Abstract
In the central nervous system, microvessel–neuron interactions appear highly coordinated. The rapid simultaneous responses of the microvasculature, neurons, and glia to focal ischemia in experimental ischemic stroke suggest that these responses could be viewed in a unitary fashion, rather than as individual components. The “neurovascular unit” consists of microvessels (endothelial cells–basal lamina matrix–astrocyte end-feet [and pericytes]), astrocytes, neurons and their axons, and other supporting cells that are likely to modulate the function of the “unit.” Each cell component generates an inflammatory response to ischemia. Matrix metalloproteinase (MMP)-9 was first associated with hemorrhagic transformation following focal ischemia in an experimental model. A series of studies of ischemic stroke patients also suggests a relationship between MMP-9 levels and several consequences of ischemic injury, including hemorrhagic transformation. Recent experimental work suggests specific cell sources for MMP-9 generation and for matrix proteases from four distinct families that could impact neurovascular unit integrity.
Keywords: matrix metalloproteinases, ischemic stroke, inflammation, matrix proteins, neurovascular unit
Introduction
Focal cerebral ischemia, thrombosis, and inflammation are intimately related. Thrombotic occlusion of a brain-supplying artery leads to focal regions of ischemia, which initiate both cellular inflammation and innate inflammatory processes. Specific responses of cerebral microvessels to focal ischemia include (i) loss of the permeability barrier that occurs rapidly, (ii) degradation of matrix components of the basal lamina, and (iii) inflammatory cell adhesion to endothelial cell leukocyte adhesion receptors in preparation for transmigration into the neuropil, with obstruction of capillaries and post-capillary venules.1–4 The cerebral microvascular endothelium, bound to the underlying basal lamina matrix (extracellular matrix, ECM), provides the interface for activation and transit of inflammatory cells.
Vascular matrix responses to focal cerebral ischemia
This interface consists of endothelial cells, astrocytes and their end-feet, and the subtending basal lamina matrix layer in capillaries that separates the two cell compartments. In the larger vessels, a myointima is encased in the basal lamina. Pericytes are also encased within the basal lamina ECM. Microvessels closer to the brain surface may display the Virchow–Robin space between the glia limitans and the myointima, which fuses as the vessels dive deep into the brain to ultimately form the capillary branches.
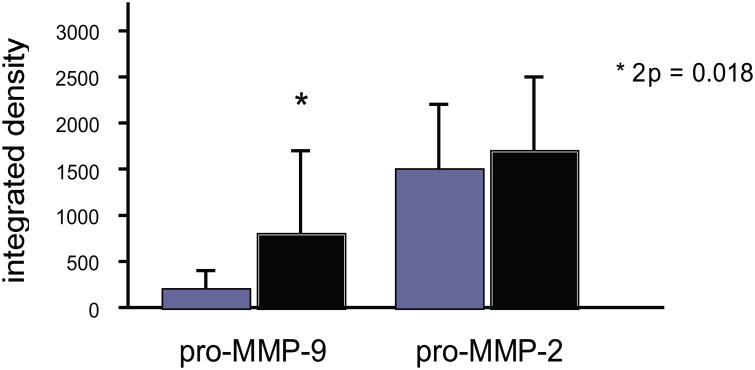
During focal cerebral ischemia, components of the microvessel basal lamina disappear. This process begins within hours of ischemia onset and is accompanied by the appearance of one or both gelatinases (depending upon the model system studied). In the nonhuman primate, for instance, pro-MMP-2 is up-regulated, along with urokinase (u-PA), cathepsin L, and heparanase within 1–2 h following the onset of focal ischemia.2,5–7 pro-MMP-9 appears in association with hemorrhagic transformation (Fig. 1). A number of laboratories have studied the appearance of both gelatinase forms in the setting of intracerebral hemorrhage, as well as their activating proteases and substrates.
Figure 1.
