Abstract
OBJECTIVE
This study examined changes in survival among children with Down syndrome (DS) by race/ethnicity in 10 regions of the United States. A retrospective cohort study was conducted on 16 506 infants with DS delivered during 1983–2003 and identified by 10 US birth defects monitoring programs. Kaplan-Meier survival probabilities were estimated by select demographic and clinical characteristics. Adjusted hazard ratios (aHR) were estimated for maternal and infant characteristics by using Cox proportional hazard models.
RESULTS
The overall 1-month and 1-, 5-, and 20-year survival probabilities were 98%, 93%, 91%, and 88%, respectively. Over the study period, neonatal survival did not improve appreciably, but survival at all other ages improved modestly. Infants of very low birth weight had 24 times the risk of dying in the neonatal period compared with infants of normal birth weight (aHR 23.8; 95% confidence interval [CI] 18.4–30.7). Presence of a heart defect increased the risk of death in the post-neonatal period nearly fivefold (aHR 4.6; 95% CI 3.9–5.4) and continued to be one of the most significant predictors of mortality through to age 20. The postneonatal aHR among non-Hispanic blacks was 1.4 (95% CI 1.2–1.8) compared with non-Hispanic whites and remained elevated by age 10 (2.0; 95% CI 1.0–4.0).
CONCLUSIONS
The survival of children born with DS has improved and racial disparities in infant survival have narrowed. However, compared with non-Hispanic white children, non-Hispanic black children have lower survival beyond infancy. Congenital heart defects are a significant risk factor for mortality through age twenty.
Keywords: Down syndrome, survival, mortality, congenital heart defects, epidemiology, low birth weight, population-based, racial disparities
Down syndrome (DS) is the most common chromosomal disorder among live births and occurs in 1 in every 700 live births in the United States.1 DS is associated with premature mortality and an increased risk for a number of co-morbid conditions including cardiac, gastrointestinal, musculoskeletal or orthopedic, ear and hearing, ophthalmic, endocrine, leukemia, and autism.2 Even though trends in the total birth prevalence of DS have been difficult to determine because of the lack of reliable data on trends in the number of DS-related fetal deaths, the birth prevalence of DS has increased in recent decades in the United States and many other countries.3,4 These trends are due in part to an increasing proportion of births to women over age 35.3,4
Although the survival probability for children with DS has improved in recent years,5,6 the overall fatality among infants with DS remains more than 5 times higher than that of general population.7,8 Furthermore, there is evidence that survival is lower for black children with DS than for white children with DS; however, the reason for the higher fatality rates among blacks is unknown.7–10 Factors associated with premature mortality among infants and children with DS include the presence of congenital heart defects (CHD), other structural malformations,6,9,10 leukemia,7 and low birth weight.9,11 Whether variations in the prevalence of these risk factors among race/ethnicity groups explain the observed differences in survival is unclear.
In this study, we examined the long-term trends in survival for children with DS and the variation in survival probabilities by maternal and infant characteristics based on data from 10 population-based surveillance programs in the United States. Adjusted hazard ratios (aHRs) were estimated to assess the association between possible prognostic factors and mortality.
METHODS
Data Sources and Case Criteria
Infants born with DS were identified by 10 population-based birth defects monitoring programs located in Arkansas, Georgia (5 central counties of metropolitan Atlanta*), California (11 counties†), Colorado, Iowa, New York (New York City excluded), North Carolina, Oklahoma, Texas, and Utah. The birth cohorts available for each program were Arkansas, 1993–2002; Georgia, 1983–2003; California, 1983–2002; Colorado, 1989–2003; Iowa, 1983–2003; New York, 1983–2003; North Carolina, 1989–1993, 1995–2003; Oklahoma, 1994–2003; Texas, 1996–2003; and Utah, 1995–2003. Vital status was ascertained for all births with DS through December 31, 2004, for all of the regions except for Arkansas and California, the follow-up periods of which ended on December 31, 2003, and December 31, 2002, respectively. Infants with karyotype diagnosis of trisomy 21 were classified as DS by using a modified British Paediatrics Association (BPA) coding system in most regions except for New York, where infants with DS were classified by both BPA and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, and in the regions of North Carolina and Colorado, where they were classified by ICD-9-CM codes only. Deaths among children with DS were ascertained by linkage with medical records, state vital records, and the National Death Index.3
Children with DS and no death records were considered alive at the end of the study follow-up period and treated as censored observations in the survival analysis. Demographic and clinical characteristics of infants with DS from birth were also collected by the surveillance programs. Infants of <500 g, <20 weeks of gestational age, or with unconfirmed diagnosis of DS were excluded from our study.
Both demographic and clinical characteristics were included as potential risk factors for survival of infants with DS including maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), maternal age (<35 years vs ≥35 years), maternal education (<12 years, ≥12 years), sex, birth weight (<1500 g, 1500–2499 g, ≥2500 g), and region. Infants with DS were classified as having a major CHD if their records indicated the presence of ICD-9-CM or BPA codes for CHD (745.000–747.430 and 747.640). Codes for normal physiologic findings in newborns or premature infants (eg, patent foramen ovale, patent ductus arteriosus), minor conditions such as tricuspid insufficiency, or unconfirmed cardiac defects were not considered structural heart defects.
Data Analysis
The survival probabilities at 1 month, 1 year, 5 years, 10 years, and 20 years were estimated by the Kaplan-Meier product-limit method.12 Greenwood’s method was used to calculate the variance of the estimated survival probability and their 95% confidence intervals (CIs).13 For those study regions with at least 20 years of follow-up (GA, CA, IA, and NY), the infant survival probabilities and 95% CIs were estimated for 4 birth cohorts (1983–1987, 1988–1992, 1993–1997, 1998–2003), and a trend analysis for the increasing survival over birth cohorts across race/ethnicity was conducted.
