Abstract
Setting: National tuberculosis control programme, Vanuatu.
Objective: To assess tuberculosis (TB) trends, characterise sputum smear-positive patients with non-conversion at 2 months and assess their treatment outcomes.
Design: Evaluation of programme data over a 9-year period (2004–2012), comparing 2-month sputum non-converters (delayed converters) with sputum smear converters diagnosed in 2011 and 2012.
Results: Annual TB case numbers were similar over the study period, with an average TB notification rate of 58 per 100 000 population. Of 417 sputum smear-positive cases, 74 (18%) were delayed converters. Delayed converters were more likely than converters (88% vs. 79%) to have had high pre-treatment sputum smear grades (OR 2.5, 95%CI 0.97–6.45). Among delayed converters, treatment adherence was high (99% good adherence), outcomes were generally good (90% treatment success, 85% cure, 4% treatment failure) and no drug resistance was detected. Deaths were unexpectedly common among converters (11/80, 14%), with significantly more deaths in Tafea than in Shefa Province (7/58 vs. 2/80, OR 5.35, 95%CI 1.07–26.79). Tafea Province also had the greatest number of delayed converters (30/74, 40.5%) and the highest TB incidence rate.
Conclusion: Delayed sputum conversion was relatively uncommon, and was not associated with adverse outcomes or drug resistance. Regional differences require further investigation to better understand local factors that may compromise patient management.
Keywords: tuberculosis, Vanuatu, 2-month non-converters, sputum smear
Abstract
Contexte : Programme national de lutte contre la tuberculose (PNT), Vanuatu.
Objectif : Evaluer les tendances de la tuberculose (TB), caractériser les patients à crachats positifs avec une absence de conversion en 2 mois et évaluer le résultat de leur traitement.
Schéma : Evaluation des données du PNT sur une période de 9 ans (2004–2012) comparant les patients sans conversion du frottis de crachats après 2 mois (conversion retardée) avec ceux ayant eu une conversion en 2011 et 2012.
Résultats : Le nombre annuel de cas de TB est resté similaire tout au long de la période d'étude avec une moyenne de taux de notification de 58/100 000. Parmi les cas à frottis positifs, 74/417 (18%) ont eu une conversion retardée. Ces patients avaient plus souvent que les autres (88% contre 79%) des grades de frottis élevés avant leur traitement (OR 2,5 ; IC 95% 0,97–6,45). Parmi ces patients à conversion différée, l'adhésion au traitement était élevée (bonne dans 99% des cas), les résultats étaient généralement bons (90% de réussite thérapeutique, 85% de guérison, 4% d'échec thérapeutique) et aucune résistance au traitement n'a été détectée. Les décès étaient étonnamment fréquents (11/80 ; 14%) parmi les conversions et plus fréquents à Tafea qu'à Shefa (7/58 contre 2/80 ; OR 5,35 ; IC05% 1,07–26,79). La province de Tafea avait également le plus grand nombre de conversions retardées (30/74 ; 14,5%) et la plus haute incidence de TB.
Conclusion : Une conversion retardée des frottis était relativement peu fréquente et n'était pas associée à des résultats médiocres ou à une résistance aux médicaments. Les différences régionales requièrent davantage d'investigations afin de mieux comprendre les facteurs locaux susceptibles de compromettre la prise en charge des patients.
Abstract
Marco de referencia: El Programa Nacional contra la Tuberculosis (PNT) de Vanuatu.
Objetivo: Evaluar las tendencias en materia de tuberculosis (TB), caracterizar a los pacientes con baciloscopia positiva, sin conversión del esputo a los 2 meses y analizar sus desenlaces terapéuticos.
Método: Se evaluaron los datos del programa de un período de 9 años (del 2004 al 2012) y se compararon los casos sin conversión del esputo a los 2 meses (conversión tardía), con los casos que convirtieron oportunamente la baciloscopia y que se habían diagnosticado en el 2011 y el 2012.
