Abstract
A series of substituted 1H-indole-2-carboxamides structurally related to compounds Org27569 (1), Org29647 (2) and Org27759 (3) were synthesized and evaluated for CB1 allosteric modulating activity in calcium mobilization assays. Structure-activity relationship studies showed that the modulation potency of this series at the CB1 receptor was enhanced by the presence of a diethylamino group at the 4-position of the phenyl ring, a chloro or fluoro group at the C5 position and short alkyl groups at the C3 position on the indole ring. The most potent compound (45) had an IC50 value of 79 nM which is ~ 2.5 and 10 fold more potent than the parent compounds 3 and 1, respectively. These compounds appeared to be negative allosteric modulators at the CB1 receptor and dose-dependently reduced the Emax of agonist CP55,940. These analogs may provide the basis for further optimization and use of CB1 allosteric modulators.
Keywords: CB1 receptor, allosteric modulators, Org compounds, structure-activity relationship
Graphical Abstract
1. Introduction
The cannabinoid type 1 (CB1) receptor is a key component of the endocannabinoid system, which also includes the CB2 receptor, endocannabinoids and enzymes involved in the biosynthesis and degradation of these endogenous ligands.1, 2 Since the cloning of CB1 and CB2 receptors in the 1990s, considerable research efforts have been directed at understanding their physiological and pathological roles. The endocannabinoid system has been shown to be involved in a number of physiological processes, including cardiovascular regulation, appetite control, learning and memory, and pain processing.3-5 The CB1 receptor is one of the most abundant G-protein coupled receptor (GPCR) expressed in the central nervous system (CNS), and is predominantly expressed at pre-synaptic nerve terminals, where it plays a key role in inhibition of transmitter release. The CB1 receptor is also found in several peripheral tissues, but at much lower concentrations. The CB2 receptor is mainly expressed in immune cells, and is involved in modulation of cytokine release and immune cell migration. The modulation of the CB1 receptor has been targeted in the treatment of a number of disorders such as obesity, drug addiction, pain, inflammation, gastrointestinal diseases, multiple sclerosis, psychosis, schizophrenia, and osteoporosis.4, 6
A large number of selective and non-selective agonists and antagonists have been developed for the CB1 receptor to date.7-9 More recently, there is convincing evidence suggesting that the CB1 receptor also contains allosteric binding site(s) that can be modulated by endogenous and/or synthetic small molecules, and the structural requirements of allosteric ligands are distinctly different from orthosteric ligands.10-12 A number of novel compounds have been reported to be CB1 allosteric modulators, including Org27569, Org29647, Org27759 (1-3),13 PSNCBAM-1 (4),14 RTI-371 (5),15 and lipoxin A4 (6).16 Compared to orthosteric ligands, allosteric ligands generally do not disrupt physiological signaling processes and may provide improved selectivity as allosteric sites are less structurally conserved than the corresponding orthosteric site among receptor subtypes. In addition, allosteric modulators may offer a significant clinical advantage in drug safety profiles because of the “ceiling” effect, the phenomenon in which a drug reaches a maximum effect so that increasing the drug dosage does not increase its effectiveness, which results from their dependence on endogenous ligands for signaling.17-19 The small molecule CB1 allosteric modulators developed to date have been demonstrated to be allosteric enhancers of agonist binding and affinity and allosteric inhibitors of agonist signaling efficacy in several in vitro functional assays.20, 21 Interestingly, 1 did not enhance or block CB1 agonist-induced effects in several animal models in mice (antinociception, catalepsy, and hypothermia) and appeared to exhibit its anorectic effect through non-CB1 specific mechanisms.22 While we have seen similar negative results in catalepsy and antinociception in rats, Org27569 attenuated the hypothermic effects of CB1 receptor agonists CP55940 and anandamide.23 Moreover, we also found that 1 resulted in a dose-related attenuation of both cue- and drug-induced reinstatement of cocaine- and methamphetamine-seeking behavior.24 Finally, 1 showed high pharmacological selectivity against over forty GPCRs including those commonly involved in drug addictions.24 Together, these results suggest that the observed effects of 1 were likely mediated through negative modulation of CB1 receptors.
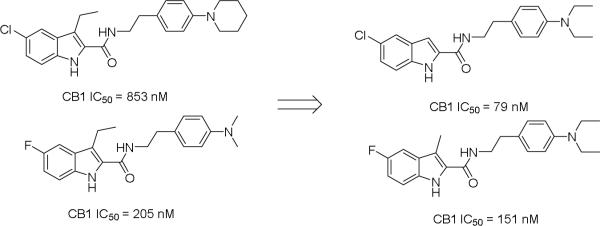
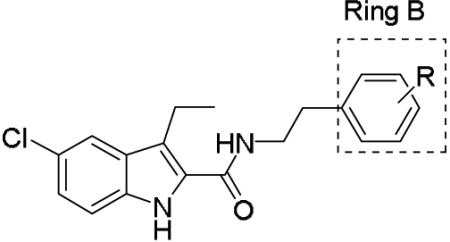
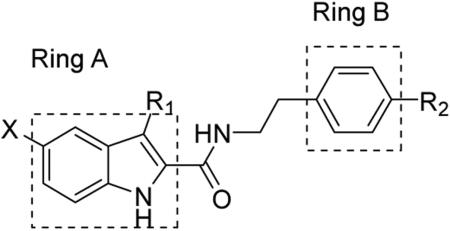
Since the discovery of 1-3, several structure-activity relationship (SAR) studies on the 1H-indole-2-carboxamide scaffold have been reported. Most of these studies have focused on modifications on the indole and phenyl rings A and B (Figure 1). Piscitelli et al examined a number of 4-substitutions on the phenyl ring B and discovered that piperidinyl or dimethylamino groups at the 4-position of the phenyl ring were preferred for CB1 activity and the carboxamide functionality was required (Figure 1).25 Subsequent work found that longer alkyl side chains with up to 9 carbon units at the C3 position of indole ring A could retain activity, whereas linkers other than an ethylene between the amide bond and the phenyl ring B resulted in total loss of activity.26-28 In an attempt to expand our understanding of the structure-activity relationship on this scaffold, we have designed additional analogs by (i) exploring different substituents at the 4-, 3-, 2-positions of the phenyl ring B, (ii) examining several rigid cyclic ring linkers between the phenyl and the indole rings, and (iii) investigating the effects of shorter alkyl side chain at the C3 position and halogenations at the C5 position of the indole ring A. Here, we report the synthesis of these 1H-indole-2-carboxamide analogs and the evaluation of CB1 and CB2 activities in fluorometric imaging plate reader (FLIPR) based calcium mobilization assays.
Figure 1.
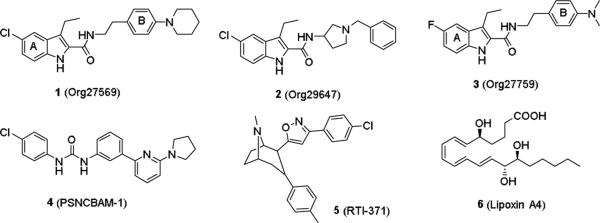
Structures of several reported CB1 allosteric modulators.
2. Methods
2.1. Chemistry
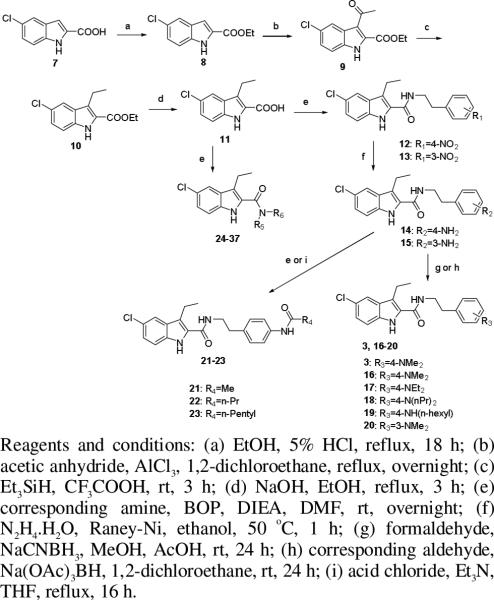
The target compounds 12, 16-37, 42-49 were synthesized following procedures depicted in Schemes 1 and 2. Scheme 1 describes the synthesis of the key intermediate 5-chloro-3-ethyl-1H-indole-2-carboxylic acid (11) and target compounds 16-37. The commercially available 5-chloro-1H-indole-2-carboxylic acid (7) was protected as ethyl ester 8 which underwent Friedel Crafts acylation to yield 9.29 Reduction of 9 by triethylsilane in trifluoroacetic acid at room temperature gave 10 which was hydrolyzed in aqueous sodium hydroxide and ethanol to furnish the intermediate 11.26, 27 Amide coupling between 11 and 4- or 3-nitrophenylethylamine in the presence of BOP provided 12 and 13 which were reduced by Raney nickel-catalyzed transfer hydrogenation using hydrazine in ethanol at 50 °C to give 14 and 15. Reductive amination of amines 14 and 15 with the corresponding aldehydes provided the target compounds 16-20. Compounds 21-23 were obtained by amide coupling of amine 14 and corresponding acids or acid chloride. Similarly, compounds 24-37 were prepared by standard amide coupling between acid 11 and the appropriate amines.
