Abstract
Background and Objective
Clinicians have difficulty assessing and monitoring early occlusal caries. Traditional clinical exam and radiographs are unable to detect the subtle alterations in enamel indicative of de- or re-mineralization, particularly under dental sealants. Although clinicians have used laser fluorescence (LF) to address this gap, this modality has demonstrated weak correlation with histology. The International Caries Detection and Assessment System (ICDAS-II) has demonstrated high sensitivity and specificity for caries detection, but since it is based on visual assessment, it is of no use in areas beneath the most commonly used dental sealants which are opaque. Optical Coherence Tomography (OCT) is an emergent assessment tool which has demonstrated great promise in detecting and quantifying caries, including areas beneath commonly used dental sealants and composites. However, OCT has not yet been widely integrated into clinical dental practice, perhaps because OCT imaging does not provide an easily accessible diagnostic outcome for clinicians. The objective of this ex vivo study was to use OCT-images of sound and carious occlusal surfaces in combination with a simple algorithm to compare the caries detection ability of OCT with tools clinicians may be more familiar with (LF and radiography), and with an established valid and reliable clinical assessment tool (ICDAS-II).
Study Design/Materials and Methods
One hundred and twenty extracted teeth with sound or naturally carious occlusal surfaces were imaged with OCT, LF, radiography, and examined clinically with the International Caries Detection and Assessment System. Teeth were randomized to one of two dental sealants recommended for use with LF. A novel simple algorithm was used to interpret OCT-based images. The accuracy of caries severity assessments of the OCT-based diagnosis, LF, ICDAS-II, and digital radiography were compared to the 4-point histological analysis gold standard.
Results
OCT and ICDAS-II caries severity assessments demonstrated high sensitivity (94.0%; 92.3%) and specificity (85.0%; 83.3%), LF demonstrated low sensitivity (65.2%) but high specificity (97.6%), and digital radiography demonstrated low sensitivity (67.1%) with moderate specificity (79.5%) on unsealed occlusal surfaces. OCT-based caries severity assessments of sealed teeth demonstrated high specificity (97.6%), sensitivity (89.9%), excellent positive predictive value (98.6%) and negative predictive value (83.3%). Despite our use of LF recommended dental sealants, in the presence of sealants, LF assessment of caries severity demonstrated high sensitivity (95.1%), but extremely low specificity (10.3%), positive predictive value (68.8%) and negative predictive value (50.0%).
Conclusion
This study found that OCT-based imaging combined with a simple diagnostic algorithm accurately assessed the severity of natural early caries on occlusal surfaces in extracted teeth both in the absence and presence of dental sealant. The findings of this study support the clinical use of OCT imaging for assessment and monitoring progression of early non-cavitated caries lesions on occlusal surfaces including areas under dental sealants.
Keywords: dental caries, dental decay, diagnostic imaging, oral diagnosis, pit and fissure sealants, tooth demineralization
INTRODUCTION
Clinicians and researchers need to be able to detect minimal changes in enamel health to accurately assess caries progression and manage early occlusal caries[1]. Traditional radiographs and clinical exam are unable to detect minimal changes in enamel indicative of early caries progression and de- or re-mineralization. Clinically accessible quantifiable assessment would provide clinicians with vital information about the efficacy and cost-effectiveness of non-invasive and minimally invasive caries prevention and management strategies such as dental sealants, enhancing very early caries management. The current lack of such capabilities may contribute to the underuse of dental sealants despite solid evidence of the efficacy of sealants for limiting progression of non-cavitated caries lesions [2–4].
Emergent caries assessment tools, such as the International Caries Detection and Assessment System (ICDAS- II), laser fluorescence (LF), and Optical Coherence Tomography (OCT) can augment the ability of clinicians to assess and predict caries progression [5–10]. ICDAS-II uses visual assessment of the tooth's natural enamel surface, taking advantage of the change in the enamel refractive index resulting from repeated demineralization challenges to detect and assess caries, including noncavitated lesions, and provides clinically meaningful measurements [11]. ICDAS-II demonstrates excellent caries detection ability on natural occlusal surfaces [12–13]. However, ICDAS-II techniques do not detect or quantify the minute changes associated with early enamel de- or re- mineralization [14], and because the methodology is based on visual assessment, it is of no use in assessing pits and fissures underneath the most commonly used dental sealants as they are opaque. DIAGNOdent (KaVo, Biberach, Germany) is a widely used non-invasive, non-ionizing laser fluorescence (LF)-based system that provides a numerical value of autofluorescence in a digital display as an indicator of caries status. This device detects the fluorescence resulting from exposure to red light (655nm wavelength) of porphyrins from cariogenic bacteria. LF has been used to quantify demineralization of tooth structure, including areas covered by unfilled clear dental sealants [15–16]. However, LF scores have demonstrated a weak correlation with histology [14], generating a high rate of false positives [17]. Optical Coherence Tomography (OCT) creates an image of tissue based on reflectivity and phase retardation related to the mineral and water content of the surface and subsurface of the tissue being imaged [18]. OCT has been used extensively in clinical medicine [19–23] but less so in dentistry [24]. This lack of use may be surprising since researchers have found that OCT can be used to assess caries severity including very early caries on occlusal surfaces [12–13, 25–29], to diagnose and even quantify changes in enamel structure associated with remineralization [9–10, 25, 30–33], with the ability to diagnose effectively even under a variety of dental sealants and composites [6, 34–36]. This lack of clinical OCT use may reflect clinicians' challenges in interpreting the OCT images[37] and the absence of a simple diagnostic read-out for clinicians [13].