Resultados: El número anual de casos de TB fue estable durante el período del estudio; se observó una tasa promedio de notificación de 58 por 100 000 habitantes. De los 417 casos con baciloscopia positiva, 74 presentaron una conversión tardía del esputo (18%). Fue más frecuente que estos casos presentaran una baciloscopia inicial de alto grado (88% contra 79%; OR 2,5; IC95% de 0,97 a 6,45). En el grupo de pacientes con conversión tardía, el cumplimiento terapéutico fue alto (99% de buena observancia), los desenlaces fueron en general favorables (90% de finalización del tratamiento, con 85% de curación y 4% de fracaso terapéutico) y no se observó farmacorresistencia. Las defunciones fueron sorprendentemente frecuentes en los pacientes con conversión oportuna del esputo (11/80; 14%), con una frecuencia significativamente mayor en la provincia de Tafea que en Shefa (7/58 comparados con 2/80; OR 5,35; IC95% de 1,07 a 26,79). La provincia de Tafea presentó además la tasa más alta de conversiones tardías (30/74; 40,5%) y la más alta tasa de incidencia de TB.
Conclusión: La conversión tardía del esputo fue relativamente infrecuente y no se asoció con desenlaces desfavorables ni farmacorresistencia. Las diferencias regionales exigen nuevas investigaciones que permitan mejorar el conocimiento de los factores locales que pueden perturbar el tratamiento de los pacientes.
Global implementation of the DOTS strategy has resulted in a decline in tuberculosis (TB) incidence rates.1 However, total patient numbers have not declined, and rates remain high in areas affected by human immunodeficiency virus (HIV) infection, poverty and drug-resistant TB.2 Despite the adoption of the DOTS strategy in 1999–2000, progress towards global TB control targets has been slow in the Pacific region, with 16 534 cases (165 per 100 000 population) notified in 2011 compared to 4017 (68/100 000) in 1990.1 TB remains a major public health threat in the region. The risk of Mycobacterium tuberculosis transmission is elevated in islands with a high population density, while vulnerability to developing TB is affected on one hand by malnutrition and food insecurity and on the other by rising rates of diabetes.3
There has been growing concern over the possible emergence of drug-resistant TB in the Pacific. To date, 11 Pacific Island countries and territories have reported cases of multidrug-resistant TB (MDR-TB), but no formal prevalence surveys have been conducted.1 A Pacific-wide supranational laboratory referral system was established in 2004 to provide ongoing surveillance for drug-resistant TB.4 The Pacific TB laboratory (PATLAB) network links countries to reference laboratories where M. tuberculosis confirmation, strain typing and comprehensive drug susceptibility testing (DST) can be performed.
Delayed sputum smear conversion has been identified as a risk factor for TB treatment failure and drug-resistant TB,5,6 and may indicate prolonged infectiousness. Sputum smear status is typically recorded at the end of the intensive phase of treatment (i.e., after 2 months), and patients who still have smear-positive sputum at this time are regarded as delayed converters. In Vanuatu, there has been concern that TB incidence rates are not declining as expected and that increasing numbers of sputum smear-positive TB patients fail to convert to smear-negative within 2 months of treatment. However, the frequency of sputum non-conversion at 2 months and the profile of these patients have not been established.
In the present study, we aimed to assess TB trends, compare the characteristics of 2-month non-converters (delayed converters) with those of converters and assess the potential contribution of drug-resistant TB.
METHODS
Study design
This retrospective cohort study included all sputum smear-positive TB cases who remained sputum smear-positive after 2 months of anti-tuberculosis treatment over a 9-year period (2004–2012). For logistical reasons, 2-month sputum non-converters (delayed converters) were compared with those with smear conversion at 2 months (converters) diagnosed during 2011–2012. Results of specimens sent to the Supranational Reference Laboratory (SRL; Queensland Mycobacterium Reference Laboratory) in Brisbane, QLD, Australia, were reviewed to assess for drug-resistant TB.