A log-rank test was used to determine whether the survival probabilities were significantly different among various levels of potential demographic and clinical risk factors.14 Cox proportional hazard models were used to estimate unadjusted and adjusted hazard ratios (aHR) for possible prognostic factors.14 The assumption of proportionality for the hazards was checked by plotting estimated log-cumulative hazard versus log of survival time for different categories of the risk factors. Possible time-dependent trends were also tested to confirm the assumption of proportionality. Final multivariate proportional hazard models were obtained, and the aHRs were estimated for the significant covariates. For the multivariate proportional hazard models, aHRs were estimated assuming survival through the preceding age period. Computations were performed by using SAS-PC (version 9.13; SAS Institute Inc, Cary, NC), and figures were generated by using S-PLUS (version 6.0; Insightful Corp, Seattle, WA). This study was approved by the Institutional Review Board of the Centers for Disease Control and Prevention.
RESULTS
From 1983 to 2003, 16 506 liveborn infants with DS were identified in the 10 regions included in our study. The number of infants ascertained in the regions ranged from 362 in Arkansas to 4686 in California with a median number of 982 infants, and the period of follow-up ranged from 9 years in Texas to 22 years in Georgia, Iowa, and New York (Table 1). Among those with comparable follow-up time (11 years), the regional variation was greatest between Arkansas (87.2%) and Utah (90.7%; P = .01).
TABLE 1.
Sample Size and Years of Observation by Region in Ascending Order of Years of Follow-up
| Region | Sample Size | Birth Years | Follow-up Years | Years of Follow-up |
|---|---|---|---|---|
| Texas | 2663 | 1996–2003 | 1996–2004 | 9 |
| Utah | 556 | 1995–2003 | 1995–2004 | 10 |
| Arkansas | 362 | 1993–2002 | 1993–2003 | 11 |
| Oklahoma | 546 | 1994–2003 | 1994–2004 | 11 |
| Colorado | 972 | 1989–2003 | 1989–2004 | 16 |
| North Carolina | 1628 | 1989–1993, 1995–2003 | 1989–2004 | 16 |
| California | 4686 | 1983–2002 | 1983–2003 | 20 |
| Iowa | 849 | 1983–2003 | 1983–2004 | 22 |
| New York | 3355 | 1983–2003 | 1983–2004 | 22 |
| Georgia | 889 | 1983–2003 | 1983–2004 | 22 |
The overall 1-month and 1-, 5-, and 20-year survival probabilities were 98%, 93%, 91%, and 88%, respectively (Tables 2 and 3), with the highest mortality occurring in the postneonatal period. Over the 20-year study period, neonatal survival did not improve appreciably, but modestly improved survival at older ages was observed for those infants with DS born after 1996 (Table 4).
TABLE 2.
Survival Probabilities for Infants With DS by Selected Maternal and Child Characteristics in 10 US Regions During 1983–2003
| Births | <28 d
|
1 y
|
5 y
|
10 y
|
20 y
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Survival % (95% CI) | Deaths | Survival % (95% CI) | Deaths | Survival % (95% CI) | Deaths | Survival % (95% CI) | Deaths | Survival % (95% CI) | ||
| Total | 16 506 | 318 | 98.1 (97.9–98.3) | 1180 | 92.9 (92.5–93.2) | 1489 | 91.0 (90.5–91.4) | 1551 | 90.7 (90.2–91.1) | 1584 | 88.1 (87.0–89.0) |
| Mother’s race/ethnicity | |||||||||||
| Non-Hispanic White | 8877 | 183 | 97.9 (97.6–98.2)a | 618 | 93.3 (92.5–93.6)a | 784 | 90.9 (90.2–91.5)a | 818 | 90.3 (89.6–90.9)a | 839 | 88.6 (87.5–89.6)a |
| Non-Hispanic Black | 1576 | 34 | 97.8 (97.0–98.4)a | 156 | 90.2 (88.6–91.6)a | 209 | 86.1 (84.2–87.8)a | 220 | 84.8 (82.8–86.6)a | 224 | 81.5 (75.7–86.1)a |
| Hispanic | 5113 | 73 | 98.6 (98.2–98.9)a | 318 | 93.8 (93.1–94.4)a | 383 | 92.2 (91.4–92.9)a | 391 | 91.8 (91.0–92.6)a | 396 | 90.2 (88.1–92.0)a |
| Other | 863 | 24 | 97.2 (95.8–98.1)a | 78 | 91.1 (89.0–92.8)a | 97 | 88.4 (86.0–90.4)a | 104 | 86.7 (84.0–89.0)a | 106 | 85.5 (82.3–88.2)a |
| Missing | 77 | 4 | — | 10 | — | 16 | — | 18 | — | 19 | — |
| Infant sex | |||||||||||
| Male | 8928 | 186 | 97.9 (97.6–98.2) | 644 | 92.8 (92.2–93.3) | 800 | 90.8 (90.1–91.3) | 833 | 90.0 (89.4–90.7) | 853 | 88.2 (87.0–89.4) |
| Female | 7577 | 132 | 98.3 (97.9–98.5) | 536 | 92.9 (92.3–93.5) | 689 | 90.6 (89.9–91.3) | 718 | 89.0 (89.0–90.5) | 731 | 87.9 (86.3–89.2) |
| Missing | 1 | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — |
| Birth wt (g) | |||||||||||
| ≥2500 | 12826 | 127 | 99.0 (98.8–99.2)a | 637 | 95.1 (94.7–95.4)a | 856 | 93.1 (92.6–93.5)a | 903 | 92.3 (91.8–92.8)a | 927 | 90.7 (89.9–91.6)a |
| 1500–2499 | 3190 | 74 | 97.7 (97.1–98.2)a | 327 | 89.8 (88.7–90.8)a | 406 | 86.9 (85.7–88.1)a | 420 | 85.9 (84.6–87.2)a | 426 | 82.7 (78.3–86.2)a |
| <1500 | 479 | 112 | 76.6 (72.6–80.2)a | 211 | 56.0 (51.4, 60.3)a | 222 | 53.3 (48.7–57.7)a | 223 | 52.6 (47.8–57.1)a | 226 | 48.0 (40.7–55.0)a |
| Missing | 11 | 5 | — | 5 | — | 5 | — | 5 | — | 5 | — |
| Maternal age (y) | |||||||||||
| <35 | 10572 | 189 | 98.2 (97.9–98.5) | 789 | 92.6 (92.0–93.0)b | 1005 | 90.2 (89.6–90.8)c | 1046 | 84.5 (88.9–90.1)b | 1071 | 87.5 (86.3–88.6)b |
| ≥35 | 5933 | 129 | 97.8 (97.4–98.2) | 391 | 93.4 (92.8–94.0)b | 484 | 91.6 (90.8–92.2)c | 505 | 90.7 (89.9–91.5)b | 513 | 89.4 (87.8–90.7)b |
| Missing | 1 | 0 | — | 0 | — | 0 | — | 0 | — | 1 | — |
—, no estimates were produced because of small numbers.