The objective of this ex vivo study was to compare current clinically used assessment tools - LF, ICDAS-II, and intraoral digital radiographs - with OCT-based diagnosis using a novel simple algorithm in extracted sound and carious teeth before and after sealant placement. Histological examination using the validated methodology proposed by Ekstrand [38] was used as the gold standard.
MATERIALS & METHODS
Protocol
One hundred twenty extracted human molars were collected by researchers at the University of Michigan. The use of extracted teeth for this study was approved by the University of Michigan IRB. The teeth were cleaned, and sterilized with ethylene oxide gas. After marking the area of interest on each tooth with a small 330 bur and indelible ink, teeth were categorized by consensus from two trained and calibrated examiners (JB and MF) according to ICDAS II criteria (0 to 4). The 120 randomly numbered samples included, 40 sound teeth and 20 teeth in each of the ICDAS II lesion categories (1–4),. Samples were photographed and then radiographed using a wireless Schick digital sensor (3 mA, 70 kVp, 0.050 seconds). The occlusal surface of teeth in each radiograph were evaluated at a later date by trained examiners (JB,EB) using the following scale : 1) Lesion presence: yes/no; and 2) Lesion depth: E=lesion in enamel; D1=outer third of dentin; D2=middle third of dentin; D3=Inner/pulpal third of dentin[38–39].
Samples were then imaged with LF (DIAGNOdent). The DIAGNOdent LF system uses two-way handpiece optical system connected to a table top base unit to measure the fluorescence within tooth structure. The handpiece is placed on the tooth, and pulses of specific wavelength of red light illuminates the surface of the tooth causing prophryns in cariogenic bacteria to fluoresce. The light emitted from the tooth's surface is transmitted through the handpiece to the base unit where it is analyzed, quantified, and a numerical value displayed The LF unit was calibrated according to the manufacturer's instructions prior to each use. A “Zero Baseline Reading” was determined for each tooth. For each specimen, the marked area of interest in the occlusal surface of the tooth was scanned with the probe by slowly rocking the wand in a pendulous motion capturing the highest reading or “the peak”. Measurements were repeated until 3 readings were within (+/−) 3 units of each other, and then recorded for that surface. Teeth were subsequently wrapped in gauze moistened with 0.1% thymol and sent to researchers at Beckman Laser Institute (BLI) at the University of California Irvine for optical coherence tomography (OCT) imaging.
At BLI, teeth were unwrapped, wicked to nearly dry with a wedge of fine filter paper, placed on a wax base with the occlusal surface horizontal, and then the area of interest was imaged with a prototype swept-source-optical coherence tomography system (SS-OCT). After initial OCT imaging, teeth within each ICDAS-II category were randomly assigned to one of two dental sealants recommended for use with LF: ClearVue (Denali Corp. denalicorporation.com) - (Group 1) or Helisoseal Clear (Ivoclar Vivadent) - (Group 2). These sealants were chosen in order to maximize the ability of LF to accurately categorize the soundness of the occlusal areas covered by dental sealant, Materials were applied according to manufacturers' instructions and cured with Elipar Freelight 2. Following sealant placement, teeth were reimaged with SS-OCT, wrapped in gauze moistened with 0.1% thymol, and returned to the University of Michigan for post-sealant LF scanning and histological analysis. Re- examination with ICDAS-II and radiographic assessments post-sealant application were not conducted. For histological analysis, individual teeth were hemisected through the marked area of interest using micro-slice machine with a 250μm thick annular blade. The plane of the section was bucco-lingual but modified by the appearance of the fissure system. Both sections of the hemisected teeth were viewed independently by two trained examiners (JB, CGC) without magnification and under a stereomicroscope (x5) measuring the extent of the lesion using the 4-point criteria as proposed by Ekstrand [38]. The 4-point criteria scores the depth of enamel and dentin demineralization scoring no enamel demineralization (0), enamel demineralization limited to the outer 50% of the enamel (1), demineralization involving between 50% of the enamel and 1/3 of the dentin (2), demineralization involving the middle 1/3 of the dentin (3) and demineralization involving the inner 1/3 of the dentin (4).
OCT Imaging
Optical Coherence tomography (OCT) is a non-invasive imaging modality that uses near-infrared light to obtain high-resolution surface and subsurface images. Several OCT systems have FDA approval for clinical use in dentistry. In vivo the image is acquired by a flexible fiber optic that is placed on the surface of the tooth to generate a real-time image of the immediate surface and subsurface tissues.
