Abstract
Alcohol (ALC) is a drug that is capable of disrupting reproductive function in adolescent humans, as well as immature rhesus monkeys and rats. Critical to determining the mechanism(s) of the effects of ALC on the pubertal process is to have a better understanding of the important events involved in the initiation of puberty. For years it has been hypothesized that there may be metabolic signals capable of linking somatic growth to the activation of the reproductive system at the time of puberty. In recent years it has been shown that insulin-like growth factor-1 (IGF-1) is one such signal that plays an early role in the pubertal process. In this review, we will describe the actions and interactions of ALC and IGF-1 on molecular and physiological processes associated with pubertal development.
Keywords: Alcohol, IGF-1, Puberty
The age at mammalian puberty is governed by central inputs controlling the synthesis and secretion of luteinizing hormone releasing hormone (LHRH) within the preoptic and hypothalamic regions of the brain. Importantly, there is a diurnal increase in the pulsatile secretion of LHRH at the time of puberty in both humans and laboratory animals. This increased release is the result of the activation of the LHRH pulse generator (Ojeda and Terasawa, 2002; Plant, 2002), resulting in the surge of prepubertal LHRH release into portal blood (Sarkar and Fink, 1979). The increased secretion of this peptide is responsible for further stimulation of pituitary gonadotropin output, resulting in ovarian maturation. The central inputs responsible for influencing LHRH secretion at this time of development are made up of both inhibitory and stimulatory components. The inhibitory components, such as gamma aminobutyric acid (GABA) and opioid peptides (Terasawa, 1999; Terasawa and Fernandez, 2001) are thought to be responsible for applying a brake on puberty during childhood. The stimulatory components, such as norepinephrine (Sarkar et al., 1981) excitatory amino acids (Claypool et al., 2000; Urbanski and Ojeda, 1990), leptin (Dearth et al., 2000), TGF-α (Ojeda et al., 1990), insulin-like growth factor-1 (IGF-1) (Hiney et al., 1991, 1996), and the kisspeptins (Navarro et al., 2004a) are responsible for driving LHRH secretion until sexual maturity. While these are all endogenous substances that influence peripubertal LHRH secretion, there are also exogenous influences such as drugs of abuse like alcohol (ALC) (Dissen et al., 2004; Hiney and Dees, 1991), and environmental substances like manganese (Lee et al., 2007), that can also act as inhibitors or stimulators, respectively, on LHRH secretion and pubertal development.
As we will detail herein, IGF-1 is beginning to stand out as one of the excitatory components playing an early, perhaps initiatory role, in events leading to enhanced LHRH secretion and the onset of puberty. Conversely, ALC has been shown to disrupt specific important events in the pubertal process. This is important since there has been a growing trend indicating that ALC use and abuse often occurs during early adolescence (Johnson et al., 2006). The increasing incidence of ALC use by adolescents is noteworthy because it represents a potentially vulnerable time for these youth, since their tolerance to the drug is likely low. While case reports involving ALC use by adolescent humans are limited in number and scope, it has been revealed that ALC can cause altered puberty-related hormones in adolescent boys and girls (Block et al., 1993; Diamond et al., 1986). Therefore, there is a risk for ALC to alter specific genes and hormones known to be important at a critical time of growth and development. In recent years, both rats and rhesus monkeys have been used as animal models to more closely assess the effects of ALC on puberty- related events. Thus, the purpose of this mini-review is to discuss the results of research using these animal models with regard to the effects of ALC and IGF-1 at the time of puberty. Furthermore, we will describe important novel interactions between these two substances on specific hormones and on a recently defined gene system associated with prepubertal LHRH secretion and the subsequent acquisition of reproductive competence.
EFFECTS OF ALC ON KEY HYPOTHALAMIC AND PITUITARY HORMONES AND THE TIMING OF PUBERTY
Initial studies revealed that chronic ALC exposure caused delayed puberty in both male (Ramaley, 1982) and female (Bo et al., 1982) rats, and in male mice (Anderson et al., 1981). Subsequently, immature female rats were used to correlate the ALC-induced delayed puberty with alterations in specific hormones known to be involved with sexual maturation. In this regard, vaginal opening (VO) as well as the time to first diestrus was markedly delayed, and the serum levels of growth hormone (GH) and luteinizing hormone (LH) were depressed following chronic, prepubertal ALC exposure (Dees and Skelley, 1990). Interestingly, the serum concentration of follicle stimulating hormone (FSH) was not affected, demonstrating the same differential effect of ALC on gonadotropin secretion previously shown in adult female rats (Dees and Kozlowski, 1984; Dees et al., 1985). Since GH and LH are important for ovarian maturation, it was not surprising that studies have also shown that ALC causes suppressed prepubertal levels of serum estradiol (E2) (Bo et al., 1982; Srivastava et al., 1999). An important study using immature rhesus monkeys revealed that chronic exposure to a low dose of ALC also altered critical puberty-related hormones. In this regard, the serum levels of GH were suppressed in the ALC-treated monkeys (Fig. 1A), and it was noted that this action was primarily due to blocking the pattern of elevated GH that normally occurs in the evening during late juvenile development (Dees et al., 2000). While FSH levels were not altered (Dees et al., 2000), the ALC caused suppressed serum LH levels (Fig. 1B), an effect that was associated with decreased prepubertal levels of serum E2 (Fig. 1C). Furthermore, although the age at first menstruation was not altered the interval between subsequent menstruations was twice that of the control monkeys (Fig. 1D), demonstrating that ALC alters the development of a regular monthly pattern of menstruation.
Fig. 1.
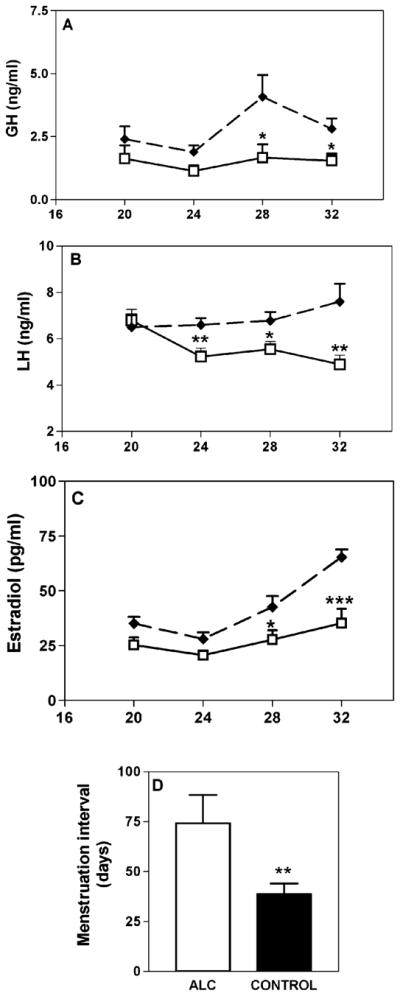
Effects of ALC on the serum levels of puberty-related hormones and on menstrual patterns in developing rhesus monkeys. Blood samples were drawn from the saphenous vein twice daily (0830 hours am and 2030 hours pm) and their averages for 5 consecutive days determined each monkey’s respective hormone level over the sampling period. Each data point represents the mean (±SEM) 5-day hormone levels from 5 control and 5 ALC-treated rhesus monkeys at each designated month. (A) GH levels were suppressed in the ALC-treated monkeys at 28 and 32 months of age. (B) LH levels were suppressed from 24 to 32 months. (C) E2 levels increased markedly between 28 and 32 months of age in the control monkeys, an action that did not occur in the ALC-treated monkeys, hence causing them to be suppressed at these time points. (D) The interval between menstruations for the ALC-treated monkeys was almost twice that of the controls. Solid and open bars represent the means (±SEM) of control and ALC-treated animals, respectively. *p < 0.05; **p < 0.01; ***p < 0.001. N = 4 to 5 animals per group. Panels A–C were assessed by ANOVA, followed by Student–Neuman–Keul’s post-hoc test. Panel D was assessed by Student’s t-test.
