Abstract
Active marijuana produces significant subjective, psychomotor, and physiological effects relative to inactive marijuana, yet demonstrating that these effects are dose-dependent has proven difficult. This within-subject, double-blind study was designed to develop a smoking procedure to obtain a marijuana dose–response function. In four outpatient laboratory sessions, daily marijuana smokers (N = 17 males, 1 female) smoked six 5-s puffs from 3 marijuana cigarettes (2 puffs/cigarette). The number of puffs from active (≥5.5% Δ9-tetrahydrocannabinol/THC) and inactive (0.0% THC) marijuana varied according to condition (0, 2, 4, or 6 active puffs); active puffs were always smoked before inactive puffs. Subjective, physiological, and performance effects were assessed prior to and at set time points after marijuana administration. Active marijuana dose-dependently increased heart rate and decreased marijuana craving, despite evidence (carbon monoxide expiration, weight of marijuana cigarettes post-smoking) that participants inhaled less of each active marijuana cigarette than inactive cigarettes. Subjective ratings of marijuana “strength,” “high,” “liking,” “good effect,” and “take again” were increased by active marijuana compared with inactive marijuana, but these effects were not dose-dependent. Active marijuana also produced modest, non-dose-dependent deficits in attention, psychomotor function, and recall relative to the inactive condition. In summary, although changes in inhalation patterns as a function of marijuana strength likely minimized the difference between dose conditions, dose-dependent differences in marijuana’s cardiovascular effects and ratings of craving were observed, whereas subjective ratings of marijuana effects did not significantly vary as a function of dose.
Keywords: cannabis, THC, cannabinoid
Marijuana is the most widely used illicit drug in the United States and smoking is the most common route of administration (Johnston, O’Malley, Bachman, & Schulenberg, 2012). Effects of marijuana, including tachycardia, subjective reports of intoxication, as well as impaired memory and attention, have been well established in a number of controlled laboratory studies (Foltin & Fischman, 1990; Foltin, Fischman, Pedroso, & Pearlson, 1987; Hart, van Gorp, Haney, Foltin, & Fischman, 2001; Nemeth-Coslett, Henningfield, O’Keeffe, & Griffiths, 1986). Although marijuana’s subjective effects are primarily mediated by Δ9-tetrahydrocannabinol (THC) (Chait, 1989; Heishman, Arasteh, & Stitzer, 1997; Kelly, Foltin, Emurian, & Fischman, 1997), demonstrating a dose–effect relationship between marijuana strength (THC concentration) and its elicited subjective and physiological effects (Azorlosa, Heishman, Stitzer, & Mahaffey, 1992; Chait, 1989; Heishman, Stitzer, & Yingling, 1989) has proven difficult.
Possible explanations for the difficulty observing marijuana dose dependence in early studies could be that the range of marijuana strengths tested (0.2% to 0.8% THC) was too narrow (Cappell, Kuchar, & Webster, 1973) or that marijuana administration was ad libitum as opposed to controlled (Ashton, Golding, Marsh, Millman, & Thompson, 1981; Herning, Hooker, & Jones, 1986). Unlike drugs administered orally or intravenously, smoked administration is reliant on inhalation strength (Azorlosa, Greenwald, & Stitzer, 1995), and participants alter their smoking topography as a function of marijuana potency. One such study tested a range of marijuana potencies (0.0% to 4.0% THC) using a timed smoking procedure, in which puff duration was measured and participants held the inhaled smoke in their lungs for a fixed amount of time. Although subjective and cardiovascular measures were not dose-dependent, expired carbon monoxide (CO) levels, an index of smoke inhalation, showed a significant inverse relationship to THC content, demonstrating that participants reduced their smoke intake as strength of the marijuana increased (Nemeth-Coslett et al., 1986). These observations have been replicated in later studies showing that CO expiration, as well as puff duration, are inversely related to marijuana strength (Chait, Fischman, & Schuster, 1985; Cooper & Haney, 2009; Kelly, Foltin, & Fischman, 1993). Thus, even while using controlled smoking procedures, smoke inhalation decreased with increasing strengths of marijuana. Consequently, marijuana exposure is reduced at higher strengths, which decreases the likelihood of observing dose-dependent marijuana effects.
In an effort to account for dose-dependent titration, Heishman and colleagues used a procedure that controlled for smoking topography (Azorlosa et al., 1992; Heishman et al., 1997) as well as THC content (1.75% to 3.55% THC). In order to deliver uniform amounts of marijuana smoke per puff, a computer-based smoking topography system monitored puff volume, inhalation volume, lung exposure duration, and interpuff interval. Although this procedure produced sensitive differences in plasma THC levels as a function of dose condition, only considerably different dose conditions produced significantly different subjective effects, that is, the lowest and highest number of puff conditions (4 vs. 16 or 25 puffs). Thus, even procedures that carefully control smoke inhalation and produce distinct plasma THC concentrations demonstrate that dose-dependent marijuana effects are difficult to observe.