P < .0001.
P < .05.
P < .01.
TABLE 3.
Survival Probabilities for Infants With DS by Selected Maternal and Child Characteristics in 10 US Regions During 1983–2003
| Births | <28 d
|
1 y
|
5 y
|
10 y
|
20 y
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Survival, % (95% CI) | Deaths | Survival, % (95% CI) | Deaths | Survival, % (95% CI) | Deaths | Survival, % (95% CI) | Deaths | Survival, % (95% CI) | ||
| Maternal education (y) | |||||||||||
| ≥12 | 11437 | 220 | 98.1 (97.8–98.3) | 787 | 93.1 (92.7–93.6) | 995 | 91.1 (90.5–91.6) | 1035 | 90.3 (89.7–90.9) | 404 | 88.9 (87.9–89.8) |
| <12 | 4132 | 66 | 98.4 (98.0–98.7) | 309 | 92.6 (91.7–93.3) | 381 | 90.4 (89.4–91.9) | 399 | 89.3 (88.3–90.3) | 1056 | 86.0 (81.3–89.6) |
| Missing | 937 | 32 | — | 84 | — | 113 | — | 117 | — | 124 | — |
| Plurality | |||||||||||
| Singleton | 16048 | 308 | 98.1 (97.9–98.3) | 1129 | 93.0 (92.6–93.4)a | 1425 | 90.8 (90.4–91.3)a | 1486 | 90.1 (89.6–90.5)a | 1518 | 88.2 (87.2–89.1)a |
| Multiple | 440 | 10 | 97.7 (95.8–98.8) | 50 | 88.6 (85.3–91.2)a | 61 | 86.0 (82.3–88.9)a | 62 | 85.5 (81.8–88.6)a | 63 | 84.4 (79.9–88.0)a |
| Missing | 18 | 0 | — | 1 | — | 3 | — | 3 | — | 3 | — |
| CHDs | |||||||||||
| No | 8576 | 176 | 97.9 (97.6–98.2) | 343 | 96.0 (95.5–96.4)b | 417 | 95.0 (94.5–95.5)b | 444 | 94.4 (93.8–94.9)b | 460 | 93.0 (92.0–93.9)b |
| Yes | 7930 | 142 | 98.2 (97.9–98.5) | 837 | 89.6 (88.9–90.2)b | 1072 | 86.0 (85.2–86.7)b | 1107 | 85.0 (84.1–85.8)b | 1124 | 82.2 (80.1–84.1)b |
| Missing | — | — | 0 | — | — | — | |||||
| Birth cohort | |||||||||||
| 1983–1989 | 2454 | 51 | 97.9 (97.2–98.4) | 213 | 91.3 (90.0–92.4)b | 292 | 88.1 (86.8–89.3)b | 310 | 87.4 (86.0–88.6)b | 334 | 85.7 (84.1–87.1)b |
| 1990–1996 | 5441 | 109 | 98.0 (97.6–98.3) | 477 | 91.2 (90.5–92.0)b | 589 | 89.2 (88.3–90.0)b | 624 | 88.4 (87.6–89.3)b | — | — |
| 1997–2003 | 8611 | 158 | 98.2 (97.9–98.4) | 490 | 94.3 (93.8–94.8)b | 608 | 92.5 (91.9–93.0)b | — | — | — | — |
—, no estimates were produced because of small numbers.
P < .001.
P < .0001.
TABLE 4.
