Abstract
Background
Practicing high-frequency yoga breathing (HFYB) induced a hypermetabolic state in a single subject during the practice but the effect has not been studied in multiple practitioners.
Material/Methods
Healthy male volunteers (n=47, group mean age ±S.D., 23.2±4.1 years) were recruited as an experimental group and another twenty volunteers were recruited as a control group. The experimental group practiced either HFYB (Breath rate 1.0 Hz) or breath awareness (BAW) on two separate days. The sequence was reversed for alternate participants. The control group was assessed under similar conditions while sitting at ease. The breath rate (RR), tidal volume (VT), ventilation (VE), VO2, VCO2, arterial PCO2 and energy expenditure (EE Kcal/day) were assessed for 35 minutes using an open circuit oxygen consumption analyzer. The assessment period was divided into before, during and after conditions. Repeated measures analyses of variance (ANOVA) were used to compare data recorded during and after the two practices with data recorded before. Before-After comparisons in the control group were with paired t-tests.
Results
The most relevant significant changes were increases in VE, VO2, VCO2 and EE during HFYB, while the same variables decreased during the control period. However after HFYB there was no change in VO2 or EE, although VE decreased as it did after the control period.
Conclusions
HFYB induces a hypermetabolic state for the duration of the practice which returns to baseline after HFYB suggesting a possible application for HFYB in hypometabolic states.
MeSH Keywords: High-Frequency Ventilation, Metabolism, Oxygen Consumption, Respiration, Ventilation, Yoga
Background
A hypermetabolic state with increased oxygen uptake is followed by a period with increased resting ventilation [1]. Most often a hyper-metabolic state due to physiological conditions such as hyperthermia [2], muscular exercise [3], and pathological conditions such as thyrotoxicosis [4] was followed by an increase in resting ventilation and metabolic rate. The opportunities to evaluate the ventilatory responses to these states under controlled conditions are few.
One such example was a study which assessed ventilation, chemosensitivity, and blood PH after experimental carbohydrate and protein feeding intended to increase the metabolic rate [1]. The test meals of carbohydrate and protein, amounting to 1000 calories, were given to 6 normal subjects. The baseline oxygen consumption (VO2) increased from 237±11.3 ml/min STPD to 302±19.4 ml/min STPD (a 27.4% increase) 2 hours later and 303±18.5 ml/min STPD (an increase of 27.9%) 3 hours after the nasogastric feeding. The mechanism appears to be related to changes in chemosensitivity and carotid body responses to altered chemical stimuli following the breakdown of carbohydrates and protein.
The ancient science of yoga includes physical postures, voluntarily regulated breathing, and meditation, among other techniques [5]. Practicing yoga can bring positive effects on well-being and human health with respect to biological and physiological parameters [6]. Voluntarily regulated yoga breathing techniques have been found to increase the oxygen consumption (and correspondingly the metabolic rate) both as an immediate effect [7,8] and as a longitudinal effect [9]. Certain yoga voluntarily regulated breathing techniques offer an opportunity to study the effects of changes in the respiratory pattern on metabolism [10]. Voluntary regulation of breathing in yoga alters autonomic activity with an improvement in cardiovascular and psychological health [11]. An example is High-Frequency Yoga Breathing (HFYB) called kapalabhati in Sanskrit. In this technique the breath frequency is increased to 1.0–2.0 Hz and exhalation is performed as an active process by contraction of the anterior abdominal muscles. When seventeen advanced practitioners performed HFYB at the rate of 2.0 Hz for three 5 minutes periods, cardiovascular changes were found during and after the practice [12]. Baroreceptor-cardiac reflex sensitivity (BRS, derived from the mean modulus calculated between systolic blood pressure and the R-R interval of the electrocardiogram) reduced during HFYB causing a decrease in cardiac vagal tone. The respiratory rate decreased after HFYB. A separate study reported that HFYB when practiced at the rate of 2.0 Hz increased sympathetic activity [13]. Based on these effects of HFYB on autonomic variables, HFYB may also influence metabolic variables, since an earlier study showed that changes in functioning of the autonomic nervous system can influence energy expenditure and oxygen consumption [14].
In an early study of HFYB and energy expenditure, assessments were made on a single subject in repeat sessions using the Krogh closed-circuit spirometer [15]. During HFYB, there was a 10–14% increase in oxygen consumed (VO2) compared to the resting state, while after the practice there was a decrease in VO2 by 3–7%. Hence, HFYB is also a physiologically-induced hypermetabolic state. However, the changes after the practice appear to be different from those associated with other hypermetabolic states [1–4]. This could possibly be due to a relaxed state following yoga breathing [15]. The study cited above on HFYB [15] was conducted on a single individual in repeat sessions using a closed-circuit apparatus. The present study was intended to assess the effects of HFYB during and after the practice in a group of trained practitioners using an open-circuit apparatus.
A closed-circuit apparatus has several practical disadvantages. It is known that breathing through a closed-circuit apparatus makes it difficult to breathe normally [16] and the accuracy of measurement is dependent on the ability of the person to breathe regularly. Apart from this, in a closed-circuit system, breathing is from a reservoir of 100% oxygen and the resistance offered by the apparatus is high and the rate of carbon dioxide removal by absorption may not be adequate for accurate results [17]. Also, when breathing in a closed-circuit system, the resistance to breathing is increased, inspiratory time is prolonged, and the work of breathing may be increased by 10.0% [18]. In contrast, the open-circuit apparatus is considered more accurate because the person breathes ambient air and the apparatus does not offer resistance to airflow [19].