Yet, establishing a procedure that produces dose-dependent marijuana effects would greatly inform drug interaction studies. For example, assessing how medications or other drugs of abuse shift the marijuana dose-response curve would elucidate the mechanism of drug interaction. Thus, the objective of this within-subject, double-blind, placebo-controlled study was to develop a smoking procedure to characterize marijuana dose-dependence by keeping participants blind to the marijuana “dose” and thereby minimizing the expectation of a particular drug effect. Non-treatment-seeking marijuana smokers smoked 6 puffs of marijuana in each session, but the number of active marijuana puffs smoked varied from 0 to 6. Participants smoked active puffs of marijuana prior to inactive puffs because it was hypothesized that this would better maintain the blind regarding the different dose conditions. The duration of inhalation and the amount of time the smoke was held in the lungs was controlled. We measured the time course of marijuana’s subjective, physiological (heart rate, blood pressure, expired carbon monoxide), and performance effects, as well as the amount of marijuana smoked from each cigarette as a function of the number of active marijuana puffs smoked.
Methods
Participants
Volunteers, 21 to 45 years of age, were recruited through newspaper advertisements. Those meeting inclusion/exclusion criteria after an initial phone screen were invited to the laboratory for further screening. Participants were accepted into the study if they were healthy, as determined by a physical examination, electrocardiogram, and urine and blood chemistry. Marijuana use was confirmed by urine toxicology and self-report. To be eligible for participation, volunteers had to report smoking at least three marijuana cigarettes four times a week for the previous month before screening. Repeated use of other drugs, with the exception of nicotine, alcohol, or caffeine, as determined by urine toxicology and self-report, and/or current use of over-the-counter or prescription medication was exclusionary, as was alcohol dependence. Those who met Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; American Psychiatric Association, 2000) revised criteria for current Axis I psychopathology according to a psychiatric examination were not eligible for participation. Females were excluded if they were pregnant, nursing, or not using contraception.
This study was part of an eight-session study assessing the effects of naltrexone, a mu-opioid antagonist, on marijuana. Prior to consent, volunteers were told that (a) the study objective was to determine if commonly prescribed medications altered marijuana’s effects on mood and physiology, (b) they would receive a capsule containing placebo or one of three medications listed on the consent form, and (c) active or inactive marijuana would be smoked after capsule administration according to instructions from the research staff. Participants were admitted into the study only after written informed consent was obtained and eligibility was verified. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accordance with the Declaration of Helsinki.
Drugs
Marijuana cigarettes (0, 5.5, or 6.2% THC; ca. 800 mg) were provided by the National Institute on Drug Abuse. Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 hr prior to the session. Because of limited supply of the highest strength of marijuana (6.2% THC), the first 13 participants smoked 6.2% THC for the active puffs, and the final five participants smoked 5.5% THC; marijuana strength was consistent within participants. Size 00 opaque capsules with lactose filler or naltrexone (12 mg) were prepared by the New York State Psychiatric Institute Research Pharmacy.
Study Design and Procedures
The study included eight outpatient sessions over the course of 3 to 6 weeks at the New York State Psychiatric Institute. Sessions, which were separated by at least 48 hr to prevent medication carryover effects, began around 9:00 a.m., and were about 6 hr in duration. After study consent was obtained and prior to the first session, participants were familiarized with computerized tasks and study procedures with one to two training sessions. During the training session, capsules and marijuana were not administered.
During each of the outpatient sessions, participants smoked a total of 6 puffs from three marijuana cigarettes (2 puffs from each cigarette). Marijuana administration occurred 45 min after capsule administration. The experimenter rolled the marijuana cigarette ends, and one end was inserted into a plastic cigarette holder so that the participants could not distinguish active from placebo marijuana based on visual cues. The number of active versus inactive cigarettes smoked during each session varied according to active puff conditions (i.e., 0 puffs = 3 inactive cigarettes; 2 puffs = 1 active + 2 inactive cigarettes; 4 puffs = 2 active + 1 inactive cigarettes; 6 puffs = 3 active cigarettes). Cigarettes were smoked according to a cued smoking procedure that has been shown to produce reliable increases in heart rate and plasma THC levels (Foltin et al., 1987). During marijuana smoking, the experimenter observed the participant behind a one-way mirror and used an intercom to guide the participant as to which color-coded cigarette to light, to “inhale” (5 s), “hold smoke in lungs” (10 s) and “exhale,” with a 40-s interval between each inhalation. After 2 puffs, the participant was instructed to extinguish the cigarette and light the next. Participants continued to smoke according to this procedure until 2 puffs from each cigarette had been smoked. Cigarettes were color coded, indicating the order in which they were to be smoked, and active cigarettes were always smoked before inactive. A randomized, within-subject design was used in which all participants were exposed to each of the puff conditions.
