Abstract
Background
Cnidium lactone is a natural coumarin compound that can inhibit a variety of cancer cell proliferation and induce cancer cell apoptosis. This experiment investigated the effect of cnidium lactone on molecular marker expression in prostate cancer nude mice to study its effect in inducing apoptosis.
Material/Methods
We randomly and equally divided 30 male BALB/C nude mice inoculated with human prostate cancer cells PC-3 into a negative control group, a cyclophosphamide group (500 mg/Kg), and cnidium lactone groups at 3 doses (280 mg/Kg, 140 mg/Kg, and 70 mg/Kg). The mice were weighed at 2 weeks after administration. Tunnel assay was applied to test the nude mice tumor cell apoptosis. ELISA was performed to detect serum AMACR, CD147, mutant P53, BCL-2, AND BAX expression levels. Tumor tissue was separated and weighed.
Results
Mice weight did not change significantly in the groups receiving 3 different doses of cnidium lactone(p>0.05), while it decreased obviously in the cyclophosphamide group (p<0.05). Tumor weight, CD147, mutant P53, and BCL-2 levels were significantly lower in the groups receiving 3 different doses of cnidium lactone than in the negative control group (p<0.05). Among them, the abovementioned indexes decreased markedly in the 280 mg/Kg and 140 mg/Kg dose groups than in the cyclophosphamide group (p<0.05). AMACR and BAX levels showed no significant difference in the cnidium lactone group or the cyclophosphamide group (p>0.05).
Conclusions
Cnidium lactone may induce prostate cancer cell apoptosis and inhibit its proliferation through regulating CD147, mutant P53, and BCL-2 expression in nude mice.
MeSH Keywords: Antigens, CD147; Bufexamac; Prostatic Neoplasms
Background
Prostate cancer is a common male urinary system disease that is difficult to diagnosis because of its long incubation period, complex etiology, and hidden pathogenic site [1]. The pathogenesis of prostate cancer is still not fully understood. The factors influencing the biological characteristics of prostate cancer are complex, which is directly related to the occurrence and development of cancer. Tumor cells’ rapid and unlimited proliferation is the most important characteristic. There is a balance between proliferation and apoptosis in normal cells; once this balance is broken, hyperplasia and even malignant tumors can appear. Therefore, much research is currently focussed on how to correct the abnormal growth cycle in tumor tissue and defining the main factors influencing it. Existing research [2,3] suggests that some drugs have certain effects in cell proliferation cycle, in particular, in inducing cell apoptosis. There is still a lack of research on drugs for treatment of prostate cancer invasion and inducing apoptosis. Cnidium lactone is a natural coumarin compound extracted from fruit of the umbelliferous plant Cnidium monnieri. In traditional Chinese medicine the fruit is characterized as acrid and bitter, warm in nature, and belong to the kidney and spleen channel. It has multiple pharmacological effects, including anti-inflammation, anti-tumor, anti-arrhythmia, decreasing blood pressure, and enhancing immunity. Experiments [4,5] showed that cnidium lactone exhibits good antitumor activity in vivo and in vitro with no adverse reactions at normal drug doses. Its main mechanism is to inhibit cancer cell proliferation by acting on cancer cell cycle or inducing apoptosis, such as in liver cancer, lung adenocarcinoma, colorectal cancer, and cervical cancer [6]. In this study, a BALB/c nude mouse prostate cancer model was used to test 3 different doses of cnidium lactone. The mice were euthanized at 2 weeks after inoculation and tumors were weighed. Serum alpha-methylacyl coenzyme A racemase (AMACR), immune glycoprotein gene (CD147), mutant P53 tumor suppressor gene, BCL-2, and BAX levels were detected. We aimed to explore the therapeutic effect of different doses of cnidium lactone on prostate cancer and its related mechanism.
Material and Methods
Animals
We obtained 30 SPF grade male BALB/c nude mice aged 4 to 6 weeks and weighing 15–20 g from the Experimental Animal Center in Shanghai. The mice were feed in super clean laminar flow cabinet under the SPF condition of SPF.
Mice were used for all experiments and all procedures were approved by the Animal Ethics Committee of Provincial Hospital affiliated to Shandong University.
