Abstract
Prevention of thromboembolic complications in atrial fibrillation remains a tremendous clinical challenge. Knowledge that the left atrial appendage (LAA) is the most common anatomical origin of cardioembolic strokes1 has been the main motivation to develop clinical and procedural strategies to exclude the LAA from the circulation, either surgically or percutaneously. This review discusses the rationale behind these strategies, their relative merits, and future prospects for LAA exclusion strategies.
Keywords: atrial fibrillation, left atrial appendage, stroke, anticoagulants

M. Valderrábano, M.D.
Left Atrial Appendage as a Source of Thromboembolism
Atrial fibrillation (AF) leads to loss of contractility in the fibrillating tissue. In the left atrial appendage (LAA), local stasis can lead to thrombus formation that may then embolize into the systemic circulation. Support for this premise derives from the finding that > 90% of thrombi found in patients with nonvalvular AF and stroke were found in the LAA.1 Other associated findings in line with the idea of LAA as a source of cardioembolism include low Doppler inflow velocities, spontaneous echocardiographic contrast, and the presence of thrombus in the LAA, all of which have been associated with high stroke risk in AF patients.2 Stroke risk is, however, influenced by a multiple other factors. It is important to recognize that not all strokes in AF can be prevented by LAA-targeted therapies, since up to 25% of strokes in AF patients can be linked to intrinsic cerebrovascular disease,3 and AF is often associated with other, LAA-independent risk factors for stroke. The CHADS2 or CHA2DS2VASc scores 4,5 can estimate the annual risk of thromboembolic events and select patients that benefit from anticoagulation,6 yet they do not include any parameters of LAA function or anatomy.
These facts are important when interpreting clinical trial results. Even a technically flawless, complication-free, perfect LAA exclusion cannot be expected to provide complete elimination of stroke risk in all AF patient populations since risk factors for stroke in AF include the risk of non-LAA-related stroke. Additionally, oral anticoagulation may provide stroke protection beyond its effects on LAA thrombi. With these caveats in mind, the LAA remains a worthwhile target to prevent strokes in patients with AF.
Exclusion of the LAA via Surgical Approaches
Surgical resection of the LAA to prevent arterial embolization in AF was proposed by Madden decades ago.7 While various forms of surgical ligation or excision have become routine, residual flow may lead to embolism recurrence. The pilot Left Atrial Appendage Occlusion Study (LAOOS) assessed closure efficacy after various LAA ligation strategies and found that 34% of patients had residual flow into the LAA after surgical exclusion,8 although it was least frequent with LAA excision.9–12 Correlations of surgical LAA closure with stroke reduction have provided conflicting results,12,13 and a large randomized trial is currently ongoing.14
The AtriClip® Left Atrial Appendage Exclusion System (AtriCure, Inc., West Chester, OH) is a surgically implanted clamp of the LAA.15 In the EXCLUDE study, complete LAA closure was achieved in 95% of patients who completed 3-month imaging follow-up, but stroke prevention data are lacking.16 Further evaluation using a stand-alone thoracoscopic17 implantation of the AtriClip are ongoing in the AtriCure Stroke Feasibility Study.
Percutaneous LAA Occlusion Devices
PLAATO
The percutaneous LAA occlusion (PLAATO) device was the first device designed for percutaneous LAA closure.18 It was made of a nitinol cage covered with a polytetrafluoroethylene membrane (Figure 1).18 In a multicenter registry of 64 high-risk patients with contraindications to warfarin,19 the procedure success was high (residual flow ≤ 3 mm in 98%); it also seemed to protect against stroke in that the annual incidence of stroke or transient ischemic attack was 3.8% compared with an expected rate of 6.6%, based on the CHADS2 score of the study population. This device was not evaluated further, but it provided proof-of-concept for device occlusion of the LAA for stroke prevention.
Figure 1.
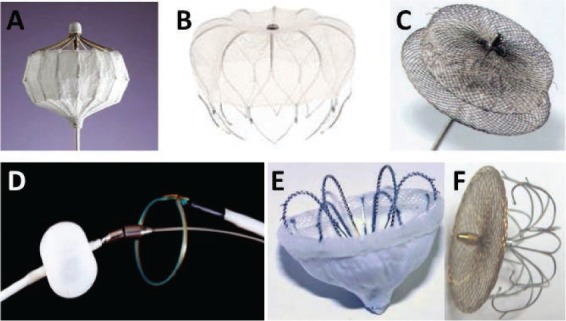
Percutaneous devices for left atrial appendage occlusion. (A) PLAATO device; (B) WATCHMAN device; (C) Amplatzer Cardiac Plug; (D) Lariat device; (E) WaveCrest Coherex device; (F) LAmbre device.