With this background, the present study intended to: (i) assess the metabolic changes associated with HFYB in trained participants, and (ii) to evaluate the effects on ventilation, oxygen uptake (VO2), and carbon dioxide output (VCO2) using an open-circuit apparatus.
Material and Methods
Participants
Seventy-one healthy male participants of Indo-Aryan ethnicity belonging to the north of India, specifically from Haryana state, were included in the study. The criteria for inclusion were: (i) persons with normal health based on a routine medical examination, (ii) persons with a minimum of 3 months experience in HFYB and BAW, and (iii) those with an ability to maintain the breath rate between 50 and 70 breaths per minute for the required time. The exclusion criteria were health disorders, with special emphasis on the respiratory system (persons with obstructive or restrictive disorders) and conditions influencing metabolism such as thyroid dysfunction and other endocrine disorders. None of the participants had to be excluded for these reasons. The total number of participants was 71; 47 participants were able to perform HFYB correctly and were included in the trial. Twenty-four participants could not perform HFYB and were excluded from the study. Hence, the number of participants for inclusion in analysis was 47 in the experimental group. The ability to practice HFYB correctly was not a necessary requirement to be included in the Control group, hence this group (n=20) was not tested for HFYB practice. The other exclusion criterion was the use of medicine or other wellness strategies, including herbal preparations. Participants’ ages ranged between 17 and 35 years (group mean ±S.D., 23.2±4.1 years). The study was not a randomized controlled study, but, in addition to the 47 participants who formed the experimental group, there were 20 participants (group mean age ±S.D., 22.3±4.9 years) recruited as a control group to evaluate the effect of retesting after no intervention. This group also had experience in the yoga breathing practices (3–180 months). Details of the baseline characteristics of the experimental and control groups are given in Table 1. They were all staying in a yoga institute in the north of India for a minimum of 3 months prior to the study. Participants were recruited by notices on the institution’s notice boards. Participants did not receive any incentive to take part in the study. The study on the experimental group was conducted between August 2009 and January 2010 and the data of the control group was taken in October 2011 in the same institution. There was no attempt to statistically calculate the sample size prior to the experiment. However post-hoc calculations following the repeated measures ANOVA, based on changes within the experimental group, in partial pressure of carbon-dioxide in the artery [PaCO2 (during) versus PaCO2 (pre)], with an effect size=1.65, showed that the power was 1.00 [20].
Table 1.
Baseline characteristics of the experimental and control group.
| Groups | Age (mean ±S.D. years) | B.M.I (mean ±S.D. Kg/m2) | Current profession | Health status | Medication | Smoking | Diet | Experience in yoga (mean ±S.D.) | Education |
|---|---|---|---|---|---|---|---|---|---|
| Experimental group (n=47) | 23.2±4.1 | 22.5±2.9 | Security personnel | Healthy | NIL | Non smokers | Vegetarian | 30.7±39.8 months | 15 years or more |
| Control group (n=20) | 22.3±4.9 | 21.2±2.9 | Security personnel | Healthy | NIL | Non Smokers | Vegetarian | 22.5±29.8 months | 15 years or more |
The signed informed consent was taken from the participants and the study was approved by the Ethics Committee of Patanjali Research Foundation, which adhered to the Indian Council of Medical Research Code and the Statement of General Principles on research using human participants in biomedical research [21].
Design
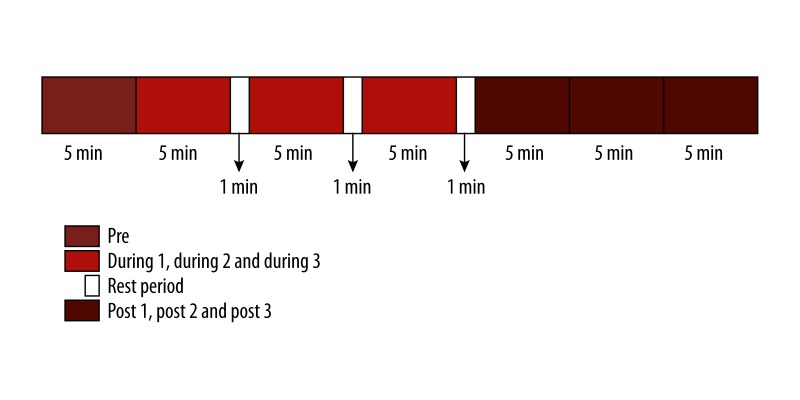
The study design was a prospective observational cohort study with a basic control group. The study design was explained to the participants. Every participant in the experimental group was assessed in 2 sessions on separate days at the same time of the day. On these 2 days the participants practiced 2 different yoga techniques – high-frequency yoga breathing (HFYB) and breath awareness (BAW). Testing the same individuals in sessions of 2 active interventions reduced inter-individual variability. The control group was separate for comparison and to assess the re-test effect. The sequence of practices was reversed for every alternate participant. The assessment of each session lasted for 35 minutes (with 3 minutes for rest in between periods of practice). Both sessions were divided into 3 periods – “Pre”, “During”, and “Post”. The “Pre” period was recorded for 5 minutes. In the “During” period, each participant practiced either high-frequency yoga breathing (HFYB) or breath awareness (BAW) for 18 minutes, interspersed with 1 minute of rest after every 5 minutes of practice. Hence, the “During” period of 15 minutes was divided into 3 sub-periods of 5 minutes each – “During 1”, ‘During 2”, and “During 3” and 3 minutes of rest in between. This “During” period was followed by 15 minutes of a “Post” period, which was also divided into 3 periods – “Post 1”, “Post 2”, and “Post 3” – of 5 minutes each. In the Post period, the participants breathed normally. This has been schematically presented in Figure 1.