Drugs and reagents
Cnidium lactone (purity >99%, HPLC detection) and cyclophosphamide (purity >98%, HPLC detection) were supplied by the China Institute of Pharmaceutical and Biological Products Verification. Human prostate cancer cells PC-3 were provided by the Chinese Academy of Sciences, Shanghai Institute of Cell Biology. The cells were maintained in F12 medium with 10% fetal bovine serum and 1% penicillin-streptomycin (10 000 U and 10 mg/ml, respectively) at 37°C and 5% CO2. The cells were digested by a mixture of 0.25% (W/V) trypsin and 0.02% EDTA for passage. Fetal bovine serum was obtained from Beijing Solarbio Technology Co., LTD; F12 medium was obtained from Shanghai Hybio Technology Co., LTD; and 2406-2 type CO2 incubator was obtained from SHELLAB Co. (USA).
Modeling
After adaptation under SPF conditions for 3 to 5 days, the BALB/c nude mice were anesthetized by 1% pentobarbital sodium (75 mg/kg) intraperitoneal injection. A 1-cm transverse incision was made at the center of the lower abdomen and the bladder and seminal vesicle gland were exposed, then 20 μl PC-3 cell suspension (about 1.5×106 cells) was injected into the prostate. The abdomen was closed and the mice were set back to the cage after waking [7]. The tumor diameter was measured using vernier calipers for length (a) and width (b) every 2 days, and the tumor volume was calculated as V=1/2×a×b×b. After the tumor reached 200 mm3, the mice were grouped for administration.
Administration
Cnidium lactone and cyclophosphamide powders were dissolved in physiological saline to get the suspension with the concentration of 28 mg/ml and 1.875 mg/ml, respectively.
Thirty male BALB/C nude mice inoculated with human prostate cancer cells PC-3 were randomly and equally divided into a negative control group, a cyclophosphamide group (500 mg/Kg, every two days), and cnidium lactone groups at 3 doses (280 mg/Kg, 140 mg/Kg, and 70 mg/Kg, once a day). Intragastric administration was applied.
Detection
The mice were continuously treated for 2 weeks. The tumor size was measured by vernier caliper on days 0, 5, 10, and 15 after administration. The tumor volume was calculated as v=a×b2/2. After being euthanized, mice were weighed and the tumor weight was measured. After removing the eye, the blood was collected and centrifuged at 1000 rpm/min for 15 min. ELISA was performed to detect serum AMACR, CD147, mutant P53, BCL-2, and BAX expression levels according to the manual. All ELISA kits were obtained from IBL Co. (Germany). A BIO-RAD model1680 type microplate reader was obtained from Shanghai Tiancheng Biotech Co., LTD (China).
TUNEL method was used for detection. After drug delivery for 15 consecutive days, the nude mouse tumor tissue was cut into paraffin sections. Sections were dewaxed by dimethyl benzene and hydrated with a gradient of ethanol (100%, 95%, 85%, 70%, and 50%). Twenty μg/ml proteinase K with no DNase was added at 20–37°C for 20 min. After washing in PBS or HBSS 3 times, hematoxylin was used to stain and the section was closed. The section was observed under an optical microscope according to the TUNEL kit manual (Promega Company).
Statistical analysis
All statistical analyses were performed using SPSS20.0 software (Chicago, IL). Numerical data are presented as means and standard deviation (±SD). One-way ANOVA was used for multiple means comparisons. P<0.05 was considered to indicate a statistically significant result.
Results
Nude mice weight, tumor weight, and volume size comparison
Compared with the control, mouse weight did not change significantly in the groups receiving 3 different doses of cnidium lactone (p>0.05), while it decreased obviously in the cyclophosphamide group (p<0.05). Compared with the control, tumor weight in the groups receiving 3 different doses of cnidium lactone decreased obviously (P<0.01), of which, the 280 mg/Kg and 140 mg/Kg dose cnidium lactone groups exhibited significantly lower tumor weight than the cyclophosphamide group (p<0.05) (Table 1).
Table 1.
