Abstract
Background
Pituitary adenoma is a common intracranial tumor in neurosurgery. Some pituitary adenomas have the characteristics of invasive growth make them unable to be removed completely by surgery leading to easy relapse. Discoidin domain receptor l (DDR1) is a new kind of tyrosine kinase receptor on the cell surface. DDR1 can be activated by tumor microenvironment signal in tumorigenesis, increasing MMP-2/9 expression and promoting the invasive ability of tumor cells. Anoxia can promote tumor growth and metastasis. This study investigated the impact of anoxic environment DDR1 expression in pituitary adenoma.
Material/Methods
A primary hypoxia pituitary adenoma cell model was established and treated with DDR1 inhibitor nilotinib. Real-time PCR and Western blot were used to detect DDR1 mRNA and protein expression. ELISA was used to detect MMP-2/9 changes. MTT method was used to detect pituitary adenoma cell proliferation. We used a transwell chamber to determine pituitary adenoma cell invasion ability.
Results
DDR1 mRNA and protein were significantly overexpressed under hypoxia (P<0.05). MMP-2 and MMP-9 expression was obviously increased in supernatant (P<0.05). Pituitary adenoma cell proliferation and invasive ability improved markedly under hypoxia (P<0.05). Nilotinib could reduce DDR1 expression, decrease MMP-2 and MMP-9 expression, and inhibit pituitary adenoma cells proliferation and invasion.
Conclusions
Hypoxia can increase DDR1 expression in pituitary adenoma cells, leading to improved MMP-2 and MMP-9 secretion, and promoting pituitary adenoma cell proliferation and invasion.
MeSH Keywords: ACTH-Secreting Pituitary Adenoma; Addresses; Hypoxia-Ischemia, Brain
Background
Pituitary adenoma is a common intracranial tumor in neurosurgery. Some pituitary adenomas have the characteristics of invasive growth. It may cause headache, cranial nerve palsy, and vision loss, depending on different invasive areas. Surgery cannot remove the invasive tumor completely and may lead to postoperative complications and it easily relapses [1,2]. The mechanism of pituitary adenoma occurrence and invasion is still not understood.
Extra cellular matrix (ECM) degradation is an important mechanism of tumor invasion. Tumor cell adhesion, ECM degradation, and neovascularization may promote tumor invasion [3,4]. Matrix metalloproteinase (MMP) belongs to the calcium zinc dependent endogenous protease family that can degrade all of the extracellular matrix and basement membrane except polysaccharides, and thus is in maintaining the physiological ECM dynamic balance [5,6]. As important members of the MMPs family, MMP-2 and MMP-9 participate in ECM and basement membrane degradation. Numerous studies confirmed that MMP-2 and MMP-9 participate in occurrence of multiple cancer invasion and migration processes such as laryngeal cancer, lung cancer, and liver cancer [7–9]. MMP-2 and MMP-9 were reported to be overexpressed in pituitary adenoma, and especially in invasive pituitary adenoma, which suggests its important role in pituitary adenoma [10]. But its specific mechanism has not been fully elucidated. Discoidin domain receptor l (DDR1) is a newly identified tyrosine kinase receptor on the cell surface. DDR1 could be activated by tumor microenvironment signal in tumorigenesis. Its location on the cell membrane indicates it can receive extracellular signals. Its specific ligand is collagen. DDR1 could be activated by tumor microenvironment signals in tumorigenesis, so as to increase MMP-2/9 expression and promote invasive ability of tumor cells [11,12].
Hypoxia can promote abnormal cytokine expression and cause nerve-endocrine-immune system disorder, thus promoting tumor growth and metastasis. This effect is particularly significant in solid tumor [13,14]. However, the effect of hypoxia on pituitary adenoma is still unknown. This study investigated the impact of anoxia on DDR1 expression in pituitary adenoma.
Material and Methods
Reagents and instruments
DMEM medium, fetal bovine serum (FBS), EDTA, and penicillin-streptomycin were purchased from Hyclone; dimethyl sulfoxide and MTT were purchased from Gibco; trypsin-EDTA was purchased from Sigma; PVDF membrane was purchased from Pall Life Sciences; Western blot-related chemical reagents were from Shanghai Beyotime biotechnology Co. LTD; ECL reagent was purchased from Amersham Biosciences; DDR-1 monoclonal antibody and HRP tagged IgG secondary antibody were purchased from Cell Signaling; nilotinib and transwell chambers were purchased from Sigma; MMP-2 and MMP-9 ELISA kits were purchased from R&D; other reagents were purchased from Shanghai Sangon Biotechnology Co., LTD; and Labsystem Version1.3.1 microplate reader was purchased from Bio-rad.