Study setting
The study was conducted in the Republic of Vanuatu, a Pacific Island nation consisting of 83 islands organised into six administrative districts (provinces). The total population is estimated at 234 023.7 Most health care services, including TB care, which is provided by the National TB Control Programme (NTP), are government-sponsored. The DOTS strategy was first implemented at two main referral hospitals in 1999, and was gradually extended to cover the entire country by 2003.8 Sputum smear microscopy and anti-tuberculosis treatment are provided free of charge. Despite routine human immunodeficiency virus (HIV) testing of TB patients since 2010, TB-HIV co-infection has not been recorded. All sputum smear-positive TB patients are routinely hospitalised at one of five hospitals (Vila Central Hospital, Lenakel Hospital, Norsup Hospital, Lolowai Hospital and the Northern Provincial Hospital) during the initial intensive phase of treatment. Standard anti-tuberculosis treatment consists of four drugs during the 2-month intensive phase, using fixed-dose combination tablets for rifampicin (RMP) and isoniazid (INH) and separate formulations of pyrazinamide and ethambutol (EMB), followed by a 4-month continuation phase using fixed-dose combination tablets of RMP and INH.8,9 Before hospital discharge, appropriate treatment partners are identified and educated on how to provide directly observed treatment (DOT). In most cases, these are local nurses, but where a nurse is not available village health workers or relatives perform the task. Local TB officers (zone nurses) monitor DOT provision throughout the continuation phase of treatment.
Patients who are diagnosed with sputum smear-positive pulmonary TB are retested after 2 months of anti-tuberculosis treatment (i.e., before hospital discharge) to assess whether smear conversion has occurred; repeat testing is performed after 5–6 months to confirm cure. Patients who remain sputum smear-positive at 2 months remain hospitalised and receive an additional month of intensive phase treatment. If the sputum smear is still positive at the end of 3 months, a specimen is submitted to the SRL, and the patient moves to the continuation phase of treatment, pending results. Routine screening for diabetes (fasting blood glucose) and HIV infection have been encouraged since 2010; before this time, it was performed at the discretion of the treating physician.
Definitions
Patients who were still smear positive after 2 months of anti-tuberculosis treatment were defined as ‘delayed converters’, while those whose 2-month smear was negative were defined as ‘converters’. Treatment success was defined as cure (two negative sputum smears, one within the final month of treatment) or treatment completion (more than 80% of treatment doses received, but no final smear performed and no adverse outcome recorded). Adverse outcome was defined as death, default (interruption of anti-tuberculosis treatment for ⩾2 consecutive months) or failure (persistent positive sputum smear at end of treatment). Treatment adherence was defined as complete if 100% of doses were supervised, good if more than 80% of doses were supervised and poor if fewer than 80% of the doses were supervised.
Data collection and analysis
Data were collected during routine NTP monitoring and evaluation visits to each of the provinces during 2012 and 2013. Data sources included provincial TB registers, patient treatment cards and laboratory registers. Individual patient notes were reviewed to supplement missing data or resolve discrepancies. De-identified data were collected on a standard proforma and entered into an Excel spreadsheet (Microsoft, Red-mond, WA, USA) by provincial TB managers. Data were personally reviewed for completeness and accuracy by the principal investigator. Data were analysed using EpiData version 2.2.2.178 (EpiData Association, Odense, Denmark), with the level of significance set at 5%. For comparative analysis, categorical variables were compared using the χ2 test, with odds ratios (ORs) and 95% confidence intervals (CIs) where appropriate.
Ethics
Formal ethics approval was obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France, with local approval and support provided by the Vanuatu Ministry of Health (study number 89/12).
RESULTS
The Figure reflects TB trends over the study period for different patient categories. The total number of TB cases diagnosed per year showed little variation (118 patients in 2005 and 114 in 2012), and more than 50% of cases were diagnosed with pulmonary TB. The proportion of all TB patients with sputum smear-positive TB was 40%. The average TB notification rate over the period was 52/100 000, using unadjusted 2009 census data as the denominator.
FIGURE.
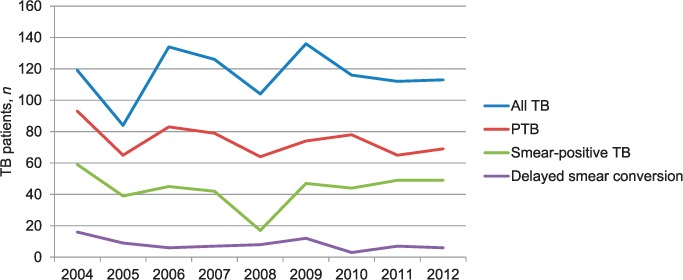
TB notification trends in Vanuatu, 2004–2012. A total of 1040 TB patients were notified during the study period. Delayed smear conversion refers to being sputum smear-positive after 2 months of anti-tuberculosis treatment. TB = tuberculosis; PTB = pulmonary TB.