Scheme 1.
Synthesis of the key intermediate 11 and target compounds 12, and 16-37.
Scheme 2.
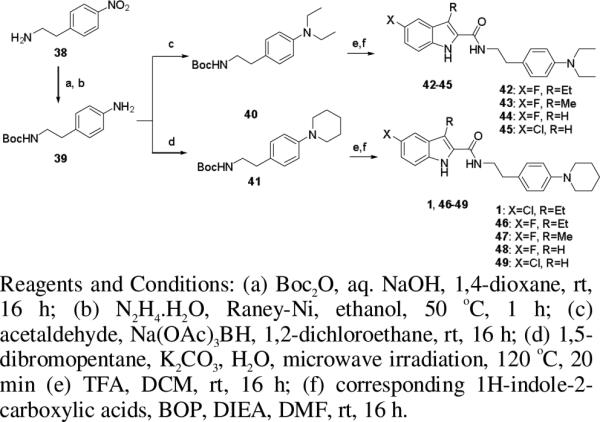
Synthesis of compounds 1, and 42-49.
The N,N-diethylphenylethylamine (40) and 4-piperidinylphenethylamine (41) used to synthesize compounds 1 and 42-49 were prepared according to Scheme 2. 4-Nitrophenethylamine (38) underwent Boc-protection and Raney nickel-catalyzed reduction with hydrazine hydrate in ethanol at 50 °C to give 39, which was then converted to 40 by reductive amination with acetaldehyde in the presence of sodium triacetoxyborohydride in 1,2-dichloroethane. Cyclization of 39 with 1,5-dibromopentane in aqueous potassium carbonate solution under microwave irradiation furnished 41.30 After removal of the Boc protective group, 40 and 41 were coupled with the corresponding 1H-indole-2-carboxylic acids to afford the target compounds 1, and 42-49.
2.2. Biological assays
The activity of the target compounds at CB1 and CB2 receptors was evaluated in FLIPR-based calcium mobilization functional assays. The calcium mobilization assays have been used routinely in our laboratory to characterize cannabinoid compounds and demonstrate correlation with other cannabinoid assays such as binding and GTP- -S.31-33 Briefly, CHO-RD-HGA16 cells stably expressing either human CB1 or CB2 receptors were loaded with Calcium 5 dye (Molecular Devices, USA), and treated with test compounds in the presence of the EC80 concentration of the agonist CP55,940 (100 nM). For the CB1 receptor, IC50 values were obtained from the plots of kinetic reduction fluorescence signals against the log of compound concentrations. For the CB2 receptor, all compounds were initially screened at 10 μM as antagonists and IC50 was obtained only if > 50% inhibition was observed. Finally, all compounds were also tested for agonist activity at 10 μM at both receptors.
3. Results and discussion
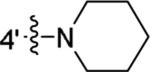
Using 1 as the lead compound (IC50 = 853 nM), we first investigated the effect of substitution on the phenyl ring B (Table 1). Previous studies have focused on 4-substitutions on this phenyl ring, particularly 4-amino groups such as piperidinyl and dimethylamino.25-27 Here we introduced a number of other amino groups at several positions. The activity of the piperidine ring-opened series (compounds 16-19) was found to be sensitive to the length of the alkyl substituent. The activity of the dialkylamino analogs increased from methyl (16) to ethyl (17) and then declined as the side chain was extended further to a propyl group (18). The hexyl analog 19 was only moderately active. Together, these implicate a limited space at this region of the CB1 binding pocket. The most potent analog was 17 with the diethylamino group at the 4-position (IC50 483 nM, 2-fold more potent than 1). All three acylated analogs (21-23) were devoid of any activity up to 10 μM. The activity also decreased with the non-substituted analog (24) or when the nitrogen was replaced by other substituents with different electronic properties (12, 24-27), in agreement with earlier findings.11 Together, these results suggest that a basic electron-accepting nitrogen was preferred at the 4-position for CB1 modulating activity.
Table 1.
Compounds 1, 12, 16-34 and their inhibitory activity at the CB1 receptor.
| |||
|---|---|---|---|
| # | R | CB1 IC50 (nM)a | CB2 % Inhibition of CP55,940b |
| 1 |
|
853 ± 130 | −16.4 ± 1.8 |
| 12 | 4’-NO2 | 5740 ± 930 | 34.8 ± 22.6 |
| 16 | 4’-NMe2 | 787 ± 110 | −12.7 ± 2.8 |
| 17 | 4’-NEt2 | 483 ± 60 | −44 ± 11 |
| 18 | 4’-NHPr2 | 1800 ± 480 | −37.4 ± 6.8 |
| 19 | 4’-NH(CH2)5CH3 | 3620 ± 790 | −35.0 ± 4.6 |
| 20 | 3’-NMe2 | >10,000 | −3.7 ± 4.3 |
| 21 | 4’-NHCOCH3 | >10,000 | −3.3 ± 11.7 |
| 22 | 4’-NHCO(CH2)2CH3 | >10,000 | 43.0 ± 3.2 |
| 23 | 4’-NHCO(CH2)4CH3 | >10,000 | 1.0 ± 0.6 |
| 24 | H | 3150±260 | −6.2 ± 25.3 |
| 25 | 4’-Cl | 2500±410 | −4.8 ± 30.0 |
| 26 | 4’-OCH3 | 2560±110 | −11.2 ± 19.6 |
| 27 | 4’-tBu | 2430±430 | −18.0 ± 35.4 |
| 28 | 2’-Cl | 5810±990 | 28.5 ± 24.0 |
| 29 | 3’-Cl | 831±190 | 24.8 ± 28.6 |
| 30 | 3’-OCH3 | 2100±170 | −6.0 ± 19.0 |
| 31 | 3’-OH | >10,000 | −0.1 ± 21.4 |
| 32 | 3’-OH,4’-OCH3 | >10,000 | −7.0 ± 19.6 |
| 33 | 3’,4’-diCl | 2170±530 | −3.2 ± 10.8 |
| 34 |
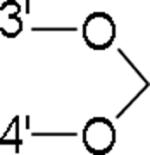
|
4320±190 | 25.8 ± 22.1 |
Against EC80 (100 nM) of CP55,940. Values are the mean ± SEM of at least three independent experiments in duplicate.
Percent inhibition of CP55,940 EC80 (100 nM). Values are the mean ± SEM of at least two independent experiments in duplicate.
While the 4-position on the phenyl ring has been previously investigated, substitutions at the other positions have not been reported. Interestingly, we found that the 4- and 3- position on the phenyl ring preferred different substituents. The 4-position appeared to favor the dialkylamino substituted series. Switching the dimethylamino group from the 4- to 3-position resulted in abolishment of CB1 inhibitory activity. 16 had an IC50 of 787 nM, whereas the 3-position substituted analog 20 displayed no potency up to 10 μM. However, the chloro group preferred a 3-position substitution pattern as 29 had an IC50 of 831 nM while 25 and 28 had IC50 values of 2500 nM and 5810 nM, respectively. Methoxy was less affected by substituent position with 26 and 30, both displaying moderate potency. This substitution difference between the 3 and 4-positions seem to suggest less steric tolerance at the 3-psotions where only a chloro, but not the dimethylamino or methoxy groups are tolerated. A combination of 3- and 4-substituents did not provide any enhancement to the potency (33 and 34), and even reduced the potency as in the case of 32 (3’-OH, 4’-OCH3), consistent with earlier observations on limited spatial pocket at these positions.
Compound 2 with a pyrrolidinyl linker between the amide bond and the phenyl ring showed significantly reduced potency compared to 1 and 3 (Table 2). Several other linkers were briefly examined and none of the phenyl (35) or piperazine (36 and 37) analogs showed CB1 inhibitory effects at concentrations up to 10 μM. Hence, it appears that a flexible alkyl linker is important to CB1 activity, which is in agreement with Khurana et al who showed that the ethylene moiety was the optimal linker.27
Table 2.
