Abstract
Introduction
The objective of this study is to identify computed tomography (CT) features of local recurrence (LR) after SBRT for lung cancer.
Methods
218 patients underwent SBRT for lung cancer from January 1st 2006–March 1st 2011. Signs of LR recorded: opacity with new bulging margin, opacification of air bronchograms, enlarging pleural effusion, new or enlarging mass, increased lung density at the treatment site.
Results
A new bulging margin at the treatment site was the only feature significantly associated with LR (p<0.005).
Conclusion
Most CT features classically associated with local recurrence following conventional RT are unreliable for predicting LR following SBRT.
Keywords: Lung cancer, Computed tomography, Stereotactic body radiation therapy, Local recurrence
Introduction
Stereotactic body radiation therapy (SBRT) is a therapeutic option for patients with stage I non-small cell lung cancer that are medically inoperable or decline surgery [1, 2]. The arrangement of radiation portals used is complex and results in the generation of a step radiation gradient, allowing the delivery of high doses of radiation to the targeted tumor volume while minimizing exposure to normal tissues [3]. The use of SBRT to treat inoperable early stage lung cancer has become widespread and has led to reported local control rates of between 80 – 100% [4].
Computed tomography (CT) is routinely used for imaging follow-up of patients following radiation therapy. Patterns of radiation injury on CT resulting from conventional radiation therapy are well described and tend to progress predictably, conforming to the radiation portal [5]. As conventional radiation induced lung injury evolves, a linear opacity with sharply demarcated borders, traction bronchiectasis and air bronchograms is typical for radiation fibrosis [3]. Once radiation fibrosis has become established, areas that demonstrate new bulging margins, increasing density, filling in of air bronchograms or the development of new parenchymal nodularity all raise suspicion for recurrent disease [3]. After the first 6 months following treatment, a new or increasing pleural effusion is also suspicious for recurrent disease [3].
In patients treated with SBRT, the complex beam arrangements can lead to the development of parenchymal lung abnormalities that differ substantially from those typically seen following conventional radiotherapy [4]. For example, mass-like fibrosis surrounding the treated tumor is a well described imaging finding following SBRT, which is not classically associated with conventional radiotherapy [6]. In this setting, detecting local recurrence (LR) becomes challenging [6,7,8]. The aim of this study is to evaluate the ability of features associated with LR on CT following conventional radiation therapy to predict LR and survival following SBRT.
Material and Methods
This study was exempt from the requirement for informed consent by our institutional review board.
Patients
218 consecutive patients underwent SBRT (total dose 4000–6000 cGy delivered in 3–5 fractions over 1–2 weeks) for local control of stage 1 non-small cell lung cancer from January 1st, 2006–March 1st, 2011. Patients were included in the analysis if they had baseline imaging and 3 follow up CTs available for review in our institution. Patients with LR, were included in analysis if the CT immediately preceding LR was performed within 6 months of the date of LR. No patient received conventional radiation therapy.
Imaging technique
CT scans were obtained with a 16–detector row (LightSpeed 16; GE Healthcare, Milwaukee) or 64–detector row (VCT; GE Healthcare Milwaukee) scanner, both of which are routinely used in our institution. Parameters for the 16–detector row scanner were as follows: tube voltage, 120 kVp; tube current, 120–380 mA; detector configuration, 16 detectors × 1.25-mm section gap; and pitch, 1.375:1. Parameters of the 64–detector row scanner were as follows: tube voltage, 120 kVp; tube current, 120–380 mA; detector configuration, 64 detectors × 0.63-mm section gap; and pitch, 0.984:1. The thoracic images were obtained with or without intravenous contrast material during a breath hold. Axial 5 × 5 mm images and coronal and sagittal plane CT 2.5 × 2.5 mm images were reconstructed and transferred to the picture archiving and communication system (PACS) server where all images are stored.
Imaging evaluation
Images were viewed on the institutional PACS (GE Healthcare, Milwaukee). Lung (width, 1500 HU; level, −500 HU) and soft tissue (width, 400 HU; level, 30 HU) window settings were used for CT evaluation. Images were independently retrospectively reviewed by two radiologists (D.H. and S.H.), who were blinded to the clinical history (other than the history of treatment with SBRT). A third radiologist (C.R.) reviewed clinical data, pathological data, and CT and positron emission tomography (PET) -CT images and reports to identify patients who had recurred.
The pre-treatment chest CT was initially evaluated. Subsequently the next 3 follow up CTs were studied in the sequence they were performed. A typical follow up imaging schedule for asymptomatic patients in our institution sees CT performed at 6, 12, 24 months after SBRT. If a patient becomes symptomatic, this schedule is altered and patients are imaged at earlier intervals. To minimize the risk of the results of the 3rd CT being false negative, patients who did not have an additional 4th CT performed more than 6 months after the 3rd follow-up study had the 3rd scan excluded from the analysis.
The following radiologic signs of LR in the SBRT field were recorded on each CT, if present: opacity with a new bulging margin, opacification of previous air bronchograms, new or enlarging pleural effusion, new/increasing mass and subjective increase in lung density in the irradiated field. A new bulging margin was defined as new or persistent convexity at the site of a previous straight margin within the treated lesion compared to the most recent CT. Opacification of previous air bronchograms was defined as new or increasing opacification of previously air-filled air airways compared to the most recent CT. A new mass was defined as a new or persistent lung lesion, separate to the treated lesion, but occurring within 2cm of the field of SBRT compared to the most recent CT. Subjective increase in lung density was defined as subjective increased attenuation in the treated lesion compared to the most recent CT.
Local failure was determined if there was evidence of recurrence as described by ACOSOG Z4099 (American College of Surgeons Oncology Group randomized phase II study assessing SBRT in stage 1 lung cancer), defined in that protocol as “the appearance of residual tumor located within the extent of the primary targeted tumor” [9]. LR was confirmed with histopathology if available, or if not available, by the results of PET/CT. PET and CT reconstructed datasets were fused and viewed on the picture archiving and communication system (PACS). PET-CT studies obtained after SBRT were compared to the baseline pre-treatment CT and PET-CT study, when available. LR was considered present if there was persistent lesional FDG avidity on PET-CT [standardized uptake value (SUV)>2.5] within an opacity at the site of treatment, more than 6 months following the completion of SBRT [10,11,12]. The date of histological confirmation was taken as the date of LR if biopsy was performed. If not, the date of PET/CT demonstrating LR was used as the date of LR.
Statistical Methods
Fisher’s exact test was used to compare features between the scans of patients with local recurrence and the scans of patients without local recurrence for the 2 readers separately. A p-value of <0.005 was assumed to be statistically significant. The inter-reader agreement on categorical feature was assessed using kappa statistic. Kappa (κ) values were interpreted as follows: 0.00–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, almost perfect agreement [13]. A test with p-value < 0.05 was considered significant. Statistical analyses were performed in software packages SAS 9.2 (SAS Institute Inc., Cary, NC, USA) and R version 2.13 (The R Foundation for Statistical Computing). All numerical values are expressed as median (range)
Results
Patients, imaging schedule and local recurrence rate
Of the 218 patients who received SBRT, 125 were excluded because their baseline CT and 3 follow up CTs were not available for review in our institution. Of the 93 patients remaining, 10 had LR. LR was confirmed in all 10 patients using FDG-PET with an additional 4 patients undergoing biopsy for pathologic confirmation Table 1. The median time to LR was 17 months (range 4–37) after SBRT.