aHRsa for Select Covariates Among Children With Down Syndrome (n = 16 418) by Age at Follow-up From 10 US Regions, 1983–2003
| 1 mo | 2–12 mo | 1–5 y | 6–10 y | 11–20 y | Overall | |
|---|---|---|---|---|---|---|
| Race/ethnicity | ||||||
| White | Referent | Referent | Referent | Referent | Referent | Referent |
| Blacks | 1.0 (0.7–1.5) | 1.4 (1.2–1.8)b | 1.9 (1.4–2.6)b | 2.0 (1.0–4.0)b | 1.7 (0.6–5.1) | 1.4 (1.0–1.6) |
| Hispanics | 0.8 (0.6–1.0)b | 1.0 (0.9–1.2) | 0.8 (0.6–1.0) | 0.6 (0.3–1.2) | 1.7 (0.6–4.7) | 0.8 (0.7–0.9)b |
| Other | 1.3 (0.8–2.0) | 1.3 (1.0–1.7) | 1.2 (0.8–2.0) | 2.4 (1.1–5.4)b | 1.9 (0.4–8.0) | 1.3 (1.1–1.6)b |
| Birth wt | ||||||
| ≥2500 | Referent | Referent | Referent | Referent | Referent | Referent |
| 1500–2499 | 2.4 (1.8–3.2)b | 1.9 (1.7–2.3)b | 1.4 (1.1–1.9)b | 1.2 (0.7–2.4) | 1.3 (0.5–3.1) | 1.8 (1.6–2.0)b |
| <1500 | 23.8 (18.4–30.7)b | 6.9 (5.6–8.6)b | 2.1 (1.2–3.9)b | 1.1 (0.2–8.2) | 8.5 (2.5–28.6)b | 8.5 (7.3–9.8)b |
| Maternal age | ||||||
| <35 | Referent | Referent | Referent | Referent | Referent | Referent |
| ≥35 y | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (0.9–1.0) | 1.0 (1.0–1.0) |
| CHD, yes vs no (referent) | 0.8 (0.6–1.2) | 4.6 (3.9–5.4)b | 4.0 (3.1–5.2)b | 1.9 (1.1–3.2)b | 2.0 (1.0–4.0)b | 2.7 (2.4–3.0)b |
| Birth cohort | ||||||
| 1983–1989 | Referent | Referent | Referent | |||
| 1990–1996 | 0.9 (0.6–1.2) | 1.0 (0.8–1.2) | 0.6 (0.5–0.8)b | — | — | — |
| 1997–2003 | 0.8 (0.5–1.1) | 0.5 (0.4–0.6)b | 0.5 (0.4–0.7)b | — | — | — |
Birth wt reference group is ≥2500. Survival probability among non-Hispanic blacks, Hispanic, and other was compared with the survival probability among non-Hispanic whites. —, no estimates were produced because of small numbers.
Adjusted for race/ethnicity, birth wt, maternal age and education, presence of a CHD, birth period, and region of birth.
Statistical difference in survival probabilities between category stratum and the referent group.
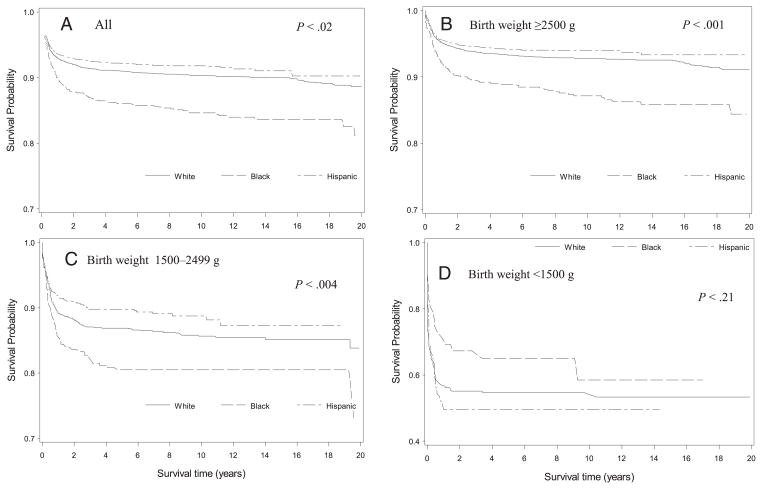
Univariate analysis demonstrated that survival varied by a number of demographic and maternal characteristics (Table 2 and 3). The greatest variation in survival to 1 year of age was by birth weight. Infants with normal birth weight had a 95% survival to 1 year compared with 56% and 90% survival to infants of very low and low birth weight, respectively. Infants born with a CHD had lower infant survival than those without (90% vs 96%). Infants born to non-Hispanic black and non-Hispanic white mothers had nearly identical neonatal survival, but thereafter infants to non-Hispanic black mothers experienced increasingly lower survival through age 20 (Fig 1). When stratified by birth weight, infants of non-Hispanic black mothers had lower overall survival only among normal and low birth weight (1500–2499 g) infants; among very low birth weight infants (<1500 g), infants of non-Hispanic black mothers had higher survival than infants of either non-Hispanic white or Hispanic mothers.
FIGURE 1.
Kaplan-Meier survival curves by race and ethnicity and birth weight for individuals born with DS in from 10 US regions, 1983–2003.
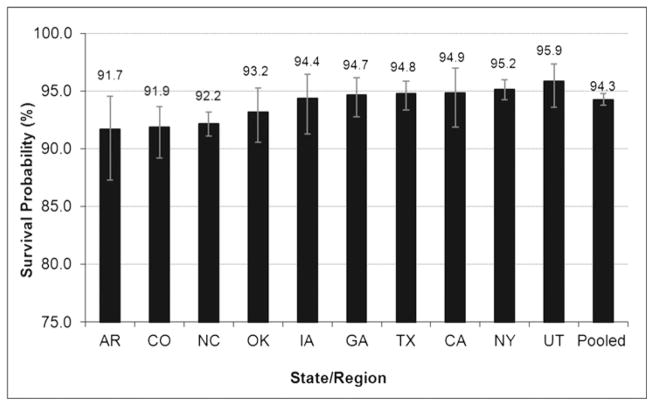
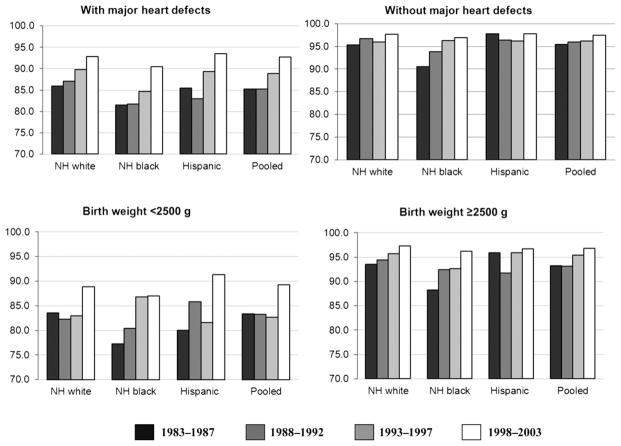
Restricting the cohort period to the years during which all regions contributed case data (1997–2003), the pooled survival probability to 1 year was 94% (Fig 2). The 1-year survival probability ranged from 92% in Arkansas to 96% in Utah (P = .02). For the regions with ≥20 years of follow-up (GA, CA, IA, and NY), an increasing trend of survival across 4 birth cohort periods was detected (P = .002) overall and for each region except for the region of Georgia (data not shown). Trends of improving survival were apparent across the 4 time periods for each major racial/ethnic groups: non-Hispanic white (P = .0002), non-Hispanic black (0.0117), and Hispanic (0.0002); however, the greatest improvement in 1-year survival trends was among infants with a major CHD or of birth weight <2500 g (Fig 3).