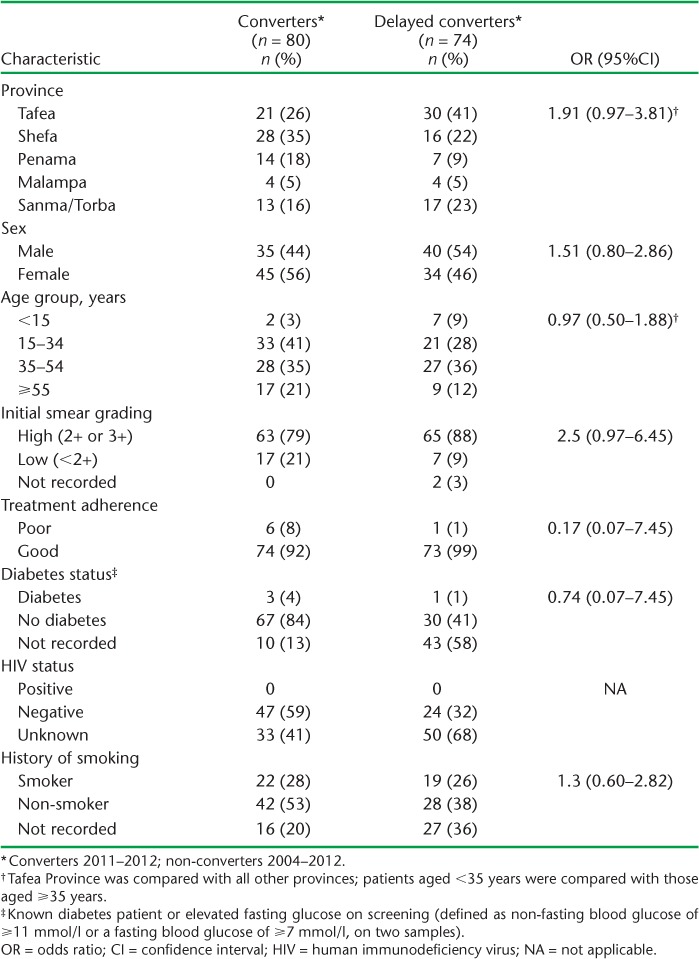
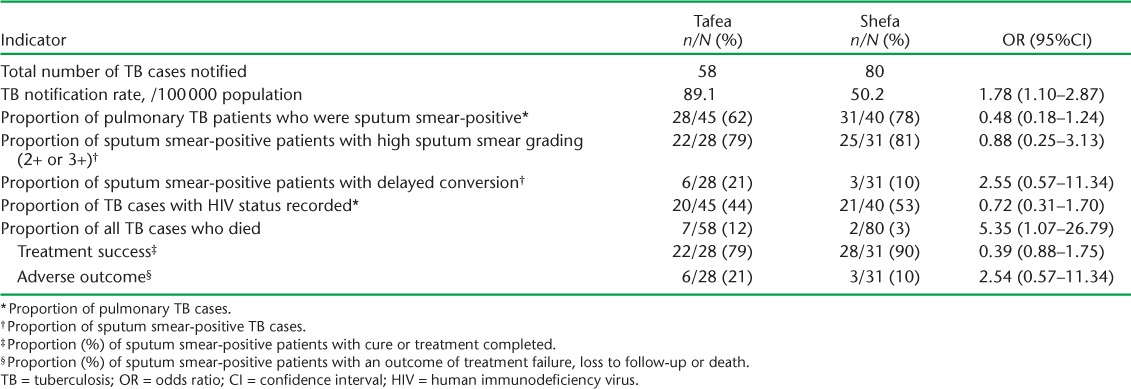
Of 417 sputum smear-positive cases, 74 (18%) failed to smear convert after 2 months of anti-tuberculosis treatment (2004–2012). Table 1 shows the characteristics of these delayed converters compared to converters (2011–2012). Tafea Province had the highest number of delayed converters (n = 30, 41%). Overall sputum smear grading was high, and delayed converters were more likely than converters to have had higher sputum smear grades (2+ or 3+) at presentation (88% vs. 79%, OR 2.5, 95%CI 0.97–6.45). There were no differences in sex or in the age groups affected. Few sputum smear-positive TB children (aged <15 years) were detected, although seven experienced delayed sputum smear conversion. Treatment adherence was good in both groups. Blood glucose and HIV testing were performed in a minority of patients; 41% of converters diagnosed in 2011–2012 failed to undergo HIV testing, although it should have been done routinely at that time.