Table 1.
The timing and SUV levels of follow–up PET/CT in 4 patients in whom a diagnosis of local recurrence was not based on biopsy.
2 of the LR patients were excluded because the most recent CT available for review was not performed within 6 months of the date of LR. 8 patients with LR and 83 patients without LR were thus included in the analysis. The CT closest to the date of LR was performed at a median of 1 month (range 0–6 months) from the date LR. Of the 83 patients without LR, 41 did not have a 4th follow-up CT more than 6 months after their 3rd follow-up CT. These patients had their 3rd follow-up CT excluded from the analysis, leaving 208 evaluable CT events without LR. Median time to the first follow up CT was 6 months (range 3–12), median time to second follow-up CT was 13 month (range 6–19) and median time to the third follow-up CT was 24 months (range 16–41).
Median patient age was 81 years (range 54–99). Tumor pathology was adenocarcinoma in 61 (66%) cases and squamous cell carcinoma in 32 (34%) cases. During the study period, 8 patients developed distant metastases (3 lung, 3 thoracic nodal, 1 adrenal and 1 osseous).
CT Features
Table 2 summarizes the CT features of recurrence which were identified on the examination closest to the diagnosis of LR. A new bulging margin at the site of treated tumor was the only feature that was statistically significantly associated with LR (p<0.005). Both readers identified this feature in 4 (50%) patients with LR. A bulging margin was identified in 19 (9%) patients without LR by reader 1 and in 21 (10%) patients by reader 2. Density increase and filled in air bronchograms were both more common in patients with LR but the presence of neither feature reached statistical significance.
Table 2.
Depicts the features of local recurrence which were identified on CT following SBRT for stage 1 non-small cell lung cancer.
| Reader 1 | Reader 2 | kappa | ||||||
|---|---|---|---|---|---|---|---|---|
| LR | No LR | LR | No LR | |||||
| N (%) | N (%) | P value | N (%) | N (%) | P value | |||
| Bulge | Present | 4 (50%) | 19 (9%) | 0.005 | 4 (50%) | 21 (10%) | 0.007 | 0.86 |
| Filled in bronchogram | Present | 2 (25%) | 19 (9%) | 0.176 | 2 (25%) | 26 (13%) | 0.278 | 0.79 |
| New effusion | Present | 1 (13%) | 74(36%) | 0.267 | 1 (13%) | 75 (36%) | 0.265 | 0.99 |
| New mass | Present | 0 (0%) | 7 (3%) | >.999 | 0 (0%) | 6 (3%) | >.999 | 0.45 |
| Density increase | Present | 5 (63%) | 68 (33%) | 0.123 | 4 (50%) | 64 (31%) | 0.264 | 0.82 |
All 3 features that were more common in patients with LR were also frequently found in patients without LR. In the group without LR, a new bulge was found in up to 10% of patients, filled in air bronchograms in up to 13% of patients and density increase in up to 33% of patients.
Inter-observer agreement
A summary of inter-observer agreement is provided in Table 2. Agreement between readers for detecting bulging margins, fill in of air bronchograms, new/enlarging pleural effusion and density increase ranged from substantial to perfect (kappa values 0.79 – 1). Inter-observer agreement for detection of a new mass was moderate (kappa value 0.45).
DISCUSSION
SBRT has been demonstrated to be safe, and effective at achieving local control in stage 1 non-small cell lung carcinoma, and is an increasingly used therapeutic option in this setting. Although local control rates are excellent, early identification of patients who do recur is vital, as options for salvage therapy such as surgery, radiofrequency ablation and cryoablation can be considered [1, 2]. Imaging is thus crucial in the follow up of these patients. In our institution, patients typically undergo imaging surveillance using CT, with positron emission tomography-CT (PET-CT) reserved as an adjunct if CT raises suspicion for LR.
The CT appearance of evolving post-radiation change seen in patients following SBRT can differ considerably from that seen following conventional radiation. The role of the radiologist in differentiating between local tumor recurrence and post radiation parenchymal distortion, becomes increasingly challenging when such atypical patterns of radiation change are present. To our knowledge this is the first statistical analysis of the diagnostic utility of classical morphologic CT features of LR in the setting of SBRT.
The results of this study demonstrate that following SBRT, a new bulging margin in an opacity at the site of treatment is significantly associated with LR. A bulging margin occurred 5 times more frequently in cases of LR than in cases without LR. However, it was only present in 50% of cases with LR and was also seen in up to 10% of cases without LR. Density increase and filled in air bronchograms were both more common in the LR cases but did not reach statistical significance. Although both of these features were more common in patients with LR, they were also frequently seen in patients without LR. For example, a density increase as the site of treatment was seen in up to 63% of cases of LR but was also present in up to 33% of cases without LR, limiting is effectiveness as a clinically useful sign. These results call into question the utility of some of the features classically associated with LR, in the post-SBRT setting.
Radiation induced lung injury is common following any form of thoracic RT and has 2 main temporal patterns [3]. Radiation pneumonitis, presenting as ground glass attenuation or consolidation, which can also be nodular or mass-like, with or without an ipsilateral pleural effusion, typically occurs within 4 to 12 weeks following completion of treatment. Subsequent to this, usually 6 to 12 months following therapy, radiation fibrosis can develop and may evolve for up to 2 years [3]. The temporal pattern of developing radiation fibrosis is similar following SBRT when compared to conventional RT [14]. There are several factors which influence the timing and extent of conventional radiation induced lung injury, including the volume of lung irradiated, the dose administered and the fractionation of dose.
The pattern of parenchymal lung abnormality following conventional radiation therapy is typically predictable, classically conforming to the treatment portal, with a sharp demarcation between normal and abnormal lung [15]. The appearance of radiation change following SBRT differs from that of conventional post radiation fibrosis, and can be classified into 3 distinct patterns [16]. A “modified conventional” pattern of fibrosis differs from the typical appearance of radiation fibrosis in that it is less extensive, and does not involve the entirety of the radiated lung field from pleural surface to pleural surface. Mass-like fibrosis occurs when consolidation and traction bronchiectasis is confined to a 2cm circumferential margin around the original tumor. Scar-like fibrosis manifests as a linear opacity with significant volume loss at the site of tumor [16]. The modified conventional pattern is most common. The pattern of post-SBRT radiation change can evolve. If it does change, it most commonly progresses from a modified conventional pattern, to either a scar-like pattern or a mass-like pattern [17]. The proportion of patients with a mass-like pattern of fibrosis increases following 2 years of follow-up [17].