Compounds 2, 35-37 and their inhibitory activity at the CB1 receptor.
| |||
|---|---|---|---|
| # | R | CB1 IC50 (nM)a | CB2 % Inhibition of CP55,940b |
| 2 |
|
4760 ± 710 | −6.2 ± 2.5 |
| 35 |

|
>10,000 | −19.2 ± 11.5 |
| 36 |
|
>10,000 | −28.0 ± 25.9 |
| 37 |
|
>10,000 | −31.1 ± 18.8 |
Against EC80 (100 nM) of CP55,940. Values are the mean ± SEM of at least three independent experiments in duplicate.
Percent inhibition of CP55,940 EC80 (100 nM). Values are the mean ± SEM of at least two independent experiments in duplicate.
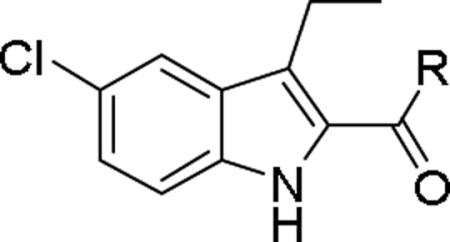
Next we turned our attention to the indole ring A where we investigated the effect of fluoro and chloro groups at the C5 position and the length of the alkyl side chain at the C3 position on the indole ring. These analogs have either a piperidinyl group as in 1 or a diethylamino group as in 17, the most potent compounds from Table 1, at the 4-position of the phenyl ring B. In general, the diethylamino analogs (17, 42-45 having IC50 values ranging from 79 to 484 nM) were more potent than the piperidinyl analogs (1, 46-49 having IC50 values from 212 to 853 nM). Compound 45 was the most potent compound in this series with an IC50 value of 79 nM, 6-fold more potent than compound 17, and 2.5-fold more potent than compound 3. The CB1 inhibitory effect seems to be sensitive to the substituent on the C3 position on the indole ring. A smaller group like H or Me is preferred to an ethyl group. On the other hand, F and Cl at the C5 position was not the determining factor in CB1 activity. While 45 and 49 with a 5-chloro were more potent than 44 and 48 with a 5-fluoro substituent, 17 and 1 were less active than their counterparts 42 and 46.
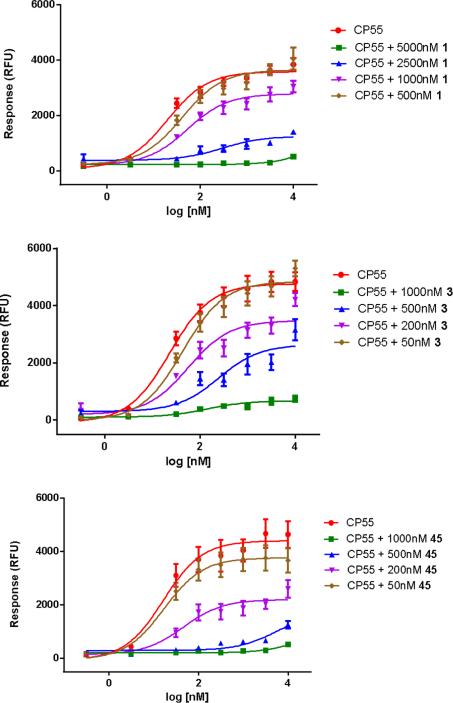
When tested at the CB2 receptor, none of the compounds showed > 35% of inhibition of the EC80 of CP55,940 at concentrations of up to 10 μM and therefore, their IC50 value was not determined (Tables 1-3). All compounds were also screened at 10 μM for agonist activity at both the CB1 and CB2 receptors. None of the compounds displayed significant agonist activity at either receptor (less than 30% of CP55,940 Emax) (Supporting Information). As depicted in Figure 2, compound 1, 3 and 45 all dose-dependently decreased the Emax of agonist CP55,940 in the same manner. Thus, this suggests that these compounds exerted their effects by an allosteric mechanism.20, 21
Table 3.
Compounds 1, 3, 17, 42-49 and their inhibitory activity at the CB1 receptor.
| |||||
|---|---|---|---|---|---|
| # | X | R1 | R2 | CB1 IC50 (nM)a | CB2 % Inhibition of CP55,940b |
| 1 | Cl | Et |
|
853 ± 130 | −16.4 ± 1.8 |
| 3 | F | Et | NMe2 | 205 ± 34 | −20.4 ± 2.4 |
| 17 | Cl | Et | NEt2 | 483 ± 60 | −44.4 ± 11.1 |
| 42 | F | Et | NEt2 | 361 ± 49 | −25.7 ± 10.7 |
| 43 | F | Me | NEt2 | 151 ± 20 | −21.8 ± 6.5 |
| 44 | F | H | NEt2 | 318 ± 53 | −23.8 ± 5.2 |
| 45 | Cl | H | NEt2 | 79 ± 7 | 0.7 ± 9.8 |
| 46 | F | Et |
|
605 ± 93 | −33.1 ± 16.3 |
| 47 | F | Me |
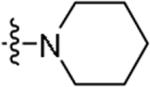
|
279 ± 51 | −8.2 ± 5.8 |
| 48 | F | H |
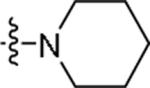
|
319 ± 76 | 20.8 ± 5.9 |
| 49 | Cl | H |
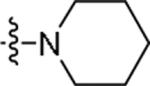
|
212 ± 41 | 15.0 ± 9.8 |
Against EC80 (100 nM) of CP55,940. Values are the mean ± SEM of at least three independent experiments in duplicate.
Percent inhibition of CP55,940 EC80 (100 nM). Values are the mean ± SEM of at least two independent experiments in duplicate.
Figure 2.
Allosteric modulation of compounds 1, 3, and 45 in the CB1 calcium mobilization assay.
4. Conclusions
The identification of the CB1 allosteric binding site and its interesting pharmacological response have prompted investigation of its function and development of molecular probes to help elucidate the pharmacology of the CB1 receptor. To this end, we have designed and synthesized a series of 1H-indole-2-carobxamide derivatives by varying substituents on the phenyl ring B, linker between the amide bond and phenyl ring B, as well as modifying the halogen substituent at the 5-position and alkyl side chain at the 3-position on the indole ring A. In general, an electron-donating group at the 4-position on the phenyl ring, as well as an ethylene linker, were important features of CB1 modulating activity. On the indole ring A, a small side chain (H or Me) was preferred while the difference in effects of the fluoro and chloro on CB1 activity was not significant. These compounds showed excellent selectivity against the CB2 receptor and did not have any significant agonist activity at either receptor in the calcium mobilization assays. Several compounds showed similar or improved potency compared to the parent compounds. Compound 45 had an IC50 of 79 nM, ~ 2.5 and 10 fold more potent than 3 and 1, respectively. In conclusion, CB1 allosteric modulators of this structural scaffold hold promising potential for further development as effective probes of the allosteric site(s) of the CB1 receptor.
5. Experimental
5.1. Chemistry
All solvents and chemicals were reagent grade. Unless otherwise mentioned, all reagents and solvents were purchased from commercial vendors and used as received. Flash column chromatography was carried out on a Teledyne ISCO CombiFlash Rf system using prepacked columns. Solvents used include hexane, ethyl acetate (EtOAc), dichloromethane, and, methanol. Microwave reactions were carried out on a CEM Discover microwave synthesizer. 1H and 13C NMR spectra were recorded on a Bruker Avance DPX-300 (300 MHz) spectrometer and were determined in chloroform-d, methanol-d4, or DMSO-d6 with tetramethylsilane (TMS) (0.00 ppm) or solvent peaks as the internal reference. Chemical shifts are reported in ppm relative to the reference signal and coupling constant (J) values are reported in hertz (Hz). Thin layer chromatography (TLC) was performed on EMD precoated silica gel 60 F254 plates and spots were visualized with UV light or iodine staining. Low resolution mass spectra were obtained using a Waters Alliance HT/Micromass ZQ system (ESI). All test compounds were greater than 95% pure as determined by HPLC on an Agilent 1100 system using an Agilent Zorbax SB-Phenyl, 2.1 mm × 150 mm, 5 μm column with gradient elution using the mobile phases (A) H2O containing 0.1% CF3COOH and (B) MeCN, with a flow rate of 1.0 mL/min.
Ethyl 5-chloro-1H-indole-2-carboxylate (8)
To a flask containing 5% HCl solution (50 ml) and ethanol (50 ml) was added 5-chloro-1H-indole-2-carboxylic acid (2 g, 10.22 mmol). The reaction was refluxed for 18 h. After that, the reaction volume was reduced to half in vacuo. The product precipitated out and was filtered off. The mother liquor was evaporated to dryness and the solid residue was washed with a mixture of acetone and water to an additional crop of the product as off-white solid (2.18 g, 95% yield). 1H NMR (CDCl3) 1.42 (t, J=7.2, 3H), 4.42 (q, J=7.2, 2H), 7.15 (m, 1H), 7.27 (m, 1H), 7.35 (m, 1H), 7.66 (s, 1H), 9.03 (br, 1H). MS (ESI) [M-H]− 222.4.