Previous studies have shown that swept-source OCT (SS-OCT) of dental tissues is able to differentiate between the reflectance signals of resin, enamel and dentin, including demineralized tooth structure [6–7, 40–43]. Figure 1 shows the SS-OCT system used in this study. Light from a swept-source laser (sweeping speed = 20 kHz, 1310nm centered wavelength, with a FWHM bandwidth of 75 nm) is directed into a 1×2 10:90 coupler, with 90% power in the sample arm and 10% power in the reference arm. The back-reflection signal from the reference arm and the backscattered signal from the sample arm are guided into a 2×2 50:50 split ratio coupler. The two signals interfere with each other to create fringe signals that are sent into a balanced amplified photodetector. The amplified signal from the detector is then digitized and processed by the computer to create cross-sectional images. The scanning system consists of 2 scanning mirrors and a focusing lens. The mirrors are synchronously controlled by the computer to direct the light beam to scan over a horizontal axis of 6mm using a 2D imaging acquiring setup or to acquire scans over an area of 6mm by 6mm with 3D setup. In the case of 3D setup, the images acquired can be stacked together to create a 3D representation of the sample. The axial resolution of the images obtained from this system is approximately 8um while the lateral resolution is around 10um. The system acquires images at a rate of 33.3 frames per second.
Figure 1.
OCT System setup
Prior to each image acquisition, the light beam was focused by a trained and experienced operator (AW) to project the narrowest line possible onto the tooth surface during each imaging scan. Samples were adjusted so that the scan line remained as perpendicular as possible to the tooth surface throughout the entire 6mm length of each scan. Sample orientation, scan localization and OCT system probe and parameters were standardized throughout the entire study to ensure comparability and reproducibility of the data obtained. Thus extensive measures were undertaken to minimize the effects of variables such as beam focusing on the data obtained, especially on the signal intensity to optical distance ratio.
In order to maximize the comparability of the images obtained and take into account tooth surface curvature, the reflection peak at the air/enamel surface in each OCT image was aligned along one horizontal pixel line in each image. After filtering for noise with an adaptive Frost filter [44–45] each 2-dimensional image (B-scan) was analyzed with simple software written by our lab (AC,PWS). The surface 15 pixel layers of the tooth were excluded from this analysis because of the permanent reflection peak at the air/tooth interface.
Diagnostic Criteria
Teeth that scored 0 on the ICDAS-II scale (no evidence of caries or change in translucency and light refraction of enamel after drying for 5 seconds) were considered sound (E0) and an ICDAS-II score of 1 (opacity or discoloration, white or brown, visible at the entrance to the pit or fissure after prolonged air drying) was considered early caries (E1). LF scores of 0–7 were considered sound (E0) and 8–14 demineralized/ early caries (E1)[46]. To determine diagnoses with OCT-based images, the 2-dimensional OCT images (B-scan) were analyzed with a software program. Locations with a log of back-scattered light intensity (BSLI) below 2.9 were scored as sound (E0), and areas equaling or exceeding 2.9 were considered to be carious (Table 1). This cut-off point was chosen based on our preliminary data in a previous pilot study. Images were also examined visually to map the physical extent of the demineralization due to caries (E1–D2). Teeth were considered radiographically sound if there was no discernible lesion present. After hemisectioning, histological analysis (gold standard) using the 4-point criteria as proposed by Ekstrand et al [38] was conducted. Teeth were categorized as sound (E0), demineralized/early caries (E1), or more severely decayed (E2, D1, D2).
Table 1.
OCT, LF, ICDAS-II, and radiograph assessment of all teeth versus Histopathology (gold standard)
| Before sealant applied | After sealant applied | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| All teeth (n=120) | OCT* % | LF % | ICDAS % | Radiograph % | OCT % | LF % |
| PPV† | 91.3 | 93.2 | 91.1 | 86.9 | 98.6 | 68.8 |
| NPV | 89.5 | 65.6 | 85.4 | 54.4 | 83.3 | 50.0 |
| Sensitivity | 94.0 | 65.2 | 92.3 | 67.1 | 89.9 | 95.1 |
| Specificity | 85.0 | 97.6 | 83.3 | 79.5 | 97.6 | 10.3 |
| False Positives (n) | 6 | 1 | 7 | 8 | 1 | 35 |
| False Negatives (n) | 4 | 21 | 6 | 26 | 8 | 4 |
|
| ||||||
| Group 1 (sealed with Clear Vue) (n=60) | ||||||
|
| ||||||
| PPV | 96.8 | 100.0 | 87.2 | 83.9 | 100.0 | 72.2 |
| NPV | 90.5 | 58.8 | 71.4 | 53.6 | 80.0 | 50.0 |
| Sensitivity | 93.8 | 65.0 | 85.0 | 66.7 | 87.5 | 92.9 |
| Specificity | 95.0 | 100 | 75.0 | 75.0 | 100.0 | 16.7 |
| False Positives (n) | 1 | 0 | 5 | 5 | 0 | 15 |
| False Negatives (n) | 2 | 14 | 6 | 13 | 5 | 3 |
|
| ||||||
| Group 2 (sealed with Helioseal) (n=60) | ||||||
|
| ||||||
| PPV | 88.1 | 97.0 | 95.0 | 90.0 | 97.3 | 65.5 |
| NPV | 88.9 | 74.1 | 100.0 | 55.2 | 87.0 | 50.0 |
| Sensitivity | 94.9 | 82.1 | 100.0 | 67.5 | 92.3 | 97.4 |
| Specificity | 76.2 | 95.2 | 90.0 | 84.2 | 95.2 | 4.8 |
| False Positives (n) | 5 | 1 | 2 | 3 | 1 | 20 |
| False Negatives (n) | 2 | 7 | 0 | 13 | 3 | 1 |
Optical Coherence Tomography (OCT), Laser Fluorescence (LF), International Caries Detection and Assessment System (ICDAS-II)
PPV = Positive Predictive Value; NPV = Negative Predictive Value
RESULTS
OCT, LF, ICDAS-II and radiograph assessments pre- and post- sealant were compared with the gold standard (histopathology) (Tables 1,2, and 3). Table 1 shows the comparison of all four assessment methods pre-sealant application, and the OCT-based and LF assessments post-sealant application. The OCT-based imaging combined with a simple diagnostics algorithm accurately assessed the severity of natural early caries on occlusal surfaces both in the absence and presence of dental sealant. Diagnostic characteristics for both dental sealants were similar (Table 1). The presence of either dental sealant on occlusal surfaces improved the specificity (SP) and PPV of OCT-based assessments, and modestly decreased sensitivity (SE) and NPV. Conversely, the presence of either dental sealant on the occlusal surface greatly improved LF SE, but dramatically reduced SP, PPV and NPV.
