Abstract
Objective
To evaluate trends in survival among children with spina bifida by race/ethnicity and possible prognostic factors in 10 regions of the United States.
Study design
A retrospective cohort study was conducted of 5165 infants with spina bifida born during 1979-2003, identified by 10 birth defects registries in the United States. Survival probabilities and adjusted hazard ratios were estimated for race/ethnicity and other characteristics using the Cox proportional hazard model.
Results
During the study period, the 1-year survival probability among infants with spina bifida showed improvements for whites (from 88% to 96%), blacks (from 79% to 88%), and Hispanics (from 88% to 93%). The impact of race/ethnicity on survival varied by birth weight, which was the strongest predictor of survival through age 8. There was little racial/ethnic variation in survival among children born of very low birth weight. Among children born of low birth weight, the increased risk of mortality to Hispanics was approximately 4-6 times that of whites. The black-white disparity was greatest among children born of normal birth weight. Congenital heart defects did not affect the risk of mortality among very low birth weight children but increased the risk of mortality 4-fold among children born of normal birth weight.
Conclusions
The survival of infants born with spina bifida has improved; however, improvements in survival varied by race/ethnicity, and blacks and Hispanics continued to have poorer survival than whites in the most recent birth cohort from 1998-2002. Further studies are warranted to elucidate possible reasons for the observed differences in survival.
Spina bifida, the most common type of neural tube defect, is defined as a protrusion of the spinal cord and/or meninges through a defect in the vertebral arches.1-5 Although the birth prevalence of spina bifida significantly decreased after the introduction of mandatory fortification of enriched grain products with folic acid in the United States,6-10 the population burden of spina bifida continues both in birth prevalence and in disparities in long-term outcomes. A birth prevalence estimate of spina bifida from 11 population-based surveillance programs in the United States was reported as 3.7 per 10 000 live births (1999-2001).11 A 1-year survival estimate of 92.1% was reported by a study of 16 US birth defect monitoring programs for 1995-2001.12 A recent long-term follow-up study of children with spina bifida in the United Kingdom estimated the survival experience at 1, 5, and 20 years of age to be 71%, 69%, and 66%, respectively.13 Furthermore, another United Kingdom study found that the 5-year survival varied considerably by severity of the lesion, with a 40% lower survival among children with open lesions compared with those with closed lesions.14
Of particular concern is an observation of a lower survival experience among non-Hispanic black children born with spina bifida than among non-Hispanic white children born with spina bifida in the metropolitan Atlanta area.15 Although the reasons for this racial/ethnic disparity in survival for children with spina bifida are unknown, this observation raises questions as to whether this finding might be evident in a national sample of children with spina bifida. As timely access to quality health care is important in reducing morbidity and mortality associated with spina bifida, it becomes important from a public health perspective to identify high-risk subpopulations of children with spina bifida, for whom targeted interventions may be made available to prevent complications and thereby improve their survival. In this study, we evaluated the long-term trend in survival among infants born with spina bifida by race/ethnicity and investigated maternal and infant characteristics associated with survival experience using data from 10 population-based surveillance programs in the United States. We also examined variations in survival probability by race/ethnicity after adjusting for other factors.
Methods
A total of 5165 infants born with spina bifida were identified through 10 population-based birth defect monitoring programs located in the following regions: Arkansas (AR) birth cohort from 1993 to 2002; Georgia (GA) births in 5 central Atlanta counties from 1979 to 2003; California (CA) births in 11 counties from 1983 to 2002; Colorado (CO) births from 1989 to 2003; Iowa (IA) births from 1983 to 2003; North Carolina (NC) births from 1989 to 2003; New York (NY) births (excluding New York City) from 1983 to 2003; Oklahoma (OK) births from 1994 to 2003; Texas (TX) births from 1996 to 2003; and Utah births (UT) from 1994-2003 (Table I; available at www.jpeds.com). We included liveborn infants with spina bifida (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 741.0 and 741.9) but excluded those with anencephaly (ICD-9-CM code 740.0), as well as those coded as possible/probable spina bifida cases based on the British Paediatric Association (BPA) codes modified by the Centers for Disease Control and Prevention. A previous report estimated the prevalence among older ages using a subgroup (n = 3390) of this study population that included those alive in 2002.16
Table I. Cohorts of children with spina bifida and 1-year survival probability from 10 US regions for various years from 1979-2003.