Ethyl 3-acetyl-5-chloro-1H-indole-2-carboxylate (9)
Acetic anhydride (1.1 ml, 8.94 mmol) was added to an ice-cooled suspension of AlCl3 (1.19 g, 8.94 mmol) in 1,2-dichloroethane (16 ml) and the reaction was stirred for 5 min under nitrogen. A solution of 7 (1 g, 4.47 mmol) in 1,2-dichloroethane (16 ml) was added dropwise and the reaction mixture was refluxed for 1 h. After cooling to room temperature, the reaction mixture was added ice water, extracted with ethyl acetate, dried over anhydrous MgSO4, and purified by column chromatography on silica (0-25% ethyl acetate in hexanes) to give the product as off-white solid (0.72 g, 60% yield). 1H NMR (CDCl3) 1.46 (t, J=7.1, 3H), 2.74 (s, 3H), 4.49 (q, J=7.2, 2H), 7.34 (m, 2H), 8.12 (s, 1H), 9.18 (br, 1H).
Ethyl 5-chloro-3-ethyl-1H-indole-2-carboxylate (10)
To a solution of 9 (0.44 g, 1.66 mmol) in trifluoroacetic aicd (4 ml) was added triethylsilane (1.1 ml, 6.64 mmol). The reaction mixture was stirred at room temperature for 3 h. It was then poured into water, extracted with ethyl acetate. The organic layer was washed with 1N NaOH, water, and brine, dried over anhydrous MgSO4, and concentrated in vacuo to give the desired product as yellow solid (0.4 g, 95% yield). 1H NMR (DMSO-d6) 1.17 (t, J=7.4, 3H), 1.35 (t, J=7.1, 3H), 3.03 (q, J=7.3, 2H), 4.35 (q, J=7.0, 2H), 7.25 (m, 1H), 7.42 (d, J=8.7, 1H), 7.74 (d, J=1.7, 1H), 11.68 (br, 1H).
5-Chloro-3-ethyl-1H-indole-2-carboxylic acid (11)
A solution of 10 (0.40 g, 1.58 mmol) and aqueous 1N NaOH (2.5 ml) in ethanol (30 ml) was refuxed for 3 h. After the reaction has completed, solvent was removed in vacuo. The residue was dissolved in 5 ml of water and pH was adjusted to 3 by 2N HCl. White precipitate formed during the reaction was filtered to give the desired product as off-white solid (0.38 g, quantitative yield). 1H NMR (DMSO-d6) 1.17 (t, J=7.3, 3H), 3.02 (q, J=7.3, 2H), 7.23 (dd, J=8.8, 1H), 7.39 (d, J=8.7, 1H), 7.71 (d, J=1.7, 1H), 11.58 (s, 1H), 13.06 (s, 1H). MS (ESI) [M-H]− 222.4.
5-Chloro-N-[2-(4-nitrophenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (12)
To a solution of 11 (0.02 g, 0.06 mmol) in DMF (2 ml) was added 4-nitrophenylethylamine (5.5 μL, 0.06 mmol), BOP (0.026 g, 0.06 mmol), and N,N-diisopropylethylamine (26 μL, 0.15 mmol). The reaction mixture was stirred at room temperature for 16 h, and then diluted with water, extracted with ethyl acetate three times. The combined organic layers were washed twice with water, and brine, dried over anhydrous MgSO4, concentrated in vacuo, and purified by column chromatography on silica to give the desired compound as white solid (0.018 g, 54% yield). 1H NMR (CDCl3) 1.19 (t, J=7.6, 3H), 2.82 (q, J=7.7, 2H), 3.10 (t, J=6.8, 2H), 3.80 (q, J=6.6, 2H), 5.99 (br, 1H), 7.21 (m, 2H), 7.24 (m, 1H), 7.31 (m, 2H), 7.42 (s, 1H), 7.58 (d, J=1.9, 1H), 8.96 (br, 1H). MS (ESI) [M+H]+ 361.3, [M-H]− 359.4.
3-Ethyl-5-chloro-N-[2-(3-nitrophenyl)ethyl]-1H-indole-2-carboxamide (13)
Prepared using the procedure for compound 12 to give the title product as white solid in 30% yield. 1H NMR (CDCl3) 1.14 - 1.23 (m, 3H), 3.10 (m, 2H), 3.68 (m, 2H), 3.81 (d, J = 6.22 Hz, 2H), 6.62 (br, 1H), 7.20 (m, 1H), 7.31 (s, 1H), 7.49 (s, 1H), 7.57 (d, J = 1.70 Hz, 2H), 8.15 (m, 2H), 9.10 (br, 1H).
N-[2-(4-Aminophenyl)ethyl]-3-ethyl-5-chloro-1H-indole-2-carboxamide (14)
To a suspension of 12 (0.71 g, 1.91 mmol) in ethanol (20 ml) was added hydrazine hydrate (1.4 ml, 28.6 mmol) and the reaction mixture was stirred at 50 °C for 15 min until the starting material is dissolved completely. Excess of Raney Nickel (0.5 g) was added to the reaction mixture and the reaction was maintained at 50 °C. After about 1 hour when gas evolution ceased, Raney Nickel was filtered under vacuum. The filtrate was concentrated in vacuo to give the desired product as white solid (0.3 g, 46% yield). 1H NMR (CDCl3) 1.12 (m, 3H), 2.72 (q, J=7.6, 2H), 2.86 (t, J=6.6, 2H), 3.76 (q, J=6.5, 2H), 5.97 (br, 1H), 6.68 (m, 2H), 7.05 (m, 2H), 7.21 (m, 1H), 7.37 (m, 1H), 7.54 (m, 1H), 8.98 (br, 1H).
N-[2-(3-Aminophenyl)ethyl]-3-ethyl-5-chloro-1H-indole-2-carboxamide (15)
Prepared using the procedure for compound 14 to give the title product as white solid in 70% yield. 1H NMR (CD3OD) 1.12 (m, 3H), 3.04 (m, 2H), 3.68 (m, 2H), 7.04 (m, 1H), 7.20 (m, 1H), 7.26 (m, 1H), 7.38 (m, 1H), 7.46 (m, 2H), 7.58 (m, 1H).
5-Chloro-N-{2-[4-(dimethylamino)phenyl]ethyl}-3-ethyl-1H-indole-2-carboxamide (16)
To a solution of 14 (0.03 g, 0.09 mmol) in methanol (1 mL), 37% formaldehyde (1 mL) was added followed by acetic acid (18 μL) and NaCNBH4 (0.027 g, 0.44 mmol). After stirring at room temperature for 16h, the reaction was quenched with 1N HCl and extracted with ethyl acetate three times. The combined organic fractions was washed with saturated NaHCO3 and brine, dried over anhydrous MgSO4, concentrated in vacuo, and purified by column chromatography on silica (0-10% MeOH in DCM) to give the desired product as yellow solid (0.015 mg, 47% yield). 1H NMR (DMSO-d6) 1.12 (t, J=7.4, 3H), 2.74 (t, J=7.3, 2H), 2.84 (s, 6H), 2.95 (q, J=7.4, 2H), 3.45 (m, 2H), 6.68 (d, J=8.7, 2H), 7.04 (d, J=2.4, 1H), 7.08 (d, J=9.0, 2H), 7.37 (m, 2H), 7.90 (m, 1H), 11.20 (s, 1H). MS (ESI) [M+H]+ 370.3, [M-H]− 368.5.
5-Chloro-N-{2-[4-(diethylamino)phenyl]ethyl}-3-ethyl-1H-indole-2-carboxamide (17)
To a solution of 14 (0.015 g, 0.044 mmol) and acetaldehyde (6.5 μL, 0.11 mmol) in 1,2-dichloroethane (2 ml) was stirred at room temperature for 30 min before Na(OAc)3BH (0.023 g, 0.11 mmol) was added. The reaction continued at room temperature for another 16 h before it was quenched with 1N NaOH. The reaction mixture was extracted with dichloromethane. The organic layer was washed water and brine, dried over anhydrous MgSO4, concentrated in vacuo, and purified by column chromatography on silica (0-10% MeOH in DCM) to give the desired product as white solid (0.012 g, 69% yield). 1H NMR (DMSO-d6) 1.06 (t, J=7.0, 6H), 1.12 (t, J=7.4, 3H), 2.72 (m, 2H), 2.95 (m, 2H), 3.29 (, q J=7.2, 4H), 3.43 (m, 2H), 6.60 (d, J=8.5, 2H), 7.04 (d, J=8.5, 2H), 7.18 (dd, J1=1.7, J2=8.7, 1H), 7.39 (d, J=8.7, 1H), 7.93 (s, 1H). MS (ESI) [M+H]+ 398.3, [M-H]− 396.5.