WATCHMAN
The WATCHMAN™ Left Atrial Appendage Closure Device (Boston Scientific, Natick, MA) consists of a self-expanding nitinol frame and a membrane cap (Figure 1) deployed in the LAA via a trans-septal puncture (Figure 2). The device has been evaluated in two randomized controlled clinical trials and one Continued Access Registry. The PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation)20 and PREVAIL (Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device In Patients with Atrial Fibrillation Versus Long Term Warfarin Therapy)21 studies were noninferiority trials that compared the WATCHMAN device with warfarin anticoagulation in AF patients. Inclusion in PROTECT-AF required a CHADS2 score ≥ 1, while PREVAIL-AF required a CHADS2 score ≥ 2 or of 1 if additional stroke risk factors were present.22 Patients were randomized to either device implantation or warfarin in a 2:1 fashion. WATCHMAN-implanted patients were treated with warfarin and aspirin for 6 weeks, at which time a follow-up transesophageal echocardiography (TEE) was performed. If the TEE findings showed no thrombus or peridevice leak < 5 mm, warfarin was discontinued and aspirin and clopidogrel prescribed for 4.5 more months followed by indefinite aspirin therapy.
Figure 2.
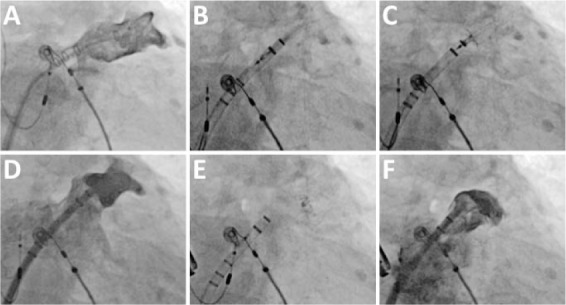
Deployment of a WATCHMAN device. (A) Initial left atrial appendage (LAA) angiogram obtained through a pigtail catheter inserted via a sheath in the LAA. (B) Sheath advancement into the LAA with the WATCHMAN inside. (C) Deployment of the WATCHMAN in the LAA. (D) LAA angiogram to verify position of initial deployment in the LAA neck. (E) Release of the WATCHMAN. (F) Final angiogram.
In PROTECT-AF, the WATCHMAN was noninferior to warfarin for the primary endpoint of cardiovascular/unexplained death, any stroke, or systemic embolism at 1065 patient-years,20 1588 patient-years,23 and 2621 patient-years of follow-up.24 At 2621 patient-years, the WATCHMAN device not only met superiority criteria but also demonstrated reduced all-cause mortality (HR 0.66; 95% CI, 0.45 to 0.98; frequentist P = 0.038) and led to improved quality-of-life measures.25There were important limitations of these analyses, including a greater rate of withdrawal in the warfarin arm, an unusually high rate of hemorrhagic stroke in the warfarin group, inclusion of patients with CHADS2 = 1 who may not require anticoagulation, and a large noninferiority margin.
In the smaller PREVAIL trial,21 the 18-month rates of the coprimary endpoint of cardiovascular death, any stroke, or systemic embolism were numerically similar between the WATCHMAN device and warfarin anticoagulation, but the device did not achieve noninferiority because the upper bound of the 95% credible interval for the 18-month rate ratio was not lower than the prespecified noninferiority margin (1.75). This finding should be interpreted in the context of a lower-than-expected event rate, particularly among the patients randomly assigned to warfarin, and the relatively short duration of follow-up.21
The WATCHMAN device did not reduce ischemic stroke compared to warfarin in either trial. However, data provided support for the mechanistic hypothesis that LAA occlusion reduces thromboembolic risk in the absence of oral anticoagulation. Some strokes in WATCHMAN-treated patients were due to air embolism. In PREVAIL, WATCHMAN implantation was noninferior to warfarin for the coprimary endpoint of ischemic stroke or systolic embolism occurring more than 7 days post randomization.