| Region | Sample size | Birth years | Follow-up years | Length of follow-up, y | Person-years | Overall 1-year survival probability |
|---|---|---|---|---|---|---|
| AR | 161 | 1993-2002 | 1993-2003 | 11 | 879 | 84.9 |
| GA | 332 | 1979-2003 | 1979-2004 | 26 | 4000 | 79.3 |
| CA | 1321 | 1983-2002 | 1983-2002 | 20 | 10 076 | 85.1 |
| CO | 233 | 1989-2003 | 1989-2004 | 16 | 1738 | 86.4 |
| IA | 361 | 1983-2003 | 1983-2004 | 22 | 3897 | 84.8 |
| NC | 684 | 1989-1993, 1995-2003 | 1989-2004 | 16 | 5031 | 86.5 |
| NY | 985 | 1983-2003 | 1983-2004 | 22 | 11 423 | 83.4 |
| OK | 207 | 1994-2003 | 1994-2004 | 11 | 1200 | 90.3 |
| TX | 747 | 1996-2003 | 1996-2004 | 9 | 3212 | 90.2 |
| UT | 134 | 1994-2003 | 1994-2004 | 11 | 719 | 87.7 |
Deaths among affected infants were ascertained by linking state vital records, medical records, and the National Death Index, and deaths were ascertained only from medical records and the National Death Index for 1998 data in CA. Patients without death records were considered alive at the end of the state’s follow-up period and treated as censored in the survival analysis. Details about the regional surveillance programs have been published.17
Long-Term Trends in Survival Probability of Infants with Spina Bifida
To examine the temporal trends in survival probabilities over the 20-year study period, we compared survival probabilities to 1 year for each birth cohort (1983-1987, 1988-1992, 1993-1997, 1998-2003) overall and by race/ethnicity (non-Hispanic whites [referred to as whites], non-Hispanic blacks [referred to as blacks], Hispanics, and other). Trends in 1-year mortality were examined using data from 4 regions (GA, CA, IA, and NY) with at least 20 years of long-term follow-up. We calculated P values to show the significant increasing trends in 1-year survival over 4 birth cohorts.
Prognostic Factors of Survival Probabilities
Maternal and infant characteristics were obtained from medical records and birth certificates. Maternal characteristics considered were maternal race/ethnicity (white, black, Hispanic, other) and maternal age (<35 years vs ≥35 years). Infant characteristics included sex (male vs female), birth weight (<1500 g, 1500-2499 g, ≥2500 g), plurality (multiple vs singleton), presence of major heart defects (yes vs no), and spina bifida lesion level (cervicothoracic vs lumbosacral). We classified infants as having a major congenital heart defect using an algorithm we developed based on ICD-9/BPA classification codes. With the exception of NC, where only 4-digit ICD-9 codes were used, all regions used the most detailed ICD-9-CM or modified BPA codes. Codes for normal physiological findings in newborns or premature infants (eg, patent foramen ovale, patent ductus arteriosus), minor conditions such as tricuspid insufficiency, or unconfirmed cardiac defects were not considered structural heart defects. For heart defect codes that lacked specificity or may have included both major and minor cardiac defects, an expert in pediatric cardiology assisted in the decision for inclusion in the major heart defect group. The distribution of each characteristic by region is presented in Table II (available at www.jpeds.com).
Table II.
Distributions of children with spina bifida in 10 US regions* by maternal and infant characteristics, 1979-2003
| Characteristics | AR (n = 161) | GA (n = 332) | CA (n = 1321) | CO (n = 233) | IA (n = 361) | NC (n = 684) | NY (n = 985) | OK (n = 207) | TX (n = 747) | UT (n = 134) | Total (N = 5165) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother’s race, n (%) | |||||||||||
| White | 128 (79.5) | 212 (63.9) | 321 (24.3) | 154 (66.1) | 345 (95.6) | 457 (66.8) | 744 (75.5) | 141 (68.1) | 235 (31.5) | 110 (82.1) | 2847 (55.1) |
| Black | 24 (14.9) | 91 (27.4) | 59 (4.5) | 4 (1.7) | 5 (1.4) | 140 (20.5) | 70 (7.1) | 11 (5.3) | 59 (7.9) | 0 (0) | 463 (9.0) |
| Hispanic | 8 (5.0) | 25 (7.5) | 860 (65.1) | 70 (30.0) | 10 (2.8) | 68 (9.9) | 69 (7.0) | 27 (13.0) | 445 (59.6) | 19 (14.2) | 1601 (31.0) |
| Other | 1 (0.6) | 3 (0.9) | 78 (5.9) | 5 (2.1) | 1 (0.3) | 19 (2.8) | 13 (1.3) | 28 (13.5) | 6 (0.8) | 5 (3.7) | 159 (3.1) |
| Missing | 0 (0) | 1 (0.3) | 3 (0.2) | 0 (0) | 0 (0) | 0 (0) | 89 (9.0) | 0 (0) | 2 (0.3) | 0 (0) | 95 (1.8) |
| Maternal age, y | |||||||||||