Table 2.
OCT, LF, ICDAS-II, and radiograph assessment of sound (E0) and early carious teeth (E1) before and after sealant application
| Before sealant applied | After sealant applied | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Histopathogy identified as E0 and E1 (All teeth n= 56) |
OCT* % (n) | LF % (n) | ICDAS % (n) | Radiograph % (n) | OCT % (n) | LF % (n) |
| E0 (n=41) | ||||||
| Scored same as histo | 82.9 (34) | 97.6 (40) | 82.9 (34) | 75.6(31) | 97.6 (40) | 9.8 (4) |
| More carious than histo | 17.1 (7) | 2.4 (1) | 17.0 (7) | 24.4 (10) | 2.4 (1) | 90.2 (37) |
| E1 (n=15) | ||||||
| Scored same as histo | 66.7 (10) | 0 | 20.0 (3) | 0 | 73.3 (11) | 0 |
| More carious than histo | 26.7 (4) | 20.0 (3) | 40.0 (6) | 40.0 (6)† | 6.7 (1) | 93.3 (14) |
| Scored as sound | 6.7 (1) | 80.0 (12) | 40.0 (6) | 60.0 (9) | 20.0 (3) | 6.7 (1) |
| Group 1 (ClearVue) (n=30) | ||||||
| E0 (n=20) | ||||||
| Scored same as histo | 90.0 (18) | 100 (20) | 75.0 (15) | 75.0 (15) | 100.0 (20) | 15.0 (3) |
| More carious than histo | 10.0 (2) | 0 | 25.0 (5) | 25.0 (5)† | 0 | 85.0 (17) |
| E1 (n=10) | ||||||
| Scored same as histo | 70.0 (7) | 0 | 30.0 (3) | 0 | 70.0 (7) | 0 |
| More carious than histo | 30.0 (3) | 10.0 (1) | 20.0 (2) | 40.0 (4)† | 10.0 (1) | 90.0 (9) |
| Scored as sound | 0 | 90.0 (9) | 50.0 (5) | 60.0 (6) | 20.0 (2) | 10.0 (1) |
| Group 2 (Helioseal) (n=26) | ||||||
| E0 (n=21) | ||||||
| Scored same as histo | 76.2 (16) | 95.2 (20) | 90.5 (19) | 76.2 (16) | 95.2 (20) | 4.8 (1) |
| More carious than histo | 23.8 (5) | 4.8 (1) | 9.5 (2) | 23.8 (5)† | 4.8 (1) | 95.2 (20) |
| E1 (n=5) | ||||||
| Scored same as histo | 60.0 (3) | 0 | 0 | 0 | 80.0 (4) | 0 |
| More carious than histo | 20.0 (1) | 40.0 (2) | 80.0 (4) | 40.0 (2)† | 0 | 100.0 (5) |
| Scored as sound | 20.0 (1) | 60.0 (3) | 20.0 (1) | 60.0 (3) | 20.0 (1) | 0 |
Optical Coherence Tomography (OCT), Laser Fluorescence (LF), International Caries Detection and Assessment System (ICDAS-II)
Radiographs did not identify any enamel lesions (E1–E2), categorizing all lesions D1–3
Table 3.