Experimental Session
Participants were instructed to not eat breakfast before each session and to not smoke marijuana or tobacco after midnight the night before each session. Upon arrival to the laboratory, CO levels were measured using a calibrated Bedfont Micro Smokerlyzer (Bedfont Scientific Ltd., Rochester, England) to confirm no recent smoking, breath alcohol levels were assessed, and use of illicit drugs (opiates, cocaine, benzodiazepines, amphetamines, methamphetamines) other than marijuana was determined by a urine toxicology screen (AllTests North America, Chandler, AZ; refer to Table 1 for schedule of session events). If CO levels indicated that the participant had smoked prior to arrival (≥8 ppm), the session did not proceed and the volunteer was sent home. Pregnancy tests were also run before the first and fifth session for female participants. A standardized breakfast was provided prior to each session.
Table 1.
Session Events
| Time (min) | Events |
|---|---|
| −90 | Urine toxicology screen, CO levels, breathalyzer, balance task, breakfast |
| −60 | Vitals, Task battery, VAS, Cigarette break |
| −45 | Capsule administration |
| −15 | CRF |
| 0 | Marijuana smoked |
| 15 | Vitals, CO, MRF, VAS, CRF |
| 30 | Vitals, CO, Task battery, MRF, VAS, CRF |
| 60 | Vitals, CO, MRF, VAS, CRF, Cigarette break |
| 90 | Vitals, CO, Task battery, MRF, VAS, CRF |
| 120 | Vitals, CO, MRF, VAS, CRF |
| 180 | Vitals, CO, Task battery, MRF, VAS, CRF |
| 210 | Vitals, CO, SE-VAS |
Note. Vitals, Heart rate and blood pressure; carbon monoxide (CO), Task Battery, repeated acquisition task, divided attention task (DAT), digit-symbol substitution task (DSST) and digit recall task (DRT); VAS, Subjective Effect-Visual Analog Scale; Capsule Rating Form (CRF), Marijuana Rating Form (MRF).
Before capsule administration, baseline subjective effects questionnaires and performance tasks were completed. Heart rate and blood pressure were also measured using a Sentry II vital signs monitor (Model 6100: NBS Medical Services, Costa Mesa, CA). Participants were then guided through the marijuana smoking procedure at 45 min after capsule administration. To determine if marijuana strength or smoking method affected inhalation behavior, CO levels were measured before smoking and throughout the session. Expired CO is an indicator of inhalation strength, with levels increasing in direct proportion to amount of marijuana smoked (Azorlosa et al., 1992). In addition, the amount of marijuana smoked in each condition was also assessed by weighing the cigarettes before and after smoking.
Vital signs (heart rate and blood pressure) were monitored at 15 to 60-min intervals throughout the session (see Table 1). A cognitive task battery and subjective ratings of mood and drug effect were also completed at specified time points after smoking. Timing of each measurement was scheduled to capture the full time course of marijuana effects. In order to minimize nicotine withdrawal symptoms, tobacco-dependent volunteers were permitted to smoke a tobacco cigarette at the same times during each session. Participants were free to leave the laboratory at the end of each session (about 3 hr after marijuana smoking), once sobriety was determined using field sobriety and balancing tasks.
Subjective-Effects Scales and Performance Tasks
Most subjective-effects ratings were measured using visual analog scales (VAS), a series of 100-mm-long lines labeled “not at all” at one end (0 mm) and “extremely” at the other end (100 mm). Participants were instructed to indicate how they felt at that particular moment.
Marijuana Rating Form (MRF)
Subjective marijuana-related effects were assessed using a five-item VAS asking participants to rate the strength of the marijuana effect, good effect, bad effect, marijuana liking, and willingness to smoke the marijuana again.