TABLE 1.
Characteristics of sputum smear-positive tuberculosis patients, comparing 2-month non-converters (delayed converters) with converters in Vanuatu
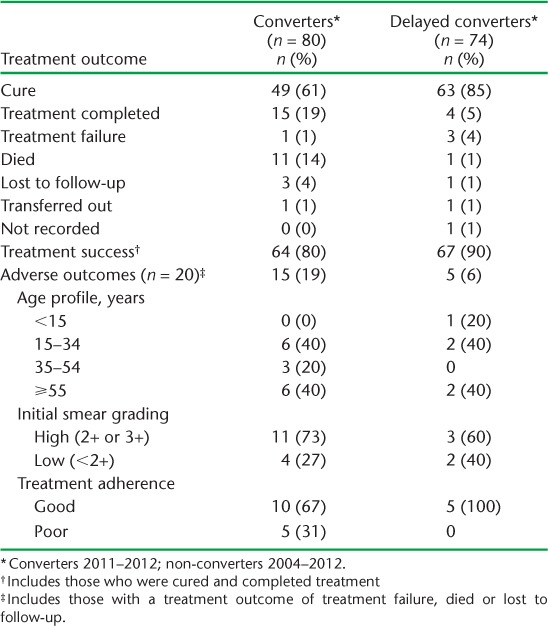
Treatment success was achieved in 90% (85% cure rate) of delayed converters and only 80% (61% cure rate) of converters (Table 2). Adverse outcomes were heavily influenced by the high number of deaths among converters compared to delayed converters (11/80, 14% vs. 1/74, 1%). Causes of death included liver failure (n = 5, 45%), septicaemia (n = 2, 36%), renal failure (n = 2, 36%), heart failure (n = 1, 19%) and cancer (n = 1, 19%); no deaths were directly related to TB. Adverse outcomes were not clustered in a particular group, and treatment adherence was rarely problematic. A small minority of delayed converters remained sputum smear-positive beyond 2 months: 22 (30%) were still positive at 3 months, 5 (7%) at 4 months and 3 (4%) at 5 months. Those who remained sputum smear-positive after 3 months went on to standard continuation phase treatment while awaiting the results of culture and DST. The three who remained smear-positive after 5 months of treatment were started on a retreatment regimen: two were cured and one died during treatment.
TABLE 2.
Treatment outcomes of sputum smear-positive tuberculosis patients in Vanuatu, comparing 2-month non-converters (delayed converters) with converters
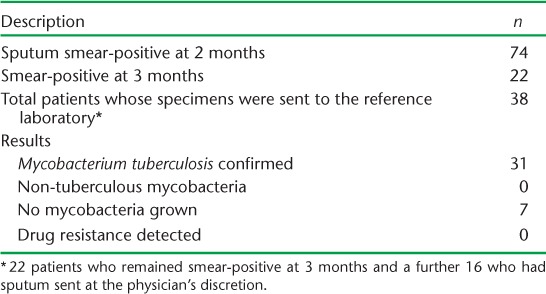
Specimens from 38 patients were sent to the supranational reference laboratory—all 22 3-month non-converters, and an additional 16 specimens sent at the physician's discretion (Table 3). In the majority of the cases (31/38, 82%), M. tuberculosis was grown from the specimen submitted (Table 3). No MDR-TB cases were detected during the study period. One patient each was identified with polydrug resistance (high-level INH, streptomycin and EMB) and low level INH monoresistance, but these were detected among relapse and default patients and not among delayed converters. No non-tuberculous mycobacteria were identified. There were some delays in receiving DST results, and these were not consistently fed back to the respective provinces.
