = p-Cl | 12 h | 63 | 91 | |
| 7 | R = p-OMe | 12 h | 83 | 94 | |
| 8 | R = o-Me | 12 h | 81 | 85 | |
| 9 |
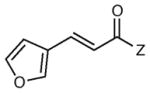
|
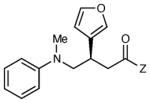
|
12 h | 83 | 91 |
Unless otherwise noted, reactions were conducted using 1.5 equiv of Michael acceptor, 2 mol% Ru(bpy)3Cl2, 15 mol% Sc(OTf)3, 20 mol% (S,S)-4c and 30 mol% Bu4N+Cl− in degassed MeCN (0.05 M) and were irradiated using a 23 W compact fluorescent light bulb.
Values represent the averaged isolated yields of two reproducible experiments.
Enantiomeric excess determined by chiral SFC analysis.
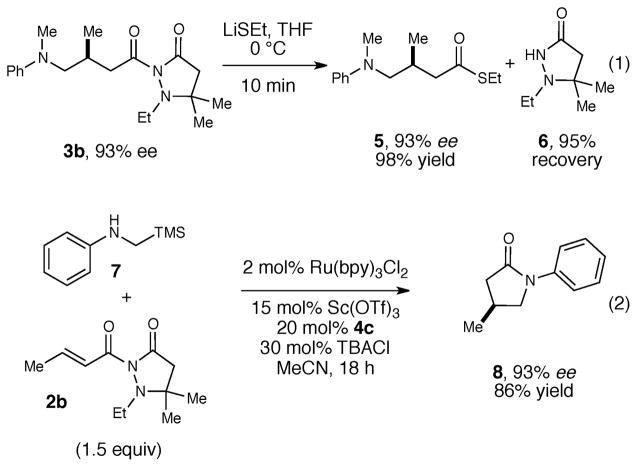
To expand the synthetic value of this method, we also investigated conditions for efficient removal of the pyrazolidinone auxiliary (Scheme 1). Standard conditions for hydrolysis and reduction of imides proved to be unselective, producing mixtures of acyl cleavage products. However, the auxiliary can be cleanly cleaved upon reaction with ethan-ethiolate, providing thioester 5 in quantitative yield with no erosion of enantioselectivity (eq 1). Importantly, the auxiliary (6) can be recovered in 95% yield after this cleavage step. Auxiliary cleavage can also be induced in an intramolecular fashion by a sufficiently nucleophilic moiety in the product.17 For example, when secondary aniline 7 is subjected to the optimized conditions, the conjugate addition product undergoes spontaneous intramolecular transacylation in situ to afford pyrrolidinone 8 in very high yield and excellent ee (eq 2).18
An intriguing unexpected result for our investigations was the observation that added chloride salts were required for optimal ee.19 We quickly ruled out the possibility of an electrolyte effect, as addition of other ammonium salts bearing non-coordinating ions had no impact on ee (Table 1, entries 11 and 12). We then examined the influence of chloride on the background, Lewis-acid-free reaction between 1 and 2b and observed no measurable change in the rate of product formation upon the addition of 30 mol% Bu4N+Cl− to a reaction conducted in the absence of Lewis acid. Thus we conclude that chloride does not have a significant impact on the photooxidation or desilylation steps leading to formation of the key α-amino radical intermediate. Instead, chloride must be interacting intimately with the Lewis acid. One would expect a scandium(III) chloride complex to be a weaker Lewis acid than its triflate analogue; indeed, a (pybox)ScCl3 complex proved to give inferior rates than the optimized triflate catalyst. However, analysis of (pybox)Sc(OTf)3-catalyzed reactions at incomplete conversions revealed that the addition of exogenous Bu4N+Cl− resulted significantly increased the rate of formation of adduct 3b under catalytic conditions.