Visual Analog Scale (VAS)
Subjective ratings of mood and physical symptoms were assessed using a 44-item VAS intended to measure marijuana-elicited affective and physical subjective effects. The VAS consisted of 44 descriptors assessing mood (e.g., “content”), medication effects (e.g., “bad effect”), and physical symptoms (e.g., “blurred vision”). The items included “I feel …” “good drug effect,” “high,” “stimulated,” “alert,” “energetic,” “mellow,” “self-confident,” “social,” “talkative,” “friendly,” “content,” “bad drug effect,” “depressed,” “irritable,” “miserable,” “clumsy,” “sedated,” “sleepy,” “withdrawn,” “unmotivated,” “tired,” “angry,” “suspicious,” “anxious,” “jittery,” “restless,” “on edge,” “confused,” “dizzy,” “forgetful,” “ “hungry,” “nauseous,” “muscle pain”; “I have…” “blurred vision,” “chills,” “stomach pain,” “an upset stomach,” “a headache,” “difficulty concentrating”; “I want…” “alcohol,” “marijuana,” “a cigarette”; “My heart is pounding or beating faster than usual”; and “Noises or sounds seem louder than usual.”
Task battery
The task battery, designed to measure attention, psychomotor ability, learning, and memory (Foltin, Fischman, Pippen, & Kelly, 1993; Haney, Comer, Ward, Foltin, & Fischman, 1997; Hart et al., 2001), consisted of a 3-min repeated acquisition task, 10-min divided attention task (DAT), 3-min digit symbol substitution task (DSST), and an immediate and delayed digit recall task (DRT). Marijuana and oral cannabinoids have been shown to modestly impair performance on these tasks relative to placebo (Bedi, Cooper, & Haney, 2012; Haney et al., 1997). Briefly, for the repeated acquisition task, four buttons corresponding to positions on the keypad were illuminated on the computer screen, and participants were required to learn and then enter a 10-response sequence as quickly as possible in a given time limit. The DAT assessed attention and required participants to track a moving target on a computer screen using a mouse while signaling when a brief stimulus appeared in one of the four corners. Accurate tracking of the target increased its speed throughout the task. Psychomotor performance was tested using the DSST, which presented the participant with nine 3 × 3 matrices of blocks, with a single blackened square in each row; below each matrix was an identifying number (1 through 9). A number appeared on the screen indicating which pattern of highlighted boxes from the above matrices should be replicated using a nine-key keypad. Performance accuracy and speed were recorded. Lastly, delayed and immediate recall were evaluated using the DRT. For this task, the participant was required to enter an eight-digit sequence that appeared on the computer screen, and again when it disappeared (immediate recall). Participants were then asked to recall and recognize one of the sequences at the end of the task battery (delayed recall/recognition).
Data Analysis
Repeated measures ANOVA with planned comparisons was used to assess the subjective, performance, and physiological effects of active marijuana, as well as amount of marijuana smoked as a function of the number of active puffs. There were two within-group factors (number of active marijuana puffs and time point). Dependent variables included subjective measures, as assessed with the MRF and VAS scales, task battery end points (refer to Foltin et al., 1993; Haney et al., 1997), expired CO, and heart rate. To ensure tobacco smoking did not influence CO levels, the data was also analyzed (not shown) excluding the four cigarette smokers who opted for cigarette breaks during sessions, and the results are consistent with the data obtained with all participants. The amount of marijuana smoked was calculated as the difference between pre- and post-smoking cigarette weights. The weight of marijuana smoked from cigarettes in active marijuana puffs condition was compared with the corresponding cigarette from the 0-puff condition. For each dependent measure, six planned comparisons were completed to determine dose dependency of marijuana’s effects (0 vs. 2, 4, and 6 puffs; 2 vs. 4 and 6 puffs; 4 vs. 6 puffs). Results were considered statistically significant when p values were ≤0.05 using Huynh-Feldt corrections.
As mentioned, this study was designed to determine whether naltrexone shifted marijuana’s dose-dependent subjective and physiological effects. However, in the absence of dose-dependent marijuana effects, it was difficult to interpret how naltrexone interacted with marijuana across the puff conditions. Because naltrexone and puff conditions were counterbalanced, and sufficient time for drug clearance was given between sessions, we chose to only report on the placebo naltrexone condition, in order to focus on the marijuana smoking procedures rather than naltrexone–marijuana interaction.
Results
Demographic Characteristics
Table 2 describes the demographic information of the 19 participants (18 males; one female) who completed the study. Due to experimental error, data from one volunteer were not included in the analysis. Seven participants reported daily cigarette smoking (4.9 ± 0.9 cigarettes/day). Four of these participants elected to smoke cigarettes during the sessions; they were permitted to smoke cigarettes twice per session, at the exact same time point in each session. Ten participants reported drinking alcohol weekly (2.0 ± 0.2 days per week, with 2.7 ± 0.4 standard drinks on each occasion). No participant reported using any over-the-counter or prescription drugs throughout the duration of the study. An additional four volunteers enrolled but did not complete the study: two left due to medication side-effects, including nausea and vomiting; one lost contact; and one tested positive for pregnancy and was discontinued from participation.