Figure 1.
Schematic representation of the study design.
Assessment procedure
One night prior to the assessment, the following precautions were taken: (i) The diet of the participants was monitored. Since the oxygen consumption was evaluated it was considered preferable to make sure that all participants had the same diet the night before testing. The quantity of the items in the meal was kept the same for all participants. The nutritional value of the diet is given in Table 2. (ii) The participants slept adjacent to the research laboratory so as to minimize activity on awakening, to come close to the basal metabolic rate. (iii) They were requested to avoid physical exertion before the assessment. The next morning the assessment was done between 05:00 and 07:30 hours. Most participants were assessed on the consecutive day at the same time of the day. The maximum interval between assessment days was 3 days. The mean ±S.D. room temperature and humidity were controlled in the laboratory and maintained at 21.9±3.8 oC and 55.3±7.5% humidity on all recording days. During the assessments, participants were requested to sit in a cross-legged posture keeping their spine straight and their eyes closed throughout the session. Assessments were taken continuously in the Pre, During 1, During 2, During 3, Post 1, Post 2, and Post 3 periods of 5 minutes each. Recordings were not done for the three 1-minute rest periods. The control group followed the same procedure of assessments except that they were instructed not to practice any yoga technique.
Table 2.
Nutritional value of the meal provided to the participants the night prior to assessments.
| Sl. No | Cooked Lentils (250 gm) | Indian Bread (100 gm) | Cooked Vegetable (250 gm) | Papaya (250 gm) | Apple (250 gm) | Cucumber (250 gm) | Milk (250 ml) | Oil (20 gm) | Total |
|---|---|---|---|---|---|---|---|---|---|
| Protein (gm) | 60 | 22.5 | 5 | 2.5 | 0 | 0 | 7.5 | 5.0 | 102.5 |
| Fat (gm) | 2.5 | 2.5 | 2.5 | 0 | 0 | 0 | 10 | 8 | 25.5 |
| Carbohydrate (gm) | 150 | 122.5 | 7.5 | 17.5 | 32.5 | 5 | 10 | 5.2 | 350.2 |
| Energy (Kcal) | 867 | 244 | 65 | 80 | 147.5 | 32.5 | 167.5 | 113.4 | 1716.9 |
Assessment
Equipment calibration
An open-circuit oxygen consumption analyzer (Quark CPET, COSMED, Italy) was used for assessment. The COSMED Quark CPET (Cardio-Pulmonary Exercise Testing) equipment is designed to perform cardio-pulmonary function tests. In this equipment the program algorithm and the presentation of measured data have been developed according to the specifications of the American Thoracic Society (ATS) and European Respiratory Society (ERS). It has an inbuilt paramagnetic gas analyzer which can analyze the amount of oxygen consumed (VO2) and volume of carbon dioxide produced (VCO2). Before starting data acquisition the flow or volume calibration of the equipment is performed using a 3-liter calibration syringe and the gas analyzer is calibrated using atmospheric air and a cylinder with fixed amount of O2 (16%) and CO2 (5%). This calibrates the equipment for ambient temperature, humidity, barometric pressure, flow rate, and gas analysis. In the present study, COSMED Quark CPET was used to measure: (i) breath rate (RR), (ii) tidal volume (VT), (iii) ventilation (VE), (iv) volume of oxygen uptake (VO2), (v) volume of carbon dioxide produced (VCO2), (vi) arterial PCO2 (estimated), and (vii) energy expenditure (EE Kcal/day) (estimated) in both the experimental and control groups.
Recording procedure
Participants were fitted with a silicone rubber face mask which has perfect adherence to any face shape and is available in 3 sizes for adults. The mask is secured with head gear fitted with Velcro. This is to ensure maximum sealing without leakage of air. After fitting the mask, participants were asked to breathe normally before commencing the test. During the recording participants were seated in the half-lotus posture, with eyes closed.
Interventions
High frequency yoga breathing (HFYB)
The experimental group practiced high-frequency yoga breathing (HFYB) for 15 minutes. While practicing HFYB, the person had to sit erect in a half-lotus posture (ardha padmasana) with the spine and neck aligned. During the practice, participants kept their eyes closed and hands on the knees. The participants breathed with a frequency of approximately 1.0 Hz, during which exhalation was forceful and involved active contraction of the anterior abdominal wall and inhalation was passive.
Breath awareness
The same group (experimental group) practiced breath awareness on a separate day for the same duration. During breath awareness, the participants sat erect in the half-lotus posture (ardha padmasana) with the spine and neck erect and maintained awareness of the breath. The attention of the participants was directed to the natural flow of air into and out of their nostrils.
Control group
The participants in the control group sat at ease with their eyes closed, spine erect, and spine and neck aligned, in the half-lotus posture (ardha padmasana) for 35 minutes. They were not given any specific instructions about how to direct their thoughts.
Data extraction
Mean values of the breath rate (RR), tidal volume (VT), ventilation (VE), volume of oxygen uptake (VO2), volume of carbon dioxide produced (VCO2), arterial PCO2 and energy expenditure (EE Kcal/day) were calculated for before (5 minutes), during (15 minutes as the 3 minutes of rest were not included) and after (15 minutes) periods. Data analysis was done separately for every period.