There is a paucity of literature evaluating the ability of CT to predict LR following SBRT. Dunlap et al evaluated a cohort of 44 patients treated with SBRT for stage 1 non-small cell lung carcinoma, 10 of whom had proven LR. Using Response Evaluation Criteria in Solid Tumors (RESCIST) 1.1 the authors predicted LR on CT in 35 of the 44 patients, yielding a false positive rate for RESCIST in this setting of 71%, and a positive predictive value of 28%. An increase in size over 3 or more consecutive follow-up scans did have a sensitivity and specificity of 100% for detection of LR [6]. Matsuo et al evaluated 37 patients, 3 of whom had LR. The authors found no difference in morphological features between patients with and without LR. Increasing size of a mass 12 months post treatment was however significantly associated with LR [18]. Takeda et al evaluated 50 patients following treatment, 20 of whom had findings suspicious for recurrence based on CT findings, but only 3 of whom ultimately had proven recurrence [7].
To our knowledge, there is only 1 previous study describing individual classical features of LR on CT, in a group of patients following SBRT [8]. No statistical analysis on the diagnostic accuracy of the features is provided in that paper, and the patient cohort is smaller than in the current study. 27 patients, 5 of whom had LR were studied. It was found that in cases of LR, bulging margins were present in 4/5 patients, filled in air bronchograms in 3/5, ipsilateral pleural effusion in 5/5, and thoracic lymphadenopathy in 3/5. All cases with LR had at least 2 of the above features.
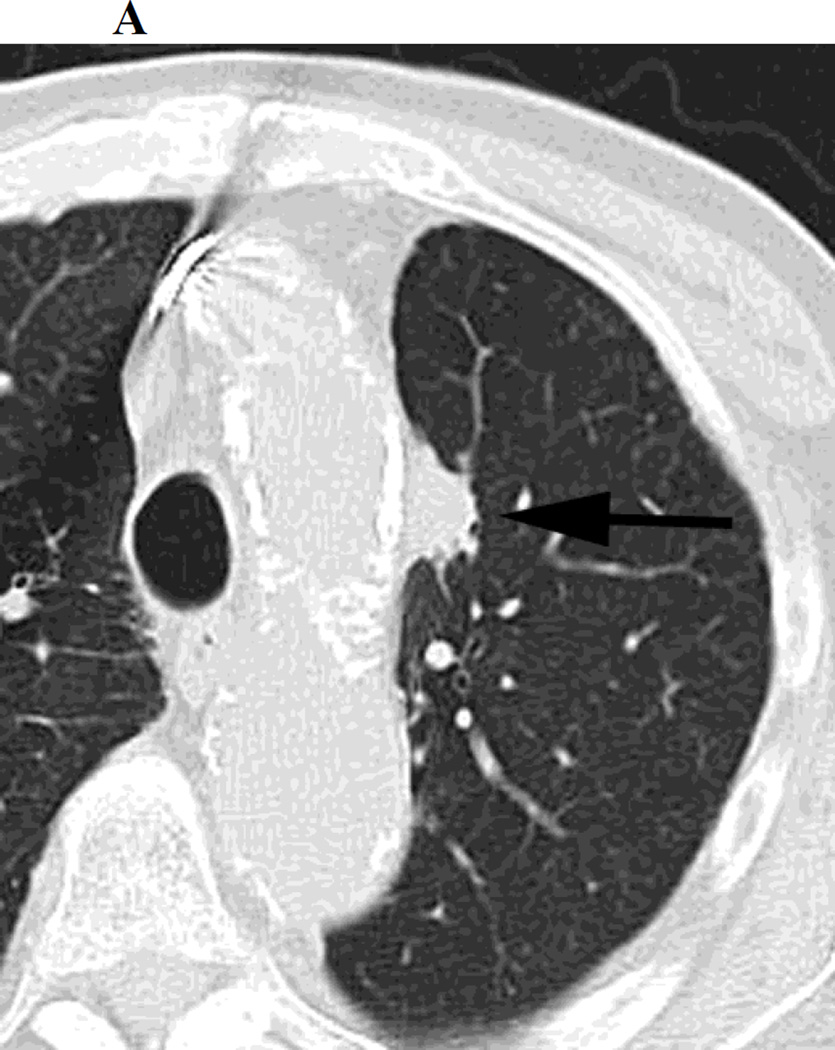
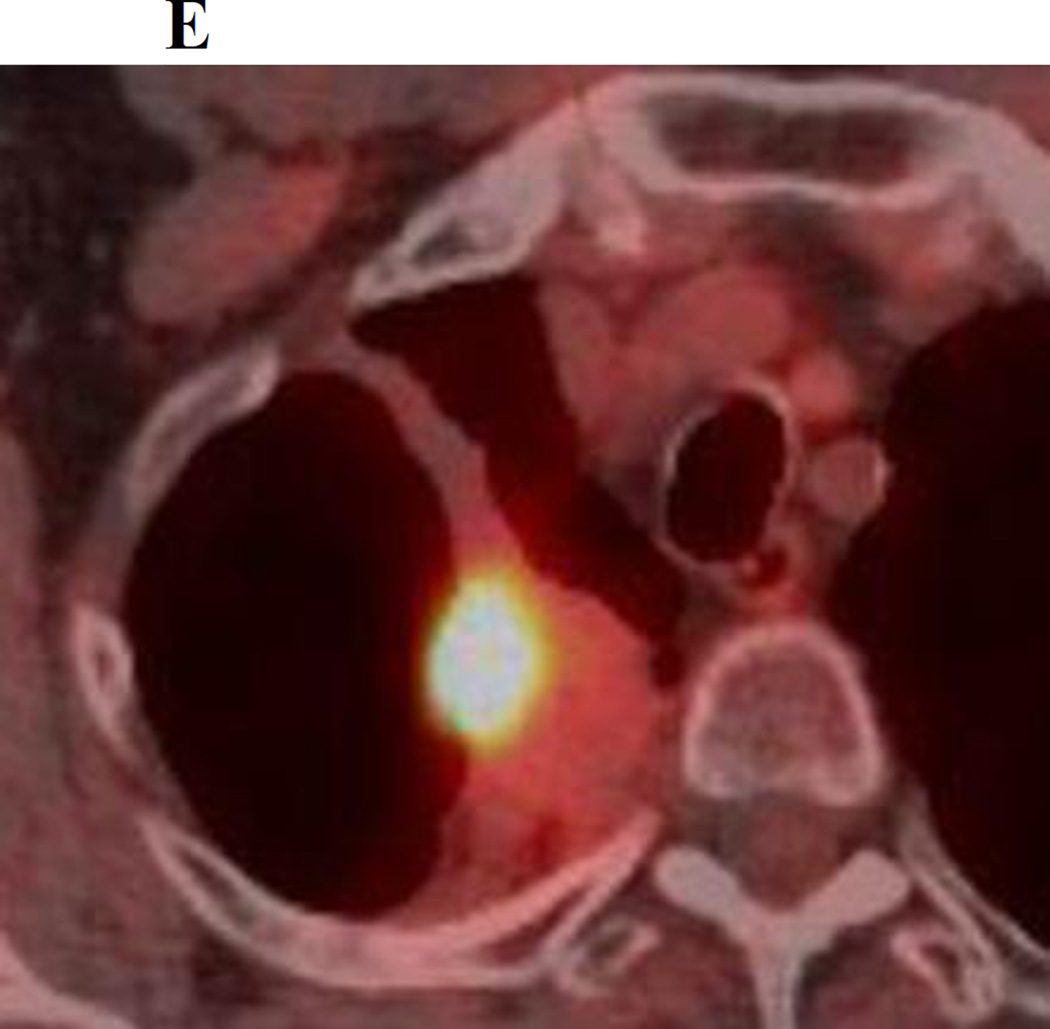
There are several factors which account for the difficulty in detecting LR following SBRT, however the unpredictable non-linear nature of post radiation change in this setting is probably the main reason for diagnostic uncertainty. The fact that post-SBRT fibrosis can evolve from a modified conventional pattern to a mass like pattern is another significant factor introducing ambiguity on surveillance CT studies. It should be emphasized that while the features we have studied may be less reliable in a post-SBRT setting, they are still likely to retain their usefulness in certain post-SBRT patients, particularly those with a “modified conventional pattern” of post-radiation change and thus should be routinely looked for. For example, Figure 1 demonstrates a left upper lobe tumor treated with SBRT, which on follow up CT scans demonstrates, increasing density, opacification of air bronchograms and a new bulging margin. Figure 2 demonstrates a right upper lobe tumor treated with SBRT which on follow up CT scans demonstrates a new bulging margin. Patients with a mass like pattern of post-SBRT parenchymal abnormality are however likely to remain a diagnostic dilemma. We feel that having a low threshold for evaluation with PET/CT is appropriate, particularly outside the first 6 months post-SBRT (when inflammatory FDG uptake can be misleading)[19].
