Background
Atopic dermatitis (AD) is a common chronic, episodic inflammatory ailment1. Recent studies have shown that AD is a life-long disease that often begins in early childhood and that persists into adulthood. AD is at least in part associated with a dysfunctional skin barrier often due to loss of function (LOF) mutations of filaggrin (FLG).2 Although the impact of FLG LOF on skin barrier function is less certain.3 While mutations of FLG are relatively common in Asian and European children with AD they were very infrequently found in those of African ancestry.2;4
The FLG gene is part of the epidermal differentiation complex (EDC).5 The EDC contains more than 60 genes many of which are involved in skin barrier function, filaggrin-2 (FLG2).6 We have shown that variation of the FLG2 gene is associated with the persistence of AD in African-Americans.7 The FLG2 gene is structurally similar to the FLG gene and the FLG2 protein is has similar biochemical and biophysical properties to the FLG protein.8 Like FLG, the production of FLG2 protein has also been shown to be diminished in those with AD and in the presence of inflammatory mediators.3;6 s1
Question addressed
Does FLG2 immunohistochemical staining vary with skin inflammatory response in those with AD?
Experimental Design
Thirty-eight archived and anonymized skin tissue specimens with a clinical diagnosis of AD were evaluated. The staining protocol and assessment are presented in the supplement.
Results
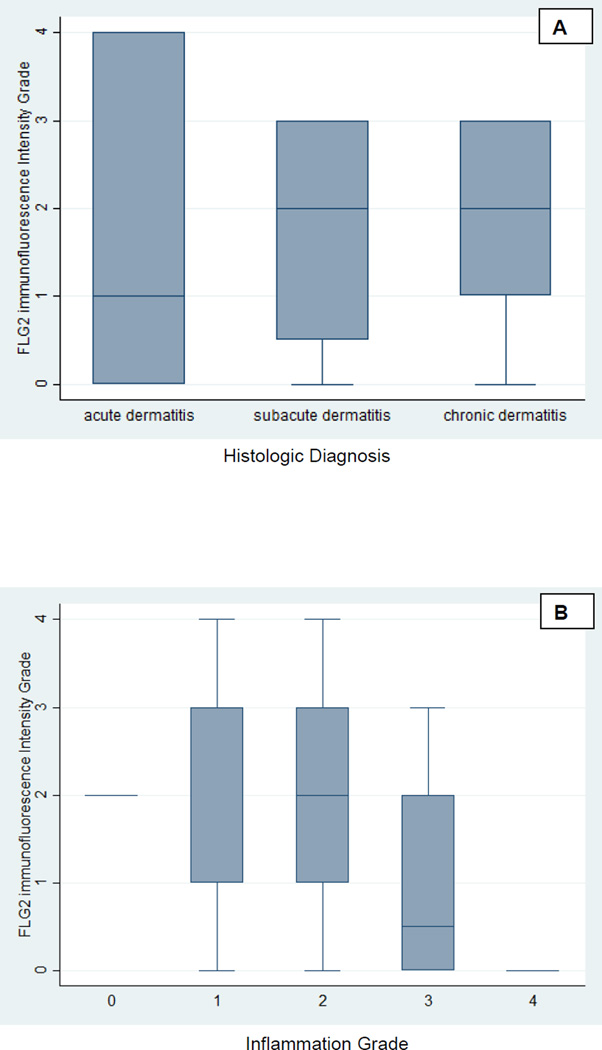
Thirty-eight specimens from 38 individuals with a clinical diagnosis were retrieved and analyzed (supplement Table 1). The most common histologic diagnosis was subacute dermatitis (58%). The median inflammation grade was 2 and the median FLG2 grade was 2 (Figure 1). FLG2 stain of 3 or greater was noted in 31.6% (N=12) subjects and an inflammation grade of 3 or greater was noted in 26.3% (10) subjects (Figure 1). The intensity of FLG2 staining was inversely associated with the histologic diagnosis as it varied from acute to subacute to chronic (p=0.031, i.e., less FLG2 staining in those with acute dermatitis) (Figure 2). The intensity of FLG2 staining was inversely associated with local inflammation (odds ratio 0.14 (0.024, 0.78) p=0.025, i.e., less FLG2 staining in those with more inflammatory cells). No correlation was noted between histologic race and FLG2 staining (p=0.687).