In PROTECT-AF, the rate of the major safety endpoint (excessive bleeding or a procedure-related complication) at 18 months was greater in the patients randomly assigned to the WATCHMAN compared with warfarin (RR 1.69; 95% CrI 1.01-3.19), determined by pericardial effusion requiring treatment and procedure-related ischemic stroke.20,26 Most safety events in the device arm occurred within the first 7 days of the procedure.26 However, over the longer-term, the difference in the cumulative safety events narrowed between treatment groups due to bleeding events in the warfarin arm, so that at 2621 patient-years of follow-up, there was no significant safety difference between the WATCHMAN and warfarin (RR 1.17, 95% CrI 0.78-1.96).24
In PREVAIL, safety events related to the procedure, including the incidence of serious pericardial effusions and procedural stroke, were significantly reduced compared with PROTECT-AF.21 This improved safety profile was consistent with the findings of the prospective Continued Access Registry that followed the PROTECT-AF trial.26
The ASAP (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) was an observational study of 150 AF patients who were ineligible for warfarin therapy27 predominantly due to prior bleeding. After WATCHMAN implantation, patients received clopidogrel for 6 months and aspirin indefinitely. At 14.4 ± 8.6 months, the observed rate of stroke or systemic embolism was 2.3% per year, significantly less than the expected rate of 7.3% per year based on CHADS2 score.
Amplatzer Cardiac Plug
The AMPLATZER™ Cardiac Plug (ACP) (St Jude Medical, Minneapolis, MN) is a self-expanding nitinol mesh that consists of a distal lobe and proximal disk, each with a sewn polyester patch, connected by a short central waist (Figure 1).28 The distal lobe acts as an anchor within the LAA, and the proximal disk covers the mouth of the LAA from the LA side, therefore the mechanism of LAA occlusion differs from that of the WATCHMAN, which occludes the LAA from within the appendage itself.
Clinical data with the ACP derive from several small observational studies, many of which are retrospective in design or involve a single center or operator.28–33 Most of the patients enrolled in these studies had intolerance or contraindications to oral anticoagulation and were treated with aspirin and clopidogrel during the postprocedural period. The most frequent safety events appear to be pericardial effusions and device embolization occurring at similar rates as the WATCHMAN experience. A randomized trial is necessary to robustly assess safety and efficacy in preventing thromboembolic events, as the mechanism of implantation and of closure differs from that of the WATCHMAN device. Moreover, most of the published studies of the ACP do not include patients who are candidates for oral anticoagulation. A large randomized clinical trial of the ACP compared with oral anticoagulation was recently halted, likely due to the presumed eminent U.S. Food and Drug Administration (FDA) approval of the WATCHMAN device, which would make patient enrollment in such a trial difficult.
Lariat Procedure
The LARIAT® Suture Delivery Device (SentreHEART, Inc., Redwood City, CA) is designed to ligate the LAA through the delivery of a surgical suture via a combined trans-septal and subxiphoid approach (Figure 3).34,35 The system has FDA approval (510K) for “suture placement and knot-tying for use in surgical applications where soft tissue are (sic) being approximated.” However, its design is conceived for and clinically applied to LAA ligation. LAA anatomy has to be favorable as assessed by preprocedural cardiac computed tomography (CT); an LAA diameter > 40 mm, the presence of lobes behind the pulmonary artery, or a posteriorly-oriented appendage should all be avoided. A micropuncture or 17-G epidural needle is used to advance a guidewire and then a 14-Fr sheath into the pericardial space. A magnet-tipped guidewire is advanced into the anterior aspect of the LAA, and a second magnet-tipped guidewire is advanced into the pericardium toward the LAA. The magnets snap together to form a rail, over which the LARIAT snare is advanced and closed at the mouth of the LAA using TEE and fluoroscopic guidance. This snare contains a preloaded surgical knot (Figure 1D).
Figure 3.