FIGURE 2.
Survival probability to 1 year among children with DS, 1997–2003.
FIGURE 3.
Racial/ethnic variation in one year survival of children with Down syndrome in four states/regions (CA, GA, IA, NY) by select clinical characteristics, 1983–2003.
For those infants with no missing values for the covariates included in the Cox proportional hazard models (n = 16 418), factors associated with an increased risk of death for children with DS were race/ethnicity, maternal age, birth weight, multiple births, and presence of CHD (Table 4). The only factor associated with an increased risk of neonatal death was birth weight. Infants of very low birth weight had 24 times the risk of dying compared with infants of normal birth weight (aHR 23.8; 95% CI 18.4–30.7), and infants of low birth weight had nearly 2.5-fold increased risk (aHR 2.4; 95% CI 1.8–3.2). Among those who survived the neonatal period, birth weight continued to be associated with an increased risk for death by age 1 for very low (aHR 1.9; 95% CI 1.7–2.3) and low (aHR 6.9; 95% CI 5.6–8.6) birth weight infants. The presence of a heart defect increased the risk of death in the postneonatal period nearly fivefold (aHR 4.6; 95% CI 3.9–5.4) and continued to be one of the most significant predictors of mortality through age 20. The aHR among non-Hispanic blacks at age 1 was 1.4 (95% CI 1.2–1.8) compared with non-Hispanic whites and increased to 2.0 (95% CI 1.0–4.0) by age 10. Infants born to Hispanic mothers had a lower aHR at 1 month of age (aHR 0.8; 95% CI 0.6–1.0) and had an overall reduced risk for mortality (aHR 0.8; 95% CI 0.7–0.9) compared with infants born to non-Hispanic white mothers.
DISCUSSION
Survival among individuals with DS has improved in the United States over time, and survival to 1 year now averages 94% among the 10 regions included in this study. With the exception of the neonatal period, survival in the United States improved over time for all age points, and the most significant gains were among infants born of low birth weight or with a major CHD. Although slight racial and ethnic disparities in survival persist, these have decreased markedly over time. Survival estimates to 1 year varied from 92% to 96% among the 10 regions included in the study. Reasons for state variation are unclear but might be the result of different population characteristics, variations in case ascertainment, state-level policies governing eligibility criteria for health care coverage, or variations in the amount and type of public services available.
This is the largest population-based survival study for people with DS using birth defects surveillance programs, which provide a unique source of quality data ascertained from multiple sources for high sensitivity and diagnostic accuracy in the United States. Previous survival estimates in the United States have been limited to individual surveillance programs.9,15,16 Data for most of those studies were subsumed by this study to produce the most nationally representative survival estimates available to date that can serve as a benchmark as well as a comparison point for reports from other countries.
Linkage of surveillance data with the National Death Index ensured the most accurate determination of the vital status of children even if they died out of state and adds significantly to the credibility of the findings17; however, the assumption that a child was alive if there was no record of death found from the three data sources could be a potential limitation of the study. Additionally, no information on surgeries, clinical management, or barriers to health care as potential contributors to the long-term survival of children with DS was examined.
Factors most associated with an increased risk of mortality among individuals with DS were race/ethnicity, low birth weight, and the presence of CHD. Although some studies have suggested a survival advantage among male children,6,18 our study confirmed findings from other studies that found no survival differences by sex9,10,19–21 Although previous reports have found an increased risk associated with low birth weight,6,9,21 only 1 other population-based study was found that examined very low birth weight infants (<1500 g) as a distinct group, possibly because of relatively low study population sizes.22 This study found that among infants with very low birth weight those with DS had 2.5 times the risk of death compared with very low birth weight infants with no birth defects. The percent of infants with DS in our study population that were born with birth weight <2500 g was 22%, a >2.5-fold increased risk compared with the general population, and these infants were twice as likely to be born <1500 g.23 Recognition of the high risk of mortality associated with very low birth weight for infants with DS is important to increase vigilance among health care providers for this vulnerable group.
Two previous reports in the United States were inconsistent with regard to racial and ethnic disparities in survival9,15; however, the discrepancy is likely the result of 2 study attributes. First, each used non overlapping time periods of observation, so that the later study (Vendola et al) might reflect the narrowing survival gap as evidenced by the decreasing trend in disparities seen in this study. The second and more compelling reason for the differences in the study findings was that Rasmussen et al presented overall hazard ratios up to 20 years of age, whereas Vendola et al restricted their analysis to the first year of life. Our study showed that association between race and mortality increased with increasing age.