The suppressed prepubertal levels of GH and LH prompted experimentation to discern whether the site of ALC action to block entry into the peripubertal period was at the hypothalamic or pituitary level. In immature female rats the hypothalamic content of the GH inhibiting hormone, somatostatin, was not altered following chronic ALC administration; however, there was a significant increase in the hypothalamic content of GH-releasing hormone (GHRH), an action that was accompanied by a marked decrease in the serum concentration of GH (Dees et al., 1990). In those same animals, ALC caused an increase in the hypothalamic content of LHRH with a concomitant decrease in LH, but not FSH (Dees et al., 1990). The increased content of the hypothalamic releasing hormones suggested that the ALC suppressed their secretion; hence, indicating a hypothalamic action of chronic ALC exposure to cause decreased pituitary hormone secretion. Further evidence for a hypothalamic action was derived from pituitary response studies. GH and LH secretory responsiveness to their respective hypothalamic releasing peptides has been shown to be unaltered by ALC in immature animals. This was best revealed in prepubertal female rhesus monkeys following chronic ALC exposure (Dissen et al., 2004). In this regard, the site of action to suppress LH secretion was assessed by hypothalamic and pituitary responsiveness to N-methyl D-L aspartic acid (NMA) and LHRH, respectively. The hypothalamic test showed that NMA stimulated LH release in control monkeys, an action that was blocked in the monkeys that received ALC. This is important since NMA is known to induce LH release by acting within the hypothalamus to stimulate LHRH secretion and does not act at the pituitary level. Three weeks later, the pituitary test was conducted using the same animals and the results showed that LHRH stimulated LH equally well in both control and ALC-treated monkeys. These results in combination with in vivo and in vitro studies in female rats showing that ALC suppresses hypothalamic LHRH secretion further demonstrates that the detrimental action of ALC is at the hypothalamic level (Ching et al., 1988; Hiney and Dees, 1991; Hiney et al., 2003; Nyberg et al., 1993).
The fact that ALC alters prepubertal GH and LH secretions is important, since these two hormones are involved in the control of growth and reproduction. For many years it has been hypothesized that somatic development may influence sexual maturation through metabolic signals that can act centrally to modify LHRH neuronal function (Plant, 1988). The fact that IGF-1 synthesis by the liver is controlled by GH, and that this peptide is a messenger carrying out the actions of GH, sparked our interest in assessing whether the IGF-1 peptide plays a role as a metabolic signal influencing the timing of puberty, and if so, determining whether IGF-1 is a target for ALC to disrupt the pubertal process.
INFLUENCES OF IGF-1 AT PUBERTY
IGF-1 is a 70 amino acid polypeptide mitogen that plays an essential role in mammalian growth, as well as cellular differentiation and survival. The peptide is produced in many tissues including brain, but most predominantly in the liver (Sara and Hall, 1990). IGF-1 binds to type 1 IGF receptors (IGF-1R) in numerous tissues (Delafontaine, 1998; Vendola et al., 1999; Werther et al., 1989), including brain (Bondy et al., 1992) where the greatest concentration of IGF-1R resides in the median eminence (ME) of the hypothalamus. The serum levels of IGF-1 increase strikingly during puberty in rodents (Crawford et al., 1993; Handelsman et al., 1987), ruminants (Jones et al., 1991; Roberts et al., 1990), and primates (Copeland et al., 1982, 1985) including humans (Anders et al., 1994; Tam et al., 2006). Thus, while it was hypothesized that this trophic factor may link somatic growth to the activation of the reproductive hypothalamus, it was critical in this regard to first determine whether the peptide was capable of acting centrally to induce prepubertal LHRH secretion.
Effects of IGF-1 on Puberty-Related Hormones and Events
An initial study examined the ability of IGF-1 to stimulate the secretion of LHRH from MEs removed from immature female rats and incubated in vitro (Hiney et al., 1991). The results showed that the peptide elicited a dose-related increase in LHRH release into the medium, an effect that occurred at a 10-fold lower dose than that required by insulin and IGF-2 to stimulate LHRH release. This information prompted us to conduct a follow-up study to assess the in vivo actions of IGF-1 in 30- to 37-day-old female rats, a time frame representing late juvenile development and continuing through the peripubertal period to first diestrus (Hiney et al., 1996). IGF-1 gene expression did not change across the peripubertal period in the preoptic area (POA) or medial basal hypothalamus (MBH); however, a developmental increase in liver IGF-1 gene expression was observed on the day of first proestrus, a change that was accompanied by elevated serum levels of IGF-1, along with the serum levels of LH and E2 (Fig. 2A– C). Importantly, the increase in serum IGF-1 was accompanied by an increase in IGF-1 receptor gene expression in the median eminence. Furthermore, the central administration of IGF-1 induced LH release in juvenile and peripubertal female rats, an action that was blocked by prior immunoneutralization of hypothalamic LHRH. Finally, to mimic the peripubertal afternoon increase in LHRH/LH secretion (Andrews and Ojeda, 1981; Urbanski and Ojeda, 1985), we administered IGF-1 (20 ng) into the third ventricle of immature animals at 1500 and 1700 hours each afternoon. This protocol resulted in the advanced onset of female puberty (Fig. 2D). Subsequently, studies by two groups have confirmed the positive influence IGF-1 on the pubertal process. Systemic IGF-1 administration was shown to advance first ovulation in rhesus monkeys (Wilson, 1998), and replacement of IGF-1 advanced puberty in GH-receptor knockout mice expressing very low levels of the peptide (Danilovich et al., 1999). Collectively, these studies provide compelling evidence supporting an important role for IGF-1 at puberty. Conversely, two other studies did not show earlier puberty following IGF-1; however, in those studies the method and duration of central IGF-1 delivery was different, and much higher doses of the peptide were used (Gruaz et al., 1997; Zeinoaldini et al., 2006).
Fig. 2.