A reasonable interpretation consistent with these results is that chloride is involved in accelerating the turnover of the Lewis acid catalyst.20 Thus, we do not believe that chloride alters the intrinsic stereoselectivity of the (pybox)Sc(OTf)3-catalyzed conjugate addition. Rather, the addition of chloride aids the enantioselective Lewis acid-mediated pathway to out-compete a slower but still significant rate of racemic background radical addition. This conclusion highlights an important conceptual distinction between this method and the asymmetric [2+2] cycloaddition recently reported by our laboratory: in this new reaction, the Lewis acid is not directly involved in the photoinduced electron transfer step. Rather, the chiral Lewis acids control the rate and selectivity of a step independent of the photoredox process itself. Thus, this study provides compelling evidence that the combination of photoredox and chiral Lewis acid catalysis might be broadly applicable to the design of enantioselective reactions involving the increasingly wide range of reactive intermediates known to be readily generated via photoredox catalysis.
In summary, we have developed the first highly enantioselective intermolecular reaction of α-amino radicals. This process showcases the ability of chiral Lewis acid catalysts to control the reactivity of these photogenerated nucleophilic intermedidates, and we expect that the combination of photoredox and chiral Lewis acid catalysis will provide an approach to control the stereochemistry of a wide variety of photoinitiated organic reactions. Studies to expand this concept to other synthetically useful transformations are currently underway in our laboratory.
Supplementary Material
Scheme 2.
Auxiliary removal
Acknowledgments
We thank Brian Dolinar and Dr. Ilia Guzei of the UW–Madison Molecular Structure Laboratory for performing X-ray crystallography. Funding for this research was provided by the NIH (GM095666) and the Sloan Foundation.
Footnotes
Supporting Information. Experimental details, characterization data for all new compounds, and crystallographic data (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hoffmann N. Chem Rev. 2008;108:1052. doi: 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]; (b) Bach T, Hehn JP. Angew Chem Int Ed. 2011;50:1000. doi: 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]
- 2.(a) Inoue Y. Chem Rev. 1992;92:741. [Google Scholar]; (b) Svoboda J, König B. Chem Rev. 2006;106:5413. doi: 10.1021/cr050568w. [DOI] [PubMed] [Google Scholar]
- 3.(a) Nicewicz DA, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nagib DA, Scott ME, MacMillan DWC. J Am Chem Soc. 2009;131:10875. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shih HW, Vander Wal MN, Grange RL, MacMillan DWC. J Am Chem Soc. 2010;132:13600. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiRocco DA, Rovis T. J Am Chem Soc. 2012;134:8094. doi: 10.1021/ja3030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. J Am Chem Soc. 2013;135:17735. doi: 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]
- 6.Du J, Skubi KL, Schultz DM, Yoon TP. Science. 2014;344:392. doi: 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto H, editor. Lewis Acids in Organic Synthesis. 1 and 2 Wiley VHC; New York: 2000. [Google Scholar]
- 8.Ruiz Espelt L, Wiensch EM, Yoon TP. J Org Chem. 2013;78:4107. doi: 10.1021/jo400428m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Brumfield MA, Quillen SL, Yoon UC, Mariano PS. J Am Chem Soc. 1984;106:6855. [Google Scholar]; (b) Hasegawa E, Xu W, Mariano PS, Yoon UC, Kim JU. J Am Chem Soc. 1988;110:8099. [Google Scholar]; (c) Xu W, Yoon TJ, Hasegawa E, Yoon UC, Mariano PS. J Am Chem Soc. 1989;111:406. [Google Scholar]; (d) Zhang X, Yeh SR, Hong S, Frecero M, Albini A, Falvey DE, Mariano PS. J Am Chem Soc. 1994;116:4211. [Google Scholar]; (e) Su Z, Mariano PS, Falvey DE, Yoon UC, Oh SW. J Am Chem Soc. 1998;120:10676. [Google Scholar]