| <35 | 146 (90.7) | 294 (88.6) | 1176 (89.0) | 211 (90.6) | 332 (92.0) | 621 (90.8) | 885 (89.8) | 192 (92.8) | 671 (89.8) | 117 (87.3) | 4645 (90.0) |
| ≥35 | 15 (9.3) | 38 (11.4) | 143 (10.8) | 22 (9.4) | 29 (8.0) | 63 (9.2) | 100 (10.2) | 15 (7.2) | 76 (10.2) | 17 (12.7) | 518 (10.0) |
| Missing | 0 (0) | 0 (0) | 2 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0.0) |
| Maternal education, y | |||||||||||
| ≥12 | 123 (76.4) | 60 (18.1) | 530 (40.1) | 65 (27.9) | 299 (82.8) | 481 (70.3) | 766 (77.8) | 119 (57.5) | 429 (57.4) | 109 (81.3) | 2916 (56.5) |
| <12 | 35 (21.7) | 20 (6.0) | 572 (43.3) | 159 (68.2) | 54 (15.0) | 201 (29.4) | 187 (19.0) | 47 (22.7) | 294 (39.4) | 22 (16.4) | 1656 (32.1) |
| Missing | 3 (1.9) | 252 (75.9) | 219 (16.6) | 9 (3.9) | 8 (2.2) | 2 (0.3) | 32 (3.2) | 41 (19.8) | 24 (3.2) | 3 (2.2) | 593 (11.5) |
| Plurality | |||||||||||
| Singleton | 157 (97.5) | 328 (98.8) | 1278 (96.7) | 227 (97.4) | 347 (96.1) | 659 (96.3) | 946 (96.0) | 198 (95.7) | 721 (96.5) | 126 (94.0) | 4987 (96.6) |
| Multiple | 4 (2.5) | 4 (1.2) | 43 (3.3) | 6 (2.6) | 14 (3.9) | 25 (3.7) | 39 (4.0) | 7 (3.4) | 21 (2.8) | 8 (6.0) | 171 (3.3) |
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.0) | 5 (0.7) | 0 (0) | 7 (0.1) |
| Birth weight, g | |||||||||||
| ≥2500 | 132 (82.0) | 274 (82.5) | 1119 (84.7) | 191 (82.0) | 308 (85.3) | 532 (77.8) | 811 (82.3) | 176 (85.0) | 625 (83.7) | 100 (74.6) | 4268 (82.6) |
| <2500 | 28 (17.4) | 58 (17.5) | 199 (15.1) | 42 (18.0) | 53 (14.7) | 151 (22.1) | 159 (16.1) | 31 (15.0) | 121 (16.2) | 33 (24.6) | 875 (16.9) |
| Missing | 1 (0.6) | 0 (0) | 3 (0.2) | 0 (0) | 0 (0) | 1 (0.1) | 15 (1.5) | 0 (0) | 1 (0.1) | 1 (0.7) | 22 (0.4) |
| Infant sex, n (%) | |||||||||||
| Female | 80 (49.7) | 158 (47.6) | 700 (53.0) | 127 (54.5) | 181 (50.1) | 354 (51.8) | 533 (54.1) | 112 (54.1) | 375 (50.2) | 65 (48.5) | 2685 (52.0) |
| Male | 80 (49.7) | 173 (52.1) | 621 (47.0) | 105 (45.1) | 180 (49.9) | 330 (48.2) | 452 (45.9) | 95 (45.9) | 371 (49.7) | 69 (51.5) | 2476 (47.9) |
| Missing | 1 (0.6) | 1 (0.3) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 4 (0.1) |
| Presence of major heart defects, n (%) | |||||||||||
| No | 145 (90.1) | 323 (97.3) | 1243 (94.1) | 222 (95.3) | 346 (95.8) | 632 (92.4) | 946 (96.0) | 195 (94.2) | 691 (92.5) | 117 (87.3) | 4860 (94.1) |
| Yes | 16 (9.9) | 9 (2.7) | 78 (5.9) | 11 (4.7) | 15 (4.2) | 52 (7.6) | 39 (4.0) | 12 (5.8) | 56 (7.5) | 17 (12.7) | 305 (5.9) |
| Lesion level† | |||||||||||
| Cervicothoracic | 21 (13.0) | 57 (17.2) | 193 (14.6) | 5 (2.1) | 27 (7.5) | 37 (5.4) | 81 (8.2) | 17 (8.2) | 69 (9.2) | 17 (12.7) | 524 (10.1) |
| Lumbosacral | 45 (28.0) | 206 (62.0) | 942 (71.3) | 65 (27.9) | 177 (49.0) | 206 (30.1) | 210 (21.3) | 41 (19.8) | 539 (72.2) | 109 (81.3) | 2540 (49.2) |
| Missing | 95 (59.0) | 69 (20.8) | 186 (14.1) | 163 (70.0) | 157 (43.5) | 441 (64.5) | 694 (70.5) | 149 (72.0) | 139 (18.6) | 8 (6.0) | 2101 (40.7) |
AR, GA (5 metropolitan Atlanta counties), CA (11 counties), CO, IA, NC, NY (New York City excluded), OK, TX, and UT.
The 40.7% lesion level information is missing during this period.
One-Year Survival Probabilities for Infants with Spina Bifida by Selected Characteristics
For children born during 1997 to 2003 (n = 2259), Kaplan-Meier 1-year survival probabilities were estimated and Greenwood’s method was used to calculate the variance and their 95% CIs.18 The log-rank test was used to determine whether 1-year survival functions were significantly different among different levels of maternal and infant characteristics.19 Adjusted hazard ratios were estimated using Cox proportional hazard models, stratified by survival time (1 month, 1 year, 5 years, and 8 years, which is the maximum years of follow-up for the limited cohort).20 The assumption of proportionality was checked by plotting the estimated log-cumulative hazard versus the log of survival time to examine if they were parallel for different categories of the risk factors. Possible time-dependent trends were also tested to check the assumption of proportionality. Possible interactions between the significant unadjusted risk factors were also examined. Computations were performed using SAS-PC (version 9.13; SAS Institute, Cary, North Carolina).