Data analysis
Experimental group: Repeated measures analyses of variance (RMANOVA)
Repeated measures analyses of variance (ANOVA) followed by post-hoc analyses with Bonferroni adjustment were done to compare data recorded during and after the two practices with data recorded before the 2 practices, using PASW Version 18.0. There were 2 within-subjects factors – Sessions (HFYB and Breath awareness sessions) and Periods (Before, During, and After).
Control group: t-tests for paired group
The control group data recorded during the control period and after it were compared to the pre-control values with the paired t-test.
Comparisons between experimental and control groups: 2-factor ANOVA
A between groups comparison was carried out with a 2-factor ANOVA where factor 1 was groups [3 levels (HFYB, BAW and control)] and factor 2 was states [7 levels (Pre, During 1–3, and Post 1–3)]. This was done for each of the variables separately.
Results
Experimental group assessed using repeated measures analysis of variance (RMANOVA):
The experimental group was assessed in 2 sessions (HFYB, BAW) and each had 3 states (Pre, During, and Post). The difference between Sessions and States was assessed with a RMANOVA followed by Bonferroni adjusted post-hoc tests.
The ANOVA values for the Within-Subjects factor (Sessions), Within-Subjects factor (States) and interaction between the 2 for the different variables are provided in Table 3. A significant interaction between Sessions and States for any variable suggests that the 2 are interdependent.
Table 3.
ANOVA table for the respiratory variables of the experimental group.
| Sl. no. | Factors | Variable | F | df | Huynh-Feldt ɛ | P |
|---|---|---|---|---|---|---|
| I | Within subjects I (Sessions) | Rf b/min | 1575.63 | 1, 46.0 | 1 | 3.08133E-37 |
| VT l | 22.33 | 1, 46.0 | 1 | 2.19437E-06 | ||
| VE l/min | 353.72 | 1, 46.0 | 1 | 3.13719E-23 | ||
| VO2 ml/min | 62.18 | 1, 46.0 | 1 | 4.36222E-10 | ||
| VCO2 ml/min | 80.94 | 1, 46.0 | 1 | 1.05187E-11 | ||
| PaCO2 (mmHg) | 274.36 | 1, 46.0 | 1 | 5.17640E-21 | ||
| Eekc kcal/day | 79.15 | 1, 46.0 | 1 | 1.46182E-11 | ||
| II | Within subjects II (States) | Rf b/min | 1176.94 | 2.28, 104.63 | 0.379 | 5.35789E-75 |
| VT l | 1.893 | 2.19, 100.51 | 0.364 | 0.152 | ||
| VE l/min | 321.56 | 1.53, 70.21 | 0.254 | 7.55600E-33 | ||
| VO2 ml/min | 45.32 | 1.95, 89.66 | 0.325 | 1.93921E-38 | ||
| VCO2 ml/min | 105.92 | 1.78, 81.70 | 0.296 | 4.06334E-22 | ||
| PaCO2 (mmHg) | 478.11 | 2.65, 122.04 | 0.442 | 1.39456E-64 | ||
| Eekc kcal/day | 85.77 | 2.0, 91.95 | 0.333 | 9.66235E-22 | ||
| III | Interaction (Sessions × States) | Rf b/min | 1296.61 | 1.99, 91.50 | 0.332 | 9.11124E-68 |
| VT l | 15.26 | 1.69, 77.69 | 0.281 | 9.14205E-06 | ||
| VE l/min | 358.01 | 1.5, 69.19 | 0.251 | 7.87464E-34 | ||
| VO2 ml/min | 64.95 | 2.15, 98.76 | 0.358 | 1.74504E-19 | ||
| VCO2 ml/min | 92.68 | 1.82, 83.90 | 1 | 5.50850E-21 | ||
| PaCO2 (mmHg) | 507.76 | 2.14, 98.63 | 0.357 | 7.08274E-54 | ||
| Eekc kcal/day | 115.49 | 2.34, 107.79 | 0.391 | 8.19004E-30 |
Post-hoc analyses (RMANOVA)
Significant increases were observed in respiratory rate, minute ventilation, volume of oxygen uptake, volume of carbon-dioxide produced, and energy expenditure during HFYB compared with before HFYB (P<0.001). There was a significant decrease in ventilation (P<0.001) and volume of carbon dioxide produced (P<0.05), after HFYB compared to before HFYB. There was a significant decrease in arterial PCO2 during HFYB (P<0.001) and after HFYB (P<0.05) compared to before HFYB. The arterial PCO2 decreased significantly (P<0.001), after BAW compared to before BAW. Details of within-group comparisons are given in Table 4.
Table 4.
Values of the respiratory variables of the three groups (within group comparisons). Values are group mean ±S.D.