Objects
We enrolled 10 pituitary adenoma patients (6 males and 4 females) from neurosurgery between January 2014 and December 2014. All the patients underwent surgery. The mean age of the selected patients was 33.5±9.4 (26–62) years. All patients’ clinical symptoms were consistent with the diagnostic criteria. The diagnosis was confirmed by clinical imaging, surgery, and pathology. No patients received growth hormone inhibitors before the surgery. The general clinical information showed no statistically significant differences (P>0.05). All subjects signed informed consent and the research was approved by ethics committee in our hospital.
Methods
Primary cell culture and hypoxia model
Pituitary adenoma cells were extracted by mechanical digestion and enzyme digestion method for primary culture. Tumor specimens were divided into small pieces at 1 mm3 under aseptic condition. After digested by 0.25% trypsin-EDTA at 37°C for 30 min, the cells were filtered, centrifuged, and maintained in DMEM medium (containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin) at 37°C and 5% CO2. The medium was changed on the next day and cells were passaged every 2–3 days. Cells in logarithmic phase were divided into control group, hypoxia group, and DDR1 inhibitor group. According to protocol of previous report [15], cells in the hypoxia group were maintained in 95% N2 and 5% CO2 incubator, while cells in the DDR1 inhibitor group were treated with 1 μM nilotinib under hypoxia.
Real-time PCR
The cDNA was synthesized with 1 μg RNA from the samples. The primers used are as follows: DDR-1 forward primer: 5′-AAGCTACGGACAGCAATTCT-3′, reverse primer: 5′-TGGCCATCTGACTCCGTCA-3′; β-actin: forward primer: 5′-AGTCTAGTTGCTGGGTACC-3′, reverse primer: 5′-TAATAGGAT GTCTGGACTCCG-3′. The cycling conditions consisted of an initial, single cycle of 1 min at 52°C, followed by 35 cycles of 30 s at 90°C, 50 s at 58°C, and 35 s at 72°C. PCR amplifications were performed in 3 duplicates for each sample. Gene expression levels were quantified relative to the expression of GAPDH using an optimized comparative Ct (ΔΔCt) value method.
Western blot
The tissue was digested with lysis buffer. Total protein was separated by denaturing 10% SDS-polyacrylamide gel electrophoresis. Detection was performed with chemiluminescence and calculated with Quantity One. Antibody dilutions were 1: 500 for DDR1. Protein levels were normalized to β-actin and changes were determined by 4 replications.
ELISA
The cell supernatant was stored at −80°C. ELISA kits were used to detect MMP-2 and MMP-9 expression changes according to the manual. Major steps included: put 50 μl diluted standard product into the corresponding reaction holes to prepare the standard curve. Add 50 μl samples to each hole. After washing the plate 5 times, 50 μl enzyme reagent was added. The plate was washed 5 times again after incubation at 37°C for 30 min. We inserted 50 μl agent A and 50 μl agent B into to each hole and the plate was incubated at 37°C for 10 min. The reaction was terminated after adding 50 μl terminates liquid. The plate was measured at 450 nm wavelength to get the absorbance value (OD value). The sample concentration was calculated according to the OD value and standard curve.
MTT assay
After culturing for 48 h, the cells in each group were digested and counted, then the cells were seeded to the 96-well plate at 3000 cells/well with 5 replicates. We added 20 μl of 5 g/L MTT solution to each well and cultured at 37°C and 5% CO2 for 4 h. After adding 150 μl DMSO for 10 min, the absorbance (OD) value of each well was detected by microplate reader at wavelength of 570 nm to calculate the cell proliferation rate.
Transwell assay
We added 300 μg/ml Matrigel to the surface of upper chamber at 4°C under sterile conditions and incubated at 37°C for 3 h. DMEM medium containing 15% FBS was added to the lower chamber. The cells were pretreated with FBS-free DMEM medium for 12 h and then seeded in the upper chamber. After 20-h incubation, the membrane was fixed in 70% formaldehyde for 30 min. Crystal violet was used for staining and the cells were counted under the microscope.