Comparison of Photographic, Radiographic, OCT, and Histologic images
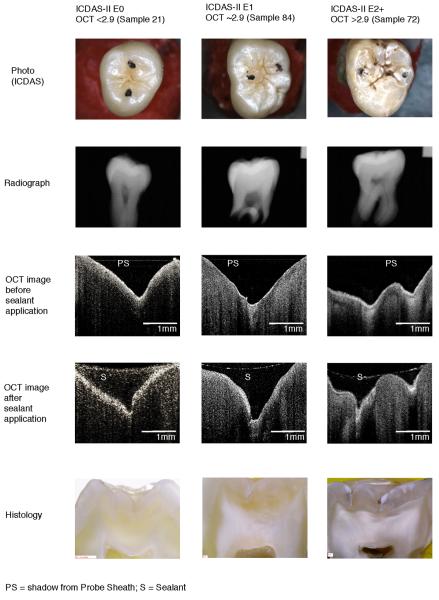
|
Table 1 shows pre-sealant application, OCT-based and ICDAS-II assessments demonstrated similarly good SE (94.0%; 92.3%) and SP (85.0%;83.3%), and positive (91.3%;91.1%) and negative (89.5%; 85.4%) predictive values on unsealed occlusal surfaces. Pre-sealant LF assessment demonstrated particularly high SP(97.6%) and positive predictive value (93.2%), but low SE (65.2%) and negative predictive value (65.6%). Pre-sealant radiograph assessment did not detect any E1 lesions, demonstrating generally low SE (67.1%), SP (79.5%), positive predictive value (86.9%) and particularly low negative predictive value (54.4%). These findings are consistent with previous studies [47] Post-sealant OCT-based assessment demonstrated high SP (97.6%) and PPV (98.6%), a modest increase in the number of false negatives (8/70) from pre-sealant assessments, and modest reductions of SE (89.9%), NPV (83.3%), and the number of false positives (1/56). Conversely, post-sealant LF assessment demonstrated lower (SP) (10.3%), PPV (68.8%), and NPV (50.0%), a modest decrease in the number of false negatives (4/79), with large increases in SE (95.1%) and the number of false positives (35/56).
Table 2 shows the OCT-based, LF, ICDAS-II and radiograph assessments and post-sealant OCT-based and LF assessments of unsealed sound (E0) and very early carious (E1) teeth compared with the gold standard. Pre-sealant OCT-based assessments correctly identified 82.9% of sound teeth and 66.7% of early caries, whilst pre-sealant LF assessment correctly identified 97.6% of sound teeth but no early caries, mischaracterizing 80% of early caries as sound and 20% as more severe caries. Post-sealant OCT-based assessment correctly categorized 97.6% of sound and 73.3% of early carious lesions compared with post-sealant LF assessments which correctly categorized 9.8% of sound but no early carious lesions, mischaracterizing 93.3% of early carious lesions as more severe than staged by the gold standard.
Representative images of 1 subject from histological categorizations E0, E1 and E2 are shown in Table 3. Table 3 compares photographs, radiographs, OCT images pre- and post- sealant, and histologic images of selected sample teeth determined by ICDAS-II to be E0, E1 and more severe than E2.
DISCUSSION
Although in this study sealants were used that are specifically recommended as compatible with LF, this modality was found to be inconsistent in it's assessments of caries under sealants, misdiagnosing caries as sound and sound teeth as carious [12, 15, 48]. This finding is consistent with other studies which found that sealant material attenuates LF signals, reducing diagnostic accuracy [49], doing so to a lesser degree in unfilled sealants than in densely filled materials. Moreover, sealants themselves may exhibit intrinsic fluorescence, confounding the diagnostic signal and perhaps contributing to the low SP of LF when sealants are present [50]. The recommended cutoffs- range for interpreting LF measurements add to the uncertainty of the diagnostic decision[51] The cutoffs used in this study, 0–7 indicating sound (E0) and 8–14 indicating demineralized/ early caries (E1), were chosen to provide maximal sensitivity and specificity[46], These cutoffs resulted in LF demonstrating a higher SP and but lower SE than other studies which used 0–11 to indicate sound or demineralized areas and 12–16 indicated enamel caries [51]; or 0–9 to indicate sound and 10–17 to indicate enamel caries [52]. Though a tooth identified by LF as sound is likely sound teeth identified by LF as carious were over 90% more severe, despite our using cutoffs to maximize SP and SE. LF would result in unnecessary dental treatment and mischaracterize truly effective non-invasive and minimally invasive caries prevention and management strategies as ineffective. [49].
ICDAS-II was identified as a reliable tool for evaluating the occlusal surfaces of natural, unsealed teeth [13], however it is not useful when sealants are present because of the need to closely examine the naked tooth surface visually, under carefully controlled conditions. As described in other studies, radiographs were unable to detect early occlusal enamel caries, regardless of the presence or absence of sealants [12, 38, 50, 53–54].
This study confirmed the findings of previous research that OCT-based imaging combined with a simple diagnostic algorithm is able to accurately assess the severity of natural early caries in the absence and presence of dental sealants[6, 35, 55]. Visual assessments, LF and radiographs are less able to do so with a lesser degree of accuracy. The numerical OCT-based assessments demonstrated excellent sensitivity (SE) and specificity (SP). Areas of interest with a log of BSLI below 2.9 were accurately identified as sound locations regardless of whether sealant was present or not. Over 90% of sound and early caries sites under dental sealants were diagnosed accurately vs the gold standard, histology. The findings of this study are significant because integrating a numerical value into OCT imaging may address a critical barrier to use of OCT into clinical practice -clinicians' difficulty interpreting OCT images to assess caries depth [56]- despite the evidence of OCT's value [9–10, 12–13, 25–33]. This capacity could improve caries management and strengthen the use of sealants and other preventive measures by enabling clinicians to detect potential failure and caries progression quickly and accurately. Moreover this capability would permit direct, inexpensive quick and effective monitoring and evaluation of management strategies in defined at risk populations.