Relative tissue contents of pro-MMP-2 and pro-MMP-9 following middle cerebral artery occlusion (MCA:O) in the nonhuman primate striatum and their association with hemorrhagic transformation in situ. Significantly increased pro-MMP-9 production is seen in samples with hemorrhage (solid bars), but not in samples without hemorrhage (hatched bars). No correlation was observed for pro-MMP-2 (n = 27 subjects).2
Ischemic stroke and (pro-)MMP-9
Montaner et al. described the relationship between MMP-9 antigen expression in peripheral blood and hemorrhagic transformations.8–14 Correlations with intracerebral hemorrhage, ischemic cerebral hemorrhage, and edema were also described. Those studies have suggested that MMP-9 is associated with cerebral injury of all types. However, an explanation for the appearance of MMP-9 in all of these settings, and its specific role(s), has not been provided.
Ischemia and the gelatinases
Curiously, pro-MMP-9 is associated with focal ischemia in rodents, but only hemorrhagic transformation in primates.2,15 Several reports have associated MMP-9 with perivascular astrocytes during ischemia or hemorrhage in the rodent. The finding of MMP-8 and MMP-9 in polymorphonuclear (PMN) leukocytes suggests that the involvement of inflammatory cells during evolving brain ischemia could provide another source of this gelatinase. This would fit the interrelationship of thrombosis, ischemia, and inflammation. However, to date, the exact contribution of MMP-9 from this source, if applicable, has not been worked out.
Biochemical interrelationships among matrix proteases
Activation of the latent gelatinases pro-MMP-2 and pro-MMP-9 is related. Expression of MT-1 MMP and MT3-MMP (activators of pro-MMP-2) and u-PA (an activator of plasminogen) are generated swiftly after the onset of ischemia (Fig. 2). Plas-min and MMP-2 can convert pro-MMP-9 to active MMP-9. Although both gelatinases have been found in tissues and plasma in animal models of focal ischemia and hemorrhage, the amount of the active forms have been variable and inconsistent. Nonetheless, both gelatinases are known to proteolyze collagen type IV, fibronectin, aggrecan, elastin, and vitronectin. Plasmin and MMP-2 can cleave the laminins, and plasmin can also degrade collagen type IV, fibronectin, and myelin basic protein. Heo et al. have shown in electronmicrographic studies in the Sprague–Dawley the loss of electron density of the basal lamina in cerebral microves-sels in the ischemic territory following focal ischemia and that this loss relates to the appearance of pro-MMP-9.16,17
Figure 2.
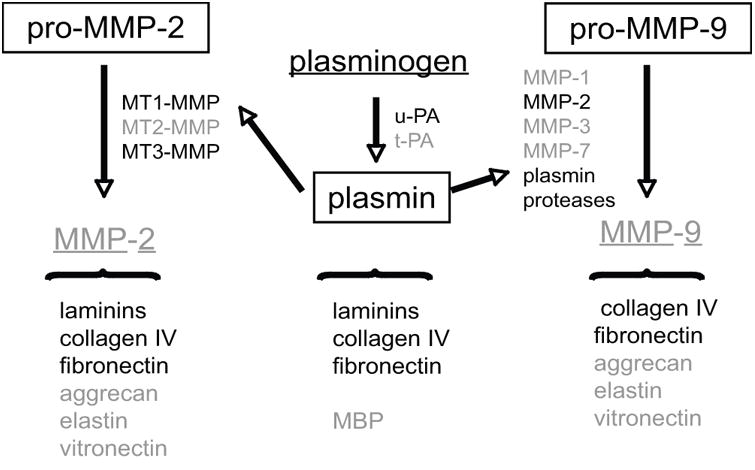
Generation of gelatinase activities and related matrix proteases following middle cerebral artery (MCA) occlusion. Proteases and ligands in black have been documented in the striatum of the nonhuman primate following MCA occlusion, while those in gray have not been directly observed or do not change in content (e.g., t-PA). pro-MMP-2 and pro-MMP-9 represent the latent inactive forms observed. Those proteases in boxes have been quantified. The ligands (black) are associated with the cerebral microvasculature. MMP, matrix metalloproteinase; MT-MMP, membrane-type MMP; u-PA, urokinase plasminogen activator; t-PA, tissue-type plasminogen activator; MBP, myelin basic protein.2,5–7
Hypothesis
These findings suggest the hypothesis that the integrity of the neurovascular unit, in particular the microvessel basal lamina and neurons, can be affected by proteases of all four families (select MMPs, serine proteases, select cysteine proteases, and heparanase). These proteases are expressed in association with both microvascular and neuronal structures. The sources of these proteases have not entirely been worked out. Furthermore, the activation of the latent forms of matrix metalloproteinases has been speculated upon, but the sources of the activators have not been described. Work here suggests that the glial compartment, astrocytes, and microglia, may be contributors to vascular matrix degradation.