Nude mice weight, tumor weight and volume size comparison (χ̄±s).
| Groups | N | Weight/g | Tumor weight/mg |
|---|---|---|---|
| Negative control | 6 | 23.80±0.35& | 356.0±5.8*,& |
| Cnidium lactone 280 mg/Kg group | 5 | 22.36±0.68 | 123.8±8.4*,# |
| Cnidium lactone 140 mg/Kg group | 6 | 22.75±0.56 | 154.0±7.2*,# |
| Cnidium lactone 70 mg/Kg group | 6 | 23.75±0.28 | 195.0±8.6* |
| Cyclophosphamide group | 5 | 16.52±0.57o | 194.0±6.9#,& |
P<0.01, compared with control;
P<0.05, compared with cyclophosphamide group;
P<0.05, compared with control.
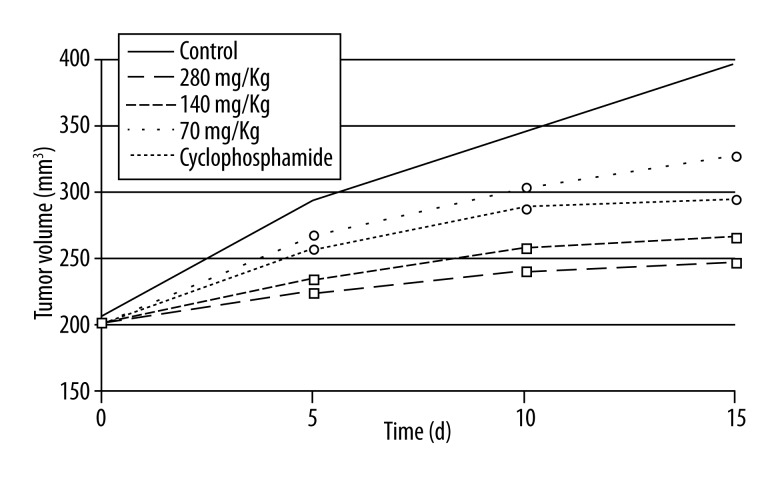
We found that the tumor volume in all of the drug-treated groups showed markedly lower level than in the control group (p<0.05). The 280 mg/Kg and 140 mg/Kg dose cnidium lactone groups exhibited significantly lower tumor volume than the cyclophosphamide group (p<0.05). The tumor volume growth trends are shown in Figure 1.
Figure 1.
Tumor volume growth trends. ○ P<0.05, compared with control; □ P<0.05, compared with negative control and cyclophosphamide group.
TUNEL assay for cell apoptosis comparison
TUNEL assay was applied to test cell apoptosis. Cells with deep stained nucleus were TUNEL-positive and TUNEL-positive cells were considered apoptosis. The results revealed that, compared with negative control, the 3 dosages of cnidium lactone groups all clearly promoted tumor cell apoptosis in the nude mice. Among them, the 280 mg/Kg cnidium lactone group showed the strongest ability to induce cell apoptosis, followed by the cyclophosphamide group and the 140 mg/Kg and 70 mg/Kg cnidium lactone groups (Figure 2).
Figure 2.
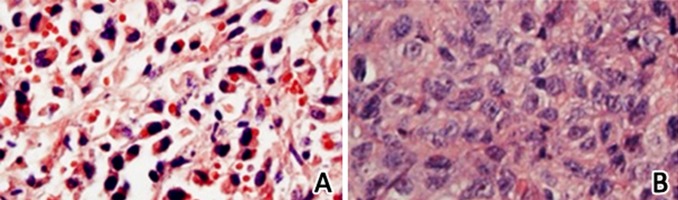
TUNEL assay for cell apoptosis comparison. (A) Nude mouse tumor cell apoptosis in the 280 mg/Kg cnidium lactone group; (B) Nude mouse tumor cell apoptosis in negative controls.