5-Chloro-N-{2-[4-(dipropylamino)phenyl]ethyl}-3-ethyl-1H-indole-2-carboxamide (18)
Prepared using the procedure for compound 17 to give the desired product as white solid in 65% yield. 1H NMR (CDCl3) 0.92 (t, J=7.3, 6H), 1.06 (t, J=7.9, 3H), 1.60 (m, 4H), 2.71 (m, 2H), 2.85 (t, J=6.6, 2H), 3.22 (t, J=7.5, 4H), 3.76 (q, J=6.2, 2H), 5.99 (s, 1H), 6.62 (d, J=8.5, 2H), 7.08 (d, J=8.5, 2H), 7.21 (m, 1H), 7.29 (m, 1H), 7.54 (s, 1H), 9.11 (s, 1H). MS (ESI) [M]+ 426.3.
5-Chloro-N-{2-[4-(hexylamino)phenyl]ethyl}-3-ethyl-1H-indole-2-carboxamide (19)
Prepared using the procedure for compound 17 to give the desired product as white solid in 32% yield. 1H NMR (CDCl3) 0.91 (t, J=7.5, 3H), 1.10 (t, J=7.3, 3H), 1.35 (m, 6H), 1.62 (m, 2H), 2.71 (q, J=7.8, 2H), 2.85 (t, J=6.5, 2H), 3.10 (t, J=7.1, 2H), 3.76 (q, J=6.0, 2H), 5.98 (s, 1H), 6.59 (d, J=7.7, 2H), 7.06 (d, J=8.3, 2H), 7.21 (m, 2H), 7.31 (m, 1H), 7.54 (s, 1H), 8.96 (s, 1H). MS (ESI) [M]+ 426.3.
5-Chloro-N-{2-[3-(dimethylamino)phenyl]ethyl}-3-ethyl-1H-indole-2-carboxamide (20)
Prepared using the procedure for compound 17 to give the title product as light yellow solid in 54% yield. 1H NMR (CDCl3) 1.07 (t, J = 7.6, 3H), 2.70 (d, J = 7.5, 2H), 2.84 (s, 6H), 2.95 (t, J = 6.6, 2H), 3.80 (m, 2H), 5.86 (br, 1H), 6.77 - 6.90 (m, 3H), 7.15 - 7.38 (m, 4H), 7.54 (d, J = 1.9, 1H), 9.04 (br, 1H). MS (ESI) [M+H]+ 370.3.
N-[2-(4-Acetylphenyl)ethyl]-5-chloro-3-ethyl-1H-indole-2-carboxamide (21)
To a solution of 14 (0.020 g, 0.058 mmol) in THF (0.3 ml) was added triethylamine (12 μL, 0.088 mmol) and acetyl chloride (6 μL, 0.088 mmol). The reaction mixture was refluxed for 4 h. The solvent was removed under vacuum. The residue was dissolved in dichloromethane. The solution was then washed with 1N HCl and brine, dried over anhydrous MgSO4, and concentrated in vacuo to give the desired product as white solid in 59% yield. 1H NMR (CDCl3) 1.13 (t, J=7.7, 3H), 2.19 (s, 3H), 2.76 (m, 2H), 2.94 (t, J=6.5, 2H), 3.79 (q, J=6.2, 2H), 5.97 (s, 1H), 7.13 (m, 1H), 7.22 (d, J=9.0, 2H), 7.29 (m, 1H), 7.47 (d, J=8.3, 2H), 7.54 (s, 1H), 8.91 (s, 1H). MS (ESI) [M+H]+ 384.4, [M-H]− 382.4.
N-[2-(4-Butanoylphenyl)ethyl]-5-chloro-3-ethyl-1H-indole-2-carboxamide (22)
Prepared using the procedure for compound 12 to give the title product as white solid (0.012 g, 50% yield). 1H NMR (CD3OD) 0.99 (t, J=7.3, 3H), 1.19 (t, J=7.7, 3H), 1.71 (m, 2H), 2.33 (t, J=7.3, 2H), 2.94 (m, 4H), 3.63 (t, J=7.3, 2H), 7.17 (d, J=8.7, 1H), 7.24 (d, J=8.1, 2H), 7.33 (d, J=8.7, 1H), 7.49 (d, J=8.3, 2H), 7.58 (s, 1H). MS (ESI) [M+H]+ 412.4, [M+Na]+ 434.3, [M-H]− 410.6.
5-Chloro-3-ethyl-N-[2-(4-hexanoylphenyl)ethyl]-1H-indole-2-carboxamide (23)
Prepared using the procedure for compound 12 to give the title product as white solid in 60% yield. 1H NMR (CDCl3) 0.89 (t, J=7.7, 3H), 1.11 (m, 5H), 1.69 - 1.81 (m, 2H), 2.36 (t, J = 7.54 Hz, 2H), 2.67 - 2.89 (m, 4H), 2.94 (t, J = 6.69 Hz, 2H), 3.63 (br, 1H), 3.73 - 3.83 (m, 2H), 5.96 (br, 1H), 6.68 (d, J=8.3, 1H), 7.08 (d, J=9.0, 2H), 7.23 (m, 1H), 7.49 (d, J=8.3, 2H), 7.54 (s, 1H), 8.91 (s, 1H). MS (ESI) [M+H]+ 440.2, [M+Na]+ 462.5, [M-H]− 438.5.
5-Chloro-3-ethyl-N-(2-phenylethyl)-1H-indole-2-carboxamide (24)
Prepared using the procedure for compound 12 to give the title product as white solid in 60% yield. 1H NMR (CDCl3) 0.99 (t, J=7.6, 3H), 2.62 (q, J=7.0, 2H), 2.93 (t, J=6.6, 2H), 3.76 (t, J=6.2, 2H), 5.89 (s, 1H), 7.15 (m, 1H), 7.19-7.30 (m, 6H), 7.47 (s, 1H), 9.10 (s, 1H). MS (ESI) [M+H]+ 327.3, [M+Na]+ 349.4, [M-H]− 325.3.
5-Chloro-N-[2-(4-chlorophenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (25)
Prepared using the procedure for compound 12 to give the title product as white solid in 50% yield. 1H NMR (CDCl3) 1.14 (t, J = 7.68 Hz, 3H), 2.71 - 2.82 (m, 2H), 2.97 (t, J = 6.78 Hz, 2H), 3.82 (q, J = 6.56 Hz, 2H), 6.04 (br. s., 1H), 7.26 - 7.39 (m, 6H), 7.58 (d, J = 1.41 Hz, 1H), 9.30 (br. s., 1H). MS (ESI) [M-H]− 359.5.
5-Chloro-N-[2-(4-methoxyphenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (26)
Prepared using the procedure for compound 12 to give the title product as white solid in 50% yield. 1H NMR (CDCl3) 1.09 (t, J=7.7, 3H), 2.71 (q, J=7.7, 2H), 2.92 (t, J=6.7, 2H), 3.80 (m, 5H), 5.98 (s, 1H), 6.89 (d, J=8.8, 2H), 7.18 (d, J=8.7, 2H), 7.22 (d, J=1.9, 1H), 7.31 (d, J=8.7, 1H), 7.55 (s, 1H), 9.28 (br, 1H). MS (ESI) [M+H]+ 357.2, [M-H]− 355.4.
5-Chloro-N-[2-(4-tert-butylphenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (27)
Prepared using the procedure for compound 12 to give the title product as white solid in 87% yield. 1H NMR (CDCl3) 1.01 (t, J=7.7, 3H), 2.66 (q, J=7.7, 2H), 2.94 (m, 2H), 3.84 (q, J=6.3, 2H), 5.99 (s, 1H), 7.19 (m, 3H), 7.34 (m, 3H), 7.53 (d, J=1.9, 1H), 9.73 (s, 1H). MS (ESI) [M+H]+ 383.3, [M-H]− 381.5.
5-Chloro-N-[2-(2-chlorophenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (28)
Prepared using the procedure for compound 12 to give the title product as white solid in 34% yield. 1H NMR (CDCl3) 1.15 (t, J=7.7, 3H), 2.79 (q, J=7.7, 2H), 3.14 (t, J=6.8, 2H), 3.85 (q, J=6.6, 2H), 6.01 (br, 1H), 7.20-7.23 (m, 3H), 7.28-7.33 (m, 2H), 7.56 (d, J=1.9, 1H), 9.23 (br, 1H). MS (ESI) [M+Na]+ 382.5, [M-H]− 359.3.