TABLE 3.
Overview of sputum specimens from Vanuatu sent to the supranational reference laboratory in Brisbane, Queensland, Australia, during the study period (2004–2012)
When comparing NTP performance indicators in Tafea and Shefa Provinces (Table 4), the average TB incidence rate for 2011–2012 was found to be higher in Tafea (89.1 vs. 50.2/100 000, OR 1.78, 95%CI 1.10–2.87). There were also significantly more deaths among TB patients in Tafea than in Shefa Province (7/58, 12% vs. 2/80, 3%, OR 5.35, 95%CI 1.07–26.79), with lower treatment success in sputum smear-positive patients (79% vs. 90%, P = 0.239). Both provinces recorded very high rates of sputum smear positivity among pulmonary TB cases (~80%), while routine HIV testing was performed in only ~50%. None of the other parameters evaluated demonstrated major differences in programme performance.
TABLE 4.
Indicators of National TB Control Programme performance in Tafea and Shefa Provinces, Vanuatu, 2011–2012
DISCUSSION
This study provides the first comprehensive description of the characteristics, management and treatment outcomes of TB patients with delayed sputum smear conversion in Vanuatu. Of sputum smear-positive patients, 18% demonstrated delayed conversion over the study period. Studies from various countries reported non-conversion rates of 5–32%.10–19 No drug-resistant TB cases were detected, although this might be an important consideration in other settings. Higher bacillary load at diagnosis has consistently been associated with delayed smear conversion at 2 months,10,11,14–17,20 as suggested by the higher sputum smear grades observed among delayed converters in our study. Other factors associated with delayed sputum smear conversion that we were unable to demonstrate include evidence of cavitation on chest X-ray,11,13,14,19 age at diagnosis,11,13,16,19 location of health facility,15,21 sex12,16 and treatment with non-standardised regimens or missed doses of anti-tuberculosis drugs.10,14
In Vanuatu, delayed conversion occurred despite good treatment adherence and in the absence of drug resistance. Although reported adherence may not be a reliable measure of true adherence, DOT by a qualified health care worker was strictly observed after discharge, and the presence of drug resistance was ruled out with a high degree of certainty with the assistance of the regional reference laboratory. The fact that a small subgroup of patients with fully drug-susceptible TB remained sputum smear-positive after 2 months of adherent treatment is intriguing, especially as the excreted bacilli were still viable. Treatment outcomes were not adversely affected by sputum non-conversion at 2 months, consistent with a recent study in Sri Lanka,10 where the delayed conversion rate was 16%. The higher cure rate achieved in delayed converters might be attributable to heightened vigilance in obtaining a smear specimen at the end of anti-tuberculosis treatment. In clinical practice, treatment adherence and drug resistance remain important considerations in TB patients with persistently positive sputum smears.
TB case notification rates were largely unchanged over the study period, although pronounced reductions in case notification rates were observed in 2005 and 2008. In 2005, the NTP coordinator left at short notice, and there was a period of suboptimal reporting before a new coordinator was hired. The sharp decline in notifications in 2008 coincided with the end of Round 2 of Global Fund funding in 2007; Round 7 started in August 2008. The uncertainties resulting from the transition led to poor coordination of TB activities during this period. The majority of cases occurred in the most populous provinces, Shefa and Tafea, but Tafea Province had higher TB notification rates, along with lower rates of sputum smear conversion after 2 months of treatment and lower treatment success. A recent qualitative study that explored the role of traditional healers in Tafea Province found that traditional beliefs about the causes of TB prevail, and that consultation with a traditional healer is frequently the first point of contact when patients develop symptoms suggestive of TB, which could prolong diagnostic delay (Viney et al., unpublished data). However, this was not supported by the observation that smear grades were high among sputum smear-positive cases in both provinces, and were even higher in Shefa than Tafea (81% vs. 79%). Overall sputum smear-positive rates are high, suggesting that smear-negative cases may have been missed. A high percentage (12%) of non-HIV-infected patients in Tafea died while on anti-tuberculosis treatment, and four died from acute liver failure. The possibility that this might be due to the concomitant use of anti-tuberculosis treatment and traditional remedies requires further investigation.21
The study was limited by that fact that it was retrospective and dependent on the quality of routine programme data, with single data entry. However, every effort was made to triangulate findings from multiple data sources, with strict scrutiny of the final data set by the author. Routine culture and radiography would have enabled better phenotyping of the patients, but these are not routinely available in resource-limited settings such as Vanuatu. Access to the SRL allowed M. tuberculosis confirmation and DST in cases with persistently positive sputum smears. This is a major strength that allowed accurate assessment of potential drug resistance. The fact that patient groups used in the comparison were recruited over different time periods, i.e., 9 years for 2-month non-converters and 2 years for converters, may have introduced bias related to changes in practice over time, such as the introduction of routine HIV testing in 2010. However, these changes would not have had any material influence on the study findings. This was moreover the only feasible design, given the small number of delayed converters identified, while patient management practices remained mostly unchanged throughout the study period. We believe that the comparative analysis presented here represents a useful overview and that the key messages are valid, despite the differences in the time periods assessed.