Results
The cohorts of children with spina bifida from the 10 regions varied in size, birth years, follow-up years, and length of follow-up, with some data available representing birth years from 1979 to 2003 (Table I). The maternal and child characteristics of each regional cohort are shown in Table II. Although maternal white was the predominant racial/ethnic group for all regions combined, the relative proportions of blacks and Hispanics varied among regions. Infants of Hispanic mothers comprised >50% of the spina bifida cohorts in CA and TX, and infants of black mothers comprised >20% of the spina bifida cohorts in GA and NC. The proportion of mothers 35 years or older was similar across regions. For maternal education, there was a fair amount of variation in the proportion of children with spina bifida, with missing information for some regions (ie, GA and CA). There were no major regional differences in spina bifida cohorts with respect to sex, maternal age, or plurality. There was some variation in the proportion of children with spina bifida who also had congenital heart defects, ranging from 2.7% in GA to 12.7% in UT. However, this variation was not statistically significant. For lesion level, 41% of children with spina bifida had missing information.
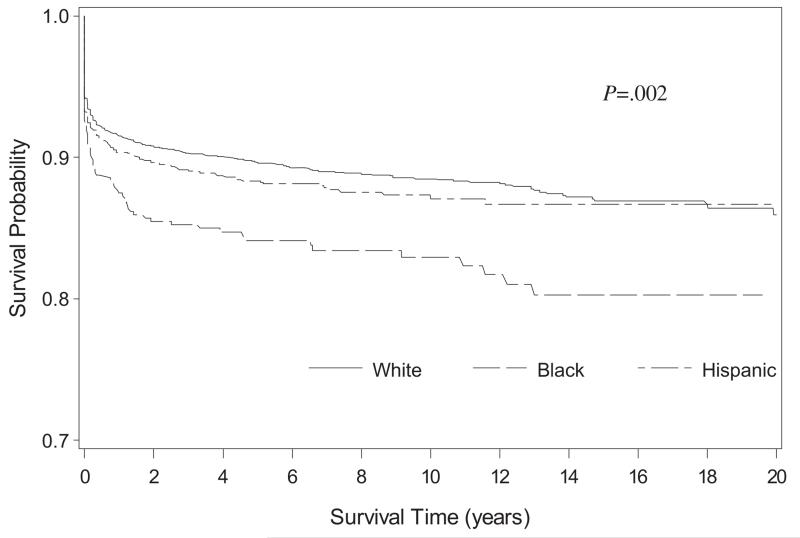
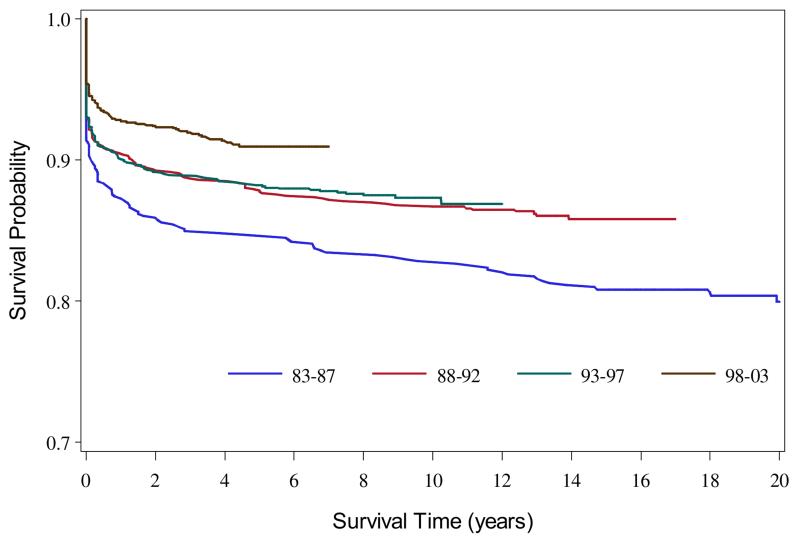
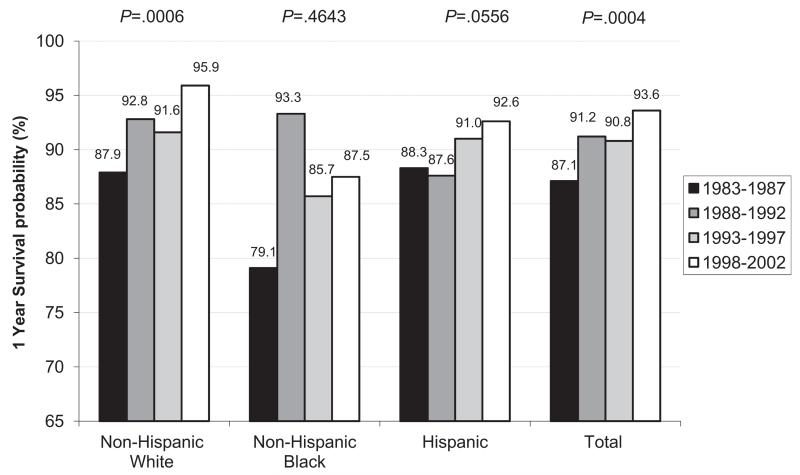
The overall survival estimates for 1983-2003 to 1, 5, and 20 years were 90.8%, 88.7%, and 85.2%, respectively, and differed by race/ethnicity (P = .002) (Figure 1). The overall survival improved among more recent birth cohorts (Figure 2; available at www.jpeds.com). In the 4 regions with at least 20 years of data (GA, CA, IA, NY, n = 2699), the 1-year survival probability during 1983-2002 showed an improving trend from 87.1% to 93.6% that was statistically significant (P = .0004) (Figure 3). This improving trend was evident for whites (from 87.9% to 95.9%) (P = .006) and Hispanics (from 88.3% to 92.6%) (P = .0556). The survival among blacks improved from 79.1% to 87.5%, a 10.6% increase, which was the largest increase among the racial/ethnic groups; however, the statistical test for trend was not significant (P = .4643) because of an unusually high spike in the survival probability for the second earliest birth cohort. Despite the sharp increase in survival, the survival probability for blacks in the most recent birth cohort remained lower than those for other groups.