- 10.(a) Pandey G, Kumaraswamy G, Bhalerao UT. Tetrahedron Lett. 1989;30:6059. [Google Scholar]; (b) Pandey G, Reddy GD, Kumaraswamy G. Tetrahedron. 1994;50:8185. [Google Scholar]
- 11.(a) McNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miyake Y, Ashida Y, Nakajima K, Nishibayashi Y. Chem Commun. 2012;48:6966. doi: 10.1039/c2cc32745g. [DOI] [PubMed] [Google Scholar]; (c) Kohls P, Jadhav D, Pandey G, Reiser O. Org Lett. 2012;14:672. doi: 10.1021/ol202857t. [DOI] [PubMed] [Google Scholar]; (d) Miyake Y, Nakajima K, Nishibayashi Y. J Am Chem Soc. 2012;134:3338. doi: 10.1021/ja211770y. [DOI] [PubMed] [Google Scholar]; (e) Zhu S, Das A, Bui L, Zhou H, Curran DP, Rueping M. J Am Chem Soc. 2013;135:1823. doi: 10.1021/ja309580a. [DOI] [PubMed] [Google Scholar]; (f) Miyake Y, Ashida Y, Nakajima K, Nishibayashi Y. Chem Eur J. 2014;20:6120. doi: 10.1002/chem.201304731. [DOI] [PubMed] [Google Scholar]; (g) Zuo Z, MacMillan DWC. J Am Chem Soc. 2014;136:5257. doi: 10.1021/ja501621q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zuo Z, Ahneman D, Chu L, Terrett J, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Jung YS, Mariano PS. Tetrahedron Lett. 1993;34:4611. [Google Scholar]; (b) Pandey G, Reddy GD, Chakrabarti D. J Chem Soc Perkin Trans. 1996;1:219. [Google Scholar]; (c) Pandey G, Chakrabarti D. Tetrahedron Lett. 1996;37:2285. [Google Scholar]; (d) Pandey G, Kapur M. Synthesis. 2001:1263. [Google Scholar]
- 13.Bauer A, Westkämper F, Grimme S, Bach T. Nature. 2005;436:1139. doi: 10.1038/nature03955. [DOI] [PubMed] [Google Scholar]
- 14.For recent reviews of enantioselective conjugate additions using chiral Lewis acids, see: Evans DA, Rovis T, Johnson JS. Pure Appl Chem. 1999;71:1407.Sibi MP, Manyem S. Tetrahedron. 2000;56:8033.Christoffers J, Koripelly G, Rosiak A, Rössle M. Synthesis. 2007:1279.
- 15.(a) Sibi MP, Ji J. J Am Chem Soc. 1996;118:9200. [Google Scholar]; (b) Sibi MP, Ji J, Sausker JB, Jasperse CP. J Am Chem Soc. 1999;121:7517. [Google Scholar]; (c) Sibi MP, Zimmerman J. J Am Chem Soc. 2006;128:13346. doi: 10.1021/ja0648108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wu JH, Radinov R, Porter NA. J Am Chem Soc. 1995;117:11029. [Google Scholar]; (e) Wu JH, Zhang GR, Porter NA. Tetrahedron Lett. 1997;38:2067. [Google Scholar]; (f) Porter NA, Feng H, Kavrakova IK. Tetrahedron Lett. 1999;40:6713. [Google Scholar]
- 16.Mariano PS, editor. Advances in Electron Transfer Chemistry. Jai Press; Greenwich, Connecticut: 1994. [Google Scholar]
- 17.Sibi has previously described the cleavage of a pyrazolidinone auxiliary using an intramolecular transacylation process: Sibi MP, Liu M. Org Lett. 2001;3:4181. doi: 10.1021/ol016807t.
- 18.During the preparation of this manuscript, Nishibayashi reported the photocatalytic synthesis of racemic pyrrolidinones by a conceptually similar desilylative conjugate addition–cyclization process: Nakajima K, Kitagawa M, Ashida Y, Miyake Y, Nishibayashi Y. Chem Commun. 2014;50:8900. doi: 10.1039/c4cc03000a.
- 19.For an excellent review of dramatic halide effects in catalytic reactions, see: Fagnou K, Lautens M. Angew Chem Int Ed. 2002;41:26. doi: 10.1002/1521-3773(20020104)41:1<26::aid-anie26>3.0.co;2-9.
- 20.For discussion of chloride-catalyzed ligand substitution processes, see: Boukhalfa H, Crumbliss AL. Inorg Chem. 2001;40:4183. doi: 10.1021/ic010050k.Biruš M, Krznaric G, Pribanić M, Ursic S. J Chem Res, Synop. 1985:4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.