5-Chloro-N-[2-(3-chlorophenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (29)
Prepared using the procedure for compound 12 to give the title product as white solid in 37% yield. 1H NMR (CDCl3) 1.12 (t, J=7.7, 3H), 2.74 (q, J=7.6, 2H), 2.96 (t, J=6.8, 2H), 3.81 (q, J=6.4, 2H), 5.96 (br, 1H), 7.15 (m, 1H), 7.22 (m, 2H), 7.31 (m, 3H), 7.56 (d, J=2.1, 1H), 9.07 (br, 1H). MS (ESI) [M+H]+ 361.3, [M-H]− 359.5.
5-Chloro-N-[2-(3-methoxyphenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (30)
Prepared using the procedure for compound 12 to give the title product as white solid in 25% yield. 1H NMR (CDCl3) 1.07 (t, J = 7.6, 3H), 2.70 (d, J = 7.5, 2H), 2.95 (t, J = 6.6, 2H), 3.72 - 3.96 (m, 5H), 5.86 (br, 1H), 6.77 - 6.90 (m, 3H), 7.15 - 7.38 (m, 4H), 7.54 (d, J = 1.9, 1H), 9.04 (br, 1H). MS (ESI) [M+H]+ 357.2.
5-Chloro-N-[2-(3-hydroxyphenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (31)
Prepared using the procedure for compound 12 to give the title product as white solid in 29% yield. 1H NMR (CDCl3) 1.07 (t, J=7.6, 3H), 2.69 (q, J=7.6, 2H), 2.89 (t, J=6.6, 2H), 3.79 (m, 2H), 6.06 (s, 1H), 6.79 (m, 3H), 7.18 (m, 3H), 7.51 (s, 1H), 9.28 (s, 1H). MS (ESI) [M+H]+ 343.5, [M-H]− 341.5.
5-Chloro-N-[2-(3-hydroxy-4-methoxyphenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (32)
Prepared using the procedure for compound 12 to give the title product as white solid in 36% yield. 1H NMR (CDCl3) 1.09 (t, J=7.6, 3H), 2.73 (m, 2H), 2.90 (t, J=6.7, 2H), 3.79 (m, 2H), 3.85 (s, 3H), 6.76 (m, 2H), 6.89 (d, J=8.5, 1H), 7.21 (m, 1H), 7.31 (m, 1H), 7.55 (s, 1H), 9.28 (br, 1H). MS (ESI) [M+H]+ 373.2, [M-H]− 371.4.
5-Chloro-N-[2-(3,4-dichlorophenyl)ethyl]-3-ethyl-1H-indole-2-carboxamide (33)
Prepared using the procedure for compound 12 to give the title product as white solid in 23% yield. 1H NMR (CDCl3) 1.16 (t, J = 7.82 Hz, 3H), 2.79 (q, J = 7.79 Hz, 2H), 3.10 (m, 2H), 3.85 (q, J = 6.53 Hz, 2H), 6.01 (br. s., 1H), 7.29 - 7.35 (m, 1H), 7.44 (d, J = 8.67 Hz, 2H), 7.57 (s, 1H), 8.21 (d, J = 8.29 Hz, 2H), 9.01 (br. s., 1H). MS (ESI) [M]+ 394.1.
N-[2-(2H-1,3-Benzodioxol-5-yl)ethyl]-5-chloro-3-ethyl-1H-indole-2-carboxamide (34)
Prepared using the procedure for compound 12 to give the title product as white solid in 33% yield. 1H NMR (CDCl3) 1.13 (t, J=7.6, 3H), 2.74 (q, J=7.6, 2H), 2.89 (t, J=7.0, 2H), 3.77 (q, J=6.2, 2H), 5.96 (s, 2H), 6.71 (m, 1H), 6.77 (m, 2H), 7.21 (m, 1H), 7.30 (m, 1H), 7.55 (d, J=1.9, 1H), 8.99 (br, 1H). MS (ESI) [M+H]+ 371.4, [M-H]− 369.5.
5-Chloro-N-{3-[6-(dimethylamino)pyridin-2-yl]phenyl}-3-ethyl-1H-indole-2-carboxamide (35)
Prepared using the procedure for compound 12 to give the title product as white solid in 40% yield. 1H NMR (CDCl3) 1.46 (t, J = 7.63 Hz, 3H), 3.12 (q, J = 7.72 Hz, 2H), 3.18 (s, 6H), 6.52 (d, J = 8.29 Hz, 1H), 7.07 (d, J = 7.16 Hz, 1H), 7.24 (d, J = 2.07 Hz, 1H), 7.32 - 7.38 (m, 1H), 7.47 (t, J = 8.01 Hz, 1H), 7.54 (dd, J = 7.54, 8.48 Hz, 1H), 7.65 (d, J = 2.07 Hz, 1H), 7.78 (dd, J = 1.22, 8.01 Hz, 1H), 7.84 (d, J = 6.22 Hz, 2H), 8.20 (t, J = 1.79 Hz, 1H), 9.09 (br. s., 2H). MS (ESI) [M+H]+ 419.7, [M-H]− 417.4.
5-Chloro-3-ethyl-2-(4-phenylpiperazine-1-carbonyl)-1H-indole (36)
Prepared using the procedure for compound 12 to give the title product as white solid in 45% yield. 1H NMR (CDCl3) 1.31 (t, J=7.6, 3H), 2.81 (q, J=7.5, 2H), 3.22 (m, 2H), 3.86 (m, 2H), 6.94 (d, J=7.9, 2H), 7.22-7.32 (m, 5H), 7.63 (d, J=1.7, 1H), 8.46 (br, 1H). MS (ESI) [M+H]+ 368.1, [M-H]− 366.6.
5-Chloro-2-[4-(4-chlorophenyl)piperazine-1-carbonyl]-3-ethyl-1H-indole (37)
Prepared using the procedure for compound 12 to give the title product as white solid in 80% yield. 1H NMR (CDCl3) 1.30 (t, J=7.6, 3H), 2.79 (q, J=7.6, 2H), 3.18 (m, 4H), 3.84 (m, 4H), 6.84 (d, J=9.0, 2H), 7.18-7.29 (m, 4H), 7.61 (s, 1H), 8.78 (s, 1H). MS (ESI) [M]+ 402.3.
tert-Butyl N-[2-(4-aminophenyl)ethyl]carbamate (39)
To a solution of 2-(4-nitrophenyl)ethan-1-amine (38, 0.3 g, 1.48 mmol) in 1,4-dioxane (4 ml) and water (1.5 ml), 1N NaOH was added and the reaction mixture was cooled to 0 °C. Upon addition of Boc2O, the reaction was stirred at room temperature for 1h. After no starting material was detected on TLC, solvent was removed in vacuo and the solid residue was dissolved in dichloromethane, washed with water and brine, dried over anhydrous MgSO4, and concentrated in vacuo to give the desired product as white solid (0.386 g, 98% yield). 1H NMR (CDCl3) 1.43 (s, 9H), 2.92 (t, J=6.6, 2H), 3.41 (t, J=6.6, 2H), 4.56 (br, 1H), 7.36 (d, J=8.5, 2H), 8.17 (d, J=8.7, 2H). This intermediate underwent the reduction procedure for 14 to give the title product as white solid in 85% yield. 1H NMR (CDCl3) 1.43 (s, 9H), 2.68 (t, J=6.9, 2H), 3.32 (t, J=6.2, 2H), 3.59 (br, 2H), 6.64 (d, J=8.3, 2H), 6.98 (d, J=8.1, 2H).
tert-Butyl N-{2-[4-(diethylamino)phenyl]ethyl}maximumcarbamate (40)
Prepared using the procedure for 17 to give the title product as colorless oil in 85% yield. 1H NMR (CDCl3) 1.14 (t, J=7.1, 6H), 1.43 (s, 9H), 2.67 (t, J=6.9, 2H), 3.33 (m, 6H), 4.56 (br, 1H), 6.63 (d, J=8.7, 2H), 7.03 (d, J=8.7, 2H).
tert-Butyl N-{2-[4-(piperidin-1-yl)phenyl]ethyl}carbamate (41)
A microwave vial containing 39 (0.05 g, 0.21 mmol), 1,5-dibromopentane (31 μL, 0.23 mmol), potassium carbonate (0.032 g, 0.23 mmol) in water (2 ml) was irradiated at 120 °C for 20 min (maximum pressure: 17 psi, power: 200W, PowerMax: off, ramp: 2 min). The reaction mixture was extracted with ethyl acetate, dried over anhydrous MgSO4, and concentrated in vacuo to give the desired product as yellow solid (0.03 g, 47% yield). 1H NMR (CDCl3) 1.43 (s, 9H) 1.57 (m, 2H), 2.17 (m, 4H), 2.70 (t, J=6.9, 2H), 3.12 (m, 4H), 3.32 (m, 2H), 6.88 (d, J=8.7, 2H), 7.07 (d, J=8.5, 2H).