Figure 1.
Box plot of the graded FLG2 immunostaining intensity using standard DAB technique versus the histologic diagnosis (A) and the graded FLG2 immunostaining intensity using standard DAB technique versus the inflammation grade (B).
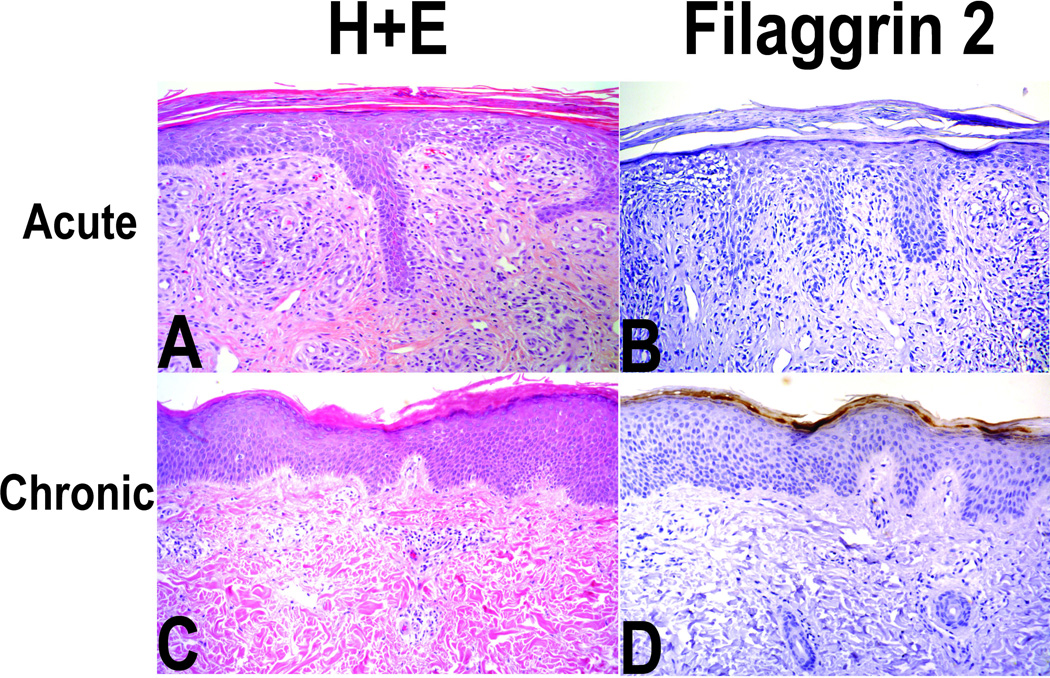
Figure 2. Typical histology (H & E and FLG2 immunostaining) of an individual with acute (panels A and B) and an individual chronic dermatitis (panels C and D).
Panel A (H & E) magnification 100X; acute spongiotic findings with grade 3 inflammation; Panel B (FLG2 staining) grade 1 FLG2 staining; Panel C chronic dermatitis with grade 1 inflammation; Panel D (FLG2 staining) grade 3/4 staining. Grading of FLG2 staining was based on the intensity of immunostaining using standard DAB technique.
Conclusions
FLG protein has long been known to have an important role in skin barrier function with respect to the final assembly of keratin as it becomes part of the fully differentiated terminal barrier skin barrier. Like FLG, FLG2 has an important role in skin barrier function.6;8 Unlike FLG, FLG2 has not been as thoroughly studied. In our study we are able to demonstrate that FLG2 is variably expressed in the stratum corneum of dermatitic skin. Its expression is minimized in those with acute spongiotic dermatitis and in association with a brisk inflammatory infiltrate. Others have also shown that FLG expression regardless of the presence of FLG LOF mutations is also diminished in those producing Th2 cytokines found in acute inflammatory responses6.