Serum AMACR and CD147 expression level comparison
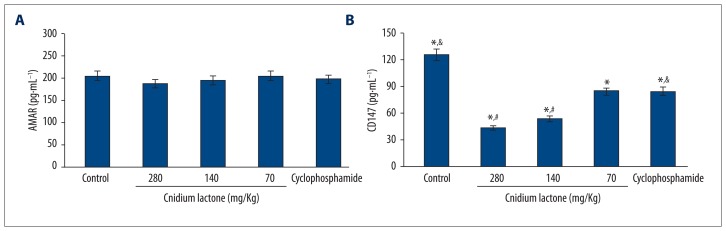
Compared with negative controls, CD147 decreased significantly in all of the groups receiving 3 different doses of cnidium lactone and cyclophosphamide group (p<0.05). Among them, it reduced markedly more in the 280 mg/Kg and 140 mg/Kg dose groups than in the cyclophosphamide group (p<0.05). AMACR failed to show significant difference among the groups (p>0.05) (Table 2 and Figure 3).
Table 2.
Serum AMACR and CD147 expression level comparison (χ̄±s).
| Groups | N | AMACR/pg·mL−1 | CD147/pg·mL−1 |
|---|---|---|---|
| Negative control | 5 | 205.3±0.65 | 126.0±2.8*,& |
| Cnidium lactone 280 mg/Kg group | 6 | 187.6±0.72 | 43.9±2.4*,# |
| Cnidium lactone 140 mg/Kg group | 6 | 195.2±0.54 | 54.1±3.2*,# |
| Cnidium lactone 70 mg/Kg group | 5 | 203.9±0.78 | 85.0±2.6* |
| Cyclophosphamide group | 5 | 196.4±0.67 | 84.5±3.4#,& |
P<0.01, compared with control;
P<0.05, compared with cyclophosphamide group;
P<0.05, compared with control.
Figure 3.
Serum AMACR and CD147 expression level comparison. (A) Serum AMACR expression level comparison; (B) Serum CD147 expression level comparison. * P<0.05, compared with controls; # P<0.05, compared with cyclophosphamide group; & P<0.05, cyclophosphamide group compared with controls.
Mutant P53, BCL-2 and BAX expression levels comparison
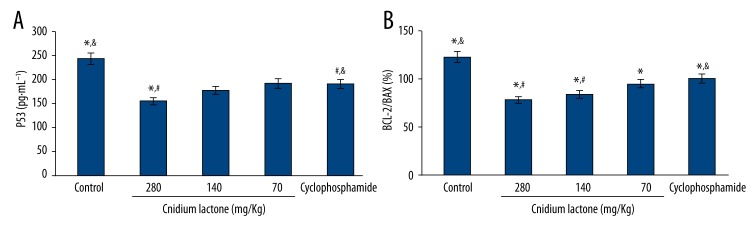
Compared with controls, the cnidium lactone group showed significantly lower levels of mutant P53 and BCL-2 (p<0.05) but not BAX (p>0.05), while the 280 mg/Kg and 140 mg/Kg dose cnidium lactone groups exhibited obviously lower mutant P53 and BCL-2 levels than in the cyclophosphamide group (p<0.05).
After calculating the ratio of BCL-2 and BAX, we found that the BCL-2/BAX ratio was lower in all of the groups receiving 3 different doses of cnidium lactone than in the controls (p<0.05), and it was markedly lower in the 280 mg/Kg and 140 mg/Kg dose cnidium lactone groups than in the cyclophosphamide group (p<0.05) (Table 3 and Figure 4).
Table 3.
Mutant P53, BCL-2 and BAX expression levels comparison (χ̄±s).
| Groups | N | Mutant P53 (pg·mL−1) | BCL-2 (pg·mL−1) | BAX (pg·mL−1) | BCL-2/BAX |
|---|---|---|---|---|---|
| Negative control | 6 | 245.4±4.37*,& | 93.0±2.8*,& | 85.4±1.3 | 123.3%*,& |
| Cnidium lactone 280 mg/Kg group | 5 | 156.5±6.38*,# | 54.0±2.4*,# | 69.8±4.1 | 78.3%*,# |
| Cnidium lactone 140 mg/Kg group | 6 | 179.5±5.26*,# | 60.2±2.2*,# | 71.0±3.2 | 84.5%*,# |
| Cnidium lactone 70 mg/Kg group | 6 | 193.8±6.51* | 69.7±1.5* | 73.2±3.6 | 95.2%* |
| Cyclophosphamide group | 5 | 192.0±5.72#,& | 75.4±1.6#,& | 74.0±2.8 | 101.3%#,& |
P<0.01, compared with control;
P<0.05, compared with cyclophosphamide group;
P<0.05, compared with control.