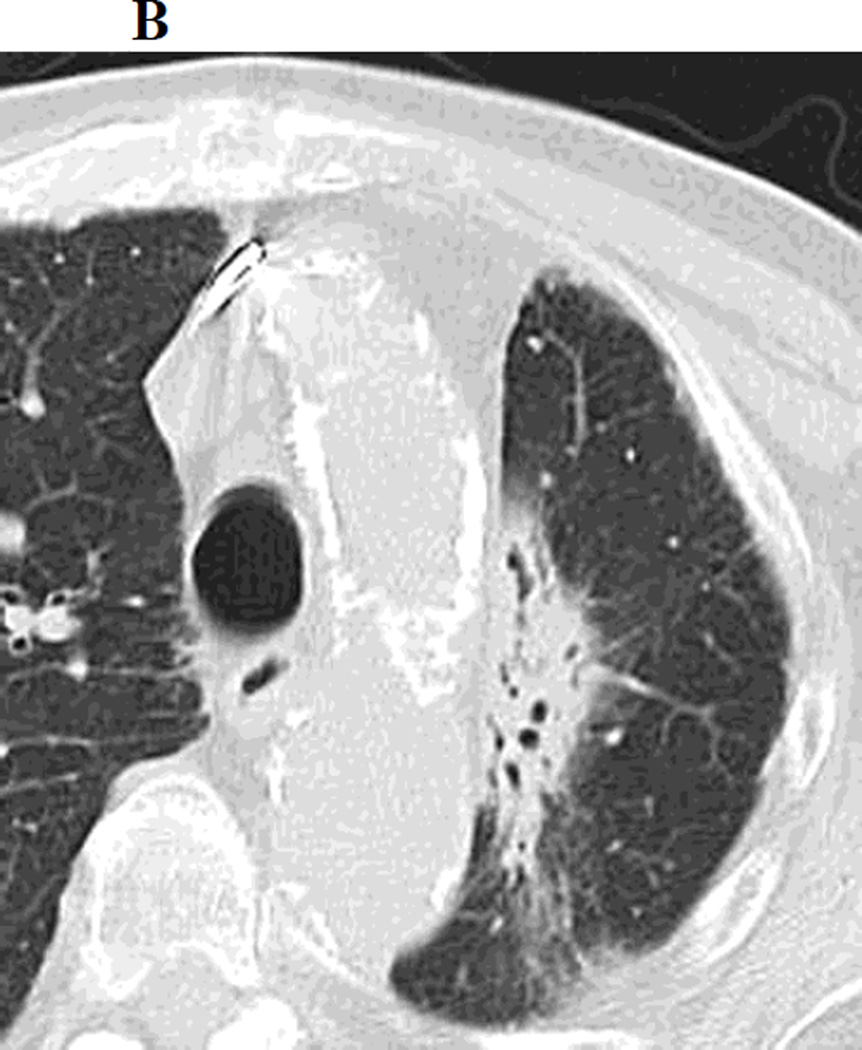
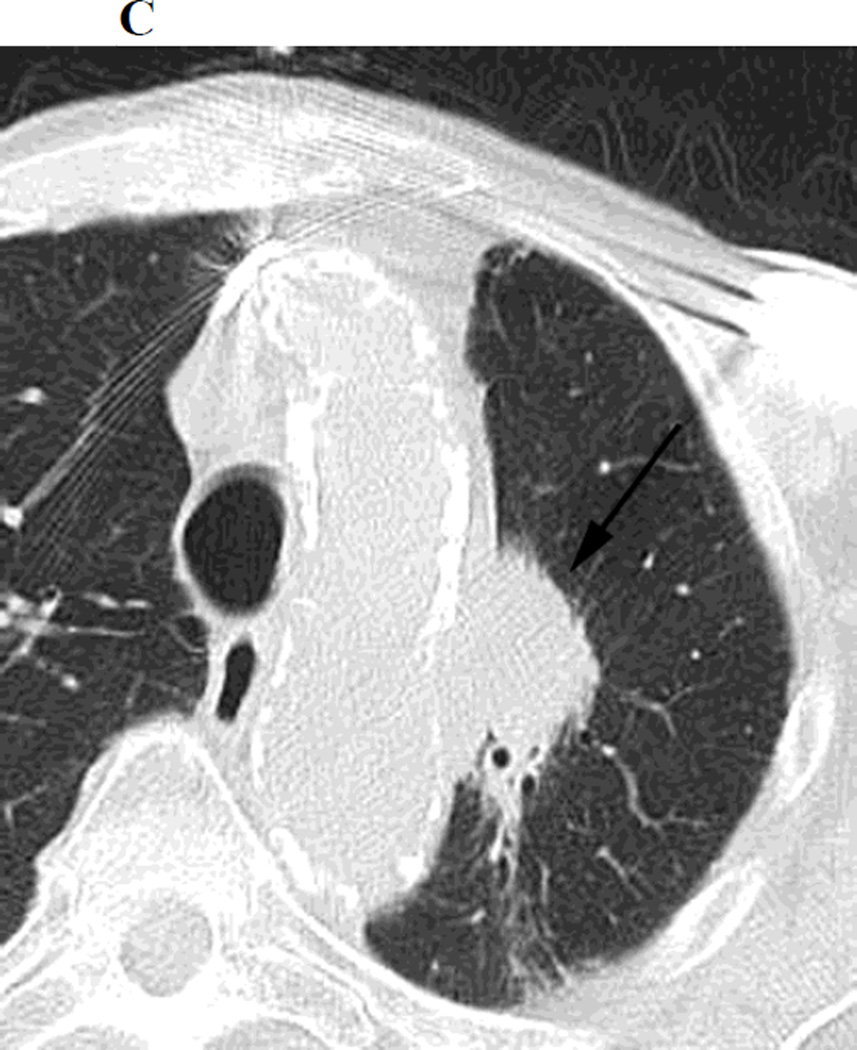
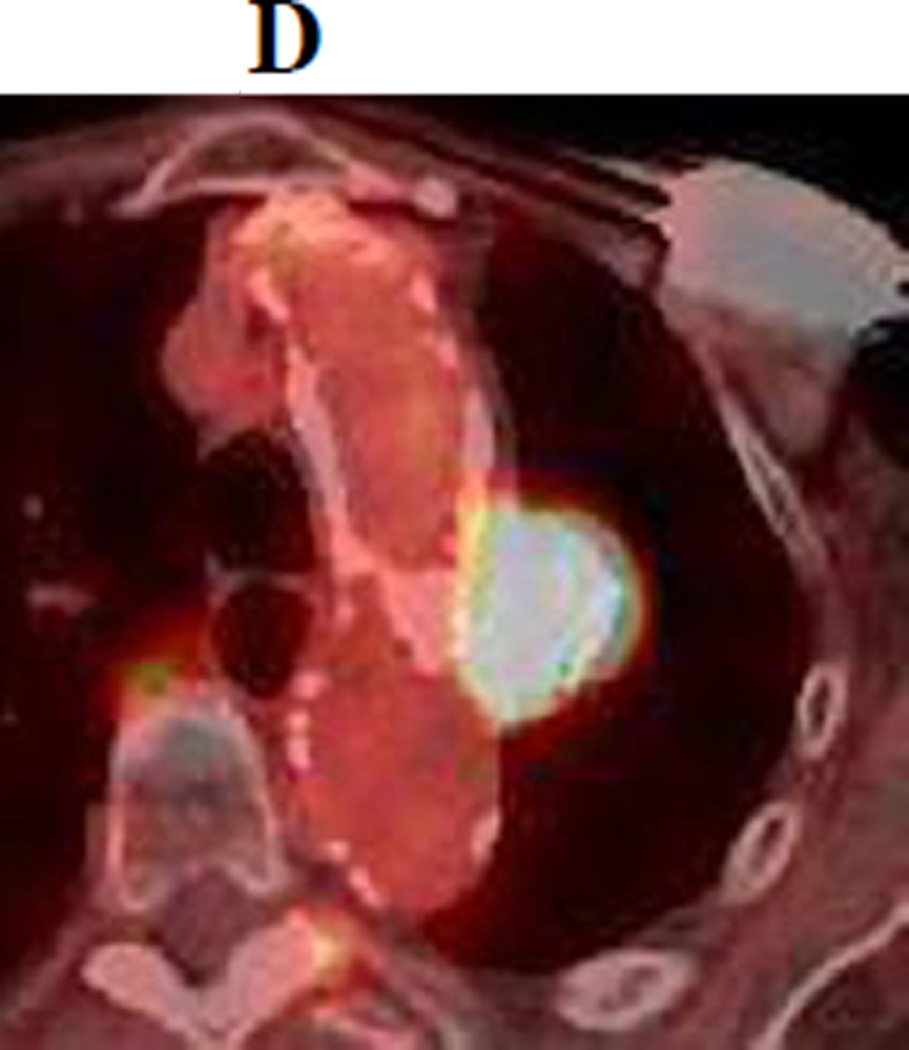
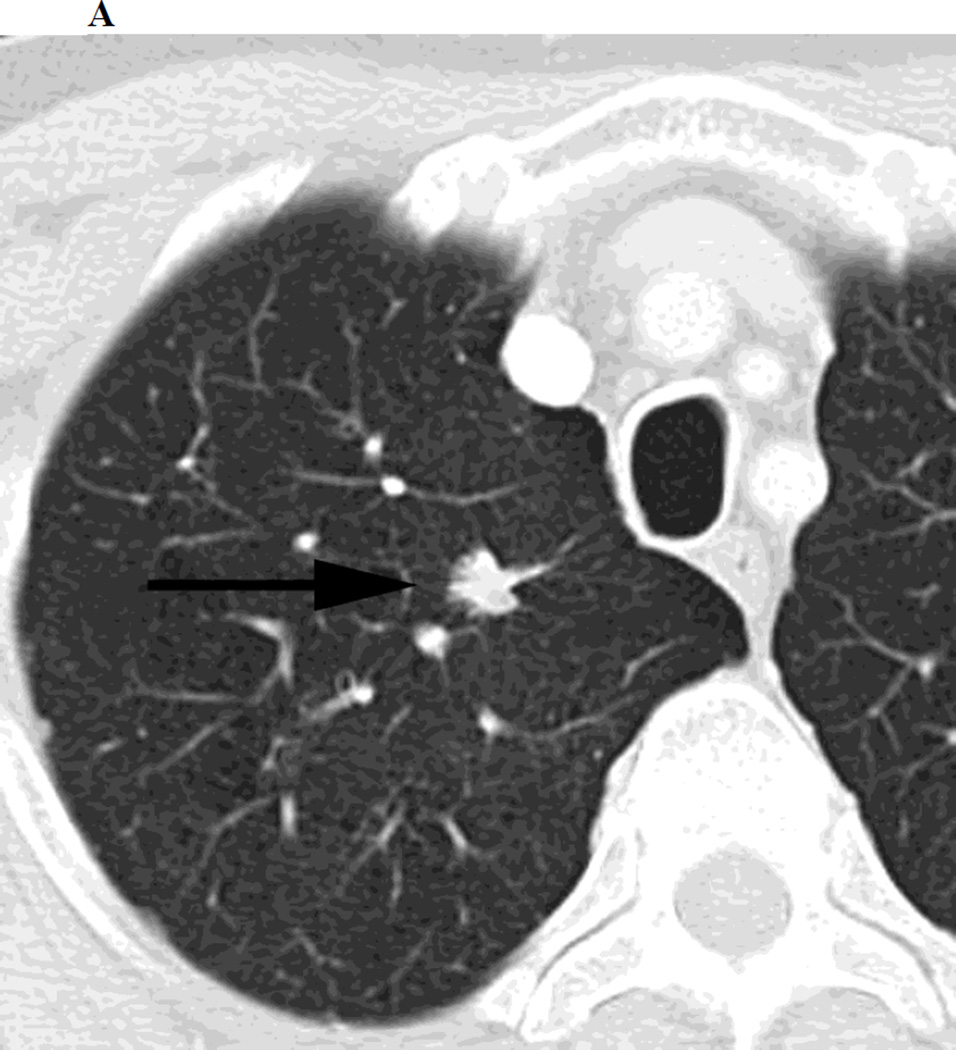
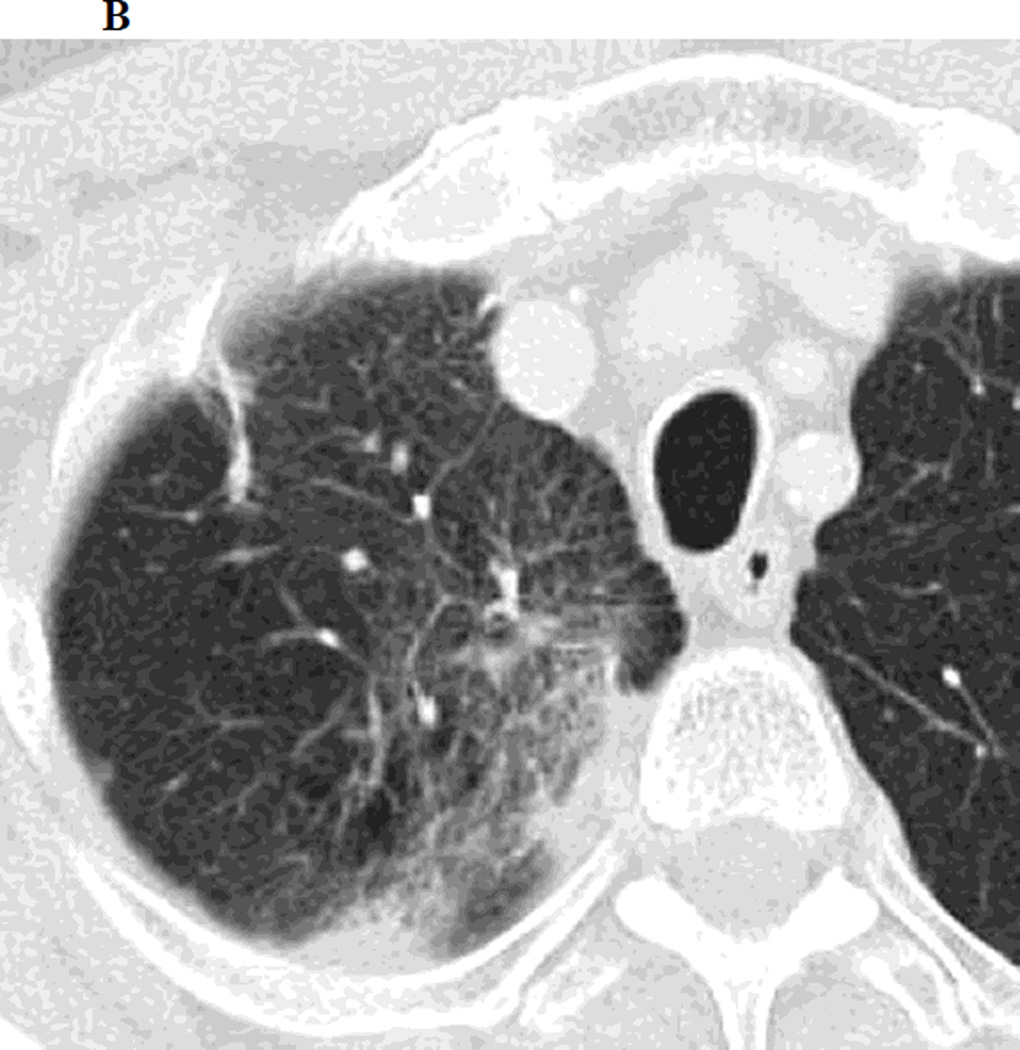
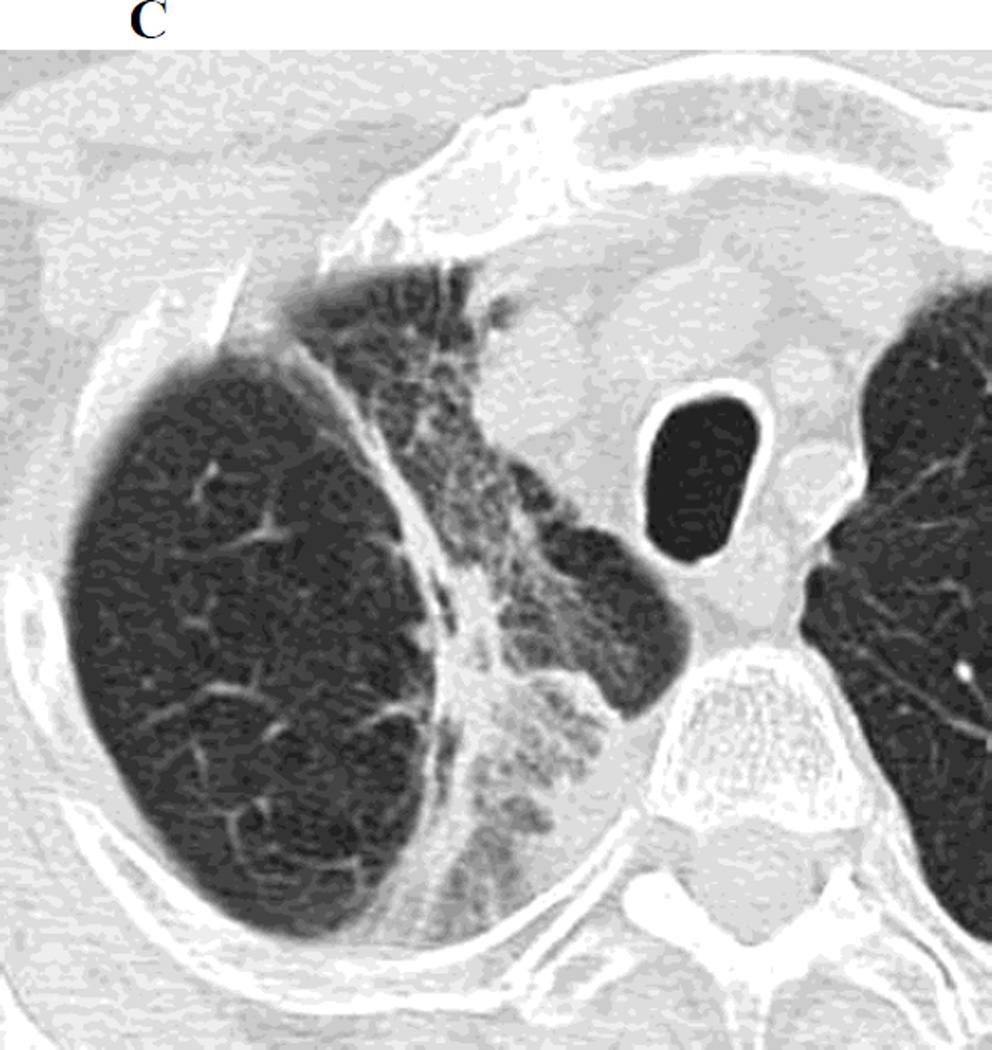
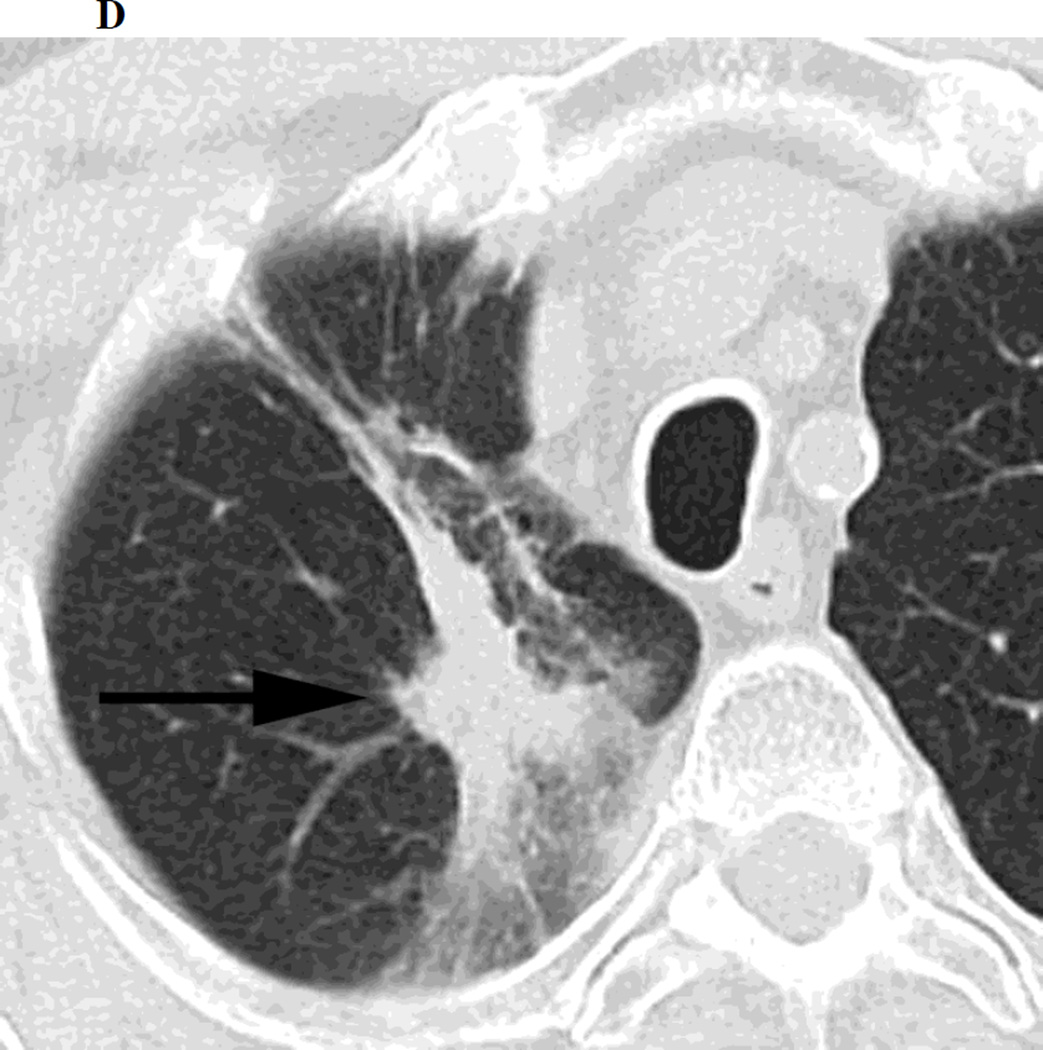
Figure 1.
a. Chest CT, on lung windows demonstrates left upper lobe lung carcinoma (arrow) pre-SBRT.
b. Chest CT, on lung windows obtained 6 months following SBRT demonstrating a linear opacity with air-bronchograms at the site of treatment consistent with a modified conventional pattern of radiation fibrosis.
c. Chest CT, on lung windows obtained 20 months following SBRT demonstrating increasing opacity at the treatment site, filling in of air- bronchograms and a small bulge in the contour of the opacity, suspicious for recurrence.