Table 2.
Demographic Characteristics of Study Participants
| Age (years old) | 27 ± 4 |
| Sex (M/F) | 17/1 |
| Race (B/W) | 10/8 |
| Years regular marijuana use | 8.9 ± 0.8 |
| Days/wk use marijuana | 6.3 ± 0.2 |
| $/wk on marijuana | 50.7 ± 6.7 |
| Marijuana cigarettes/day | 5.4 ± 1.0 |
Note. Data are presented as means (± SD) or as frequency. Sex is indicated as female (F) and male (M); race is indicated as Black (B) and White (W).
Subjective-Effect Ratings
MRF
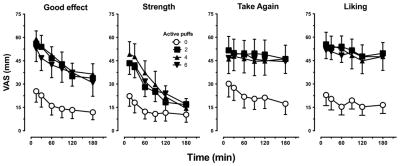
Figure 1 depicts representative subjective ratings of marijuana’s “strength,” “good effect,” “take again,” and “liking” as a function of active puff condition and session time point. All active puff conditions increased ratings of “strength,” “good effect,” “take again,” and “liking” compared with 0 active puffs (p ≤ .001). However, these effects were not dose-dependent; active puff conditions did not systematically differ from one another.
Figure 1.
Representative subjective ratings of marijuana quality as a function of number of active puffs and time as measured by the MRF (0 mm = not at all; 100 mm = extremely). Data are presented as average group values for each post-smoking time point (± SEM). Active marijuana increased subjective ratings (0 puffs < 2, 4, or 6 puffs; p ≤ .001); however, dose-dependent effects were not observed.
VAS
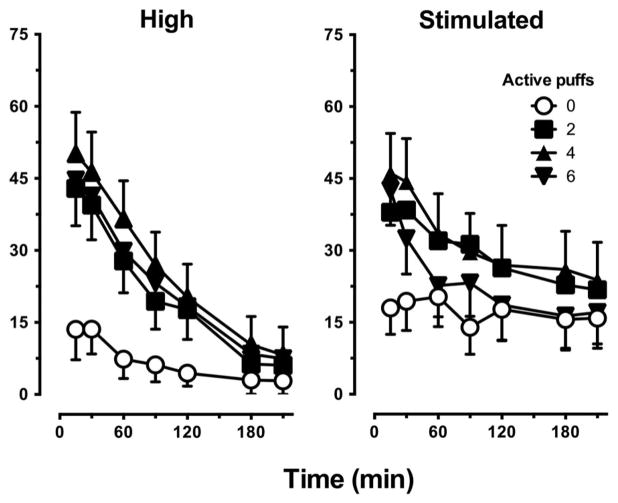
Figure 2 portrays ratings of subjective drug effects, including “high” and “stimulated” as a function of active puff condition and session time point. Two, 4, and 6 puffs of active marijuana increased ratings of both subjective effects relative to 0 active puffs (“high,” p ≤ .001; “stimulated,” p ≤ .05). However, these effects were not dose-dependent.
Figure 2.
Representative subjective ratings of drug effect as a function of number of active puffs and time as measured by the SE-VAS (0 mm = not at all; 100 mm = extremely). Data are presented as average group values for each post-smoking time point (± SEM). Active marijuana increased subjective ratings (0 puffs < 2, 4, or 6 puffs; p ≤ .001), but not dose-dependently.
Marijuana craving
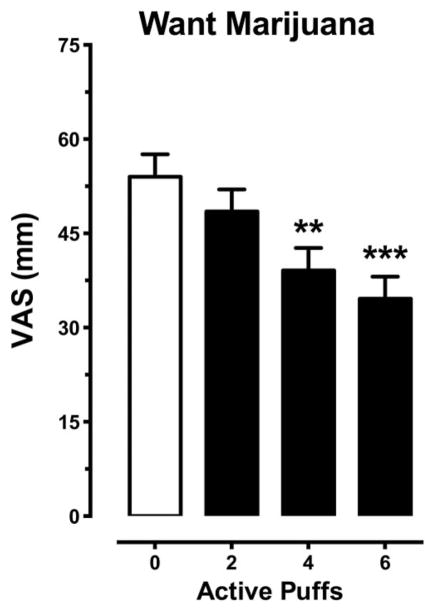
Figure 3 depicts ratings of marijuana craving averaged across session time points as a function of active marijuana puffs. The higher active puff conditions decreased marijuana craving relative to 0 active puffs (p ≤ .01). Marijuana craving dose dependently decreased with increasing number of active marijuana puffs (0 puffs >4 and 6 puffs, p ≤ .01; 2 puffs > 4 and 6 puffs, p ≤ .05).