Figure 4.
Mutant P53, BCL-2 and BAX expression levels comparison. (A) Serum P53 expression levels comparison. * P<0.05, compared with controls; # P<0.05, compared with cyclophosphamide group; & P<0.05, cyclophosphamide group compared with controls; (B) Serum BCL-2/BAX expression levels comparison. * P<0.05, compared with controls; # P<0.05, compared with cyclophosphamide group; & P<0.05, cyclophosphamide group compared with controls.
Discussion
In recent years, the incidence of prostate cancer in China is obviously rising [8–10]. Cnidium lactone is reportedly a type of coumarin compound with antitumor activity, but its effect in prostate cancer cell apoptosis has not been investigated. In vitro experiments suggested that cnidium lactone may inhibit human prostate cancer DU145 cell proliferation through inducing apoptosis [11]. It can significantly increase mutant P53 and Bax expression in human breast cancer MDA – MB435 cells, while inhibiting Bcl-2 expression to induce MDA – MB435 apoptosis [12]. It can act on the G2/M stage in lung adenocarcinoma A549 to induce apoptosis and inhibit metastasis through the PI3K/Akt pathway [13]. It also can induce liver cancer cell apoptosis by regulating NF-κB activity [14]. The cell proliferation process is determined by a series of gene regulations, while apoptosis is the only way for cell renewal. The P53 tumor suppressor gene is divided into wild and mutant types – the wild-type P53 gene can inhibit tumor cell abnormal growth, while the mutant P53 gene can promote cancer cell growth. Studies have shown that the positive rate of mutant P53 protein in the biopsy of prostate cancer patients is significantly higher than in the hyperplasia tissue [15,16]. Excessive mutant P53 gene expression has a direct influence on a variety of tumor occurrence and metastasis, such as ovarian cancer and breast cancer [17,18]. Research indicated that Bcl-2 can increase cell resistance to most of the DNA damage factors and inhibit most of the target cell apoptosis caused by chemotherapy drugs [19]. The Bax gene can promote cell apoptosis, in contrast to the role of Bcl-2. Calculating the ratio of the Bcl-2/Bax in cells can more clearly reflect the main factors that influence cell apoptosis.
Prostate cancer PC-3 is a kind of hormone-independent cancer cell [20–22]. This study used a BALB/c nude mouse model inoculated with human PC-3 cells to simulate prostate cancer growth in vivo. After different doses of cnidium lactone were administered by lavage, we found that mouse weight was not significantly changed compared with the negative control group, but cyclophosphamide can significantly decrease mouse weight. The tumor weight in the 3 different doses of cnidium lactone groups was significantly lower than in the negative controls (p<0.01), and it was obviously lower in the 280 mg/Kg and 140 mg/Kg dose groups compared with the cyclophosphamide group (p<0.05), indicating that cnidium lactone can effectively inhibit tumor cell growth and its effect was significantly better than that of cyclophosphamide. Importantly, the damage to the nude mice caused by cnidium lactone under this dose is relatively small. Its expression in the 3 different doses of cnidium lactone groups was obviously lower than in the negative controls (p<0.05), and the 280 mg/Kg and 140 mg/Kg dose cnidium lactone groups also showed significantly lower expression than in the cyclophosphamide group (p<0.05), showing that cnidium lactone can inhibit mutant P53 and CD147 gene expression, thus affecting the tumor proliferation process. P53 gene mutations occur when the normal human cells were stimulated and mutant P53 gene can promote cancer cells abnormal growth. Mutant P53 gene expression can be detected in serum [23,24]. Cnidium lactone is clearly more effective than cyclophosphamide in reducing mutant P53 gene expression level. We speculate that it may promote the cancer cell death by inducing p21 expression, inhibiting Cdk activity, and making the damaged DNA lose repair ability. This may be because the mutant P53 tumor-suppressor gene can indirectly act on vascular endothelial growth factor expression to inhibit tumor angiogenesis and prostate cancer cell proliferation. The CD147 gene exists in a variety of cancer cells, including glioma, lymphoma, and breast tumor cells. It is mainly involved in the process of tumor cells invasion and metastasis. Compared with the negative controls, cnidium lactone can significantly inhibit its expression to decrease prostate cancer invasion and metastasis and further prevent the progression of prostate cancer.