Statistical analysis
All statistical analyses were performed using SPSS16.0 software (Chicago, IL). Numerical data are presented as means and standard deviation (±S). Differences between multiple groups were analyzed using 1-way ANOVA or LSD test. P<0.05 was considered as a significant difference.
Results
Hypoxia impact on DDR1 mRNA expression
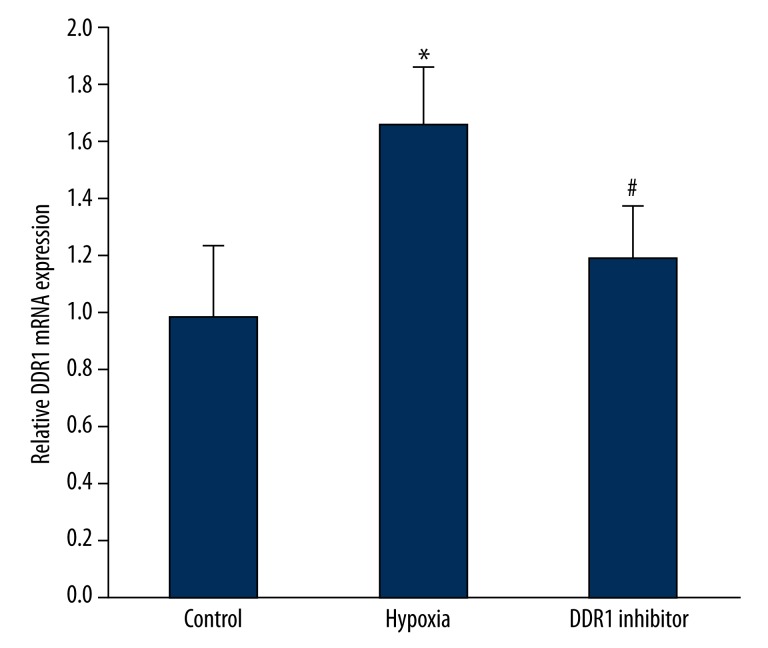
Real-time PCR was used to detect hypoxia impact on DDR1 mRNA expression. It was found that DDR1 mRNA expression in pituitary adenoma increased significantly under hypoxia (P<0.05). DDR1 inhibitor treatment can obviously reduce DDR1 mRNA overexpression caused by hypoxia (P<0.05) (Figure 1).
Figure 1.
Hypoxia impact on DDR1 mRNA expression. * P<0.05, compared with control; # P<0.05, compared with hypoxia group.
Hypoxia impact on DDR1 protein expression
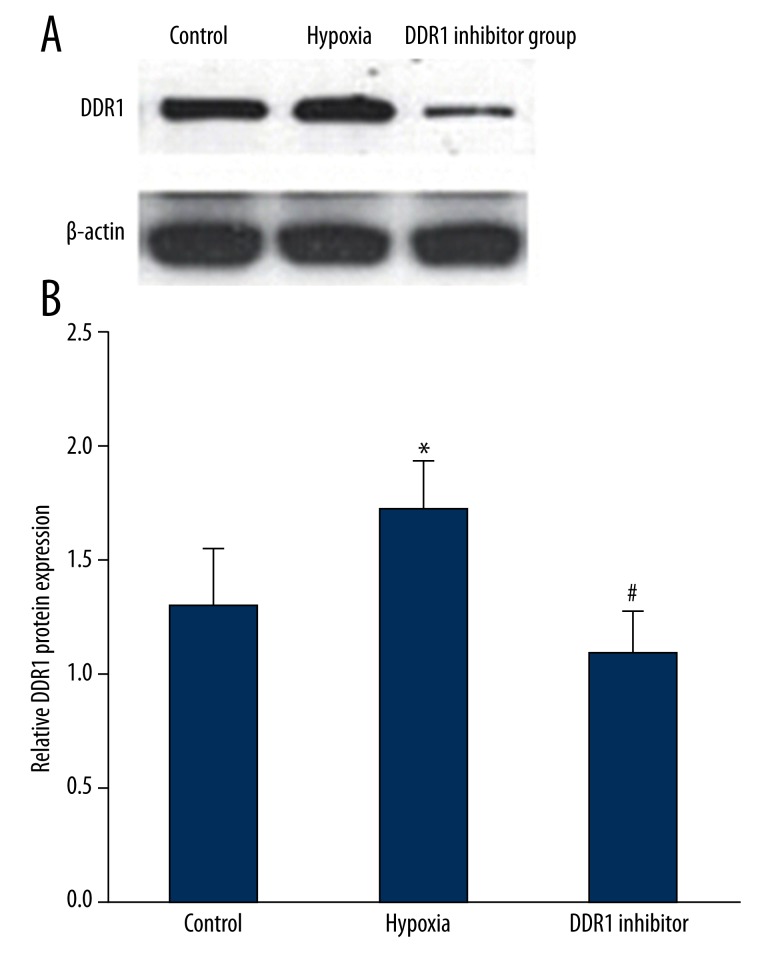
Western blot was used to measure hypoxia impact on DDR1 protein expression. The results showed that, similar to DDR1 mRNA expression, DDR1 protein was markedly up-regulated under hypoxia (P<0.05). DDR1 inhibitor treatment can decrease DDR1 protein overexpression caused by hypoxia (P<0.05) (Figure 2).