Several studies observed that the greatest black-white disparity occurred among infants with no major CHD, suggesting that more undiagnosed CHD among non-Hispanic blacks might be contributing to the disparity.8,9,24,25 Non-Hispanic black infants without a CHD had lower 1-year survival than non-Hispanic whites from 1983 to 1989 (91.3% vs 95.8%), but the disparity nearly disappeared by the 1997–2003 period (97.4% vs 97.7%). To evaluate the potential role of undiagnosed CHD in the black-white disparity, we examined the percent of infants with DS and a CHD by race and over time. During both the earliest and most recent time periods, a greater percent of non-Hispanic blacks with DS was diagnosed with a heart defect compared with non-Hispanic whites: 44% among non-Hispanic blacks versus 41% among non-Hispanic whites during 1983–1989 and 53% among non-Hispanic blacks versus 50% among non-Hispanic whites during 1997–2003. Furthermore, although less severe CHD such as muscular ventricular septal defects are reported less frequently among non-Hispanic blacks compared with non-Hispanic whites,26 there appear to be little racial/ethnic difference in the birth prevalence of more severe heart defects that would have a greater influence on survival, such as atrioventricular septal defect, the most common heart defect among infants with DS.26,27
Unique to this study was the examination of the changing impact across the life span of factors that contribute to mortality. Goldman et al found age variations in the contribution by specific factors to mortality, but the study was limited to the first year of life.22 Our analysis showed that very low and low birth weight conferred the greatest risk of mortality during the neonatal period, but the associated risk lessened with increasing age. CHDs are the leading cause of death among infants with DS8 and conferred a risk of mortality that was highest during the post-neonatal period and was associated with the greatest risk relative to the other factors from age 1 through 10. The mortality risk associated with non-Hispanic black race increased with increasing age even after adjusting for the presence of a heart defect and low birth weight, possibly reflecting inadequate access or utilization of health care services.
The population-based approach of this study is a substantial strength in that it ensures the most complete live-born population of infants with DS. The limitation of this and most survival studies is the lack of accounting for the prenatal experience. Attitudes toward prenatal testing and the use of it vary by a number of maternal factors, including sociodemographic factors, race, and ethnicity.28 The proportion of elective terminations of a fetus with DS in the United States ranged from 7% to 37% with variations by time period and regional racial/ethnic composition.29–32 A recent review of population-based studies of pregnancies with a positive prenatal diagnosis of DS estimated that 67% of pregnancies were terminated, but a temporal analysis found that the proportion of electively terminated pregnancies has consistently decreased in recent years.33 Although information on frequency of termination is scarce and difficult to interpret,34,35 evidence suggests that elective terminations might have a significant impact on the epidemiology of live born infants with DS.36,37 If the presence of severe comorbid conditions such as major CHD factor into the decision to terminate, the observed decreasing trends in the decision to terminate a pregnancy with a positive diagnosis of DS could be the result of more fetuses with less complicated health profiles being carried to term. This potential scenario would lead to a live birth population with an overall higher likelihood of survival, but the extent to which this might contribute to the recent improvements in survival among children with DS is not clear.
Survival in the United States compares favorably for both short- and long-term survival of individuals with DS. The 93% infant survival in the United States was higher than that reported in the United Kingdom (88%),38 Australia (92%),6 Ireland (88%),20 Italy (80%),21 and Denmark (85%).39 The 10-year survival of 91% in the United States was also higher than estimates elsewhere, which ranged from 82% to 85%,6,20,37 and the 20-year survival rate of 88% was higher than reported elsewhere.37 International comparisons should be made with some caution because differences in ascertainment methods, the time period of observation, and disposition for elective terminations could account for some of the observed differences in infant survival.4,40 However, comparison of the longer-term survival estimates that are likely to be less affected by such factors can become particularly important in planning for health services to address specialized health and social services needs of children, adolescents, and adults with DS. In particular, about half of children with DS have major comorbid conditions such as CHD for which access to timely and quality health care services is critically important not only for survival but also to ensure a reasonable quality of life.
CONCLUSIONS
Survival of individuals with DS has improved over the past 20 years, and the racial and ethnic disparities have diminished, particularly during infancy, potentially as the result of improved access to health care services; however, non-Hispanic blacks still appear to be at greater risk of mortality throughout childhood and adolescence. Improved surgical and medical management of CHD and issues related to low birth weight have contributed to the overall improved survival of those with DS, yet significant risks are still associated with these factors. Population-based survival analyses to date have been limited to the evaluation of demographic and clinical factors present at birth. Linkage of population-based birth defects surveillance data with additional data sources such as hospital discharge data would provide a powerful tool to examine the impact of health care access, quality, and utilization on health outcomes of individuals with DS.
WHAT’S KNOWN ON THIS SUBJECT
Although survival of children born with Down syndrome has improved, unexplained racial and ethnic disparities in survival persist in the United States.
WHAT THIS STUDY ADDS
This study used population-based data from 10 birth defects monitoring programs in the United States to examine survival trends among children born with Down syndrome and to evaluate the changing influence of survival predictors over the life course.
Acknowledgments
We thank the following birth defect monitoring programs: Arkansas Reproductive Health Monitoring System (Charlotte Hobbs, Bridget S. Mosley); California Birth Defects Monitoring Program (Barbara Warmerdam, Marcia Ehinger); Colorado Responds to Children with Special Needs (Carol Stanton, Margaret Ruttenber); Iowa Registry for Congenital and Inherited Disorders (Bradley D. MacDowell, Paul Romitti); New York State Congenital Malformations Registry (Charlotte Druschel, Ying Wang); North Carolina Birth Defects Monitoring Program (Katie Harmsen, Robert Meyer); Oklahoma Birth Defects Registry (Kay A. Pearson); Texas Birth Defects Epidemiology and Surveillance Branch (Lisa K Marengo); and Utah Birth Defect Network (Marcia Feldkamp, Miland Palmer).