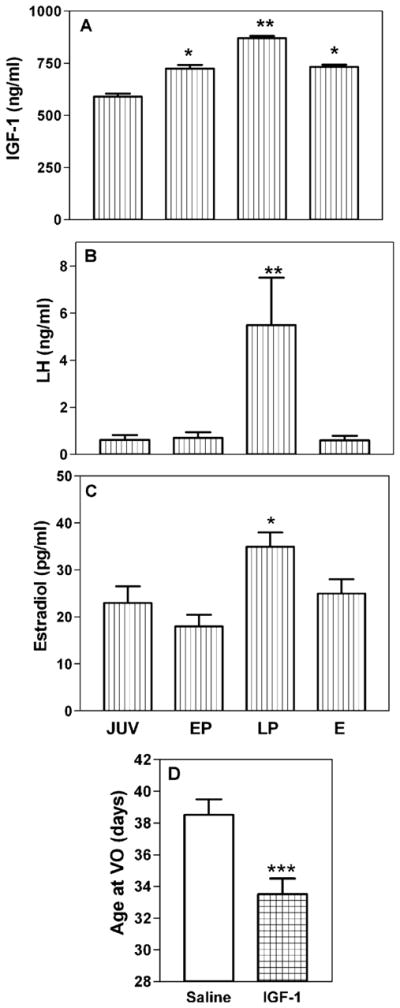
Serum IGF-1, LH, and E2 levels during pubertal development in the female rat. (A) The serum levels of IGF-1 increased during early proestrus (EP), peaked at late proestrus (LP), and remained elevated over the juvenile (JUV) levels through first estrus (E). (B and C) The serum levels of LH and E2 were also elevated during late proestrus. (D) Daily administration of IGF-1 advanced the time of vaginal opening. The respective bars represent the mean (±SEM) of an N of 25 to 40 animals per group in A–C and 6 to 7 per group in D. *p < 0.05 and **p < 0.01, by ANOVA with post-hoc testing using the Student–Newman–Keuls multiple range test; ***p < 0.001, by t-test.
The notion that IGF-1 of peripheral origin is capable of crossing the blood brain barrier and activating LHRH secretion at puberty is supported by the above mentioned studies showing that IGF-1 advanced female puberty in three species, and by the observation in female rats that there was no change in hypothalamic IGF-1 gene expression between 30 and 37 days if age. Other studies, however, suggest it is also possible that centrally derived IGF-1 may also play a role at puberty. It has been shown in both male and female mice that IGF-1 gene expression in the reproductive hypothalamus is elevated during the neonatal period, decreases through 20 days of life, and then increases by day 30 and again by day 60 (Daftary and Gore, 2003). These authors also noted that this gene expression pattern was similar to that observed for hypothalamic IGF-1 protein as assessed by immunocytochemistry. Thus, research to date indicates that hypothalamic IGF-1 gene expression increases by the end of juvenile development (Daftary and Gore, 2003), but does not increase again during the peripubertal period (Hiney et al., 1996). The serum levels of the peptide begin to increase at 20 days of age, continue to rise through juvenile development (Handelsman et al., 1987), and then show a final peak increase at first proestrus (Hiney et al., 1996). Therefore, these facts collectively suggest that increasing levels of both centrally and peripherally derived IGF-1 are available to the hypothalamus for influencing peripubertal LHRH neuronal functions.
IGF-1R Influences at Puberty
Further support for a role of IGF-1 at puberty is shown by the presence of the IGF-1R within the POA and the MBH, including the ME. In this regard, IGF-1R gene expression and protein levels have been shown to increase developmentally in the POA and MBH of male and female mice (Daftary and Gore, 2004). Additionally, those investigators also showed the presence of the IGF-1R on LHRH perikarya in the POA, septum and anterior hypothalamus. This coexpression in both the POA and ME, the principal sites within the rodent brain responsible for LHRH synthesis and release, respectively, further suggests IGF-1 may influence LHRH neuronal functions. These observations lend support to previous studies indicating that IGF-1 can stimulate LHRH gene expression in POA explants from 30-day-old female mice incubated in vitro (Daftary and Gore, 2003), and in specific LHRH neuronal cell lines (Anderson et al., 1999; Longo et al., 1998; Zhen et al., 1997). In the LHRH neuronal cell line, IGF-1 was shown to initially stimulate LHRH secretion into the incubation medium, then over time inhibited the release of the peptide, demonstrating a biphasic effect (Anderson et al., 1999; Longo et al., 1998). In female rats, we have shown there is a marked increase in IGF-1R content specifically within the ME on the day of first proestrus (Hiney et al., 1996). This increase in the ME is important for several reasons. The ME contains one of the highest concentrations of the IGF-1R in brain (Aguado et al., 1989; Bohannon et al., 1986; Lesniak et al., 1988; Marks et al., 1991; Werther et al., 1989), the structure is outside the blood brain barrier, contains the LHRH nerve terminals, and the timing of the increase corresponds to the proestrus rise in serum IGF-1. Thus, it is likely that the ME is a primary site of action for peripherally derived IGF-1 to stimulate LHRH/LH secretion at puberty. Importantly, IGF-1 is capable of stimulating LHRH release from isolated immature female ME in vitro (Hiney et al., 1991), but whether this effect is through a direct action on LHRH nerve terminals and/or requires communication with glial elements in the region is not known.
Effects of IGF-1 on Neuronal and Glial Puberty-Related Genes
In recent years it has become increasingly clear that there is an integrative participation between neuronal circuits and glial networks that facilitate LHRH secretion. It is thought that neural and glial excitatory signaling to LHRH neurons increases at a time when the inhibitory tone to those neurons decreases, and at this time the enhanced peripubertal activation of LHRH release occurs. While important progress has been made in identifying neural and glial elements involved in LHRH secretion (Ojeda et al., 2006), there has been a need for a better understanding as to what endogenous and exogenous substances control their expression. In this regard, we have recently assessed the effects of specific factors on puberty- related genes expressed in neurons and glial cells that are responsible for producing substances known to stimulate LHRH secretion. In the context of this review, we will limit the discussion below to one neuronal and one glial gene, since they have both been assessed as to the influences of IGF-1 and ALC.
The KiSS-1 metastis suppressor gene is responsible for the synthesis of kisspeptins. Neurons expressing this gene within the reproductive hypothalamus are located in two nuclei. Rostrally, the neurons are within the anteroventral periventricular nucleus (AVPV), which lies in the POA and anterior hypothalamus. Caudally, the neurons are within the arcuate nucleus, which is in the MBH. The kisspeptins are ligands of the G-protein coupled receptor 54 (GPR54), and have been shown to play a key role in the timing of puberty (Navarro et al., 2004b; Tena-Sempere, 2006). It has been shown that a mutation of the GPR54 gene in human (de Roux et al., 2003; Seminara et al., 2003), and a deletion of this gene in mice (Seminara et al., 2003) caused hypogonadotropic hypogonadism and a delay in puberty. Increases in KiSS-1 and GPR54 gene expressions have been observed during pubertal development (Navarro et al., 2004a), and the kisspeptins act at the hypothalamic level to stimulate LHRH/LH release in immature rats and rhesus monkeys (Navarro et al., 2004a; Shahab et al., 2005; Thompson et al., 2004), and advance vaginal opening in rats (Navarro et al., 2004b). Hence, in the last few years it has been widely accepted that the KiSS-1 gene is a major influence on the onset of puberty, but only recently have attempts been made to determine what governs the developmental activation of this gene. Because the actions of the kisspeptins are similar to those described above for IGF-1, we hypothesized that IGF-1 may be an upstream regulator of this gene. Our recent results revealed that IGF-1 stimulated the KiSS-1 gene in the AVPV nucleus of immature female rats (Hiney et al., 2009), an area that has previously been shown to contain the kisspeptin neurons that are more sensitive to changes in the steroid milieu at puberty (Clarkson and Herbison, 2006). The fact that this gene was induced after both central and systemic administration, and that this occurred prior to the normal rise in KiSS-1 gene expression at 30 days of age is important, and demonstrates that IGF-1 is an activator of this gene at a critical time of mammalian development. Whether this action occurs directly on the kisspeptin containing neuron, or through interneurons or glial cells that also expresses IGF-1R (Fernandez-Galaz et al., 1997; Lesniak et al., 1988) has not been determined.