Experimental Approach
A ready target for investigation is the appearance of pro-MMP-9 in relationship to ischemia-related hemorrhagic transformation. Heo et al. were the first to demonstrate a relation between pro-MMP-9 generation and hemorrhagic transformation of the evolving infarction (Fig. 1).2 The cause(s) of (pro-)MMP-9 generation in association with hemorrhage, whether it is responsible for the hemorrhage, its substrate in the setting of hemorrhage (e.g., whether vascular or pericellular), and the precise substrates of activity have not yet been worked out. The sources and results of these presumed activities have also not been clarified.
There is need to explain all aspects of the appearance of pro-MMP-9 in the clinical settings of cerebral ischemia, its relationship to innate inflammation, and the appearance of pro-MMP-9 in model systems. A unifying hypothesis and explanation for the varied levels of pro-MMP-9 in both brain tissue and plasma, as well as the relationship to inflammation in these systems, has not appeared.
Potential cellular sources of (pro-)MMP-2 and (pro-)MMP-9
A survey of experimental work in the rodent suggests that microglial cells and/or astrocytes may provide a source of pro-MMP-9 and/or pro-MMP-2, when these cells are stimulated with pro-inflammatory cytokines.18 Each cell type of the neurovascular unit can provide an inflammatory response and can participate in innate inflammation. Of these cells, microglia, as resident inflammatory sentinels in the CNS, are of interest. Broadly, stimulation of microglia can produce gelatinases under experimental conditions. These cells undergo morphologic changes, when grown on different matrix substrates in vitro and exposed to experimental ischemia. Indeed, Mabuchi et al. have demonstrated that microglial cells are activated in the face of evolving ischemic regions when vascular permeability increases.19 Although difficult to distinguish from invading macrophages, their exact position in the ischemic region and their morphology are relevant. Those observations and the events related to increased microvascular permeability suggest that when the microglia in the ischemic region are exposed to plasma proteins, enhanced activation can occur. Support for this thesis comes from models of experimental autoimmune encephalomyelitis (EAE). These suggest further that in vitro experiments with primary microglia and astrocytes, and in experiments in small animal models, where MMP-9 levels are measured, may be compounded by the presence of added serum, or extravasated plasma.
Summary
A strategy for examining the precise origins of MMP-9 in the setting of hemorrhage during ischemia should take into account the precise environment of these cells. As a background for those studies that involve the activation of innate inflammation, the following has been noted: (1) plasma matrix protease activity can disturb astrocyte–matrix adhesion, (2) pro-MMP-9 is generated by cerebral tissue in response to exposure to hemorrhage, (3) the activation and responses of specific cells in the neuropil occurs, and (4) the glial compartment is important to observations of MMP-9 generation in the setting of hemorrhagic transformation during focal ischemia. Whether the generation of (pro-)MMP-9 is causal or a result of these injuries is an unresolved question.
Acknowledgments
This work was supported in part by grants NS 026945, NS 053716, and NS 038710 from the National Institutes of Health.
Footnotes
Conflicts of interest: The author declares no conflicts of interest.
References
- 1.del Zoppo GJ, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1284. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 2.Heo JH, et al. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Okada Y, et al. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- 4.Pitzer JE, del Zoppo GJ, Schmid-Schönbein GW. Neutrophil activation in smokers. Biorheology. 1996;33:45–58. [PubMed] [Google Scholar]
- 5.Hosomi N, et al. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32:1341–1348. doi: 10.1161/01.str.32.6.1341. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda S, et al. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang DI, et al. Activation systems for matrix metalloproteinase-2 are upregulated immediately following experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- 8.Montaner J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2667. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos M, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 10.Montaner J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 11.Abilleira S, et al. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99:65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]
- 12.Rosell A, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Sabin J, et al. Temporal profile of matrix met-alloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 14.Cuadrado E, et al. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukoc Biol. 2008;84:207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
- 15.Asahi M, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Kwon I, et al. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res. 2009;87:668–676. doi: 10.1002/jnr.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Zoppo GJ, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 19.Mabuchi T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]