Results from this study will help to further improve NTP performance in Vanuatu, and particularly patient management and care in underperforming provinces. Attention could also be paid to sputum smear converters in whom cure rates were suboptimal; obtaining a sputum specimen at the end of anti-tuberculosis treatment should increase cure rates in this group. Although there was no documented evidence of HIV infection, HIV testing rates remain low, and this aspect of the NTP should be improved. Similarly, testing TB patients for diabetes mellitus and documenting other comorbidities and risk factors such as smoking should be more comprehensively implemented.
In conclusion, TB trends in Vanuatu remained unchanged over the study period. Delayed sputum smear conversion was relatively uncommon, and was not associated with adverse treatment outcomes or drug resistance. Regional differences require further investigations to better understand local factors that may delay TB diagnosis and compromise treatment outcome.
Acknowledgments
The authors wish to thank the members of the National Tuberculosis Programme in Vanuatu who assisted with data collection and the Supranational Reference Laboratory in Brisbane, QLD, Australia, for verifying DST results. This research was supported through an operational research course jointly developed by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France, and Médecins Sans Frontières, Brussels-Luxembourg, and run in the South Pacific by The Union and the Public Health Division of the Secretariat of the Pacific Community, Nouméa, New Caledonia. We would also like to thank staff from the Queensland Mycobacterium Reference Laboratory, Brisbane, QLD, Australia, for kindly assisting us with laboratory data and for providing useful comments on the manuscript.
Additional support for running the course was provided by the School of Population Health, The University of Auckland, Auckland, New Zealand; the College of Medicine, Nursing and Health Sciences, Fiji National University, Suva, Fiji; the Division of TB Elimination, Centers for Disease Control and Prevention, Atlanta, GA, USA; Regional Public Health, Hutt Valley District Health Board, Lower Hutt, New Zealand; the National TB Programme, Fiji Ministry of Health, Suva, Fiji; the Sydney Emerging Infections and Biosecurity Institute, The University of Sydney, Sydney, NSW, Australia; and the Dunedin School of Medicine, The University of Otago, Dunedin, New Zealand. Funding for the course was provided by the Global Fund to Fight AIDS, TB and Malaria, Geneva, Switzerland, the World Diabetes Foundation, Gentofte, Denmark, and the Australian Agency for International Development, Canberra, ACT, Australia.