Figure 2.
Hypoxia impact on DDR1 protein expression. (A) Hypoxia impact on DDR1 protein expression. (B) Hypoxia impact on DDR1 protein expression. * P<0.05, compared with control; # P<0.05, compared with hypoxia group.
The impact of hypoxia-induced DDR1 overexpression on MMP2/MMP9
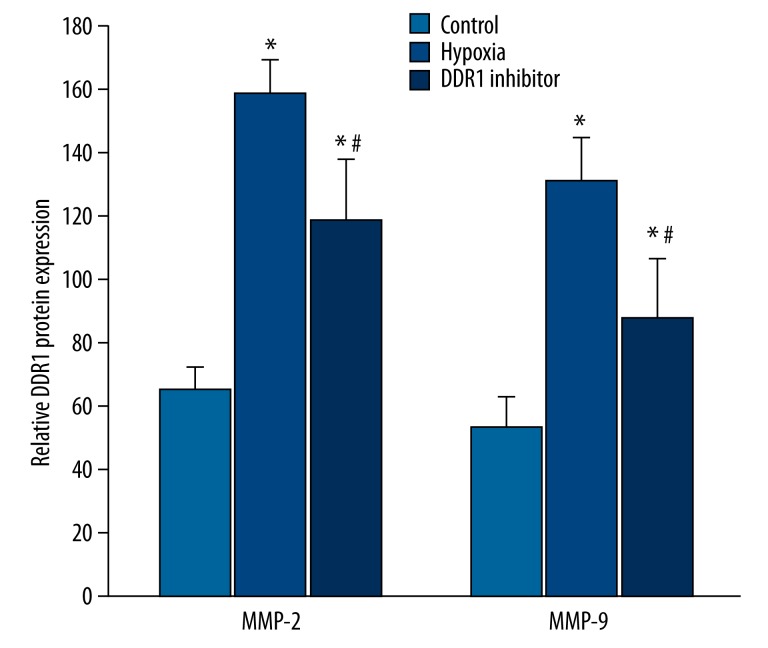
MMP2/MMP9 content in the supernatant was detected by ELISA. The results revealed that compared with control (65.12±7.28 ng/ml), MMP-2 significantly increased under hypoxia (158.27±11.25 ng/ml) (P<0.05), and MMP-9 expression (131.44±13.16ng/ml) in the hypoxia group was also higher than the in the controls (53.27±9.36 ng/ml) (P<0.05). After adding DDR1 inhibitor, MMP-2 (118.27±19.43 ng/ml) and MMP-9 (87.21±19.11 ng/ml) expression decreased compared with the hypoxia group (P<0.05) (Figure 3).
Figure 3.
The impact of hypoxia induced DDR1 overexpression on MMP2/MMP9.* P<0.05, compared with control; # P<0.05, compared with hypoxia group.