Figure 3.
Ratings for “I want marijuana” on the SE-VAS as a function of number of active puffs (0 mm = not at all; 100 mm = extremely). Data are presented as average group values for all post-smoking time points (± SEM). Active marijuana decreased subjective ratings (0 puffs > 2, 4, or 6 puffs, p ≤ .05), a moderate dose-dependent effect (2 > 4 puffs, p ≤.05; 2 > 6 puffs, p ≤ .01) was observed. Differences from the 0-puff condition are indicated as follows: * p ≤ .05; ** p ≤ .01; *** p ≤ .001.
Performance effects
Overall, active marijuana had few effects on attention, psychomotor function, and recall as measured by performance on the DAT, DSST, and DRT tasks. Active marijuana decreased the number of attempted entries on the DSST (6 vs. 2 and 0 puffs, p ≤ .05), reflecting impaired psychomotor performance, and decreased accuracy of immediate recall on the DRT (6 vs. 0, 2, and 4 puffs, p ≤ .05) compared with 0 active puffs, indicative of impaired memory (data not shown).
Heart rate, carbon monoxide levels, and weight of marijuana smoked
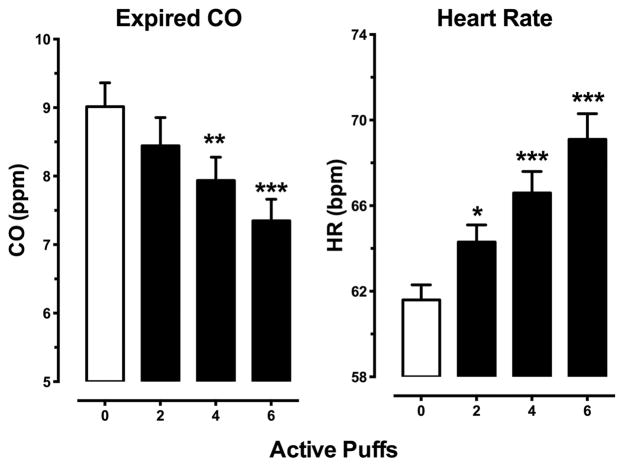
Figure 4 illustrates expired carbon monoxide and heart rate averaged across the session time points as a function of number of active marijuana puffs. Marijuana decreased expired CO at higher active puff conditions (p ≤ .01) and increased heart rate (p ≤ .001) at all active puff conditions. Both effects were dose-dependent with increasing number of puffs increasing heart rate (0 < 2, 4, and 6 puffs, p ≤ .05; 2 < 4 and 6 puffs, p ≤ .05; 4 < 6 puffs, p ≤ .05) and decreasing expired CO (0 > 4 and 6 puffs, p ≤ .05; 2 > 6 Puffs, p ≤ .01).
Figure 4.
Heart rate (beats per minutes [bpm]) and expired carbon monoxide (parts per million [ppm]) as a function of number of active puffs and time. Active marijuana dose-dependently decreased expired CO levels and increased heart rate. Data are presented as mean values (± SEM) across all post-smoking time points. Differences from the 0-puff condition are indicated as follows: * p ≤ .05; ** p ≤ .01; *** p ≤ .001.
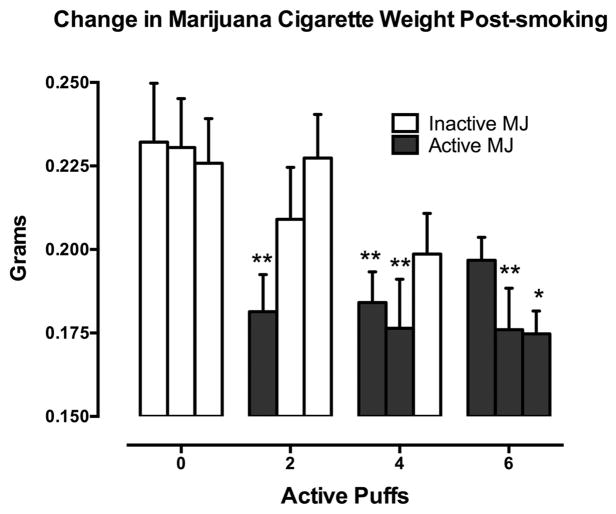
Figure 5 portrays the amount of active and inactive marijuana cigarettes smoked as a function of marijuana strength and puff condition. When comparing the weights of the first, second, and third cigarettes smoked under each active puff condition to the same numbered cigarette in the inactive marijuana condition (0 puffs), there was a significantly larger change in amount smoked for inactive versus active cigarettes: Cigarette 1, 0 > 2 and 4 puffs, p ≤ .01; Cigarette 2, 0 > 4 and 6 puffs, p ≤ 0.01, 2 > 4 puffs, p ≤ .05; Cigarette 3, 0 > 6 puffs, p ≤ .05, 2 > 6 puffs, p ≤ .01.