5-Fluoro-N-{2-[4-(diethylamino)phenyl]ethyl}-3-ethyl-1H-indole-2-carboxamide (42)
Prepared using the procedure for compound 12 to give the title product as white solid in 54% yield. 1H NMR (CDCl3) 1.08 (t, J=7.6, 3H), 1.15 (t, J=7.1, 6H), 2.69 (m, 2H), 2.86 (t, J=6.6, 2H), 3.34 (q, J=7.0, 4H), 3.78 (q, J=6.5, 2H), 6.04 (s, 1H), 6.66 (d, J=8.7, 2H), 7.02 (m, 1H), 7.10 (d, J=8.7, 2H), 7.20 (dd, J1=9.6, J2=2.4, 1H), 7.31 (dd, J1=8.9, J2=4.3, 1H), 9.40 (br, 1H). MS (ESI) [M+H]+ 382.5, [M-H]− 380.6.
5-Fluoro-N-{2-[4-(diethylamino)phenyl]ethyl}-3-methyl-1H-indole-2-carboxamide (43)
Prepared using the procedure for compound 12 to give the title product as white solid in 53% yield. 1H NMR (CDCl3) 1.15 (t, J=7.0, 6H), 2.29 (s, 3H), 2.86 (t, J=6.7, 2H), 3.34 (q, J=7.2, 4H), 3.76 (q, J=6.4, 2H), 6.06 (s, 1H), 6.66 (d, J=8.7, 2H), 7.01 (m, 1H), 7.09 (d, J=8.7, 2H), 7.19 (dd, J1=9.5, J2=2.4, 1H), 7.31 (m, 1H), 9.54 (br, 1H). MS (ESI) [M+H]+ 368.4, [M-H]−366.6.
5-Fluoro-N-{2-[4-(diethylamino)phenyl]ethyl}-1H-indole-2-carboxamide (44)
Prepared using the procedure for compound 12 to give the title product as white solid in 33% yield. 1H NMR (CDCl3) 1.17 (t, J=7.1, 6H), 2.83 (t, J=6.8, 2H), 3.35 (q, J=7.2, 4H), 3.69 (q, J=6.6, 2H), 6.19 (s, 1H), 6.67 (m, 3H), 7.02 (m, 1H), 7.08 (m, 2H), 7.25 (m, 1H), 7.37 (dd, J1=8.9, J2=4.4, 1H), 9.38 (br, 1H). MS (ESI) [M+H]+ 354.5, [M-H]− 352.4.
5-Chloro-N-{2-[4-(diethylamino)phenyl]ethyl}-1H-indole-2-carboxamide (45)
Prepared using the procedure for compound 12 to give the title product as white solid in 30% yield. 1H NMR (CDCl3) 1.17 (t, J=7.1, 6H), 2.84 (t, J=6.8, 2H), 3.35 (q, J=7.0, 4H), 3.69 (m, 2H), 6.25 (s, 1H), 6.65 (m, 3H), 7.09 (d, J=8.7, 2H), 7.22 (m, 1H), 7.38 (m, 1H), 7.58 (s, 1H), 9.78 (br, 1H). MS (ESI) [M+H]+ 370.3, [M-H]− 368.5.
5-Fluoro-3-ethyl-N-{2-[4-(piperidin-1-yl)phenyl]ethyl}-1H-indole-2-carboxamide (46)
Prepared using the procedure for compound 12 to give the title product as white solid in 95% yield. 1H NMR (CDCl3) 1.08 (t, J=7.7, 3H), 1.58 (m, 2H), 1.71 (m, 4H), 2.70 (q, J=7.7, 2H), 2.89 (t, J=6.6, 2H), 3.12 (m, 4H), 3.79 (q, J=6.4, 2H), 6.02 (s, 1H), 6.91 (d, J=8.7, 2H), 7.01 (m, 1H), 7.14 (d, J=8.7, 2H), 7.20 (dd, J1=9.4, J2=2.4, 1H), 7.31 (m, 1H), 9.46 (br, 1H). MS (ESI) [M+H]+ 394.6, [M-H]− 392.7.
5-Fluoro-3-methyl-N-{2-[4-(piperidin-1-yl)phenyl]ethyl}-1H-indole-2-carboxamide (47)
Prepared using the procedure for compound 12 to give the title product as white solid in 96% yield. 1H NMR (CDCl3) 1.59 (m, 2H), 1.72 (m, 4H), 2.28 (s, 3H), 2.88 (t, J=6.6, 2H), 3.13 (m, 4H), 3.77 (q, J=6.3, 2H), 6.02 (s, 1H), 6.92 (d, J=8.5, 2H), 7.03 (m, 1H), 7.13 (d, J=8.5, 2H), 7.19 (dd, J1=9.5, J2=2.2, 1H), 7.31 (m, 1H), 9.41 (br, 1H). MS (ESI) [M+H]+ 380.3, [M-H]− 378.5.
5-Fluoro-N-{2-[4-(piperidin-1-yl)phenyl]ethyl}-1H-indole-2-carboxamide (48)
Prepared using the procedure for compound 12 to give the title product as white solid in 55% yield. 1H NMR (CDCl3) 1.26 (m, 2H), 1.72 (m, 4H), 2.85 (t, J=6.8, 2H), 3.13 (m, 4H), 3.70 (q, J=6.6, 2H), 6.14 (s, 1H), 6.63 (d, J=1.3, 1H), 6.93 (d, J=8.7, 2H), 7.04 (m, 1H), 7.12 (d, J=8.7, 2H), 7.24 (m, 1H), 7.36 (m, 1H), 9.21 (br, 1H). MS (ESI) [M+H]+ 366.5, [M-H]− 364.6.
5-Chloro-N-{2-[4-(piperidin-1-yl)phenyl]ethyl}-1H-indole-2-carboxamide (49)
Prepared using the procedure for compound 14 to give the title product as white solid in 30% yield. 1H NMR (CDCl3) 1.26 (m, 2H), 1.72 (m, 4H), 2.85 (t, J=6.8, 2H), 3.15 (m, 4H), 3.70 (q, J=6.6, 2H), 6.15 (s, 1H), 6.61 (d, J=1.5, 1H), 6.92 (d, J=8.7, 2H), 7.12 (d, J=8.7, 2H), 7.23 (m, 1H), 7.36 (d, J=8.7, 1H), 7.59 (d, J=1.9, 1H), 9.32 (br, 1H). MS (ESI) [M+H]+ 382.5, [M-H]− 380.5.
5.2. Biological assays
5.2.1. CB1 Calcium Mobilization Assay
CHO-RD-HGA16 cells (Molecular Devices, USA) stably expressing the human CB1 receptor were used. The day before the assay, cells were plated into 96-well black-walled assay plates at 25,000 cells/well in Ham's F12 supplemented with 10% fetal bovine serum, 100 units of penicillin, 100 units of streptomycin, and 100 μg/mL g/mL Normocin Normocin™. The cells were incubated overnight at 37°C, 5% CO2. Prior to the assay, Calcium 5 dye (Molecular Devices, USA) was reconstituted according to the manufacturer's instructions. The reconstituted dye was diluted 1:40 in pre-warmed (37°C) assay buffer (1X HBSS, 20 mM HEPES, 2.5 mM probenecid, pH 7.4 at 37°C). Growth medium was removed and the cells were gently washed with 100 μL of pre-warmed (37°C) assay buffer. The cells were incubated for 45 minutes at 37°C, 5% CO2 in 200 μL of the diluted Calcium 5 dye. For antagonist (IC50) assays, the EC80 concentration of CP55,940 was prepared at 10× the desired final concentration in 0.25% BSA/0.5% DMSO/0.5% EtOH/assay buffer, aliquoted into 96-well polypropylene plates, and warmed to 37°C. Serial dilutions of the test compounds were prepared at 10× the desired final concentration in 2.25% BSA/4.5% DMSO/4.5% EtOH/assay buffer. After the dye-loading incubation period, the cells were pre-treated with 25 μL of the test compound serial dilutions and incubated for 15 min at 37°C. After the pre-treatment incubation period, the plate was read with a FLIPR Tetra (Molecular Devices, USA). Calcium-mediated changes in fluorescence were monitored every 1 second over a 90 second time period, with the Tetra adding 25 μL of the CP55,940 EC80 concentration at the 10 second time point (excitation at 470-495 nm, detection at 515-575 nm). FLIPR traces were normalized by trace alignment at the last time point before agonist addition (ScreenWorks software, Molecular Devices, USA). Relative fluorescence units (RFU) were plotted against log compound concentration. Data were fit to a three-parameter logistic curve to generate IC50 values (GraphPad Prism 6.0, GraphPad Software, Inc., San Diego, CA). For the modulation experiments, the above procedure was followed except that cells were pre-treated with a single concentration of test compound (prepared at 10× the desired concentration in 2.25% BSA/4.5% DMSO/4.5% EtOH/assay buffer) and the Tetra added serial dilutions of CP55,940 (prepared at 10× the desired concentration in 0.25% BSA/0.5% DMSO/0.5% EtOH/assay buffer). For agonist screens, the above procedure was followed except that cells were pre-treated with 2.25% BSA/4.5% DMSO/4.5% EtOH/assay buffer and the Tetra added single concentration dilutions of the test compounds prepared at 10× the desired final concentration in 0.25% BSA/0.5% DMSO/0.5% EtOH/assay buffer. Test compound RFUs were compared to the CP55,940 Emax RFUs to generate % Emax values.