The impact of hypoxia-induced DDR1 overexpression on pituitary adenoma proliferation
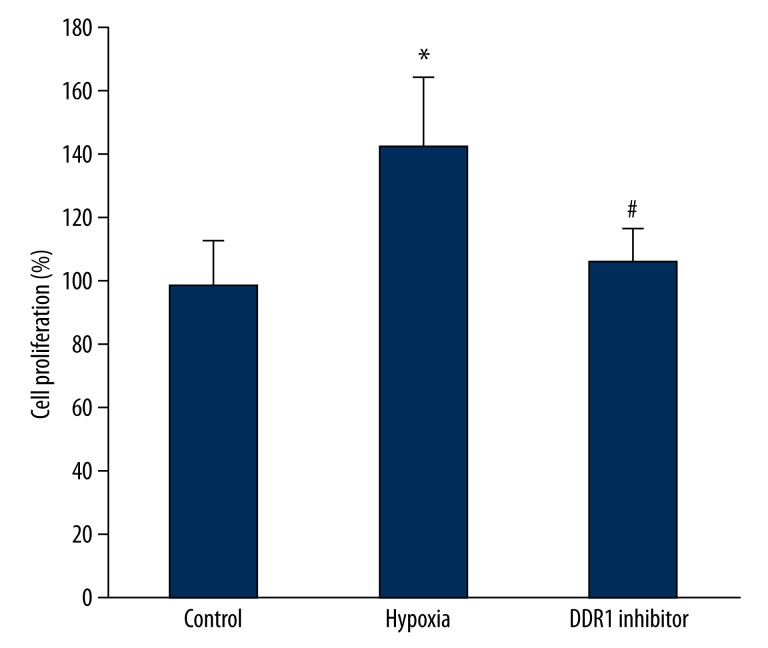
Pituitary adenoma proliferation was determined by MTT method. We found that cell proliferation obviously increased under hypoxia compared with controls (P<0.05). DDR1 inhibitor could decrease DDR1 expression and inhibit cell proliferation significantly compared with the hypoxia group (P<0.05) (Figure 4).
Figure 4.
The impact of hypoxia induced DDR1 overexpression on pituitary adenoma proliferation. * P<0.05, compared with control; # P<0.05, compared with hypoxia group.
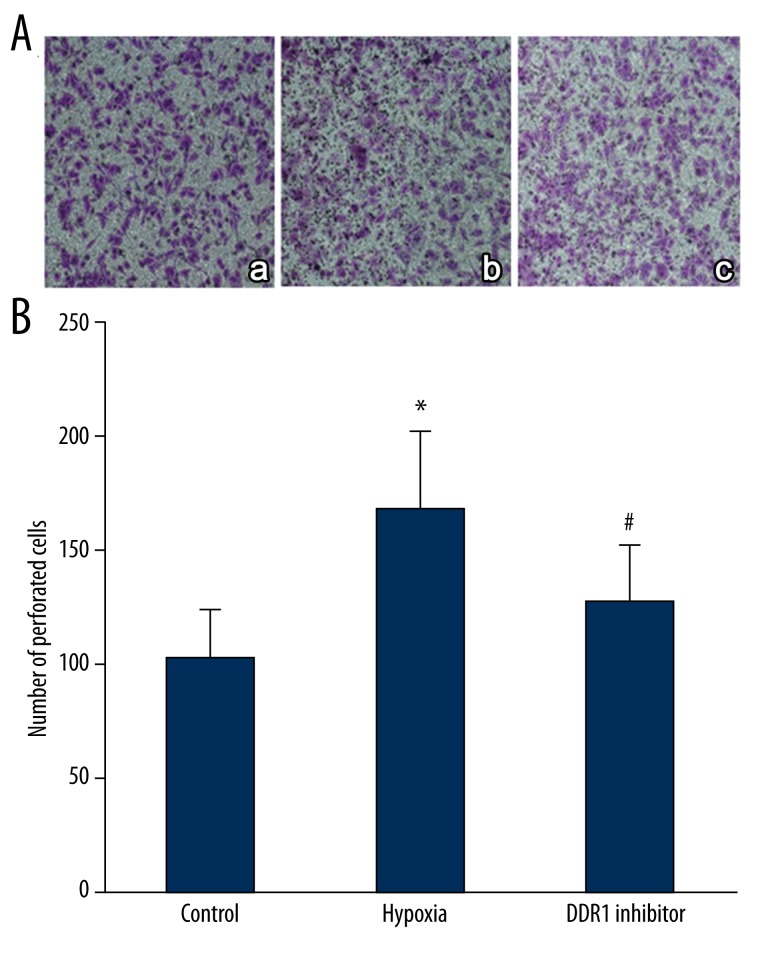
The impact of hypoxia induced DDR1 overexpression on pituitary adenoma invasion
Transwell assay was performed to detect the impact of hypoxia-induced DDR1 overexpression on pituitary adenoma invasion. The results suggest that cell invasive ability rose significantly after hypoxia treatment (P<0.05). DDR1 inhibitor obviously decreased the number of perforated cells compared with the hypoxia group (P<0.05) (Figure 5).