| Group, sample size and statistics | States | Rf (breaths/min) | VT (Liters) BTPS | VE (liters/min) BTPS | VO2 (ml/min) STPD | VCO2 (ml/min) STPD | PaCO2 (mmHg) STPD | EEkc (kcal/day) |
|---|---|---|---|---|---|---|---|---|
| HFYB (n=47) post-hoc tests following ANOVA | Pre | 16.31±2.78 | 0.51±0.13 | 7.98±1.75 | 240.77±54.52 | 182.79±52.80 | 35.79±2.23 | 1636.49±385.78 |
| During 1 | 60.20±7.20*** | 0.40±0.09*** | 23.42±6.06*** | 318.20±68.13*** | 345.11±96.14*** | 26.80±2.40*** | 2347.24±402.67*** | |
| During 2 | 61.11±7.17*** | 0.36±0.08*** | 21.79±5.00*** | 326.56±70.07*** | 294.67±67.57*** | 25.79±2.19*** | 2302.92±436.88*** | |
| During 3 | 62.62±7.25*** | 0.36±0.07*** | 21.82±4.78*** | 327.68±67.79*** | 285.04±64.26*** | 25.24±2.26*** | 2291.81±412.21*** | |
| Post 1 | 15.72±3.11 | 0.48±0.14 | 7.20±1.67*** | 244.53±45.34 | 154.09±45.30*** | 35.05±2.14* | 1605.86±308.98 | |
| Post 2 | 16.38±3.25 | 0.49±0.13 | 7.59±1.59 | 237.29±46.99 | 163.02±42.76** | 35.08±2.14* | 1582.32±324.06 | |
| Post 3 | 16.43±3.39 | 0.50±0.14 | 7.71±1.58 | 237.04±46.48 | 166.93±42.47* | 35.14±2.12* | 1587.95±323.13 | |
| Cohen’s d | 0.044 | 0.150 | 0.285 | 0.023 | 0.445 | 0.321 | 0.126 | |
| BAW (n=47) post-hoc tests following ANOVA | Pre | 15.56±3.54 | 0.51±0.18 | 7.43±1.63 | 229.56±56.54 | 174.89±47.79 | 36.35±1.93 | 1561.38±386.81 |
| During 1 | 14.46±4.03 | 0.60±0.31 | 7.54±1.89 | 228.70±57.52 | 184.85±60.97 | 36.26±2.86 | 1574.44±411.55 | |
| During 2 | 14.81±4.06 | 0.57±0.29 | 7.45±1.71 | 225.63±56.75 | 177.06±53.61 | 35.85±2.85 | 1543.84±397.44 | |
| During 3 | 14.59±4.17 | 0.60±0.33 | 7.51±1.74 | 225.70±57.13 | 178.89±55.52 | 35.68±2.71 | 1547.52±404.35 | |
| Post 1 | 16.08±3.45 | 0.49±0.14 | 7.42±1.64 | 222.84±55.34 | 165.90±45.69 | 35.55±1.99*** | 1508.81±376.84 | |
| Post 2 | 16.27±3.29 | 0.50±0.14 | 7.67±1.62 | 228.40±54.52 | 172.14±48.10 | 35.53±1.96*** | 1550.17±378.18 | |
| Post 3 | 16.30±3.70 | 0.52±0.15 | 7.80±1.73 | 231.80±56.07 | 175.14±47.87 | 35.39±2.10*** | 1574.06±386.05 | |
| Cohen’s d | 0.187 | 0.041 | 0.121 | 0.033 | 0.080 | 0.436 | 0.044 | |
| Control (n=20) t-test for paired data | Pre | 15.16±3.56 | 0.53±0.13 | 7.62±1.26 | 244.65±44.33 | 195.37±32.63 | 37.77±2.43 | 1680.05±296.21 |
| During 1 | 14.41±3.68 | 0.51±0.13* | 7.05±1.18** | 221.09±39.36*** | 178.51±32.29*** | 37.72±2.26 | 1521.72±267.16*** | |
| During 2 | 14.73±3.53 | 0.51±0.13 | 7.02±1.20*** | 216.58±36.94*** | 175.48±27.83** | 37.59±2.27 | 1491.77±246.10*** | |
| During 3 | 14.59±3.32 | 0.48±0.10** | 6.76±1.10*** | 208.07±37.07*** | 166.81±29.60*** | 37.65±1.94 | 1430.02±250.15*** | |
| Post 1 | 14.90±3.03 | 0.48±0.10* | 6.94±1.06** | 211.85±35.54*** | 169.10±24.90*** | 37.53±2.17 | 1454.67±234.98*** | |
| Post 2 | 15.23±2.89 | 0.48±0.09* | 7.00±1.05** | 213.23±38.02*** | 169.88±25.44*** | 37.33±2.12 | 1463.57±248.84*** | |
| Post 3 | 15.05±3.37 | 0.50±0.12 | 6.99±1.02** | 213.91±35.43*** | 172.00±26.84*** | 37.16±2.04 | 1471.02±236.66*** | |
| Cohen’s d | 0.030 | 0.371 | 0.559 | 0.784 | 0.858 | 0.189 | 0.8.09 |
P<0.05, post-hoc analyses with Bonferroni adjustment and t-test for paired data;
P<0.01, post-hoc analyses with Bonferroni adjustment and t-test for paired data;
P<0.001, post-hoc analyses with Bonferroni adjustment compared with pre and t-test for paired data.
Control group assessed with t-tests for paired data
The control group data recorded during the control period of quiet sitting and after it were compared to the pre-control values with the paired t-test.
In the control group there was a significant reduction in the volume of oxygen uptake, volume of carbon dioxide produced, ventilation, and energy expenditure during (P<0.001) and after (P<0.001) the control non-intervention period compared with before. The ventilation (P<0.01) and arterial PCO2 (P<0.05) reduced significantly in before-after comparisons. There was also a significant reduction in tidal volume during (P<0.05) and after (P<0.05) compared with before.
Comparisons between experimental and control groups based on 2-factor ANOVA:
To compare between Experimental and Control groups a 2-factor ANOVA (Factor 1=Groups; Factor 2=States, i.e., Pre, During, Post) was carried out.