Figure 5.
Amount of marijuana cigarettes smoked depicted as difference between pre-smoking and post-smoking cigarette weights. Significant difference between cigarettes from active puff groups and corresponding 0-puff cigarette indicated as * p ≤ .05; ** p ≤ .01.
Discussion
An objective of the current study was to establish a method to characterize the dose–effect function of marijuana’s subjective and physiological effects. This novel procedure, which was designed to blind participants to the amount of active marijuana they smoked in each condition, did not prevent participants from adjusting their inhalation patterns as a function of marijuana strength. Both carbon monoxide expiration (an indication of inhalation strength) and measures of the amount of each cigarette smoked demonstrate that inhalation strength was inversely proportional to marijuana strength; this finding is consistent with earlier studies showing that expired CO decreases as the number of active marijuana puffs (Chait, 1989; Herning et al., 1986) or marijuana strength (Cooper & Haney, 2009) increases. Participants’ evaluation of how marijuana made them feel (e.g., “I feel high,” “I liked the dose,” “The dose was strong”) was not dose-dependent, but the procedure did produce dose-dependent changes in ratings of marijuana craving and heart rate. Thus, despite the participants’ attempts to titrate dose by decreasing smoke inhalation as the number of active puffs increased, the heart rate and craving data suggest that plasma THC levels increased with increasing number of active puffs.
As to why marijuana craving ratings and heart rate were dose-dependent, although evaluation of marijuana’s direct effects were not, previous studies have demonstrated that subjective responses are more sensitive to expectancy effects than physiological effects (Camí, Guerra, Ugena, Segura, & de la Torre, 1991). That is, in experienced marijuana smokers, marijuana-associated cues (i.e., the act of smoking, marijuana cigarette appearance, taste, smell) and the expectation of its effects contribute to the subjective response to the drug (Chait et al., 1988; Fillmore, Mulvihill, & Vogel-Sprott, 1994; Kirk, Doty, & De Wit, 1998). For example, inactive marijuana administration produced marijuana-like subjective drug effect ratings when participants were instructed that the marijuana administered was active (Metrik et al., 2009). In the current procedure, active marijuana cigarettes were always smoked before inactive cigarettes in order to mask the dose conditions. We hypothesize that under active puff conditions, the direct effects of the first two puffs increased the expectancy that subsequent puffs were also active. As such, participants rated 2, 4, and 6 puffs as producing a similar level of intoxication, even though the cardiovascular data suggest that the dose conditions resulted in distinct plasma THC levels.
In terms of potential study limitations, the sample was mostly male, daily marijuana smokers, and, as discussed previously (Cooper & Haney, 2010), the inclusion of an equal number of females could potentially reveal sex-dependent differences in marijuana’s effects. Additionally, selecting for a population with less marijuana exposure may yield findings that vary from the current results. Heavy marijuana users develop tolerance to the subjective and performance-impairing effects of marijuana, so they may have difficulty distinguishing between subtle subjective effects within a narrow range of active marijuana doses. The methodology employed to establish marijuana’s dose dependency could also be refined to alter the impact of stimulus and outcome expectancy.
In summary, the study provided a comprehensive assessment of the effects of smoked marijuana within a precisely controlled dose range while attempting to account for confounds represented by differences in smoking behavior, such as dose titration. The findings demonstrate the difficulties in designing a marijuana-smoking paradigm that produces dose–response relationships for subjective effects. Ratings of marijuana’s subjective effects may be particularly influenced by expectancy, whereas physiological measures and ratings of craving may better reflect the amount of marijuana exposure.
Acknowledgments
This research was supported by the U.S. National Institute on Drug Abuse (Margaret Haney: DA09236; Ziva D. Cooper: DA027755).