d. PET-CT obtained 21 months following SBRT demonstrating intense 18F-FDG consistent with local recurrence.
Figure 2.
a. Chest CT, on lung windows demonstrates a right upper lobe lung carcinoma (arrow).
b. Chest CT, on lung windows obtained 6 months following SBRT demonstrating patchy ground glass opacity in the right lung apex at the site of treatment.
c. Chest CT, on lung windows obtained 18 months following SBRT demonstrating a developing linear opacity at the site of treatment consistent with radiation fibrosis.
d. Chest CT, on lung windows obtained 24 months following SBRT demonstrating a new focal bulge in the contour of the opacity (arrow) suspicious for local recurrence.
e. PET/CT obtained 25 months following SBRT demonstrating intense 18F-FDG corresponding to the focal bulge, consistent with local recurrence.
Several studies have evaluated the diagnostic utility of PET/CT following SBRT. It has been demonstrated that PET/CT improves the detection of regional recurrent disease post SBRT and increases the likelihood that eligible patients will be able to undergo salvage therapy. In a cohort of 35 patients with lung cancer, at a median follow up of 12.8 months, Ebright et al detected 10 cases of regional recurrence using PET/CT in comparison to 0 cases using CT alone [10]. Nakajima et al demonstrated that a combination of mass like consolidation and moderate to intense SUVmax was seen on PET/CT in 91% of patients with LR. When these two findings were detected after 12 months following treatment, they had a sensitivity and specificity of 100% for predicting LR [20]. Several authors have suggested that the presence of SUVmax >5, more than 6 months following treatment should be treated as suspicious for tumor recurrence [21, 22]. It should be emphasized that primary tumors can have varying SUV values at baseline and therefore a low SUVmax in the presence of concerning CT features should still be treated with suspicion. Because of the potential for varying levels of SUVmax in the primary tumor we feel that a PET/CT prior to treatment should be strongly considered in every patient undergoing SBRT. This would allow a baseline level of metabolic activity to be established and would render the results of any subsequent PET/CTs more useful.