BCL-2 expression levels in the groups receiving 3 different doses of cnidium lactone were significantly lower than in the negative controls (p<0.05), and its levels in the 280 mg/Kg and 140 mg/Kg dose groups were obviously lower than in the cyclophosphamide group (p<0.05). In contrast, BAX level showed no significant differences in the cnidium lactone group and cyclophosphamide group when compared with the negative controls (p>0.05). This suggests that cnidium lactone can accelerate prostate cancer cells apoptosis by reducing BCL-2 expression and up-regulating the BCL-2/BAX ratio to abate the antagonism between BCL-2 and BAX. Its effect on BCL-2 expression is significantly higher than cyclophosphamide. This may be because BCL-2 can promote cytochrome C release by mitochondria and increase caspase protease activated by cytochrome C, thus weakening its inhibitory effect on cell apoptosis. In addition, as a kind of proto-oncogene, the excessive expression of Bcl-2 may produce an antagonism effect on cell apoptosis induced by the wild-type P53 gene. This indicates that cnidium lactone may also achieve the effect of promoting prostate cancer cell apoptosis by decreasing wild-type P53 gene level through acting on the Bcl-2 expression. Cnidium lactone showed no significant effect on BAX expression in the nude mice.
Conclusions
Above all, cnidium lactone can effectively inhibit CD147, mutant P53, and BCL-2 expressions in a nude mouse prostate cancer model and induce prostate cancer cell apoptosis. It exhibited certain therapeutic effects for the treatment of hormone-dependent prostate cancer, but the mechanism of its induction of prostate cancer cell apoptosis still needs further in-depth study.
Footnotes
Source of support: Departmental sources
References
- 1.Choudhury Y, Yang Z, Ahmad I, et al. AMP-activated protein kinase (AMPK) as a potential therapeutic target independent of PI3K/Akt signaling in prostate cancer. Oncoscience. 2014;1:446–56. doi: 10.18632/oncoscience.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai CF, Yeh WL, Chen JH, et al. Osthole suppresses the migratory ability of human glioblastoma multiforme cells via inhibition of focal adhesion kinase-mediated matrix metalloproteinase-13 expression. Int J Mol Sci. 2014;15:3889–903. doi: 10.3390/ijms15033889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarzab A, Grabarska A, Kielbus M, et al. Osthole induces apoptosis, suppresses cell-cycle progression and proliferation of cancer cells. Anticancer Res. 2014;34:6473–80. [PubMed] [Google Scholar]
- 4.Chen Z, Mao X, Liu A, et al. Osthole, a natural coumarin improves cognitive impairments and BBB dysfunction after transient global brain ischemia in C57 BL/6J mice: involvement of Nrf2 pathway. Neurochem Res. 2015;40:186–94. doi: 10.1007/s11064-014-1483-z. [DOI] [PubMed] [Google Scholar]
- 5.Ninio-Many L, Grossman H, Levi M, et al. MicroRNA miR-125a-3p modulates molecular pathway of motility and migration in prostate cancer cells. Oncoscience. 2014;1:250–61. doi: 10.18632/oncoscience.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LY, Huang WJ, Ho FM, et al. N-Hydroxycinnamide derivatives of osthole inhibit cell migration and invasion by suppressing Smad2 and Akt pathways in human colorectal adenocarcinoma cells. Chem Biol Interact. 2014;217:1–8. doi: 10.1016/j.cbi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Chao X, Zhou X, Zheng G, et al. Osthole induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Pharm Biol. 2014;52:544–50. doi: 10.3109/13880209.2013.850517. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Zhan T, Duffy D, et al. A pilot phase II Study of digoxin in patients with recurrent prostate cancer as evident by a rising PSA. Am J Cancer Ther Pharmacol. 2014;2:21–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Wei SM, Fei JX, Tao F, et al. Anti-CD27 antibody potentiates antitumor effect of dendritic cell-based vaccine in prostate cancer-bearing mice. Int Surg. 2015;100:155–63. doi: 10.9738/INTSURG-D-14-00147.