Figure 1.
Kaplan-Meier survival curves for children born with spina bifida by race/ethnicity, 1983-2003.
Figure 2.
Kaplan-Meier survival curves for children born with spina bifida by birth cohort, 1983-2003.
Figure 3.
Trends in survival probability to 1 year by race/ethnicity (GA [5 metropolitan Atlanta counties], CA [11 counties], IA, NY [New York City excluded]: non-Hispanic white 1543, non-Hispanic black 205, Hispanic 951) and birth cohort in 4 regions during 1983-2002. P values for increasing trend based on an unadjusted proportional hazard model.
Characteristics found to be significantly associated with decreased 1-year survival probability for children with spina bifida during 1997-2003 in the 10 US regions were maternal black race, multiple births, low birth weight, presence of major heart defects, and cervicothoracic lesions (Table III). Because of the high proportion of missing data for maternal education and lesion level, these variables were not considered in subsequent analyses.
Table III. One-year survival probabilities for infant with spina bifida by selected maternal and infant characteristics in 10 US regions* during 1997-2003.
| Characteristics | No. of births of infants with spina bifida | No. of deaths (%) | Survival probabilities (%) with 95% CIs | Log-rank test, P |
|---|---|---|---|---|
| Total | 2259 | 162 (7.2%) | 92.8 (91.7-93.8) | |
| Mother’s race/ethnicity, n | ||||
| White | 1110 | 65 (5.9%) | 94.1 (92.6-95.4) | .0103‡ |
| Black | 205 | 25 (12.2%) | 87.8 (82.5-91.6) | |
| Hispanic | 876 | 68 (7.8%) | 92.2 (90.3-93.8) | |
| Other | 64 | 4 (6.3%) | 93.8 (84.2-97.6) | |
| Missing | 4 | 0 (0%) | ||
| Maternal education, y | ||||
| ≥12 | 1474 | 98 (6.6%) | 93.4 (92.0-94.5) | .4083 |
| <12 | 709 | 54 (7.6%) | 92.4 (90.2-94.1) | |
| Missing | 76 | 10 (13.2%) | ||
| Maternal age, y | ||||
| <35 | 1978 | 141 (7.1%) | 92.9 (91.6-93.9) | .8347 |
| ≥35 | 280 | 21 (7.5%) | 92.5 (88.7-95.0) | |
| Missing | 1 | 0 (0%) | ||
| Plurality, n | ||||
| Singleton | 2172 | 148 (6.8%) | 93.1 (92.0-94.2) | .0002‡ |
| Multiple | 80 | 14 (17.5%) | 82.5 (72.2-89.2) | |
| Missing | 7 | 0 (0%) | ||
| Infant sex, n | ||||
| Male | 1118 | 68 (6.1%) | 91.8 (90.1-93.3) | .0544 |
| Female | 1140 | 93 (8.2%) | 93.9 (92.3-95.2) | |
| Missing | 1 | 1 (100%) | ||
| Birth weight, g | ||||
| <1500 | 99 | 59 (59.6%) | 40.4 (30.7-49.9) | <.0001‡ |
| 1500-2499 | 311 | 36 (11.9%) | 88.4 (84.3-91.5) | |
| ≥2500 | 1845 | 67 (3.6%) | 96.4 (95.4-97.1) | |
| Missing | 4 | 0 (0%) | ||
| Presence of major heart defects, n | ||||
| No | 2082 | 130 (6.2%) | 93.8 (92.6-94.7) | <.0001‡ |
| Yes | 177 | 32 (18.1%) | 81.9 (75.4-86.8) | |
| Lesion level† | ||||
| Cervicothoracic | 203 | 33 (16.3%) | 83.7 (77.9-88.2) | <.0001‡ |
| Lumbosacral | 1471 | 77 (5.2%) | 94.8 (93.4-95.8) | |
| Missing | 585 | 52 (8.9%) | ||
AR, GA (5 metropolitan Atlanta counties), CA (11 counties), CO, IA, NC, NY (New York City excluded), OK, TX, and UT.