The Oct-2 POU homeodomain genes are associated with hypothalamic development and are expressed in glial cells. Members of this gene family activate TGF-α, one of the key factors involved in the process by which glial products facilitate LHRH release at puberty (Ojeda et al., 1999). Because the expression of Oct-2 increases in the hypothalamus just before puberty (Ojeda et al., 1999), we assessed whether IGF- 1 could influence the expression of two isoforms of this gene. We demonstrated that early central administration of IGF-1 is capable of precociously inducing Oct-2c in the POA, and both Oct-2a and Oct-2c in the MBH prior to their normally occurring prepubertal increase (Dees et al., 2005). This indicates that IGF-1 is an upstream regulator of Oct-2 expression, and hence, by subsequently activating TGF-α, may represent an early event in enhanced cell–cell communications facilitating LHRH secretion at the time of puberty.
The above comments demonstrating that IGF-1 is capable of activating neuronal (KiSS-1) and glial (Oct-2) genes involved in the control of prepubertal LHRH secretion is important with regard to assessing sites and mechanisms of IGF-1 action to influence puberty. There are numerous genes expressed in neurons and glial cells within the POA and MBH regions of the brain that may develop an extensive and interrelated neuronal and glial communication network at puberty. This is a new and complicated area of study and identifying key specific genes and what controls them will no doubt help us gain a better understanding of events leading to mammalian puberty.
INTERACTIONS BETWEEN ALC AND IGF-1
The above section provided evidence demonstrating that IGF-1 is an important excitatory influence on LHRH secretion and the pubertal process. It was also pointed out that ALC alters key puberty-related hormones and delays signs of pubertal development. Because of these actions, we hypothesized that IGF-1 may be a substance through which ALC may affect pubertal development.
Effects of ALC on IGF-1 Synthesis
In immature female rats, chronic ALC exposure over the late juvenile-peripubertal period of development caused a decrease in the hepatic expression of both IGF-1a (Fig. 3A) and IGF-1b (not shown) gene transcripts; effects that were associated with altered growth and suppressed levels of serum IGF-1 and LH (Fig. 3B, C) (Srivastava et al., 1995). In those same rats, there were no ALC-induced changes observed in IGF-1 mRNA in either the POA or MBH, nor were any changes detected in IGF-1R mRNA within the ME. Importantly, similar results regarding growth and the serum levels of IGF-1 and LH have been observed in prepubertal female rhesus monkeys following chronic ALC exposure (Dees et al., 2000). Earlier studies using adult male rats also revealed suppressed serum IGF-1 levels following chronic ALC exposure (Sonntag and Boyd, 1988; Soszynski and Frohman, 1992). Because IGF-1 mediates the growth promoting effects of GH (Isaksson et al., 1987; Lowe et al., 1988), and because of the IGF-1 influence on prepubertal LHRH/LH secretion (Hiney et al., 1991, 1996) it is likely that ALC exerts at least some of its detrimental effects on growth, LH secretion and the pubertal process by suppressing hepatic IGF-1 synthesis, and subsequently, resulting in decreased serum levels of the protein.
Fig. 3.
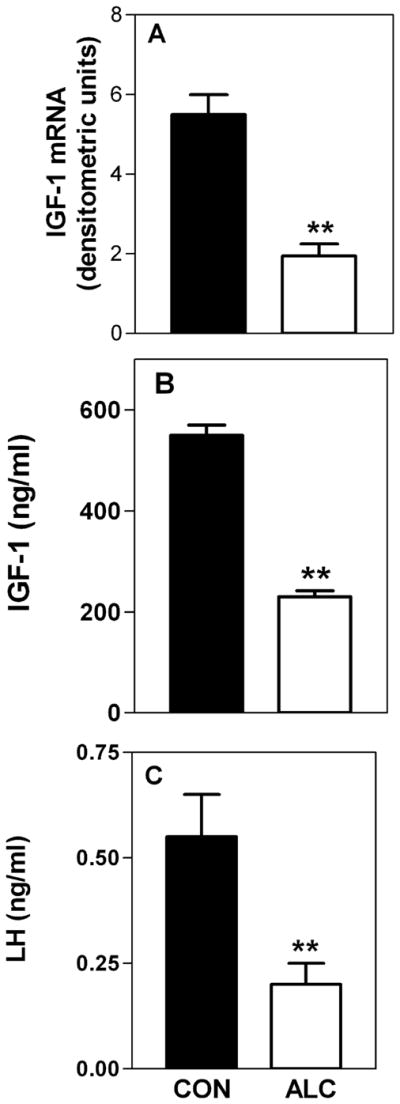
Effects of chronic ALC administration on IGF-1 gene expression in the liver and on the serum levels of IGF-1 and LH. (A) Densitometric quantitation of bands generated by RNase protection showing that liver IGF-1a mRNA expression was markedly suppressed in the ALC-treated animals. Similar results were also observed for IGF-1b expression (not shown). (B and C) ALC exposure also concomitantly suppressed serum levels of both IGF-1 and LH. The respective bars represent the mean (±SEM) of an N of 7 to 8 animals per group in A, and 26 to 36 per group in B and C. **p < 0.001.
The ALC-induced reduction of hepatic IGF-1 gene expression appears to be due, at least partially, to an ALC induced suppression of serum GH, since this hormone regulates IGF-1 gene expression in the liver. However, other possibilities are that ALC may be causing an alteration in GH receptors and / or to a direct action on the hepatocyte. To assess whether there was any GH-independent effect of ALC within the liver itself, we used transgenic mice that overexpress GH; hence, the circulating GH levels were held constant, and thereby, allowed for the assessment of potential direct actions of ALC at the level of either the GH-receptor or the synthesizing machinery within the hepatocyte. The expression of GH in these mice is controlled by the promoter and not by the hypothalamic releasing and inhibiting hormones. This is important because, as stated earlier, the ALC-induced suppression in GH is due to a hypothalamic, not pituitary, action. Since ALC was not expected to affect the promoter, we considered this mouse model a unique approach to differentiate between GH-dependent and GH-independent effects of ALC on the ability of the liver to synthesize and secrete IGF-1. The transgenic mice exposed to ALC showed a decrease in hepatic IGF-1 transcripts, as well as serum protein levels, compared to transgenic controls. The ALC did not alter serum GH levels held constant by the promoter, and did not affect mouse GH receptor protein levels. Thus, in addition to ALC altering IGF-1 synthesis by suppressing the hypothalamic–pituitary–GH axis, this study using transgenic mouse model has demonstrated that the drug can also alter IGF-1 gene expression by acting directly within the hepatocyte in a GH-independent manner (Srivastava et al., 2002).