Footnotes
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2012. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 2.Raviglione M, Marais B, Floyd K et al. Scaling up of interventions to achieve global tuberculosis control: progress, new developments and update. Lancet. 2012;379:1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 3.Lönnroth K, Jaramillo E, Williams B G et al. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Dawson D. Anti-TB drug resistance in the Pacific Island countries and territories 2010–2011. World Health Organization, Western Pacific Region Office, Manila, The Philippines: Pacific TB Laboratory Initiative; 2012. [Google Scholar]
- 5.Feng-Zeng Z, Levy M H, Sumin W. Sputum microscopy results at two and three months predict outcome of tuberculosis treatment. Int J Tuberc Lung Dis. 1997;1:570–572. [PubMed] [Google Scholar]
- 6.Caminero J A. Multidrug-resistant tuberculosis: epidemiology, risk factors and case finding. Int J Tuberc Lung Dis. 2010;14:382–390. [PubMed] [Google Scholar]
- 7.Vanuatu National Statistics Office. National census of population and housing 2009. Vanuatu. Port Vila, Vanuatu: Ministry of Finance and Economic Management, Vanuatu National Statistics Office; 2009. [Google Scholar]
- 8.National TB Control Programme, Vanuatu. Vanuatu technical guideline and policies for tuberculosis control. Port Vila, Vanuatu: Ministry of Health, NTP Vanuatu; 2011. [Google Scholar]
- 9.World Health Organization. Treatment of tuberculosis guidelines. 4th ed. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 10.Jayakody W, Harries A D, Malhotra S et al. Characteristics and outcomes of tuberculosis patients who fail to smear convert at two months in Sri Lanka. Public Health Action. 2013;3:26–30. doi: 10.5588/pha.12.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singla R, Osman M M, Khan N et al. factors predicting sputum smear positivity among pulmonary tuberculosis patients 2 months after treatment. Int J Tuberc Lung Dis. 2003;7:58–64. [PubMed] [Google Scholar]
- 12.Dembele S M, Ouedraogo H Z, Combary A et al. Conversion rate of two-month follow-up of smear-positive tuberculosis patients in Burkina Faso. Int J Tuberc Lung Dis. 2007;11:1339–1344. [PubMed] [Google Scholar]
- 13.Dominguez-Castellano A, Muniain M A, Rodriguez-Bano J et al. Factors associated with time to sputum smear conversion in active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7:432–438. [PubMed] [Google Scholar]
- 14.Wang J-Y, Lee L-N, Yu C-J et al. Factors influencing time to smear conversion in patients with smear-positive pulmonary tuberculosis. Respirology. 2009;14:1012–1019. doi: 10.1111/j.1440-1843.2009.01598.x. [DOI] [PubMed] [Google Scholar]
- 15.Parikh R, Nataraj G, Kanade S et al. Time to sputum conversion in smear positive pulmonary TB patients on Category 1 DOTS and factors delaying it. J Assoc Physicians India. 2012;60:22–26. [PubMed] [Google Scholar]
- 16.Caetano-Mota P, Carvalho A, Valente I et al. Predictors of delayed sputum smear and culture conversion among a Portuguese population with pulmonary tuberculosis. Rev Port Pneumol. 2012;18:72–79. doi: 10.1016/j.rppneu.2011.12.005. [Article in English, Portuguese] [DOI] [PubMed] [Google Scholar]
- 17.Bouti K, Aharmin M, Marc K et al. Factors influencing sputum conversion among smear-positive pulmonary tuberculosis patients in Morocco. ISRN Pulmonology. 2013;(2013) Article ID 486507. http://dx.doi.org/10.1155/2013/486507 Accessed January 2014. [Google Scholar]
- 18.Kayigamba F R, Bakker M I, Mugisha V et al. Sputum completion and conversion rates after intensive phase of tuberculosis treatment: an assessment of the Rwandan control program. BMC Res Notes. 2012;5:357. doi: 10.1186/1756-0500-5-357. http://www.biomedcentral.com/1756-0500/5/357 Accessed January 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Kuyp F, Mahan C S. Prolonged positivity of sputum smears with negative vultures during treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2012;16:1663–1667. doi: 10.5588/ijtld.12.0238. [DOI] [PubMed] [Google Scholar]
- 20.Tiwari S, Kumar A, Kapoor S K. Relationship between sputum smear grading and smear conversion rate and treatment outcome in the patients of pulmonary tuberculosis undergoing DOTS—a prospective cohort study. Indian J Tuberc. 2012;59:135–140. [PubMed] [Google Scholar]
- 21.Auerbach B J, Reynolds S J, Lamorde M et al. Traditional herbal medicine use associated with liver fibrosis in rural Rakai, Uganda. PLOS ONE. 2012;7:e41737. doi: 10.1371/journal.pone.0041737. [DOI] [PMC free article] [PubMed] [Google Scholar]