In this study, a prototype laboratory SS-OCT system was used. Several commercially available mobile OCT systems are approved for dental use by trained personnel, even in a non-dental environment, requiring only a source of electricity. A simple software algorithm can easily be added to existing computer systems to facilitate OCT-based assessments' integration into electronic medical record systems. The use of a numerical output in addition to the OCT image improves diagnostic assessments particularly in clinical situations even following calibration[57]. Although ICDAS-II was able to accurately assess caries severity in this study. ICDAS-II may be less accurate in a traditional clinical situation with non-experts [58], who demonstrate much lower diagnostic accuracy [58]. While ICDAS-II training is available online (https://www.icdas.org/courses), it but does not calibrate users in a difficult and multi-factorial evaluation process. Another challenge in a non-dental screening setting is LF and ICDAS-II both require that teeth be air dried for assessment. OCT can be used without air drying, since teeth can be imaged after being wicked dry as in this study. Thus OCT imaging is eminently suitable for community dentistry in non-traditional settings or teledentistry, facilitating the monitoring of clinically suspicious surfaces over time, even by different providers, and at different locations. This capacity becomes particularly valuable in communities where there is limited access to dental care, where vulnerable or disabled populations are unable to receive care in traditional dental care facilities, or for individuals whose dental treatment is particularly complicated or difficult (e.g. hemophilia, special needs). The initial expense of investing in an OCT system may be counterbalanced by quicker and more accurate dental diagnosis, resulting in more effective prevention and less intervention. Communication between clinicians in situations where different clinicians may be involved in patient care, and a valuable benchmark for quality assurance may be some of the additional benefits of this approach[59]. Clinical studies are now indicated to translate these ex vivo data to the clinical environment.
CONCLUSION
OCT-based imaging combined with a simple diagnostic algorithm can accurately assess the severity of natural early caries on occlusal surfaces in extracted teeth both in the absence and presence of dental sealant. The findings of this study support the clinical use of OCT imaging for assessment and monitoring progression of early non-cavitated caries lesions on occlusal surfaces including areas under dental sealants.
ACKNOWLEDGMENTS
This work was supported by grants from NIH/NIBIB 5R03EB014852-02 and the Beckman Foundation.
Footnotes
The authors declare no potential conflicts of interests with respect to the authorship and/or publication of this article.
REFERENCES
- 1.Ekstrand KR, et al. Detection, diagnosing, monitoring and logical treatment of occlusal caries in relation to lesion activity and severity: an in vivo examination with histological validation. Caries Res. 1998;32(4):247–54. doi: 10.1159/000016460. [DOI] [PubMed] [Google Scholar]
- 2.San Martin L, et al. Dental sealant knowledge, opinion, values and practice of Spanish dentists. BMC Oral Health. 2013;13:12. doi: 10.1186/1472-6831-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tellez M, et al. Sealants and dental caries: dentists' perspectives on evidence-based recommendations. J Am Dent Assoc. 2011;142(9):1033–40. doi: 10.14219/jada.archive.2011.0324. [DOI] [PubMed] [Google Scholar]
- 4.Beauchamp J, et al. Evidence-based clinical recommendations for the use of pit-and-fissure sealants: a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139(3):257–68. doi: 10.14219/jada.archive.2008.0155. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira Zandona A, et al. The natural history of dental caries lesions: a 4-year observational study. J Dent Res. 2012;91(9):841–6. doi: 10.1177/0022034512455030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtzman JS, et al. Ability of optical coherence tomography to detect caries beneath commonly used dental sealants. Lasers Surg Med. 2010;42(8):752–9. doi: 10.1002/lsm.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada Y, et al. Validation of swept-source optical coherence tomography (SS-OCT) for the diagnosis of occlusal caries. J Dent. 2010;38(8):655–65. doi: 10.1016/j.jdent.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Kuhnisch J, et al. Occlusal caries detection in permanent molars according to WHO basic methods, ICDAS II and laser fluorescence measurements. Community Dent Oral Epidemiol. 2008;36(6):475–84. doi: 10.1111/j.1600-0528.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones RS, et al. Imaging artificial caries on the occlusal surfaces with polarization-sensitive optical coherence tomography. Caries Res. 2006;40(2):81–9. doi: 10.1159/000091052. [DOI] [PubMed] [Google Scholar]