Taken together, the research to date indicates that there are both GH-dependent and GH-independent effects of ALC at the hepatic level to depress IGF-1 synthesis. The overall detrimental effect of these actions is decreased circulating levels of IGF-1, and therefore, less peptide available to hypothalamus to influence prepubertal LHRH release.
Effects of ALC on IGF-1 Induced LHRH Release
A study conducted a decade ago using both in vivo and in vitro methods was the first to demonstrate that ALC can act at the hypothalamic level to alter the ability of IGF-1 to stimulate LHRH/LH secretion in immature female rats (Hiney et al., 1998). In the first part of this study, a single injection of ALC or saline was administered via gastric gavage, and at the end of a 1.5-hour absorption phase, each rat was administered IGF-1 into a surgically implanted cannula within the brain third ventricle. The results indicated that IGF-1 stimulated LH secretion between 20 and 30 minutes postinjection in the controls, an action confirming a previous report (Hiney et al., 1996). However, this effect of IGF-1was blocked in the rats that were exposed to ALC. Subsequently, ME explants were removed from the base of the brain and the nerve terminals were incubated in the presence or absence of ALC in vitro. IGF-1 stimulated the release of prostaglandin E2 (PGE2) and LHRH from incubates in the absence of ALC. The presence of ALC in the medium, however, blocked the secretion of both hormones. In an earlier study, it was shown that PGE2 replacement to medium containing ALC resulted in the stimulation of LHRH secretion (Hiney and Dees, 1991). Thus, these combined results indicate that these central actions of ALC are due in part to diminished formation of PGE2, a known stimulator of LHRH secretion (Ojeda et al., 1979).
Effects of ALC on IGF-1-Induced Neuronal and Glial Puberty-Related Genes
Earlier we discussed the importance of the neuronal KiSS-1/kisspeptin system regarding LHRH secretion and the onset of puberty, and documented the ability of IGF-1 to stimulate expression of the prepubertal KiSS-1 gene (Hiney et al., 2009). Recently, we began a series of studies in order to gain a better understanding of potential substances that may control or alter the expression of the KiSS-1 gene at puberty. Since IGF-1 is a regulator of the KiSS-1 gene, and since ALC causes suppressed circulating levels of IGF-1, we considered it possible that the drug may interfere with the central action of IGF-1 to up-regulate the KiSS-1 gene. In this regard, we recently showed that chronic ALC exposure caused a marked suppression in basal KiSS-1 gene expression in the AVPV and arcuate (ARC) nuclei (Srivastava et al., 2009). These nuclei, the latter included within the MBH, represent the two principal areas of KiSS-1 gene expression in the reproductive hypothalamus. In that same study, we subsequently assessed the potential for ALC to affect IGF-1 signaling. While no changes were observed following ALC on hypothalamic IGF-1 receptor gene and protein expression (not shown), we did detect a marked decrease in phosphorylated-Akt protein expression in both AVPV and ARC brain regions. Table 1 demonstrates these respective effects of ALC on IGF-1 signaling and KiSS-1 expression in both brain regions of prepubertal female rats. Hence, because KiSS-1 gene regulation is critical for kiss-peptin production and its influence on LHRH release at puberty, the fact that chronic ALC exposure is capable of affecting basal levels of KiSS-1 expression is relevant with regard to identifying the mechanism by which ALC suppresses prepubertal LHRH secretion and alters the pubertal process.
Table 1.
Effects of ALC on IGF-1 Signaling and KiSS-1 Gene Expression in the Anteroventral Periventricular (AVPV) and Arcuate (ARC) Nuclei
| Control | ALC | |
|---|---|---|
| AVPV nucleus | ||
| IGF-1R | 0.197 ± 0.016 | 0.2 ± 0.03 |
| p-Akt | 0.43 ± 0.024 | 0.31 ± 0.03* |
| KiSS-1 | 7.95 ± 1.02 | 1.72 ± 0.198*** |
| ARC nucleus | ||
| IGF-1R | 0.243 ± 0.03 | 0.247 ± 0.02 |
| p-Akt | 0.39 ± 0.03 | 0.23 ± 0.03** |
| KiSS-1 | 2.56 ± 0.18 | 1.56 ± 0.10*** |
IGF-1R and phosphorylated (p)-Akt values represent densitometric quantification of Western Blots and are reported as their respective mean (±SEM) protein/β-actin ratio. KiSS-1 gene expression was determined by real-time PCR and is reported as mean (±SEM) relative expression. N = 6 to 8 for IGF-1R and p-Akt, and N = 11 to 13 for KiSS-1.
p < .05;
p < .01;
p < 001.
Studies assessing the effects of ALC on glial connections with LHRH secretion have also been conducted. The ability of IGF-1 to regulate Oct-2 genes, and the relationship of these genes regarding glial to neuronal interactions that were discussed above, lead investigators to assess whether ALC exposure altered the expression of Oct-2 transcripts in immature rats. In one study, the effect of ALC on the ability of IGF-1 to induce Oct-2 gene expression was assessed. Results indicated that acute ALC administration blocked the central action of IGF-1 to induce both transcripts of this gene in the POA and MBH, an action that was correlated with suppressed serum levels of LH (Dees et al., 2005). Another study has shown that chronic ALC exposure also causes suppressed hypothalamic Oct-2 gene expression (Kim et al., 2005). Therefore, these studies suggest that the Oct-2 gene is a target by which ALC can interfere with peripubertal glial to neuronal communications associated with facilitating LHRH secretion.
This is a new area of investigation showing that ALC can affect the regulation of important puberty-related neuronal and glial genes involved in the prepubertal LHRH secretory pathway. Obviously, more research is needed to identify other puberty-related genes, determine their endogenous regulators, and discern any effects of ALC on their basal and induced levels of expression.
CONCLUSIONS
Case reports from humans and research with experimental animals show that ALC can cause detrimental effects on puberty- related hormones at this critical time of growth and development. In this review we have discussed the influence of ALC on puberty using rats and rhesus monkeys as animal models. We described herein that ALC exposure causes suppressed serum levels of GH, LH, and E2, hence resulting in delayed vaginal opening in rats and markedly altering the development of a regular monthly pattern of menstrual activity in rhesus monkeys. While this basic information was important, it became clear that in order to define the mechanisms of ALC action on hormones and pubertal development we needed to gain a better understanding of factors or substances initiating and/or involved in early pubertal processes.
The existence of metabolic signals capable of linking somatic development to the activation of the LHRH/LH releasing system at puberty has been considered for several years. The trophic factor IGF-1 is particularly suited in this regard since it increases in serum as puberty approaches and provides the growth promoting action of GH. Therefore, we hypothesized that IGF-1 may be a signal capable of acting centrally to stimulate LHRH release at puberty. This review reveals the evidence available to date showing that IGF-1 can stimulate prepubertal LHRH release, and when administered early, can advance signs of puberty in female rodents and rhesus monkeys. Additionally, IGF-1 administration has been shown to precociously up-regulate specific puberty-related genes, including KiSS-1. This gene is important for producing kisspeptins, key peptides involved in LHRH release at puberty. Collectively, this information demonstrates that IGF-1 plays an important role at puberty and thus, suggests that this peptide may be a target through which ALC may affect pubertal development.