We also thank Centers for Disease Control and Prevention staff members Dr Tiffany Riehle-Colarusso, Mike Atkinson, and Don Gambrell for their help in classification, data linkages, as well as all Metropolitan Atlanta Congenital Defects Program staff for their help in data collection and analysis and for their constructive suggestions. There were no external funds used for this study, and there were no financial conflicts of interest among Centers for Disease Control and Prevention staff.
ABBREVIATIONS
- aHR
adjusted hazard ratios
- BPA
British Paediatrics Association
- CHD
congenital heart defect
- CI
confidence interval
- DS
Down syndrome
Footnotes
Clayton, Cobb, DeKalb, Fulton, and Gwinnett.
San Francisco and Santa Clara (1983–2003); Stanislaus (1984–2003); Merced (1985–2003); Fresno, Tulare, and San Joaquin (1986–2003); Kern, Kings, and Madera (1987–2003); Los Angeles (1990–2003, excluding 1998).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Mr Kucik conceptualized and designed the study, carried out the analyses, drafted the initial manuscript, and approved the final manuscript as submitted; Dr Shin conceptualized and designed the study, carried out the initial analyses, contributed to the initial draft of the initial manuscript, and approved the final manuscript as submitted; Dr Siffel conceptualized and designed the study, carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted; Ms Marengo coordinated data submission from 1 of the study sites, contributed to the initial draft of the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted; and Dr Correa conceptualized and designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted.
This work was presented in part at the annual meeting of the National Birth Defects Prevention Network; February 11–13, 2008; Washington, DC; and at the annual meeting of the 21st Society for Pediatric and Perinatal Epidemiologic Research; June 23–24, 2008; Chicago, IL.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING: No external funds were used for this study, and there were no financial conflicts of interest among Centers for Disease Control and Prevention staff.
References
- 1.Parker SE, Mai CT, Canfield MA, et al. National Birth Defects Prevention Network. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Bull MJ Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- 3.Shin M, Besser LM, Kucik JE, Lu C, Siffel C, Correa A Congenital Anomaly Multistate Prevalence and Survival Collaborative. Prevalence of Down syndrome among children and adolescents in 10 regions of the United States. Pediatrics. 2009;124(6):1565–1571. doi: 10.1542/peds.2009-0745. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi G, Gualdi S, Bower C, et al. International trends of Down syndrome 1993–2004: Births in relation to maternal age and terminations of pregnancies. Birth Defects Res A Clin Mol Teratol. 2010;88(6):474–479. doi: 10.1002/bdra.20666. [DOI] [PubMed] [Google Scholar]
- 5.Irving C, Basu A, Richmond S, Burn J, Wren C. Twenty-year trends in prevalence and survival of Down syndrome. Eur J Hum Genet. 2008;16(11):1336–1340. doi: 10.1038/ejhg.2008.122. [DOI] [PubMed] [Google Scholar]
- 6.Leonard S, Bower C, Petterson B, Leonard H. Survival of infants born with Down’s syndrome: 1980–96. Paediatr Perinat Epidemiol. 2000;14(2):163–171. doi: 10.1046/j.1365-3016.2000.00252.x. [DOI] [PubMed] [Google Scholar]
- 7.Day SM, Strauss DJ, Shavelle RM, Reynolds RJ. Mortality and causes of death in persons with Down syndrome in California. Dev Med Child Neurol. 2005;47(3):171–176. doi: 10.1017/s0012162205000319. [DOI] [PubMed] [Google Scholar]
- 8.Shin M, Kucik JE, Correa A. Causes of death and case fatality rates among infants with down syndrome in metropolitan Atlanta. Birth Defects Res A Clin Mol Teratol. 2007;79(11):775–780. doi: 10.1002/bdra.20414. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen SA, Wong LY, Correa A, Gambrell D, Friedman JM. Survival in infants with Down syndrome, Metropolitan Atlanta, 1979–1998. J Pediatr. 2006;148(6):806–812. doi: 10.1016/j.jpeds.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359(9311):1019–1025. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 11.Boghossian NS, Hansen NI, Bell EF, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Survival and morbidity outcomes for very low birth weight infants with Down syndrome. Pediatrics. 2010;126(6):1132–1140. doi: 10.1542/peds.2010-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 13.Greenwood M. The natural duration of cancer. Rep Public Health Med Subj (Lond) 1926;33:1–26. [Google Scholar]
- 14.Cox DR, OD . Analysis of Survival Data. London, UK: Chapman & Hall; 1984. [Google Scholar]
- 15.Vendola C, Canfield M, Daiger SP, et al. Survival of Texas infants born with trisomies 21, 18, and 13. Am J Med Genet A. 2010;152A(2):360–366. doi: 10.1002/ajmg.a.33156. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Hu J, Druschel CM, Kirby RS. Twenty-five-year survival of children with birth defects in New York State: a population-based study. Birth Defects Res A Clin Mol Teratol. 2011;91(12):995–1003. doi: 10.1002/bdra.22858. [DOI] [PubMed] [Google Scholar]