- 10.Kang H, et al. Nondestructive Assessment of Early Tooth Demineralization Using Cross-Polarization Optical Coherence Tomography. IEEE J Sel Top Quantum Electron. 2010;16(4):870–876. doi: 10.1109/JSTQE.2009.2033610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail AI, et al. Rational and evidence for the International Caries Detection and Assessment System (ICDAS II). In: Stookey GK, editor. Clinical Models Workshop: Remin-Demin, Precavitation, Caries; Proceedings of the 7th Indiana Conference; Indianapolis, Indiana: Indiana University School of Dentistry; 2005. [Google Scholar]
- 12.Rodrigues JA, et al. Performance of fluorescence methods, radiographic examination and ICDAS II on occlusal surfaces in vitro. Caries Res. 2008;42(4):297–304. doi: 10.1159/000148162. [DOI] [PubMed] [Google Scholar]
- 13.Gomez J, et al. In vitro performance of different methods in detecting occlusal caries lesions. J Dent. 2013;41(2):180–6. doi: 10.1016/j.jdent.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Jablonski-Momeni A, et al. Performance of laser fluorescence at tooth surface and histological section. Lasers Med Sci. 2011;26(2):171–8. doi: 10.1007/s10103-010-0768-y. [DOI] [PubMed] [Google Scholar]
- 15.Diniz MB, et al. The influence of pit and fissure sealants on infrared fluorescence measurements. Caries Res. 2008;42(5):328–33. doi: 10.1159/000151327. [DOI] [PubMed] [Google Scholar]
- 16.Lussi A, et al. Performance and reproducibility of a laser fluorescence system for detection of occlusal caries in vitro. Caries Res. 1999;33(4):261–6. doi: 10.1159/000016527. [DOI] [PubMed] [Google Scholar]
- 17.Achilleos EE, et al. Evaluation of a new fluorescence-based device in the detection of incipient occlusal caries lesions. Lasers Med Sci. 2013;28(1):193–201. doi: 10.1007/s10103-012-1111-6. [DOI] [PubMed] [Google Scholar]
- 18.Colston BW, Jr., et al. Imaging of the oral cavity using optical coherence tomography. Monogr Oral Sci. 2000;17:32–55. doi: 10.1159/000061643. [DOI] [PubMed] [Google Scholar]
- 19.Tadrous PJ. Methods for imaging the structure and function of living tissues and cells: 1. Optical coherence tomography. J Pathol. 2000;191(2):115–9. doi: 10.1002/(SICI)1096-9896(200006)191:2<115::AID-PATH589>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Sattler E, Kastle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18(6):061224. doi: 10.1117/1.JBO.18.6.061224. [DOI] [PubMed] [Google Scholar]
- 21.Adhi M, Duker JS. Optical coherence tomography--current and future applications. Curr Opin Ophthalmol. 2013;24(3):213–21. doi: 10.1097/ICU.0b013e32835f8bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfonso F, et al. Optical coherence tomography: from research to clinical application. Minerva Med. 2012;103(6):441–64. [PubMed] [Google Scholar]
- 23.Burns JA. Optical coherence tomography: imaging the larynx. Curr Opin Otolaryngol Head Neck Surg. 2012;20(6):477–81. doi: 10.1097/MOO.0b013e3283582d7d. [DOI] [PubMed] [Google Scholar]
- 24.Wilder-Smith P, et al. Optical diagnostics in the oral cavity: an overview. Oral Dis. 2010;16(8):717–28. doi: 10.1111/j.1601-0825.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le MH, Darling CL, Fried D. Automated analysis of lesion depth and integrated reflectivity in PS-OCT scans of tooth demineralization. Lasers Surg Med. 2010;42(1):62–8. doi: 10.1002/lsm.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louie T, et al. Clinical assessment of early tooth demineralization using polarization sensitive optical coherence tomography. Lasers Surg Med. 2010;42(10):738–45. doi: 10.1002/lsm.21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner A, et al. Polarization-sensitive optical coherence tomography of dental structures. Caries Res. 2000;34(1):59–69. doi: 10.1159/000016571. [DOI] [PubMed] [Google Scholar]
- 28.Douglas SM, Fried D, Darling CL. Imaging Natural Occlusal Caries Lesions with Optical Coherence Tomography. Proc Soc Photo Opt Instrum Eng. 2010;7549:75490N. doi: 10.1117/12.849344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang H, et al. Imaging early demineralization with PS-OCT. Proc Soc Photo Opt Instrum Eng. 2010;7549 doi: 10.1117/12.849343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones RS, Fried D. Remineralization of enamel caries can decrease optical reflectivity. J Dent Res. 2006;85(9):804–8. doi: 10.1177/154405910608500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong SL, Darling CL, Fried D. Nondestructive measurement of the inhibition of demineralization on smooth surfaces using polarization-sensitive optical coherence tomography. Lasers Surg Med. 2007;39(5):422–7. doi: 10.1002/lsm.20506. [DOI] [PubMed] [Google Scholar]
- 32.Can AM, Darling CL, Fried D. High-resolution PS-OCT of Enamel Remineralization. Proc Soc Photo Opt Instrum Eng. 2008;6843:68430T1–68430T7. doi: 10.1117/12.778787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amaechi BT, et al. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J Biomed Opt. 2003;8(4):642–7. doi: 10.1117/1.1606685. [DOI] [PubMed] [Google Scholar]
- 34.Fried D, et al. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. J Biomed Opt. 2002;7(4):618–27. doi: 10.1117/1.1509752. [DOI] [PubMed] [Google Scholar]
- 35.Jones RS, Staninec M, Fried D. Imaging artificial caries under composite sealants and restorations. J Biomed Opt. 2004;9(6):1297–304. doi: 10.1117/1.1805562. [DOI] [PubMed] [Google Scholar]