The ANOVA values for between groups comparisons for all states are given in Table 5.
Table 5.
Two factor ANOVA table for between group comparisons.
| Sl. no. | Comparisons | Variables | F | df | P |
|---|---|---|---|---|---|
| 1 | Pre (HFYB vs. BAW vs. Control) | Rf b/min | 1.019 | 2, 111 | 0.340 |
| VT l | 0.216 | 2, 111 | 0.806 | ||
| VE l/min | 1.367 | 2, 111 | 0.259 | ||
| VO2 ml/min | 0.767 | 2, 111 | 0.467 | ||
| VCO2 ml/min | 1.308 | 2, 111 | 0.275 | ||
| PaCO2 (mmHg) | 5.969 | 2, 111 | 0.003 | ||
| Eekc kcal/day | 0.869 | 2, 111 | 0.422 | ||
| 2 | During 1 (HFYB vs. BAW vs. Control) | Rf b/min | 947.712 | 2, 111 | <0.0001 |
| VT l | 9.981 | 2, 111 | 0.0001 | ||
| VE l/min | 209.583 | 2, 111 | <0.0001 | ||
| VO2 ml/min | 32.795 | 2, 111 | <0.0001 | ||
| VCO2 ml/min | 65.492 | 2, 111 | <0.0001 | ||
| PaCO2 (mmHg) | 205.664 | 2, 111 | <0.0001 | ||
| Eekc kcal/day | 57.835 | 2, 111 | <0.0001 | ||
| 3 | During 2 (HFYB vs. BAW vs. Control) | Rf b/min | 978.805 | 2, 111 | <0.0001 |
| VT l | 13.336 | 2, 111 | <0.0001 | ||
| VE l/min | 244.945 | 2, 111 | <0.0001 | ||
| VO2 ml/min | 41.329 | 2, 111 | <0.0001 | ||
| VCO2 ml/min | 59.896 | 2, 111 | <0.0001 | ||
| PaCO2 (mmHg) | 251.752 | 2, 111 | <0.0001 | ||
| Eekc kcal/day | 53.616 | 2, 111 | <0.0001 | ||
| 4 | During 3 (HFYB vs. BAW vs. Control) | Rf b/min | 1031.833 | 2, 111 | <0.0001 |
| VT l | 14.272 | 2, 111 | <0.0001 | ||
| VE l/min | 267.247 | 2, 111 | <0.0001 | ||
| VO2 ml/min | 46.108 | 2, 111 | <0.0001 | ||
| VCO2 ml/min | 53.346 | 2, 111 | <0.0001 | ||
| PaCO2 (mmHg) | 294.222 | 2, 111 | <0.0001 | ||
| Eekc kcal/day | 56.999 | 2, 111 | <0.0001 | ||
| 5 | Post 1 (HFYB vs. BAW vs. Control) | Rf b/min | 0.927 | 2, 111 | 0.399 |
| VT l | 0.048 | 2, 111 | 0.953 | ||
| VE l/min | 7.705 | 2, 111 | 0.497 | ||
| VO2 ml/min | 4.046 | 2, 111 | 0.020 | ||
| VCO2 ml/min | 1.274 | 2, 111 | 0.284 | ||
| PaCO2 (mmHg) | 10.154 | 2, 111 | <0.0001 | ||
| Eekc kcal/day | 1.832 | 2, 111 | 0.165 | ||
| 6 | Post 2 (HFYB vs. BAW vs. Control) | Rf b/min | 0.982 | 2, 111 | 0.378 |
| VT l | 0.197 | 2, 111 | 0.821 | ||
| VE l/min | 1.434 | 2, 111 | 0.243 | ||
| VO2 ml/min | 1.712 | 2, 111 | 0.185 | ||
| VCO2 ml/min | 0.559 | 2, 111 | 0.574 | ||
| PaCO2 (mmHg) | 8.495 | 2, 111 | 0.0003 | ||
| Eekc kcal/day | 0.874 | 2, 111 | 0.420 | ||
| 7 | Post 3 (HFYB vs. BAW vs. Control) | Rf b/min | 1.158 | 2, 111 | 0.318 |
| VT l | 0.207 | 2, 111 | 0.813 | ||
| VE l/min | 2.032 | 2, 111 | 0.136 | ||
| VO2 ml/min | 1.572 | 2, 111 | 0.212 | ||
| VCO2 ml/min | 0.440 | 2, 111 | 0.645 | ||
| PaCO2 (mmHg) | 6.864 | 2, 111 | 0.002 | ||
| Eekc kcal/day | 0.890 | 2, 111 | 0.414 |
Please note: where P< 0.0001 it is mentioned as P<0.0001. Otherwise actual values are provided.
Post-hoc analyses (2-factor ANOVA)
Since HFYB involved manipulation of RF which changes VE the difference between HFYB, BAW and control for RF and VE are not presented here because these values would be different.
The oxygen consumption was significantly lower during both BAW and control sessions compared to HFYB, which is not surprising because during HFYB, exhalation is an active process requiring more oxygen utilization.
The level of VCO2 was significantly lower during (i) BAW compared to HFYB and (ii) control compared to HFYB. This too may be expected because with active exhalation in HFYB, the amount of CO2 exhaled during the practice of HFYB would be higher than tidal breathing.
Energy expenditure was significantly lower during BAW and control intervention periods compared to HFYB.