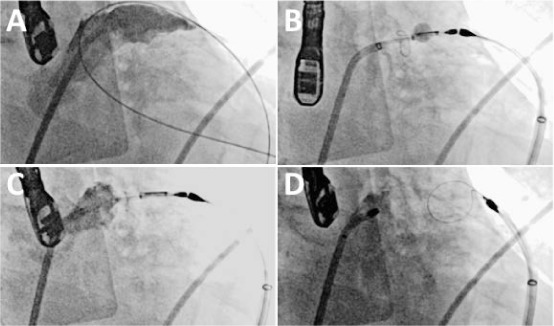
Deployment of a Lariat left atrial appendage (LAA) suture. (A) Initial LAA angiogram. (B) Endocardial magnet-tipped wire snapped with the epicardial magnet-tipped wire. The endocardial wire has a balloon over it for echocardiographic guidance. The snare has been advanced over the wires and is closed over the LAA. (C) Contrast injection in the left atrium shows complete occlusion. (D) After the suture is delivered, the snare is opened and retracted. Contrast injection shows a closed stump.
To date, the safety and efficacy of LAA closure with the LARIAT has been limited to small observational studies.36–39 The first reported series included 92 patients who were poor candidates or ineligible for warfarin therapy.36 Successful closure (residual leak < 1 mm) was achieved in 96% of cases. Significant pericardial effusions occurred in three patients, and pericarditis occurred in two patients. At 1-year follow-up, 55% of the patients remained on warfarin therapy and there were no thromboembolic events. Price et al.38 compiled retrospectively collected data from eight sites in the United States and a total of 154 unselected patients. In nine patients, the LARIAT device was not deployed due to access or delivery issues. Of the remaining 145, successful LAA ligation was achieved acutely in 92%, which was 86% of the attempted patients. Follow-up post-discharge imaging of the LAA was available in 63 patients, of whom 79% had persistent complete LAA ligation. Significant procedural complications occurred, including major bleeding (9%) as well as right and left ventricular perforations (3 total) that required surgery. On post-discharge follow-up, strokes occurred in two patients, and six pericardial and pleural effusions also occurred (three of each). A total of four deaths occurred following the procedure.
These data highlight that despite comparable rates of acute success with LAA ligation, the LARIAT device is associated with higher rates of complications than previously reported when applied to an unselected population of patients deemed to be at high risk of stroke and bleeding (the standard clinical indications). Of particular concern—in the absence of efficacy studies showing stroke protection—is the occurrence of LAA stump thrombi (four cases) and the significant rate of incomplete LAA closure (up to 21%). Similar results were reported by Miller et al.39 in a series of 41 patients from four centers. Despite achieving an acute success (complete LAA closure) in 38 patients (93%), incomplete closure was detected on follow-up imaging in 24% of the patients. Two patients required surgical repair of an LAA perforation. One patient had a transient ischemic attack, and eight developed pericardial effusions requiring pericardiocentesis. Similarly, despite the high acute technical success, the incidence of complications and significant LAA leaks raise concerns about its safety when applied to unselected populations. Thrombus at the LAA ligated stump has been reported.40–44 The real incidence remains unknown in the absence of prospective data collection sets.
In sum, from the small amount of data available, the LARIAT appears to provide high rates of acute anatomic closure, although procedural morbidity is not uncommon. Robust clinical efficacy data is absent.
Other Devices
Several other LAA closure devices are currently in development. The WaveCrest® LAA Occlusion System (Coherex Medical, Salt Lake City, UT) is unique in that device implantation is a two-step process: first, the proximal ePTFE (polytetrafluoroethylene) cap/occluder is positioned, and then the distal anchors are deployed. Incorporation of foam into the edges of the occluder could potentially enhance LAA sealing. This device currently has CE mark, and initiation of a pivotal trial within the United States is planned. The LAmbre™ LAA occluder (Lifetech Scientific Corp., Shenzhen, China)45,46 is a self-expanding nitinol device consisting of a distal hook-embedded umbrella and a proximal covering disk, both with sewn-in PET fabric. A short articulating central waist connects the umbrella and cover. The device is advanced through a relatively low-profile delivery sheath (8-10 Fr).
LAA Exclusion: Will it Ever Surpass Anticoagulation?
The role of LAA exclusion strategies in the therapeutic armamentarium for AF critically depends on their efficacy at stroke prevention and their procedural safety. Novel oral anticoagulants (NOACs) are noninferior or superior to warfarin for prevention of stroke and systemic embolism and do not require ongoing monitoring.47–50 However, inherent to OACs is a substantial ongoing hazard of major bleeding as well as noncompliance, side effects, and, in the case of the NOACs, lack of an available antidote. Currently, none of the LAA exclusion strategies has FDA approval for the indication of stroke prevention. Their final role will depend on their ability to demonstrate comparable clinical efficacy and safety to NOACs or acceptable outcomes when NOACs are contraindicated. Lariat is FDA approved for suture delivery and tissue approximation and its use for stroke prevention is considered “off-label”. Atriclip is FDA approved for surgical clipping of the left atrial appendage but not for stroke prevention. The Watchman device does not have FDA approval. At this point, compelling data are still absent.