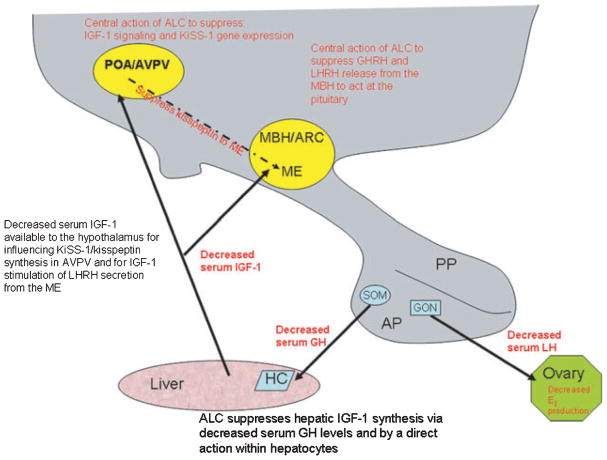
Research has shown that ALC can indeed alter specific prepubertal influences of IGF-1. The schematic drawing shown in Fig. 4 summarizes the actions and interactions of ALC and IGF-1 on critical puberty-related hormones. As shown and discussed throughout this review, ALC can act both peripherally and centrally to suppress prepubertal LHRH/LH secretion. Chronic ALC exposure has been shown to suppress basal IGF-1 synthesis in the liver, resulting in decreased circulating peptide available to the hypothalamus for facilitating LHRH secretion. Furthermore, studies designed to more critically assess specific hypothalamic actions of ALC on IGF-1 functions show that acute ALC exposure blocks IGF-1 induced LHRH secretion directly from the prepubertal ME, and also blocks IGF-1 stimulated KiSS-1 gene expression in the AVPV nucleus. Additionally, the observations that chronic ALC administration suppresses Oct-2 gene expression, and that acute exposure blocks IGF-1 induced Oct-2 expression, infers that ALC may alter prepubertal glial– neuronal interactions that influence LHRH secretion. Thus, ALC can not only act peripherally to suppress circulating levels of IGF-1 available to the hypothalamus, but can also act centrally to block important IGF-1 actions resulting in suppressed LHRH secretion and ultimately, alter signs in pubertal development.
Fig. 4.
Schematic drawing showing the actions and interactions of alcohol (ALC) and insulin-like growth factor-1 (IGF-1) related to growth and puberty. ALC not only acts peripherally to suppress IGF-1 synthesis in the liver, and hence circulating levels of the peptide available to the hypothalamus, but can also act centrally to block important IGF-1 actions. The cumulative effect of altered hypothalamic IGF-1 signaling is suppressed growth hormone releasing hormone (GHRH) and luteinizing hormone releasing hormone (LHRH) secretion at a critical time of development. Ultimately, these reductions lead to suppressed growth hormone (GH), luteinizing hormone (LH), and estradiol (E2) secretion, resulting in altered growth and pubertal development. Preoptic area (POA); anteroventral periventricular nucleus (AVPV); arcuate nucleus (ARC); median eminence (ME); medial basal hypothalamus (MBH); anterior pituitary (AP); posterior pituitary (PP); somatotroph (SOM); gonadotroph (GON); hepatocyte (HC).
Because puberty is a complex time of development, it requires utilizing multiple experimental approaches to identify specific factors controlling the timing of puberty, as well as to discern the sites and mechanisms of ALC action on LHRH secretion and the progression of pubertal processes. Research in this area is important and clearly relevant to child health from a standpoint of identifying causes, effects, and possible treatments for ALC-induced deficiencies in adolescents.
Acknowledgments
This work was supported by the NIH grant AA07216 (to WLD).
References
- Aguado J, Rodrigo J, Cacicedo L, Mellstrom B. Distribition of insulin-like growth factor-1 receptor mRNA in rat brain. Regulation of the hypothalamo-neurohypophysial system. J Mol Endocrinol. 1989;11:231–239. doi: 10.1677/jme.0.0110231. [DOI] [PubMed] [Google Scholar]
- Anders J, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-1 in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size and body mass index. J Clin Endocrinol Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Willis BR, Oswald C, Gupta A, Zaneveld L. Delayed male sexual maturation induced by chronic ethanol injestion. Fed Proc. 1981;40:825–829. [Google Scholar]
- Anderson RA, Zwain IH, Arroyo A, Mellon PL, Yen SS. The insulin-like growth factor system in the GT1-7 GnRH neuronal cell line. Neuroendocrinology. 1999;70:353–359. doi: 10.1159/000054496. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Ojeda SR. A detailed analysis of the serum LH secretory profile in conscious, free-moving female rats during the time of puberty. Endocrinology. 1981;109:2032–2039. doi: 10.1210/endo-109-6-2032. [DOI] [PubMed] [Google Scholar]
- Block GD, Yamamoto ME, Mallick A, Styche AJ. Effects on prepubertal hormones by ethanol abuse in adolescents. Alcohol Clin Exp Res. 1993;17:505. [Google Scholar]
- Bo WJ, Krueger WA, Rudeen PK, Symmes SK. Ethanol-induced alterations in the morphology and function of the rat ovary. Anat Rec. 1982;202:255–260. doi: 10.1002/ar.1092020210. [DOI] [PubMed] [Google Scholar]
- Bohannon NJ, Figlewicz DP, Corp ES, Wilcox J, Porte D, Jr, Baskin DG. Identification of binding sites for insulin-like growth factor (IGF-1) in the median eminence of the rat brain by quantitative autoradiography. Endocrinology. 1986;119:943–945. doi: 10.1210/endo-119-2-943. [DOI] [PubMed] [Google Scholar]
- Bondy C, Werner H, Roberts CT, LeRoith D. Cellular pattern of type-1 insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors 1 and 11. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Ching M, Valencia M, Negro-Villar A. Acute ethanol treatment lowers hypophyseal portal plasma LHRH and systemic plasma LH levels in orchadectomized rats. Brain Res. 1988;443:325–328. doi: 10.1016/0006-8993(88)91627-7. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin- releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool LE, Kasuya E, Saitoh Y, Marzban F, Terasawa E. N-methyl-D, L-aspartate induces the release of LHRH in the prepubertal and pubertal female rhesus monkey as measured by in vivo push-pull perfusion in the stalk-median eminence. Endocrinology. 2000;141:219–228. doi: 10.1210/endo.141.1.7231. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Eichberg JW, Parker CR, Bartke A. Puberty in the chimpanzee: somatomedin-C and its relationship to somatic growth and steroid hormone concentration. J Clin Endocrinol Metab. 1985;60:1154–1160. doi: 10.1210/jcem-60-6-1154. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Kuehl TJ, Castracane VD. Pubertal endocrinology of the baboon: elevated somatomedin-C/insulin-like growth factor 1 at puberty. J Clin Endocrinol Metab. 1982;55:1198–1201. doi: 10.1210/jcem-55-6-1198. [DOI] [PubMed] [Google Scholar]
- Crawford BA, Singh JM, Simpson JM, Handelsman DJ. Androgen regulation of circulating insulin-like growth factor-1 during puberty in male hypogonadal mice. J Endocrinol. 1993;139:57–65. doi: 10.1677/joe.0.1390057. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: Relationship to gonadotropin-releasing hormone neurons. Endocrinology. 2003;144:2034–2045. doi: 10.1210/en.2002-221025. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormone neurons during postnatal development. J Neuroendocrinol. 2004;16:160–169. doi: 10.1111/j.0953-8194.2004.01149.x. [DOI] [PubMed] [Google Scholar]