Discussion
A period of HFYB increased energy expenditure and oxygen uptake but unlike other states associated with increased energy expenditure and oxygen uptake, HFYB was followed by a period during which ventilation decreased in contrast to increased ventilation reported following periods of hypermetabolism. One difference between the 2 conditions was that ventilation increased to a greater extent during HFYB (173–193%), whereas during experimentally-induced hypermetabolism, the increase in ventilation was considerably lower (48.0–53.2%). Apart from this difference in minute ventilation, the period of HFYB was associated with changes in blood chemistry associated with the changes in breath frequency and tidal volume. During HFYB, PaCO2 was significantly reduced (25–29%). This reduction could be anticipated given the consciously regulated increase in breath frequency during HFYB despite the decrease in tidal volume. The increased ventilation associated with decreased PaCO2 did not produce any symptoms of hyperventilation based on reports taken from the subjects. This is comparable to the findings of an earlier study on 140 participants who performed HFYB but showed no evidence of hyperventilation discomfort [22] based on the Nijmegan Discomfort Questionnaire [23], which is designed to detect symptoms of over-breathing.
The changes in arterial carbon dioxide levels (PaCO2) showed that the reduction during HFYB persisted to a lesser degree (1.8–2.1%) for 15 minutes after the practice. In other conditions, such as exercise in which energy expenditure and oxygen uptake are increased to a greater degree, the compensatory changes in respiration may not have been adequate to reduce CO2 levels and, hence, ventilation would have increased after the exercise.
The decrease in arterial carbon dioxide levels (PaCO2) during (25.0–29.0%) and following HFYB (1.8–2.9%) are related to the consciously increased breath frequency with an increase in ventilation as a consequence.
On the other hand, the reduction in PaCO2 following BAW may be due to a reduction in physiological arousal after BAW, causing a reduction in blood CO2 levels. This is supported by an earlier finding that BAW increases vagal tone, as seen in the HRV with an increase in the high-frequency power and decrease in the low-frequency power of heart rate variability [24].
In summary, these findings appear to show HFYB as physiologically different from other hypermetabolic states. In most physiological conditions associated with an increase in energy expenditure, such as exercise, the energy expenditure remains high after the activity due to an oxygen deficit or debt. After HFYB, the energy expenditure did not change and neither did the VO2. This could be because of reduced CO2 levels so that the respiratory drive for ventilation remained low after HFYB. However, HFYB involves active contraction of the anterior abdominal muscles with exhalation as an active process. The return of oxygen consumption levels to the baseline after the practice suggests that the work of breathing associated with this did not cause an oxygen debt. Apart from this, HFYB is possibly associated with a relaxed mental state, as described earlier [15]. This is supported by a study by Dostalek (1991) on the EEG during and after HFYB, which reported an increase in alpha, theta, and beta1 in the EEG. After HFYB, both alpha and beta decreased but there was no change in theta. The authors interpreted this as a relative increase in slow-frequency waves after HFYB [25]. The concept of HFYB being associated with mental relaxation is further supported by a study of the heart rate variability (HRV) during HFYB, which showed a decrease in NN50, pNN50, and the mean RR interval suggestive of a shift in the autonomic balance with possible reduction in sympathetic activity [26]. In summary, HFYB caused an increase in VO2, VE, EE, and VCO2 during the practice, while after HFYB, VE and VCO2 decreased. These results suggest that HFYB may be useful to increase energy expenditure without causing other changes associated with physical and mental exertion. Hence, a possible application of HFYB is as a low-intensity activity to increase energy expenditure while also increasing mental relaxation. This makes it different from exercise requiring a constant work output. This may be clinically useful in stress-related hypometabolic conditions [27]. Practicing HFYB may also be useful for stress-related diseases, considering the effects of relaxation on nitric oxide, norepinephrine, cortisol, melatonin, and markers of inflammation, as well as immunological variables [28].
The present results are limited by the following factors: (i) The sample sizes of the intervention and the control group differed. The difference in sample sizes is regrettable and every attempt would be made in future studies to avoid such a disparity. (ii) The non-intervention group was also trained in yoga and, hence, may inadvertently have been in a yoga state of mind, with possible awareness of the breath, in the control period. However, the possibility of involvement in breath modulation/awareness in the control group seems limited because there were no significant changes in this group. (iii) The ECG was not simultaneously monitored in this study, so the effect of HFYB on the heart and autonomic nervous system (based on the HRV) were not obtained. While changes in cardiac activity are not directly related to the aims of the present study, given the close association between cardiovascular and respiratory systems, this could be a useful addition to future studies in this area.
Nonetheless, HFYB appears to be an example of a physiologically interesting breath-regulating technique that is worth exploring further.
Conclusions
High-frequency yoga breathing (HFYB; 1.0 Hz) brought about a 34.6% increase in oxygen consumption during HFYB, which stopped immediately after HFYB. This suggests that HFYB can serve as a form of low-intensity activity that does not incur a post-activity oxygen debt. This type of practice could be useful for persons who are not able to undertake rigorous exercise.