The 25.9% of lesion level information is missing during this period.
Statistical difference in survival probabilities.
In multivariable models, an increased risk of mortality was evident for black and Hispanic children with spina bifida compared with white children with spina bifida from 1 month of age and extending to 5 and 8 years of age, after adjustment for other variables (Table IV). The estimated hazard for maternal race/ethnicity and the presence of a major heart defect differed significantly across levels of birth weight; therefore, hazard ratios are stratified by birth weight. Because no deaths occurred between the ages of 5 and 8 years, hazard ratios are presented for age 8 only. Maternal race/ethnicity was not associated with an increased hazard for death among children born of very low birth weight but was associated with an increased hazard among children born of low and normal birth weight. The presence of a major heart defect was associated with a decreased risk among children born of very low birth weight and showed the highest risk for death among children born of normal birth weight, for whom there was an approximately 4-fold increase in mortality risk. The improved survival for children born with spina bifida in the more recent birth cohort became more apparent in children born of low and normal birth weight.
Table IV. The aHRs for race/ethnicity, presence of major heart defects, and low birth weight among children with spina bifida (N = 2259) in 10 US regions* by age at follow-up, 1997-2003.
| Very low birth weight (<1500 g) |
Low birth weight (1500-2499 g) |
Normal birth weight (≥2500 g) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Prognostic factor | 1 mo old | 1 y old | 8 y old† | 1 mo old | 1 y old | 8 y old† | 1 mo | 1 y old | 8 y old† |
| Race/ethnicity | |||||||||
| Blacks | 1.1 (0.5-2.2) | 1.1 (0.5-2.0) | 1.0 (0.5-1.9) | 1.5 (0.3-7.8) | 1.4 (0.4-5.4) | 2.0 (0.7-5.8) | 1.8 (0.5-6.5) | 2.1 (1.0-4.5) | 1.9 (1.0-3.7) |
| Hispanics | 1.1 (0.6-2.1) | 1.0 (0.5-1.9) | 0.9 (0.5-1.8) | 6.0 (2.3-16.0) | 4.2 (1.9-9.4) | 3.7 (1.8-7.8) | 1.1 (0.5-2.7) | 1.2 (0.7-2.0) | 1.3 (0.8-1.9) |
| Presence of major heart defects |
0.5 (0.2-1.4) | 0.7 (0.3-1.5) | 0.8 (.4-1.6) | 1.3 (0.5-3.3) | 2.1 (1.0-4.5) | 2.6 (1.3-5.0) | 3.9 (1.5-10.5) | 4.6 (2.6-8.0) | 3.6 (2.1-6.1) |
| Birth cohort | 1.1 (0.6-1.9) | 0.9 (0.5-1.6) | 1.0 (0.6-1.6) | 0.5 (0.3-1.1) | 0.6 (0.3-1.2) | 0.6 (0.3-1.1) | 0.9 (0.4-2.0) | 0.8 (0.5-1.2) | 0.7 (0.5-1.1) |
aHR, Adjusted hazard ratio.
Hazard ratios for blacks, Hispanics, and other; whites used as a reference group.
AR, GA (5 metropolitan Atlanta counties), CA (11 counties), CO, IA, NC, NY (New York City excluded), OK, TX, and UT.
No deaths occurred between ages 5 and 8 y, so the aHR at 5 years of age are identical to those at age 8 y.
Discussion
We found that the 1-year survival probability has improved over time for all children with spina bifida, but the magnitude of improvements varied by race/ethnicity. The factors most associated with a lower survival probability among children with spina bifida were low birth weight and the presence of major heart defects. We also found that children with spina bifida born to nonwhite mothers had a lower probability of survival compared with children born to white mothers, but this disparity was not present among children born of very low birth weight.
Although there were regional variations in survival in our study, the pooled survival probability at 1 year of age (92.8% in 10 US regions during 1997-2003) was comparable with those in previous studies. In particular, a study reported a 1-year survival probability for children with spina bifida of 92.1% during 1995-2001.12 Our findings and those of others highlight improved survival for children with spina bifida in recent decades. Advanced medical and surgical management including elective repair of spina bifida lesions in utero has been suggested as a contributing factor in the improvement of survival probability among children with spina bifida.1 It has also been suggested that folic acid fortification might have improved survival by reducing the risk of severe types of spina bifida.1,12,21-23 Our data suggest that survival did improve among cohorts born after fortification of folic acid (Figure 2), but it is unclear whether the improvement was caused in part by folic acid fortification or if the improvement was an extension of a previous trend toward improved survival. Such a trend may have occurred as a result of advances in the clinical treatment and management of congenital heart defects and conditions related to being born of low birth weight.