- 36.Lenton P, et al. Imaging in vivo secondary caries and ex vivo dental biofilms using cross-polarization optical coherence tomography. Dent Mater. 2012;28(7):792–800. doi: 10.1016/j.dental.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa H, et al. Validation of swept source optical coherence tomography (SS-OCT) for the diagnosis of smooth surface caries in vitro. J Dent. 2013;41(1):80–9. doi: 10.1016/j.jdent.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Ekstrand KR, Ricketts DN, Kidd EA. Reproducibility and accuracy of three methods for assessment of demineralization depth of the occlusal surface: an in vitro examination. Caries Res. 1997;31(3):224–31. doi: 10.1159/000262404. [DOI] [PubMed] [Google Scholar]
- 39.Ricketts DN, et al. Relating visual and radiographic ranked scoring systems for occlusal caries detection to histological and microbiological evidence. Oper Dent. 2002;27(3):231–7. [PubMed] [Google Scholar]
- 40.Hariri I, et al. Effects of structural orientation of enamel and dentine on light attenuation and local refractive index: an optical coherence tomography study. J Dent. 2012;40(5):387–96. doi: 10.1016/j.jdent.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Ishibashi K, et al. Swept-source optical coherence tomography as a new tool to evaluate defects of resin-based composite restorations. J Dent. 2011;39(8):543–8. doi: 10.1016/j.jdent.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Natsume Y, et al. Estimation of lesion progress in artificial root caries by swept source optical coherence tomography in comparison to transverse microradiography. J Biomed Opt. 2011;16(7):071408. doi: 10.1117/1.3600448. [DOI] [PubMed] [Google Scholar]
- 43.Amaechi BT, et al. Quantification of root caries using optical coherence tomography and microradiography: a correlational study. Oral Health Prev Dent. 2004;2(4):377–82. [PubMed] [Google Scholar]
- 44.Sowa MG, et al. A comparison of methods using optical coherence tomography to detect demineralized regions in teeth. J Biophotonics. 2011;4(11–12):814–23. doi: 10.1002/jbio.201100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frost VS, et al. A model for radar images and its application to adaptive digital filtering of multiplicative noise. IEEE Trans Pattern Anal Mach Intell. 1982;4(2):157–66. doi: 10.1109/tpami.1982.4767223. [DOI] [PubMed] [Google Scholar]
- 46.Lussi A, Hellwig E. Performance of a new laser fluorescence device for the detection of occlusal caries in vitro. J Dent. 2006;34(7):467–71. doi: 10.1016/j.jdent.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Kuhnisch J, et al. Comparison of visual inspection and different radiographic methods for dentin caries detection on occlusal surfaces. Dentomaxillofac Radiol. 2009;38(7):452–7. doi: 10.1259/dmfr/34393803. [DOI] [PubMed] [Google Scholar]
- 48.Askaroglou E, et al. Effect of sealants on laser fluorescence caries detection in primary teeth. Lasers Med Sci. 2011;26(1):29–34. doi: 10.1007/s10103-009-0745-5. [DOI] [PubMed] [Google Scholar]
- 49.Gostanian HV, et al. An in vitro evaluation of the effect of sealant characteristics on laser fluorescence for caries detection. Pediatr Dent. 2006;28(5):445–50. [PubMed] [Google Scholar]
- 50.Anttonen V, Seppa L, Hausen H. Clinical study of the use of the laser fluorescence device DIAGNOdent for detection of occlusal caries in children. Caries Res. 2003;37(1):17–23. doi: 10.1159/000068227. [DOI] [PubMed] [Google Scholar]
- 51.Burin C, et al. Occlusal caries detection: a comparison of a laser fluorescence system and conventional methods. Pediatr Dent. 2005;27(4):307–12. [PubMed] [Google Scholar]
- 52.Attrill DC, Ashley PF. Occlusal caries detection in primary teeth: a comparison of DIAGNOdent with conventional methods. Br Dent J. 2001;190(8):440–3. doi: 10.1038/sj.bdj.4800998. [DOI] [PubMed] [Google Scholar]
- 53.Lussi A. Comparison of different methods for the diagnosis of fissure caries without cavitation. Caries Res. 1993;27(5):409–16. doi: 10.1159/000261572. [DOI] [PubMed] [Google Scholar]
- 54.Shi XQ, Welander U, Angmar-Mansson B. Occlusal caries detection with KaVo DIAGNOdent and radiography: an in vitro comparison. Caries Res. 2000;34(2):151–8. doi: 10.1159/000016583. [DOI] [PubMed] [Google Scholar]
- 55.Takamori K, et al. Detection of occlusal caries under sealants by use of a laser fluorescence system. J Clin Laser Med Surg. 2001;19(5):267–71. doi: 10.1089/10445470152612008. [DOI] [PubMed] [Google Scholar]
- 56.Van Hilsen Z, Jones RS. Comparing potential early caries assessment methods for teledentistry. BMC Oral Health. 2013;13:16. doi: 10.1186/1472-6831-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agbaje JO, et al. Examiner performance in calibration exercises compared with field conditions when scoring caries experience. Clin Oral Investig. 2012;16(2):481–8. doi: 10.1007/s00784-011-0523-1. [DOI] [PubMed] [Google Scholar]
- 58.Zandona AG, et al. Student versus faculty performance using a new visual criteria for the detection of caries on occlusal surfaces: an in vitro examination with histological validation. Oper Dent. 2009;34(5):598–604. doi: 10.2341/08-082-L. [DOI] [PubMed] [Google Scholar]
- 59.Mendes FM, et al. Radiographic and laser fluorescence methods have no benefits for detecting caries in primary teeth. Caries Res. 2012;46(6):536–43. doi: 10.1159/000341189. [DOI] [PubMed] [Google Scholar]