Figure 5.
The impact of hypoxia induced DDR1 overexpression on pituitary adenoma invasion. (A) The impact of hypoxia-induced DDR1 overexpression on pituitary adenoma invasion. a, control; b, hypoxia group; c, DDR1 inhibitor group; (B) The impact of hypoxia-induced DDR1 overexpression on pituitary adenoma invasion. * P<0.05, compared with control; # P<0.05, compared with hypoxia group.
Discussion
Invasive pituitary adenoma has a complicated mechanism and interacts with the nerve-endocrine-immune network. It is affected by a variety of factors, not only genes, but also ECM [16,17]. ECM plays a physiological role in normal pituitary tissues. At the same time, its abnormal expression and rebuilding are associated with pituitary tumor occurrence and development. MMPs can degrade and reconstruct ECM. Although the specific mechanism has not been elucidated, MMP-2/9 is associated with the pituitary adenoma invasion [10].
DDR1 ligand collagen combining with DDR1 can activate the DDR1 signaling pathway. DDR1 is up-regulated after collagen protein ligand stimulation, thus promoting MMP-2/9 expression, ECM reconstruction, and invasion occurrence [12]. Hypoxia can accelerate tumor angiogenesis, induce cell apoptosis, change tumor cell invasiveness, and regulate energy metabolism [18–20]. However, DDR1 expression and its effect on pituitary adenoma under hypoxia still need further investigation. This study confirmed that DDR1 mRNA and protein are overexpressed in primary pituitary adenoma cells under hypoxia. Increased DDR1 expression can promote MMP-2 and MMP-9 expression in supernatant, thereby accelerating pituitary adenoma cell proliferation and invasion. Nilotinib treatment can reduce DDR1 expression and further inhibit MMP-2 and MMP-9 expression to suppress pituitary adenoma cells proliferation and invasion. Our results show that hypoxia can promote pituitary adenoma cell proliferation and invasion by increasing DDR1 expression in pituitary adenoma cells and improving MMP-2 and MMP-9 secretion, further promoting cell proliferation and invasion. In different environments, after binding with DDR1, DDR1 ligand collagen can promote the DDR1 signaling pathway. DDR1 overexpressed after stimulation by collagen ligand can promote MMP-2/9 expression, extracellular matrix reconstruction, and tumor invasion [12]. This study confirmed that DDR1 is importantly influenced by hypoxia and regulates pituitary adenoma cell growth, proliferation, and infiltration through MMP-2/9.
Conclusions
To sum up, as a cell-surface tyrosine kinase receptor that can bind with collagen in the ECM, DDR1 plays an important role in pituitary adenomas under hypoxia and it could be a target to inhibit pituitary adenoma cell proliferation and invasion. Further investigation is needed to discover the theoretical basis for clinical treatment.