FLG2 is a gene closely associated with FLG gene in both location and likely its physiologic activity6 S1, S3. We recently demonstrated that FLG2 stop gain variants that are likely to be LOF mutations in African-Americans with AD are associated with the persistence of AD.7 More specifically, S2377X is a single base pair substitution of T to G resulting in a premature stop codon. In African-American children, this variant has a MAF of 0.22 and children with this variant are more likely to have persistent symptoms of AD (OR = 0.44; 95% CI: 0.25, 0.46). The magnitude of this finding is similar to the effect of FLG LOF in white children.9 Our current study is consistent with this finding in that the localized cutaneous inflammation noted in acute spongiotic dermatitis diminishes the intensity of FLG2 staining. As has been shown for FLG, this effect on FLG2 is likely a direct effect of Th2 cytokines product on keratinocytes6. The effect of Th2 cytokines on the production of FLG occurs regardless of the presence of a FLG LOF mutation.6
We demonstrated that the intensity of FLG2 staining is associated with histologic findings of acute spongiotic dermatitis and the histologic presence of prominent inflammatory cell infiltrate. We do not know if AD disease activity preceded the diminished FLG2 production or if FLG2 diminished production caused the AD disease activity. Such a hypothesis will require more mechanistic studies. We also do not know if our histologic findings are correlated to genetic anomalies in the FLG2 locus. We also did not evaluate other barrier proteins. These associations need to be confirmed in future studies.
Supplementary Material
Acknowledgment
This research was supported by R01-AR0056755 from the NIAMS. JS, TD, and DJM were involved in all aspect of the study and manuscript preparation.
Footnotes
No conflicts of interest to declare.
Reference List
- 1.Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other variables: A US population-based study. Journal of Allergy & Clinical Immunology. 2013;132:1132–1138. doi: 10.1016/j.jaci.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Brown SJ, Relton CL, Liao H, et al. Filaggrin mutations and childhood atopic eczema: A population-based case-control study. Journal of Allergy Clinical Immunology. 2008;121:940–946. doi: 10.1016/j.jaci.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Drongenlen V, Alloul-Ramdhani M, Hanso MO, et al. Knock-down of filaggrin does not affect lipid organization and composition in stratum corneum of reconstructed human skin equivalents. Experimental Dermatology. 2013;22:807–812. doi: 10.1111/exd.12271. [DOI] [PubMed] [Google Scholar]
- 4.Margolis DJ, Gupta J, Apter A, et al. Exome sequencing of Filaggrin and related genes in African-American children with atopic dermatitis. Journal of Investigative Dermatology. 2014;134:2272–2274. doi: 10.1038/jid.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SJ, McLean WH. One remarkable molecule: filaggrin. Journal of Investigative Dermatology. 2012;132:751–762. doi: 10.1038/jid.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellerin L, Henry J, Hsu CY, et al. Defects in filaggrin-like proteins in both lesional and nonlesional atopic skin. Journal of Allergy & Clinical Immunology. 2013;131:1094–1102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 7.Margolis DJ, Gupta J, Apter AJ, et al. Filaggrin-2 variation is associated wtih more persistent atopic dermatitis in Arican Americans. Journal of Allergy & Clinical Immunology. 2014;133:784–789. doi: 10.1016/j.jaci.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makino T, Mizawa M, Yamakoshi T, et al. Expression of filaggrin-2 protein in the epidermis of human skin disease: A comparative analysis with filaggrin. Biochemical and Biophysical Research Communications. 2014;449:100–106. doi: 10.1016/j.bbrc.2014.04.165. [DOI] [PubMed] [Google Scholar]
- 9.Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and Filaggrin mutations in a US longitudinal cohort. Journal of Allergy & Clinical Immunology. 2012;130:912–917. doi: 10.1016/j.jaci.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.