The authors acknowledge and appreciate the exceptional assistance of Divya Lakhaney, Elyssa Berg, and Michael Harakas in data collection, and Richard Foltin for statistical assistance. Ziva D. Cooper and Margaret Haney designed the study and wrote the protocol. Authors Divya Ramesh and Ziva D. Cooper managed the literature searches, summaries of previous related work, and performed statistical analysis of results. All authors contributed and have approved the final manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Ashton H, Golding J, Marsh VR, Millman JE, Thompson JW. The seed and the soil: Effect of dosage, personality and starting state on the response to delta-9-tetrahydrocannabinol in man. British Journal of Clinical Pharmacology. 1981;12:705–720. doi: 10.1111/j.1365-2125.1981.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azorlosa JL, Greenwald MK, Stitzer ML. Marijuana smoking: Effects of varying puff volume and breathhold duration. The Journal of Pharmacology and Experimental Therapeutics. 1995;272:560–569. [PubMed] [Google Scholar]
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: Effect of varying delta-9-tetrahydrocannabinol content and number of puffs. The Journal of Pharmacology and Experimental Therapeutics. 1992;261:114–122. [PubMed] [Google Scholar]
- Bedi G, Cooper ZD, Haney M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2011.00427.x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camí J, Guerra D, Ugena B, Segura J, de la Torre R. Effect of subject expectancy on the THC intoxication and disposition from smoked hashish cigarettes. Pharmacology, Biochemistry and Behavior. 1991;40:115–119. doi: 10.1016/0091-3057(91)90330-5. [DOI] [PubMed] [Google Scholar]
- Cappell H, Kuchar E, Webster CD. Some correlates of marihuana self-administration in man: A study of titration of intake as a function of drug potency. Psychopharmacologia. 1973;29:177–184. doi: 10.1007/BF00414031. [DOI] [PubMed] [Google Scholar]
- Chait LD. Delta-9-tetrahydrocannabinol content and human marijuana self-administration. Psychopharmacology. 1989;98:51–55. doi: 10.1007/BF00442005. [DOI] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Chait LD, Fischman MW, Schuster CR. “Hangover” effects the morning after marijuana smoking. Drug and Alcohol Dependence. 1985;15:229–238. doi: 10.1016/0376-8716(85)90002-X. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug and Alcohol Dependence. 2009;103:107–113. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Opioid antagonism enhances marijuana’s effects in heavy marijuana smokers. Psychopharmacology. 2010;211:141–148. doi: 10.1007/s00213-010-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Mulvihill LE, Vogel-Sprott M. The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacology. 1994;115:383–388. doi: 10.1007/BF02245081. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The effects of combinations of intranasal cocaine, smoked marijuana, and task performance on heart rate and blood pressure. Pharmacology, Biochemistry and Behavior. 1990;36:311–315. doi: 10.1016/0091-3057(90)90409-B. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: Cardiovascular consequences. Pharmacology, Biochemistry and Behavior. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug and Alcohol Dependence. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-U. [DOI] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behavioural Pharmacology. 1997;8:101–112. [PubMed] [Google Scholar]
- Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. S0893-133X(01)00273-1[pii] [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacology, Biochemistry and Behavior. 1997;58:93–101. doi: 10.1016/S0091-3057(96)00456-X. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacology, Biochemistry and Behavior. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Herning RI, Hooker WD, Jones RT. Tetrahydrocannabinol content and differences in marijuana smoking behavior. Psychopharmacology. 1986;90:160–162. doi: 10.1007/BF00181232. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National results on adolescent drug use, overview of key findings, 2011. Bethesda, MD: National Institute on Drug Abuse; 2012. [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Are choice and self-administration of marijuana related to delta-9-THC content? Experimental and Clinical Psychopharmacology. 1997;5:74–82. doi: 10.1037/1064-1297.5.1.74. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Fischman MW. Effects of smoked marijuana on heart rate, drug ratings and task performance by humans. Behavioural Pharmacology. 1993;4:167–178. doi: 10.1097/00008877-199304000-00009. [DOI] [PubMed] [Google Scholar]
- Kirk JM, Doty P, De Wit H. Effects of expectancies on subjective responses to oral delta-9-tetrahydrocannabinol. Pharmacology, Biochemistry and Behavior. 1998;59:287–293. doi: 10.1016/S0091-3057(97)00414-0. [DOI] [PubMed] [Google Scholar]
- Metrik J, Rohsenow DJ, Monti PM, McGeary J, Cook TA, de Wit H, Kahler CW. Effectiveness of a marijuana expectancy manipulation: Piloting the balanced-placebo design for marijuana. Experimental and Clinical Psychopharmacology. 2009;17(4):217–225. doi: 10.1037/a0016502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of marijuana smoking on subjective ratings and tobacco smoking. Pharmacology, Biochemistry and Behavior. 1986;25:659–665. doi: 10.1016/0091-3057(86)90156-5. [DOI] [PubMed] [Google Scholar]