Footnotes
Source of support: Departmental sources
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Zwillich CW, Sahn SA, Weil JV. Effects of hypermetabolism on ventilation and chemosensitivity. J Clin Invest. 1997;60:900–6. doi: 10.1172/JCI108844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudio R, Jr, Abramson N. Heat-induced hyperventilation. J Appl Physiol. 1968;25:742–46. doi: 10.1152/jappl.1968.25.6.742. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya NK, Cunningham D, Goode JC, et al. Hypoxia, ventilation, PCO2, and exercise. Respir Physiol. 1970;9:329–47. doi: 10.1016/0034-5687(70)90090-3. [DOI] [PubMed] [Google Scholar]
- 4.Valtin H, Tenney SM. Respiratory adaptation to hyperthyroidism. J Appl Physiol. 1960;15:1107–12. doi: 10.1152/jappl.1960.15.6.1107. [DOI] [PubMed] [Google Scholar]
- 5.Taimini IK. The Science of Yoga. The Theosophical Publishing House; Madras, India: 1961. [Google Scholar]
- 6.Cavallera GM, Gatto M, Boari G. Personality, cognitive styles and Morningness-Eveningness disposition in a sample of Yoga trainees. Med Sci Monit. 2014;20:238–46. doi: 10.12659/MSM.889030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Telles S, Singh N. High frequency yoga breathing increases energy expenditure from carbohydrates. Med Sci Monit. 2011;17(9):LE7–8. doi: 10.12659/msm.881916. [DOI] [PubMed] [Google Scholar]
- 8.Telles S, Nagarathna R, Nagendra HR. Physiological measures during right nostril breathing. J Altern Complement Med. 1996;2:479–84. doi: 10.1089/acm.1996.2.479. [DOI] [PubMed] [Google Scholar]
- 9.Telles S, Nagarathna R, Nagendra HR. Breathing through a particular nostril can alter metabolism and autonomic activities. Indian J Physiol Pharmacol. 1994;38:133–37. [PubMed] [Google Scholar]
- 10.Telles S, Naveen KV. Voluntary breath regulation in yoga: Its relevance and physiological effects. Biofeedback. 2008;36:70–73. [Google Scholar]
- 11.Critchley HD, Nicotra A, Chiesa PA, et al. Slow breathing and hypoxic challenge: cardiorespiratory consequences and their central neural substrates. PLoS One. 2015;10:e0127082. doi: 10.1371/journal.pone.0127082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stancák A, Jr, Kuna M, Srinivasan, et al. Kapalabhati – yogic cleansing exercise. I. Cardiovascular and respiratory changes. Homeost Health Dis. 1991;33:126–34. [PubMed] [Google Scholar]
- 13.Raghuraj P, Ramakrishnan AG, Nagendra HR, Telles S. Effect of two selected yogic breathing techniques of heart rate variability. Indian J Physiol Pharmacol. 1998;42:467–72. [PubMed] [Google Scholar]
- 14.Saad MF, Alger SA, Zurlo F, et al. Ethnic differences in sympathetic nervous system-mediated energy expenditure. Am J Physiol. 1991;261:E789–94. doi: 10.1152/ajpendo.1991.261.6.E789. [DOI] [PubMed] [Google Scholar]
- 15.Miles WR. Oxygen consumption during three yoga-type breathing patterns. J Appl Physiol. 1964;19:75–82. doi: 10.1152/jappl.1964.19.1.75. [DOI] [PubMed] [Google Scholar]
- 16.Judy WV. Energy expenditure, metabolism, temperature regulation and exercise. In: Selkurt EE, editor. Basic physiology for health sciences. Little Brown and Company; Boston: 1982. [Google Scholar]
- 17.American Association of Respiratory Care. AARC clinical practice guidelines for metabolic measurement using direct calorimetry during mechanical ventilation. Respir Care. 1994;39:1170–75. [PubMed] [Google Scholar]
- 18.Branson RD. The measurement of energy expenditure: instrumentation, practical consideration, and clinical application. Respir Care. 1990;35:640–59. [Google Scholar]
- 19.Matarese LE. Indirect calorimetry: technical aspects. J Am Diet Assoc. 1997;11:154–60. doi: 10.1016/s0002-8223(97)00754-2. [DOI] [PubMed] [Google Scholar]
- 20.Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]
- 21.Indian Council of Medical research. Ethical guidelines for biomedical research on human participants. New Delhi. 2006 [Google Scholar]
- 22.Telles S, Singh N, Yadav A, Balkrishna A. Effect of yoga on different aspects of mental health. Indian J Physiol Pharmacol. 2012;56:245–54. [PubMed] [Google Scholar]
- 23.Van Doorn P, Folgering H, Colla P. Control of the end-tidal PCO2 in the hyperventilation syndrome: effects of biofeedback and breathing instructions compared. Bull Eur Physiopathol Respir. 1982;18:829–36. [PubMed] [Google Scholar]
- 24.Telles S, Mohapatra RS, Naveen KV. Heart rate variability spectrum during Vipassana mindfulness meditation. J Indian Psychol. 2005;23:1–5. [Google Scholar]
- 25.Stancak A, Jr, Kuna M, Srinivasan, Dostalek C, Vishnudevananda S. Kapalabhati – yogic cleansing exercise. II. EEG topography analysis. Homeost Health Dis. 1991;33:182–89. [PubMed] [Google Scholar]
- 26.Telles S, Singh N, Balkrishna A. Heart rate variability changes during high frequency yoga breathing and breath awareness. Biopsychosoc Med. 2011;5:4. doi: 10.1186/1751-0759-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizokami T, Wu Li A, El-Kaissi S, Wall JR. Stress and thyroid autoimmunity. Thyroid. 2004;14:1047–55. doi: 10.1089/thy.2004.14.1047. [DOI] [PubMed] [Google Scholar]
- 28.Esch T, Fricchione GL, Stefano GB. The therapeutic use of the relaxation response in stress-related diseases. Med Sci Monit. 2003;9(2):RA23–34. [PubMed] [Google Scholar]