- Danilovich V, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role for IGF-1. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- Dearth RK, Hiney JK, Dees WL. Leptin acts centrally to induce the prepubertal secretion of luteinizing hormone in the female rat. Peptides. 2000;21:387–392. doi: 10.1016/s0196-9781(00)00157-1. [DOI] [PubMed] [Google Scholar]
- Dees WL, Dissen GA, Hiney JK, Lara F, Ojeda SR. Alcohol ingestion inhibits the increased secretion of puberty-related hormones in the developing female rhesus monkey. Endocrinology. 2000;141:1325–1331. doi: 10.1210/endo.141.4.7413. [DOI] [PubMed] [Google Scholar]
- Dees WL, Kozlowski GP. Differential effects of ethanol on luteinizing hormone. Follicle stimulating hormone and prolactin in the female rat. Alcohol. 1984;1:429–433. doi: 10.1016/0741-8329(84)90017-x. [DOI] [PubMed] [Google Scholar]
- Dees WL, Rettori V, Kozlowski GP, McCann SM. Ethanol and the pulsatile release of luteinizing hormone, follicle stimulating hormone and prolactin in ovariectomized rats. Alcohol. 1985;2:641–646. doi: 10.1016/0741-8329(85)90139-9. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW. The effects of ethanol during the onset of female puberty. Neuroendocrinology. 1990;51:64–69. doi: 10.1159/000125317. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW, Hiney JK, Johnston CA. Actions of ethanol on hypothalamic and pituitary hormones in prepubertal female rats. Alcohol. 1990;7:21–25. doi: 10.1016/0741-8329(90)90055-h. [DOI] [PubMed] [Google Scholar]
- Dees WL, Srivastava VK, Hiney JK. Alcohol alters insulin-like growth factor-1 activated Oct-2 POU gene expression in the immature female hypothalamus. J Stud Alcohol. 2005;66:35–45. doi: 10.15288/jsa.2005.66.35. [DOI] [PubMed] [Google Scholar]
- Delafontaine P. Growth factors and vascular smooth muscle responses. Eur Heart J. 1998;19:G18–G22. [PubMed] [Google Scholar]
- Diamond F, Ringenberg L, MacDonald D, Barnes J, Shi Hu C, Ducket G, Sweetland M, Root A. Effects of drug and alcohol abuse upon pituitary-testicular function in adolescent males. J Adolesc Health. 1986;7:28–33. doi: 10.1016/s0197-0070(86)80091-2. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Dearth RK, Scott HM, Ojeda SR, Dees WL. Alcohol Alters prepubertal luteinizing hormone secretion in immature female rhesus monkeys by a hypothalamic action. Endocrinology. 2004;145:4558–4564. doi: 10.1210/en.2004-0517. [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Morschl E, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Role of astroglia and insulin-like growth factor-1 in gonadal hormone-dependent synaptic plasticity. Brain Res Bull. 1997;44:525–531. doi: 10.1016/s0361-9230(97)00238-4. [DOI] [PubMed] [Google Scholar]
- Gruaz NM, d’Alleves V, Charnay Y, Skottner A, Ekvarn S, Fryklund L, Aubert ML. Effects of constant infusion with insulin-like growth factor-1 (IGF-1) to immature female rats on body weight gain, tissue growth and sexual function: evidence that such treatment does not affect sexual maturation or fertility. Endocrine. 1997;6:11–19. doi: 10.1007/BF02738796. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Spaliviero JA, Scott CD, Baxter RC. Hormonal regulation of the peripubertal stage of insulin-like growth factor-1 in the rat. Endocrinology. 1987;120:491–496. doi: 10.1210/endo-120-2-491. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Dearth RK, Srivastava VK, Rettori V, Dees WL. Actions of ethanol on epidermal growth factor-receptor activated luteinizing hormone secretion. J Stud Alcohol. 2003;64:809–816. doi: 10.15288/jsa.2003.64.809. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Dees WL. Ethanol inhibits LHRH release from the median eminence of prepubertal female rats in vitro: Investigation of its actions on norepinephrine and prostaglandin E2. Endocrinology. 1991;128:1404–1408. doi: 10.1210/endo-128-3-1404. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor (IGF-1) stimulates LHRH release from the prepubertal female median eminence in vitro. Neuroendocrinology. 1991;54:420–423. doi: 10.1159/000125924. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Lara F, Dees WL. Ethanol blocks the central action of IGF-1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sci. 1998;62:301–308. doi: 10.1016/s0024-3205(97)01111-9. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor-1 (IGF-1) of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3727. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Pine MD, Dees WL. Insulin-like growth factor-1 activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384. doi: 10.1210/en.2008-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson OGP, Lindahl A, Nilsson A, Isgaard J. Mechanism of the stimulating effect of growth hormone on longitudinal bone growth. Endocrinol Rev. 1987;8:426–435. doi: 10.1210/edrv-8-4-426. [DOI] [PubMed] [Google Scholar]
- Johnson LD, O’Malley PM, Backman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–1005: Vol. 1 Secondary school students (NIH Publication no. 06–5883) NIDA; Bethesda, MD: 2006. [Google Scholar]
- Jones JE, Armstrong JD, Harvey RW. Changes in metabolites, metabolic hormones, and luteinizing hormone before puberty in Angus, Braford, Charolais, and Simmental heifers. J Anim Sci. 1991;69:1607–1612. doi: 10.2527/1991.6941607x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Sohn H, Ha M, Han JY, Kang SS, Choi WS, Cho GJ. Prepubertal chronic ethanol administration alters TTF-1 and Oct-2 expression in the hypothalamus of female rats. Mol Brain Res. 2005;136:262–266. doi: 10.1016/j.molbrainres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Lee B, Hiney JK, Pine MD, Srivastava VK, Dees WL. Manganese induces hypothalamic luteinizing hormone releasing hormone secretion in prepubertal female rats: hypothalamic site and mechanism of action. J Physiol. 2007;578:765–772. doi: 10.1113/jphysiol.2006.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak MA, Hill JM, Kiess W, Rojeski M, Candace BP, Roth J. Receptors for insulin-like growth factors 1 and 11: autoradiographic localization in rat brain and comparison to receptor for insulin. Endocrinology. 1988;123:2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- Longo KM, Sun Y, Gore AC. Insulin-like growth factor-1 effects on gonadotropin-releasing hormone biosynthesis in GT1-7 cells. Endocrinology. 1998;139:1125–1132. doi: 10.1210/endo.139.3.5852. [DOI] [PubMed] [Google Scholar]