This study is retrospective in nature and as such has an inherent weakness in terms of data collection. The low number of local recurrences in the studied population, which is in keeping with reported LR rates in the literature [4], is another limitation. 4 patients were diagnosed as locally recurrent using a combination of PET/CT and histological analysis. In 6 patients, PET/CT alone was used to diagnose LR. Ideally histological confirmation would have been available in all cases, however, as discussed PET/CT, is a robust technique for detecting LR in the post SBRT setting. Simultaneous review of all the studied features of LR may have resulted in a degree of bias in determining the diagnostic performance of each feature individually. In addition, while the only CT findings analyzed were those relating directly to LR, the presence of pulmonary metastases or obvious osseous metastases on a given image may also have introduced some bias into the analysis.
To our knowledge this study represents the largest cohort of patients studied for signs of LR on CT following SBRT for lung cancer, and is the only such study to statistically analyze the diagnostic utility of the individual imaging characteristics of LR. Although the results of this study demonstrate that a new bulging margin at the site SBRT is significantly associated with LR, we feel that the results call into question using traditional features of LR as the only method of determining LR in patients following SBRT. Radiologists face an ongoing challenge when tasked with interpreting the often complex patterns of post radiation parenchymal lung abnormality in this setting. Because of this, we feel that PET/CT has a vital role to play when recurrence is suspected clinically or radiologically. To facilitate follow-up assessment, a baseline PET/CT should be strongly considered in all patients prior to commencing SBRT.
Highlights.
CT signs which are routinely used to evaluate for local recurrence (LR) of lung cancer following conventional radiation therapy were used to detect LR in a group of 93 patients with stage 1 lung cancer who had been treated with stereotactic body radiation therapy (SBRT).
10.7% (10/93) of patients had LR.
A new bulging margin at the treatment site was the only feature significantly associated with LR. It was present in 50% of patients with LR and 10% of patients without LR.
Many of the CT signs used to detect LR in patients following SBRT may be of limited use. Because of this, we feel that PET/CT has a vital role to play when LR is suspected clinically or radiologically.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors have no conflict of interest or source of funding to declare.
References
- 1.Guckenberger M, Allgäuer M, Appold S, et al. Safety and Efficacy of Stereotactic Body Radiotherapy for Stage I Non-Small-Cell Lung Cancer in Routine Clinical Practice: A Patterns-of- Care and Outcome Analysis. J Thorac Oncol. 2013 Jun 27; doi: 10.1097/JTO.0b013e318293dc45. [DOI] [PubMed] [Google Scholar]
- 2.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage nonsmall cell lung cancer: results of a prospective trial. Lung Cancer. 2010 Apr;68(1):72–77. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Choi YW, Munden RF, Erasmus JJ, et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. Radiographics. 2004 Jul-Aug;24(4):985–997. doi: 10.1148/rg.244035160. [DOI] [PubMed] [Google Scholar]
- 4.Larici AR, del Ciello A, Maggi F, et al. Lung abnormalities at multimodality imaging after radiation therapy for non-small cell lung cancer. Radiographics. 2011 May-Jun;31(3):771–789. doi: 10.1148/rg.313105096. [DOI] [PubMed] [Google Scholar]
- 5.Libshitz HI, Shuman LS. Radiation injury after hypofractionated stereotactic radiotherapy for peripheral small lung tumors: serial changes on CT. Radiation-induced pulmonary change: CT findings. J Comput Assist Tomogr. 1984;8:15–19. doi: 10.1097/00004728-198402000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Dunlap NE, Yang W, McIntosh A, et al. Computed tomography-based anatomic assessment overestimates local tumor recurrence in patients with mass-like consolidation after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012 Dec 1;84(5):1071–1077. doi: 10.1016/j.ijrobp.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 7.Takeda A, Kunieda E, Takeda T, et al. Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumor recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 2008 Mar 15;70(4):1057–1065. doi: 10.1016/j.ijrobp.2007.07.2383. [DOI] [PubMed] [Google Scholar]
- 8.Kato S, Nambu A, Onishi H, et al. Computed tomography appearances of local recurrence after stereotactic body radiation therapy for stage I non-small-cell lung carcinoma. Jpn J Radiol. 2010 May;28(4):259–265. doi: 10.1007/s11604-009-0415-3. [DOI] [PubMed] [Google Scholar]
- 9. [Last accessed 9/1/2014]; http://atc.wustl.edu/protocols/rtog/1021/Z4099-1021_A0_05-02-2011.pdf.
- 10.Ebright MI, Russo GA, Gupta A, et al. Positron emission tomography combined with diagnostic chest computed tomography enhances detection of regional recurrence after stereotactic body radiation therapy for early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013 Mar;145(3):709–715. doi: 10.1016/j.jtcvs.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9(7):621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 12.Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg. 2005;129:639–644. doi: 10.1016/j.jtcvs.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977 Jun;33(2):363–374. [PubMed] [Google Scholar]
- 14.Guckenberger M, Heilman K, Wulf J, et al. Pulmonary injury and tumor response after stereotactic body radiotherapy (SBRT): results of a serial follow-up CT study. Radiother Oncol. 2007 Dec;85(3):435–442. doi: 10.1016/j.radonc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Logan PM. Thoracic manifestations of external beam radiotherapy. AJR Am J Roentgenol. 1998 Sep;171(3):569–577. doi: 10.2214/ajr.171.3.9725275. [DOI] [PubMed] [Google Scholar]
- 16.Trovo M, Linda A, El Naqa I, et al. Early and late lung radiographic injury following stereotactic body radiation therapy (SBRT) Lung Cancer. 2010 Jul;69(1):77–85. doi: 10.1016/j.lungcan.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Dahele M, Palma D, Lagerwaard F, et al. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol. 2011 Jul;6(7):1221–1228. doi: 10.1097/JTO.0b013e318219aac5. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo Y, Nagata Y, Mizowaki T, et al. Evaluation of mass-like consolidation after stereotactic body radiation therapy for lung tumors. Int J Clin Oncol. 2007 Oct;12(5):356–362. doi: 10.1007/s10147-007-0691-9. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Liu H, Balter P, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012 Aug 1;83(5):1558–1565. doi: 10.1016/j.ijrobp.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima N, Sugawara Y, Kataoka M, et al. Differentiation of tumor recurrence from radiation-induced pulmonary fibrosis after stereotactic ablative radiotherapy for lung cancer: characterization of 18F-FDG PET/CT findings. Ann Nucl Med. 2013 Apr;27(3):261–270. doi: 10.1007/s12149-012-0682-4. [DOI] [PubMed] [Google Scholar]
- 21.Bollineni VR, Widder J, Pruim J, et al. Residual (18)F-FDG-PET uptake 12 weeks after stereotactic ablative radiotherapy for stage I non-small-cell lung cancer predicts local control. Int J Radiat Oncol Biol Phys. 2012;83:e551–e555. doi: 10.1016/j.ijrobp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo Y, Nakamoto Y, Nagata Y, et al. Characterization of FDG-PET images after stereotactic body radiation therapy for lung cancer. Radiother Oncol. 2010;97:200–204. doi: 10.1016/j.radonc.2010.04.011. [DOI] [PubMed] [Google Scholar]