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha S, Bhanja P, Partanen A, et al. Low intensity focused ultrasound (LOFU) modulates unfolded protein response and sensitizes prostate cancer to 17AAG. Oncoscience. 2014;1:434–45. doi: 10.18632/oncoscience.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shokoohinia Y, Hosseinzadeh L, Alipour M, et al. Comparative evaluation of cytotoxic and apoptogenic effects of several coumarins on human cancer cell lines: osthole induces apoptosis in p53-deficient H1299 cells. Adv Pharmacol Sci. 2014;2014:847574. doi: 10.1155/2014/847574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SM, Tsai CF, Chen DR, et al. p53 is a key regulator for osthole-triggered cancer pathogenesis. Biomed Res Int. 2014;2014:175247. doi: 10.1155/2014/175247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YC, Lin JC, Hung CM, et al. Osthole inhibits insulin-like growth factor-1-induced epithelial to mesenchymal transition via the inhibition of PI3K/Akt signaling pathway in human brain cancer cells. J Agric Food Chem. 2014;62:5061–71. doi: 10.1021/jf501047g. [DOI] [PubMed] [Google Scholar]
- 14.Yang SM, Chan YL, Hua KF, et al. Osthole improves an accelerated focal segmental glomerulosclerosis model in the early stage by activating the Nrf2 antioxidant pathway and subsequently inhibiting NF-kappaB-mediated COX-2 expression and apoptosis. Free Radic Biol Med. 2014;73:260–69. doi: 10.1016/j.freeradbiomed.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Rossi E, Rugge M, Facchinetti A, et al. Retaining the long-survive capacity of Circulating Tumor Cells (CTCs) followed by xeno-transplantation: not only from metastatic cancer of the breast but also of prostate cancer patients. Oncoscience. 2014;1:49–56. doi: 10.18632/oncoscience.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao J, Zhang T, Xu D, et al. FBXL20-mediated Vps34 ubiquitination as a p53 controlled checkpoint in regulating autophagy and receptor degradation. Genes Dev. 2015;29:184–96. doi: 10.1101/gad.252528.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gioacchini FM, Alicandri-Ciufelli M, Rubini C, et al. Prognostic value of Bcl-2 expression in squamous cell carcinoma of the larynx: a systematic review. Int J Biol Markers. 2014 doi: 10.5301/jbm.5000116. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Choi JE, Woo SM, Min KJ, et al. Combined treatment with ABT-737 and VX-680 induces apoptosis in Bcl-2- and c-FLIP-overexpressing breast carcinoma cells. Oncol Rep. 2015;33:1395–401. doi: 10.3892/or.2015.3728. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Shi H, Ren F, et al. Misregulation of polo-like protein kinase 1, P53 and P21WAF1 in epithelial ovarian cancer suggests poor prognosis. Oncol Rep. 2015;33:1235–42. doi: 10.3892/or.2015.3723. [DOI] [PubMed] [Google Scholar]
- 20.Nishijima J, Hara T, Ikemoto K, et al. Clinical significance of ERG rearrangement subtype and its association with increased p53 expression in Japanese and German prostate cancer. Neoplasma. 2015;62(2):278–87. doi: 10.4149/neo_2015_033. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Cao LY, Cheng SJ, et al. p53-induced microRNA-1246 inhibits the cell growth of human hepatocellular carcinoma cells by targeting NFIB. Oncol Rep. 2015;33:1335–41. doi: 10.3892/or.2015.3715. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Wen J, Li R, et al. Gene expression profiling analysis of castration-resistant prostate cancer. Med Sci Monit. 2015;21:205–12. doi: 10.12659/MSM.891193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Xie PG, Lin YL, et al. Aberrant methylation of PCDH10 predicts worse biochemical recurrence-free survival in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1363–68. doi: 10.12659/MSM.891241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu WB, Gui SL, Lin YL, et al. Promoter methylation of protocadherin8 is an independent prognostic factor for biochemical recurrence of early-stage prostate cancer. Med Sci Monit. 2014;20:2584–89. doi: 10.12659/MSM.893083. [DOI] [PMC free article] [PubMed] [Google Scholar]