Footnotes
Source of support: Departmental sources
References
- 1.Manara R, Gabrieli J, Citton V, et al. Intracranial internal carotid artery changes in acromegaly: a quantitative magnetic resonance angiography study. Pituitary. 2014;17:414–22. doi: 10.1007/s11102-013-0516-y. [DOI] [PubMed] [Google Scholar]
- 2.Oklu R, Deipolyi AR, Wicky S, et al. Identification of small compound biomarkers of pituitary adenoma: a bilateral inferior petrosal sinus sampling study. J Neurointerv Surg. 2014;6:541–46. doi: 10.1136/neurintsurg-2013-010821. [DOI] [PubMed] [Google Scholar]
- 3.Swift J, Discher DE. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J Cell Sci. 2014;127:3005–15. doi: 10.1242/jcs.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wietecha MS, Cerny WL, DiPietro LA. Mechanisms of vessel regression: toward an understanding of the resolution of angiogenesis. Curr Top Microbiol Immunol. 2013;367:3–32. doi: 10.1007/82_2012_287. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Kesh K, Ganguly N, et al. Matrix metalloproteinases and gastrointestinal cancers: Impacts of dietary antioxidants. World J Biol Chem. 2014;5:355–76. doi: 10.4331/wjbc.v5.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoseini SM, Kalantari A, Afarideh M, et al. Evaluation of plasma MMP-8, MMP-9 and TIMP-1 identifies candidate cardiometabolic risk marker in metabolic syndrome: results from double-blinded nested case-control study. Metabolism. 2015;64:527–38. doi: 10.1016/j.metabol.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Uloza V, Liutkevicius V, Pangonyte D, Lesauskaite V. Characteristics of Expression of Matrix Metalloproteinases (MMP-2 and MMP-9) in Glottic Squamous Cell Carcinoma and Benign Vocal Fold Lesions. Clin Exp Otorhinolaryngol. 2015;8:57–64. doi: 10.3342/ceo.2015.8.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farha AK, Dhanya SR, Mangalam SN, Remani P. Anti-metastatic effect of deoxyelephantopin from Elephantopus scaber in A549 lung cancer cells in vitro. Nat Prod Res. 2015:1–5. doi: 10.1080/14786419.2015.1012165. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Shen Y, Cao B, et al. Elevated expression levels of androgen receptors and matrix metalloproteinase-2 and -9 in 30 cases of hepatocellular carcinoma compared with adjacent tissues as predictors of cancer invasion and staging. Exp Ther Med. 2015;9:905–8. doi: 10.3892/etm.2014.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Matsumoto Y, Okada M, et al. Matrix metalloproteinase 2 and 9 expression correlated with cavernous sinus invasion of pituitary adenomas. J Med Invest. 2005;52:151–58. doi: 10.2152/jmi.52.151. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida D, Teramoto A. Enhancement of pituitary adenoma cell invasion and adhesion is mediated by discoidin domain receptor-1. J Neurooncol. 2007;82:29–40. doi: 10.1007/s11060-006-9246-6. [DOI] [PubMed] [Google Scholar]
- 12.Toy KA, Valiathan RR, Nunez F, et al. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150:9–18. doi: 10.1007/s10549-015-3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Wang HX, Qin C, et al. Relationship between clinicopathological features and HIF-2alpha in gastric adenocarcinoma. Genet Mol Res. 2015;14:1404–13. doi: 10.4238/2015.February.13.19. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Liu Y, Bu W, et al. Radiation-/hypoxia-induced solid tumor metastasis and regrowth inhibited by hypoxia-specific upconversion nanoradiosensitizer. Biomaterials. 2015;49:1–8. doi: 10.1016/j.biomaterials.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Notte A, Rebucci M, Fransolet M, et al. Taxol-induced unfolded protein response activation in breast cancer cells exposed to hypoxia: ATF4 activation regulates autophagy and inhibits apoptosis. Int J Biochem Cell Biol. 2015;62:1–14. doi: 10.1016/j.biocel.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Shi J, Zhao D, et al. Raf kinase inhibitor protein inhibits cholangiocarcinoma cell metastasis by downregulating matrix metalloproteinase 9 and upregulating tissue inhibitor of metalloproteinase 4 expression. Oncol Lett. 2015;9:15–24. doi: 10.3892/ol.2014.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babichenko, Andriukhin MI, Pulbere S, Loktev A. Immunohistochemical expression of matrix metalloproteinase-9 and inhibitor of matrix metalloproteinase-1 in prostate adenocarcinoma. Int J Clin Exp Pathol. 2014;7:9090–98. [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Xiao Z, Zhao Y, et al. HIF-1alpha inhibition sensitizes pituitary adenoma cells to temozolomide by regulating MGMT expression. Oncol Rep. 2013;30:2495–501. doi: 10.3892/or.2013.2689. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Liu Z, Li J, et al. Relative quantitative expression of hypoxia-inducible factor 1alpha messenger ribonucleic acid in recurrent craniopharyngiomas. Neurol India. 2014;62:53–56. doi: 10.4103/0028-3886.128291. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Bi Y, Xue L, et al. Dendritic cell SIRT1-HIF1alpha axis programs the differentiation of CD4+ T cells through IL-12 and TGF-beta1. Proc Natl Acad Sci USA. 2015;112:E957–65. doi: 10.1073/pnas.1420419112. [DOI] [PMC free article] [PubMed] [Google Scholar]