- Lowe WL, Jr, Lasky SR, LeRoith D, Roberts CT., Jr Distribution and regulation of rat insulin-like growth factor-1 messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol. 1988;2:528–535. doi: 10.1210/mend-2-6-528. [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr, Baskin DG. Localization of type 1 insulin-like growth factor receptor mRNA in the adult rat brain by in situ hybridization. Mol Endocrinol. 1991;5:1158–1168. doi: 10.1210/mend-5-8-1158. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barriero ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated mRNA expression of KiSS-1 and its putitive receptor, GPR54, in rat hypothalamus and potent LH-releasing activity of KiSS-1 peptide. Endocrinology. 2004a;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004b;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg C, Hiney JK, Minks JB, Dees WL. Ethanol alters N-methyl DL-aspartic acid-induced secretion of luteinizing hormone releasing hormone and the onset of puberty in the female rat. Neuroendocrinology. 1993;57:863–868. doi: 10.1159/000126446. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Hill J, Hill DF, Costa ME, Tapia V, Cornea A, Ma YJ. The Oct-2 POU domain gene in the neuroendocrine brain: a transcriptional regulator of mammalian puberty. Endocrinology. 1999;140:3774–3789. doi: 10.1210/endo.140.8.6941. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent A, Matagne V, Mungenast AE. The neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Negro-Vilar A, McCann SM. Release of prostaglandins Es (PGEs) by hypothalamic tissue: evidence of their involvement in catecholamine- induced luteinizing hormone-releasing hormone release. Endocrinology. 1979;104:617–624. doi: 10.1210/endo-104-3-617. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Terasawa E. Neuroendocrine regulation of puberty. In: Pfaff D, Arnold A, Etgeb A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. Vol. 4. Elsevier; New York: 2002. pp. 589–659. [Google Scholar]
- Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci USA. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. Puberty in primates. In: Knobil E, Neil JD, editors. The Physiology of Reproduction. Vol. 2. Raven Press; New York: 1988. pp. 1763–1788. [Google Scholar]
- Plant T. Neurophysiology of puberty. J Adolesc Health. 2002;31:185–191. doi: 10.1016/s1054-139x(02)00484-6. [DOI] [PubMed] [Google Scholar]
- Ramaley JA. The regulation of gonadotropin secretion in immature ethanol-treated male rats. J Androl. 1982;3:248–252. [Google Scholar]
- Roberts CA, McCutcheon SN, Blair HT. Developmental patterns of insulin-like growth factor-1 concentrations in sheep. Domest Anim Endocrinol. 1990;4:457–463. doi: 10.1016/0739-7240(90)90003-i. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genen E, Carel J, Matsuda F, Chaussin J, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS-1-derived peptide receptor GRP54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Fink G. Mechanism of the first spontaneous gondotrophin surge and that induced by pregnant mare serum and effects of neonatal androgen in rats. J Endocrinol. 1979;83:339–354. doi: 10.1677/joe.0.0830339. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Smith GC, Fink G. Effect of manipulating central catecholamines on puberty and the surge of luteinizing hormone and gonadotropin releasing hormone induced by pregnant mare serum gonadotropin in female rats. Brain Res. 1981;213:335–349. doi: 10.1016/0006-8993(81)90239-0. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatizidaki E, Thresher A, Acierno J, Shagoury J, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick A, Zahn D, Dixon J, Kaiser U, Slaugenhaupt S, Gusella J, O’Rahilly S, Carlton M, Crowley W, Aparicio S, Colledge W. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara S, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Boyd RL. Chronic ethanol feeding inhibits plasma levels of insulin-like growth factor-1. Life Sci. 1988;43:1325–1330. doi: 10.1016/0024-3205(88)90588-7. [DOI] [PubMed] [Google Scholar]
- Soszynski PA, Frohman LA. Inhibitory effects of ethanol on the growth hormone (GH)-releasing hormone-GH-insulin-like growth factor-1 axis in the rat. Endocrinology. 1992;131:2603–2608. doi: 10.1210/endo.131.6.1359962. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Dearth RK, Hiney JK, Chandrashekar V, Mattison LA, Bartke A, Dees WL. Alcohol suppresses insulin-like growth factor-1 gene expression in prepubertal transgenic female mice overexpressing the bovine growth hormone gene. Alcohol Clin Exp Res. 2002;26:1697–1702. doi: 10.1097/01.ALC.0000036922.18456.EF. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Short term alcohol administration alters KiSS-1 gene expression in the reproductive hypothalamus of prepubertal female rats. Alcohol Clin Exp Res. 2009;33:1–10. doi: 10.1111/j.1530-0277.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Nyberg CL, Dees WL. Effect of ethanol on the synthesis of insulin-like growth factor-1 (IGF-1) and the IGF-1 receptor in late prepubertal female rats: a correlation with serum IGF-1. Alcohol Clin Exp Res. 1995;19:1467–1473. doi: 10.1111/j.1530-0277.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Rettori V, Dees WL. Effects of ethanol on intraovarian nitric oxide production in the prepubertal female rat. J Endocrinol. 1999;161:69–75. doi: 10.1677/joe.0.1610069. [DOI] [PubMed] [Google Scholar]
- Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006;91:4369–4373. doi: 10.1210/jc.2006-0953. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83:275–281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- Terasawa E. Hypothalamic control of the onset of puberty. Curr Opin Endocrinol Diabetes. 1999;6:44–49. [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Ojeda SR. The juvenile peripubertal transition period in the female rat: establishment of a diurnal pattern of pulsatile LH secretion. Endocrinology. 1985;117:644–649. doi: 10.1210/endo-117-2-644. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990;126:1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-1 and insulin-like growth factor-1 receptor gene expression in the primate ovary. Hum Reprod. 1999;14:2328–2332. doi: 10.1093/humrep/14.9.2328. [DOI] [PubMed] [Google Scholar]
- Werther A, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Mendelsohn FAO. Localization and characterization of IGF-1 receptors in brain and pituitary gland using in vitro autoradiography and computerized densitometry. A distinct distribution from insulin receptors. J Neuroendocrinol. 1989;1:369–377. doi: 10.1111/j.1365-2826.1989.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Premature elevation in serum insulin-like growth factor-1 advances first ovulation in monkeys. J Endocrinol. 1998;158:247–257. doi: 10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]
- Zeinoaldini S, Swarts JJM, Van de Heijning BJM. Central application of IGF-1 postpones time of vaginal opening in normally fed, but not food-restricted rats. Horm Res. 2006;66:169–174. doi: 10.1159/000094144. [DOI] [PubMed] [Google Scholar]
- Zhen S, Zakaria M, Wolfe A, Radovick S. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by insulin-like growth factor-1 in a cultured GnRH-expressing neuronal cell line. Mol Endocrinol. 1997;11:1145–1155. doi: 10.1210/mend.11.8.9956. [DOI] [PubMed] [Google Scholar]