Our findings also found racial/ethnic disparities in the improvement of survival probabilities for children with spina bifida during 1983-2002. Survival among blacks and Hispanics with spina bifida improved over time but was consistently lower compared with that of whites. In a previous study in Atlanta, the survival probability during 1979-1994 was reported to be 67.1% for blacks and 82.8% for whites,15 although this difference was not statistically significant after adjusting for other factors. In our study, poorer survival among blacks compared with whites was statistically significant only for 1-year mortality in infants of normal birth weight, although the data suggest an increased risk of mortality at all ages for each birth weight group except for those born of very low birth weight. Similarly, Hispanics born of very low birth weight had no excess risk of mortality, but Hispanic infants of low birth weight had an increased risk up to 6 times that of whites. Future studies should examine possible modifiable factors influencing race/ethnic disparities in survival, including barriers to access to health care.
Birth weight was the strongest predictor of infant mortality even after adjusting for race, and it continued to be significantly associated with poor survival through age 8. In our study, 17% of infants with spina bifida were born with very low or low birth weight compared with 8% in the general population.24 Infants born of low birth weight in our study were 4 times more likely to die in infancy compared with very low or low birth weight infants in the general US population.25 The vulnerability of this group should prompt increased clinical vigilance, particularly during infancy, to reduce the mortality risk.
In our analysis, the presence of major heart defects had a significant impact on the survival of children with spina bifida. We did not have information on treatment for congenital heart defects or on the presence of other major noncardiac defects, so it was not possible for us to examine the impact of clinical characteristics and management on survival. Further studies are warranted to examine how these factors may impact the survival of children with spina bifida.
In the univariate analyses, there was some suggestion that the higher cervicothoracic lesions were associated with a lower survival than for lumbosacral, a finding consistent with a previous study.12 However, for nearly one-half of the children with spina bifida, information on the level of the lesion was not available. Because the level of the lesion could potentially be related to both survival and the long-term quality of life of affected children, future studies of survival could fill important knowledge gaps for clinical decision making by examining detailed survival probabilities by lesion level.
Trends in the improving survival may be attributable to improved prenatal screening and differential decision making following a prenatal diagnosis.26,27 Parents may decide more often to terminate the pregnancy if their fetus has a more severe type of spina bifida or the fetus has other major associated defects. If true, this would result in a higher number of infants with less severe type of spina bifida, which in turn could impact survival estimates. Future studies to evaluate the impact on survival should include an examination of the epidemiology of prenatally diagnosed cases and patterns of terminated pregnancies.
Our study examined racial/ethnic variations in survival using a large sample size from 10 US population-based surveillance programs that encompassed a wide geographic and racial/ethnic diversity. Ascertainment of vital status was done using comprehensive data sources to minimize the underestimation of deaths among the study participants.28 One of the limitations of our study was the regional and periodic variation of maternal and infant characteristics and variations in the diagnostic coding by surveillance system. We also lacked information on any surgeries, care management, and barriers to access to health care to examine the prognostic impact of these factors on survival.
This study identified improvements in survival among children with spina bifida over the past 2 decades, noting that some racial/ethnic disparities in survival have decreased in recent years but still persist. Given the important attendant severity of conditions and chronic complications associated with spina bifida, the improved survival of children with spina bifida raises important challenges for health care providers responsible for providing prognostic information and advice to at-risk individuals, families, and communities.
Acknowledgments
The authors would like to thank the National Birth Defects Prevention Network and the following birth defects monitoring programs that participated in the Congenital Anomaly Multistate Prevalence and Survival (CAMPS) Collaborative for their conscientious and skilled data collection efforts: AR Reproductive Health Monitoring System (Charlotte Hobbs, Bridget S. Mosley), CA Birth Defects Monitoring Program (Barbara Warmerdam, Marcia Ehinger), CO Responds to Children with Special Needs (Carol Stanton, Margaret Ruttenber), IA Registry for Congenital and Inherited Disorders (Bradley D. MacDowell, Paul Romitti), NY State Congenital Malformations Registry (Charlotte Druschel, Ying Wang), NC Birth Defects Monitoring Program (Katie Harmsen, Robert Meyer), OK Birth Defects Registry (Kay A. Pearson), TX Birth Defects Epidemiology and Surveillance Branch (Lisa K Marengo), and UT Birth Defect Network (Marcia Feldkamp, Miland Palmer). We would like to thank the Centers for Disease Control and Prevention staff, Dr Tiffany Riehle-Colarusso, Mike Atkinson, and Don Gambrell for their help in classification, data linkages, and all Metropolitan Atlanta Congenital Defects Program staff for their help in data collection and analysis and for their constructive suggestions.
Glossary
- AR
Arkansas
- BPA
British Paediatric Association
- CA
California
- CO
Colorado
- GA
Georgia
- IA
Iowa
- NC
North Carolina
- NY
New York
- OK
Oklahoma
- TX
Texas
- UT
Utah
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the views of the California Department of Public Health.
The authors declare no conflicts of interest.
Portions of this study have been presented at the 1st World Congress on Spina Bifida Research and Care, Orlando, FL, March 15-18, 2009; 12th National Birth Defects Prevention Network Annual Meeting, February 23-25, 2009, Nashville, TN; 48th Teratology Society, Monterey, CA, June 30, 2008; 21st Society for Pediatric and Perinatal Epidemiologic Research, Chicago, IL, June 23-24, 2008; and 41st Society for Epidemiologic Research, Chicago, IL, June 24-27, 2008.