5.2.2. CB2 Calcium Mobilization Assay
The CB2 assay used CHO-RD-HGA16 cells (Molecular Devices, USA) stably expressing the human CB2 receptor. This assay was carried out in a similar manner as the CB1 assay except that cells were plated at a density of 30,000 cells/well.
Supplementary Material
Acknowledgments
We thank Tiffany Langston for technical assistance and David Perrey for the critical reading of the manuscript. This work was supported by National Institute on Drug Abuse, National Institutes of Health, USA (grants DA032837 and DA003672), and by the National Natural Science Foundation of China (81473089).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Agonist screens at the CB1 and CB2 receptors and HPLC analysis of target compounds.
References and notes
- 1.Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Frontiers in behavioral neuroscience. 2012;6:9. doi: 10.3389/fnbeh.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Di M, Bisogno T, De Petrocellis L. Endocannabinoids: new targets for drug development. Curr. Pharm. Des. 2000;6:1361–1380. doi: 10.2174/1381612003399365. [DOI] [PubMed] [Google Scholar]
- 4.Mackie K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- 5.Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005;7:E625–654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makriyannis A. 2012 Division of medicinal chemistry award address. Trekking the cannabinoid road: a personal perspective. J. Med. Chem. 2014;57:3891–3911. doi: 10.1021/jm500220s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 2006;30(Suppl 1):S13–18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 9.Jagerovic N, Fernandez-Fernandez C, Goya P. CB1 cannabinoid antagonists: structure-activity relationships and potential therapeutic applications. Curr. Top. Med. Chem. 2008;8:205–230. doi: 10.2174/156802608783498050. [DOI] [PubMed] [Google Scholar]
- 10.Ross RA. Tuning the endocannabinoid system: allosteric modulators of the CB1 receptor. Br. J. Pharmacol. 2007;152:565–566. doi: 10.1038/sj.bjp.0707349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross RA. Allosterism and cannabinoid CB(1) receptors: the shape of things to come. Trends Pharmacol. Sci. 2007;28:567–572. doi: 10.1016/j.tips.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Shore DM, Baillie GL, Hurst DH, Navas F, 3rd, Seltzman HH, Marcu JP, Abood ME, Ross RA, Reggio PH. Allosteric modulation of a cannabinoid G protein-coupled receptor: binding site elucidation and relationship to G protein signaling. J. Biol. Chem. 2014;289:5828–5845. doi: 10.1074/jbc.M113.478495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulation ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J. Biol. Chem. 2012;287:12070–12082. doi: 10.1074/jbc.M111.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horswill JG, Bali U, Shaaban S, Keily JF, Jeevaratnam P, Babbs AJ, Reynet C, Wong Kai In P. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 2007;152:805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro HA, Howard JL, Pollard GT, Carroll FI. Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br. J. Pharmacol. 2009;156:1178–1184. doi: 10.1111/j.1476-5381.2009.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pamplona FA, Ferreira J, Menezes de Lima O, Jr., Duarte FS, Bento AF, Forner S, Villarinho JG, Bellocchio L, Wotjak CT, Lerner R, Monory K, Lutz B, Canetti C, Matias I, Calixto JB, Marsicano G, Guimaraes MZ, Takahashi RN. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. U.S.A. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug. Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, Conn PJ, Lindsley CW. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 2012;55:1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, Westwood P, Marrs J, Thomson F, Cowley P, Christopoulos A, Pertwee RG, Ross RA. Allosteric modulation of the cannabinoid CB1 receptor. Mol. Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- 21.Baillie GL, Horswill JG, Anavi-Goffer S, Reggio PH, Bolognini D, Abood ME, McAllister S, Strange PG, Stephens GJ, Pertwee RG, Ross RA. CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol. Pharmacol. 2013;83:322–338. doi: 10.1124/mol.112.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamage TF, Ignatowska-Jankowska BM, Wiley JL, Abdelrahman M, Trembleau L, Greig IR, Thakur GA, Tichkule R, Poklis J, Ross RA, Pertwee RG, Lichtman AH. In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav. Pharmacol. 2014;25:182–185. doi: 10.1097/FBP.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Y, Qiu Y, Jing L, Thorn DA, Zhang Y, Li JX. Behavioral effects of the cannabinoid CB1 receptor allosteric modulator ORG27569 in rats. Pharmacology research & perspectives. 2014;2:e00069. doi: 10.1002/prp2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing L, Qiu Y, Zhang Y, Li JX. Effects of the cannabinoid CB(1) receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 2014;143:251–256. doi: 10.1016/j.drugalcdep.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piscitelli F, Ligresti A, La Regina G, Coluccia A, Morera L, Allara M, Novellino E, Di Marzo V, Silvestri R. Indole-2-carboxamides as allosteric modulators of the cannabinoid CB(1) receptor. J. Med. Chem. 2012;55:5627–5631. doi: 10.1021/jm201485c. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoud MM, Ali HI, Ahn KH, Damaraju A, Samala S, Pulipati VK, Kolluru S, Kendall DA, Lu D. Structure-Activity Relationship Study of Indole-2-carboxamides Identifies a Potent Allosteric Modulator for the Cannabinoid Receptor 1 (CB1). J. Med. Chem. 2013 doi: 10.1021/jm4009828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khurana L, Ali HI, Olszewska T, Ahn KH, Damaraju A, Kendall DA, Lu D. Optimization of chemical functionalities of indole-2-carboxamides to improve allosteric parameters for the cannabinoid receptor 1 (CB1). J. Med. Chem. 2014;57:3040–3052. doi: 10.1021/jm5000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn KH, Mahmoud MM, Samala S, Lu D, Kendall DA. Profiling two indole-2-carboxamides for allosteric modulation of the CB1 receptor. J. Neurochem. 2013;124:584–589. doi: 10.1111/jnc.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami Y, Watanabe T, Takahashi H, Yokoo H, Nakazawa Y, Koshimizu H, Adachi N, Kurita M, Yoshino T, Inagaki T, Ohishi M, Watanabe M, Tani M, Yokoyama Y. Fischer indolization of 2-sulfonyloxyphenylhydrazones: A new and practical approach for preparing 7-oxygenated indoles and application to the first synthesis of eudistomidin-A (Fischer indolization and its related compounds, Part 28). Tetrahedron. 1998;54:45–64. [Google Scholar]
- 30.Ju Y, Varma RS. Aqueous N-heterocyclization of primary amines and hydrazines with dihalides: microwave-assisted syntheses of N-azacycloalkanes, isoindole, pyrazole, pyrazolidine, and phthalazine derivatives. J. Org. Chem. 2006;71:135–141. doi: 10.1021/jo051878h. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Gilliam A, Maitra R, Damaj MI, Tajuba JM, Seltzman HH, Thomas BF. Synthesis and biological evaluation of bivalent ligands for the cannabinoid 1 receptor. J. Med. Chem. 2010;53:7048–7060. doi: 10.1021/jm1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulp A, Bortoff K, Zhang Y, Seltzman H, Mathews J, Snyder R, Fennell T, Maitra R. Diphenyl purine derivatives as peripherally selective cannabinoid receptor 1 antagonists. J. Med. Chem. 2012;55:10022–10032. doi: 10.1021/jm301181r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.German N, Decker AM, Gilmour BP, Gay EA, Wiley JL, Thomas BF, Zhang Y. Diarylureas as allosteric modulators of the cannabinoid CB1 receptor: structure-activity relationship studies on 1-(4-chlorophenyl)-3-{3-[6-(pyrrolidin-1-yl)pyridin-2-yl]phenyl}urea (PSNCBAM-1). J. Med. Chem. 2014;57:7758–7769. doi: 10.1021/jm501042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.