- 17.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 18.Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH. Comparative survival advantage of males with Down syndrome. Am J Hum Biol. 2003;15(2):192–195. doi: 10.1002/ajhb.10132. [DOI] [PubMed] [Google Scholar]
- 19.Dupont A, Vaeth M, Videbech P. Mortality and life expectancy of Down’s syndrome in Denmark. J Ment Defic Res. 1986;30(pt 2):111–120. doi: 10.1111/j.1365-2788.1986.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayes C, Johnson Z, Thornton L, et al. Ten-year survival of Down syndrome births. Int J Epidemiol. 1997;26(4):822–829. doi: 10.1093/ije/26.4.822. [DOI] [PubMed] [Google Scholar]
- 21.Mastroiacovo P, Bertollini R, Corchia C. Survival of children with Down syndrome in Italy. Am J Med Genet. 1992;42(2):208–212. doi: 10.1002/ajmg.1320420214. [DOI] [PubMed] [Google Scholar]
- 22.Goldman SE, Urbano RC, Hodapp RM. Determining the amount, timing and causes of mortality among infants with Down syndrome. J Intellect Disabil Res. 2011;55 (1):85–94. doi: 10.1111/j.1365-2788.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- 23.Kochanek KD, Kirmeyer SE, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2009. Pediatrics. 2012;129 (2):338–348. doi: 10.1542/peds.2011-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correa-Villaseñor A, McCarter R, Downing J, Ferencz C The Baltimore-Washington Infant Study Group. White-black differences in cardiovascular malformations in infancy and socioeconomic factors. Am J Epidemiol. 1991;134(4):393–402. doi: 10.1093/oxfordjournals.aje.a116101. [DOI] [PubMed] [Google Scholar]
- 25.Freeman SB, Taft LF, Dooley KJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80(3):213–217. [PubMed] [Google Scholar]
- 26.Kucik JE, Alverson CJ, Gilboa SM, Correa A. Racial/ethnic variations in the prevalence of selected major birth defects, metropolitan Atlanta, 1994–2005. Public Health Rep. 2012;127(1):52–61. doi: 10.1177/003335491212700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman RJ, Rasmussen SA, Botto LD, et al. The contribution of chromosomal abnormalities to congenital heart defects: a population-based study. Pediatr Cardiol. 2011;32(8):1147–1157. doi: 10.1007/s00246-011-0034-5. [DOI] [PubMed] [Google Scholar]
- 28.Kuppermann M, Learman LA, Gates E, et al. Beyond race or ethnicity and socioeconomic status: predictors of prenatal testing for Down syndrome. Obstet Gynecol. 2006;107(5):1087–1097. doi: 10.1097/01.AOG.0000214953.90248.db. [DOI] [PubMed] [Google Scholar]
- 29.Bishop J, Huether CA, Torfs C, Lorey F, Deddens J. Epidemiologic study of Down syndrome in a racially diverse California population, 1989–1991. Am J Epidemiol. 1997;145(2):134–147. doi: 10.1093/oxfordjournals.aje.a009084. [DOI] [PubMed] [Google Scholar]
- 30.Cragan JD, Gilboa SM. Including prenatal diagnoses in birth defects monitoring: Experience of the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res A Clin Mol Teratol. 2009;85(1):20–29. doi: 10.1002/bdra.20508. [DOI] [PubMed] [Google Scholar]
- 31.Forrester MB, Merz RD, Yoon PW. Impact of prenatal diagnosis and elective termination on the prevalence of selected birth defects in Hawaii. Am J Epidemiol. 1998;148(12):1206–1211. doi: 10.1093/oxfordjournals.aje.a009610. [DOI] [PubMed] [Google Scholar]
- 32.Krivchenia E, Huether CA, Edmonds LD, May DS, Guckenberger S. Comparative epidemiology of Down syndrome in two United States population, 1970–1989. Am J Epidemiol. 1993;137(8):815–828. doi: 10.1093/oxfordjournals.aje.a116743. [DOI] [PubMed] [Google Scholar]
- 33.Natoli JL, Ackerman DL, McDermott S, Edwards JG. Prenatal diagnosis of Down syndrome: a systematic review of termination rates (1995–2011) Prenat Diagn. 2012;32(2):142–153. doi: 10.1002/pd.2910. [DOI] [PubMed] [Google Scholar]
- 34.Britt DW, Risinger ST, Miller V, Mans MK, Krivchenia EL, Evans MI. Determinants of parental decisions after the prenatal diagnosis of down syndrome: bringing in context. Am J Med Genet. 2000;93(5):410–416. [PubMed] [Google Scholar]
- 35.Kramer RL, Jarve RK, Yaron Y, et al. Determinants of parental decisions after the prenatal diagnosis of Down syndrome. Am J Med Genet. 1998;79(3):172–174. doi: 10.1002/(sici)1096-8628(19980923)79:3<172::aid-ajmg4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Crider KS, Olney RS, Cragan JD. Trisomies 13 and 18: population prevalences, characteristics, and prenatal diagnosis, metropolitan Atlanta, 1994–2003. Am J Med Genet A. 2008;146(7):820–826. doi: 10.1002/ajmg.a.32200. [DOI] [PubMed] [Google Scholar]
- 37.Siffel C, Correa A, Cragan J, Alverson CJ. Prenatal diagnosis, pregnancy terminations and prevalence of Down syndrome in Atlanta. Birth Defects Res A Clin Mol Teratol. 2004;70(9):565–571. doi: 10.1002/bdra.20064. [DOI] [PubMed] [Google Scholar]
- 38.Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375(9715):649–656. doi: 10.1016/S0140-6736(09)61922-X. [DOI] [PubMed] [Google Scholar]
- 39.Mikkelsen M, Poulsen H, Nielsen KG. Incidence, survival, and mortality in Down syndrome in Denmark. Am J Med Genet Suppl. 1990;7:75–78. doi: 10.1002/ajmg.1320370714. [DOI] [PubMed] [Google Scholar]
- 40.Leoncini E, Botto LD, Cocchi G, et al. How valid are the rates of Down syndrome internationally? Findings from the International Clearinghouse for Birth Defects Surveillance and Research. Am J Med Genet A. 2010;152A(7):1670–1680. doi: 10.1002/ajmg.a.33493. [DOI] [PubMed] [Google Scholar]