References
- 1.Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet. 2004;364:1885–95. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- 2.Moore K. The developing human: clinically oriented embryology. 3rd ed. WB Saunders; Philadelphia: 1982. [Google Scholar]
- 3.Hunter A. Brain and the spina cord. Oxford University Press; New York: 1993. [Google Scholar]
- 4.Padmanabhan R. Etiology, pathogenesis and prevention of neural tube defects. Congenit Anom (Kyoto) 2006;46:55–67. doi: 10.1111/j.1741-4520.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 5.Kinsman SL, Levey E, Ruffing V, Stone J, Warren L. Beyond multidisciplinary care: a new conceptual model for spina bifida services. Eur J Pediatr Surg. 2000;10(Suppl 1):35–8. doi: 10.1055/s-2008-1072413. [DOI] [PubMed] [Google Scholar]
- 6.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–6. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 7.Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995-2002. Pediatrics. 2005;116:580–6. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson RE, Allen WP, Pai GS, Best R, Seaver LH, Dean J, et al. Decline in prevalence of neural tube defects in a high-risk region of the United States. Pediatrics. 2000;106:677–83. doi: 10.1542/peds.106.4.677. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Racial/ethnic differences in the birth prevalence of spina bifida: United States, 1995-2005. MMWR Morb Mortal Wkly Rep. 2009;57:1409–13. [PubMed] [Google Scholar]
- 10.Shin M, Besser LM, Correa A. Prevalence of spina bifida among children and adolescents in metropolitan Atlanta. Birth Defects Res A Clin Mol Teratol. 2008;82:748–54. doi: 10.1002/bdra.20530. [DOI] [PubMed] [Google Scholar]
- 11.Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Research (Part A) 2006;76:747–56. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- 12.Bol KA, Collins JS, Kirby RS. Survival of infants with neural tube defects in the presence of folic acid fortification. Pediatrics. 2006;117:803–13. doi: 10.1542/peds.2005-1364. [DOI] [PubMed] [Google Scholar]
- 13.Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375:649–56. doi: 10.1016/S0140-6736(09)61922-X. [DOI] [PubMed] [Google Scholar]
- 14.Althouse R, Wald N. Survival and handicap of infants with spina bifida. Arch Dis Child. 1980;55:845–50. doi: 10.1136/adc.55.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong LY, Paulozzi LJ. Survival of infants with spina bifida: a population study, 1979-94. Paediatr Perinat Epidemiol. 2001;15:374–8. doi: 10.1046/j.1365-3016.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- 16.Shin M, Besser LM, Siffel C, Kucik JE, Shaw GM, Lu C, et al. Prevalence of spina bifida among children and adolescents in 10 regions in the United States. Pediatrics. 2010;126:274–9. doi: 10.1542/peds.2009-2084. [DOI] [PubMed] [Google Scholar]
- 17.National Birth Defects Prevention Network State Birth Defects Surveillance Program Directory. Birth Defects Res Part A Clin Mol Teratol. 2008;82:907–61. [Google Scholar]
- 18.Greenwood M. The natural duration of cancer. Rep Public Health Med Subjects. 1926;33:1–26. [Google Scholar]
- 19.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A (Gen) 1972;135:187–207. [Google Scholar]
- 20.Cox DR, Oakes D. Analysis of survival data. Chapman & Hall; London: 1984. [Google Scholar]
- 21.Oakeshott P, Hunt GM. Long-term outcome in open spina bifida. Br J Gen Pract. 2003;53:632–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34:114–20. doi: 10.1159/000056005. [DOI] [PubMed] [Google Scholar]
- 23.Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675–6. doi: 10.1016/S0140-6736(98)00070-1. [DOI] [PubMed] [Google Scholar]
- 24.Martin JA, Hamilton BE, Sutton PD, et al. National Vital Statistics Reports. 1. Vol. 59. National Center for Health Statistics; Hyattsville (MD): 2010. Births: final data for 2008. [PubMed] [Google Scholar]
- 25.Mathews TJ, MacDorman MF. NationalVital StatisticsReports. 2. Vol. 57. National Center for Health Statistics; Hyattsville (MD): 2008. Infant mortality statistics from the 2005 periodlinked birth/infant death dataset. [PubMed] [Google Scholar]
- 26.Kucik JE, Alverson CJ, Gilboa SM, Correa A. Racial/ethnic variations in the prevalence of selected major birth defects, metropolitan Atlanta, 1994-2005. Public Health Rep. 2012;127:52–61. doi: 10.1177/003335491212700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ethen MK, Canfield MA. Impact of including elective pregnancy terminations before 20 weeks gestation on birth defect rates. Teratology. 2002;66(Suppl 1):S32–5. doi: 10.1002/tera.90008. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen SA, Wong LY, Correa A, Gambrell D, Friedman JM. Survival in infants with Down syndrome, Metropolitan Atlanta, 1979-1998. J Pediatr. 2006;148:806–12. doi: 10.1016/j.jpeds.2006.01.010. [DOI] [PubMed] [Google Scholar]