Conflict of Interest Disclosure: Dr. Valderrábano is a consultant for and conducts research on behalf of Boston Scientific Corp., St. Jude Medical, Biosense Webster, Hansen Medical, Inc.
References
- 1.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996 Feb;61(2):755–9. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 2.Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998 Jun;31(7):1622–6. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 3.Miller VT, Rothrock JF, Pearce LA, Feinberg WM, Hart RG, Anderson DC. Ischemic stroke in patients with atrial fibrillation: effect of aspirin according to stroke mechanism. Stroke Prevention in Atrial Fibrillation Investigators. Neurology. 1993 Jan;43(1):32–6. doi: 10.1212/wnl.43.1_part_1.32. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001 Jun 13;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 5.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137(2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, Rydén LE, Cannom DS et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011 Mar 15;57(11):e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Madden JL. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J Am Med Assoc. 1949 Jul 2;140(9):769–72. doi: 10.1001/jama.1949.02900440011003. [DOI] [PubMed] [Google Scholar]
- 8.Healey JS, Crystal E, Lamy A et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005 Aug;150(2):288–93. doi: 10.1016/j.ahj.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008 Sep 9;52(11):924–9. doi: 10.1016/j.jacc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Alexander JC, Pearson PJ, Feldman T. Left atrial appendage occlusion: lessons learned from surgical and transcatheter experiences. Ann Thorac Surg. 2011 Dec;92(6):2283–92. doi: 10.1016/j.athoracsur.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol. 2000 Aug;36(2):468–71. doi: 10.1016/s0735-1097(00)00765-8. [DOI] [PubMed] [Google Scholar]
- 12.Dawson AG, Asopa S, Dunning J. Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion? Interact Cardiovasc Thoracic Surg. 2010 Feb;10(2):306–11. doi: 10.1510/icvts.2009.227991. [DOI] [PubMed] [Google Scholar]
- 13.García-Fernández MA, Pérez-David E, Quiles J et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003 Oct 1;42(7):1253–8. doi: 10.1016/s0735-1097(03)00954-9. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock R, Healey J, Vincent J et al. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann Cardiothorac Surg. 2014 Jan;3(1):45–54. doi: 10.3978/j.issn.2225-319X.2013.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fumoto H, Gillinov AM, Ootaki Y et al. A novel device for left atrial appendage exclusion: the third-generation atrial exclusion device. J Thorac Cardiovasc Surg. 2008 Oct;136(4):1019–27. doi: 10.1016/j.jtcvs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Ailawadi G, Gerdisch MW, Harvey RL et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011 Nov;142(5):1002–9. doi: 10.1016/j.jtcvs.2011.07.052. 1009.e1. [DOI] [PubMed] [Google Scholar]
- 17.Benussi S, Mazzone P, Maccabelli G et al. Thoracoscopic appendage exclusion with an atriclip device as a solo treatment for focal atrial tachycardia. Circulation. 2011 Apr 12;123(14):1575–8. doi: 10.1161/CIRCULATIONAHA.110.005652. [DOI] [PubMed] [Google Scholar]
- 18.Sievert H, Lesh MD, Trepels T et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation. 2002 Apr 23;105(16):1887–9. doi: 10.1161/01.cir.0000015698.54752.6d. [DOI] [PubMed] [Google Scholar]
- 19.Block PC, Burstein S, Casale PN et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv. 2009 Jul;2(7):594–600. doi: 10.1016/j.jcin.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Holmes DR, Reddy VY, Turi ZG et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009 Aug 15;374(9689):534–42. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 21.Holmes DR, Jr, Kar S, Price MJ et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014 Jul 8;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Fuster V, Rydén LE, Cannom DS et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15;114(7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 23.Reddy VY, Doshi SK, Sievert H et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013 Feb 12;127(6):720–9. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 24.Reddy VY. Long term results of PROTECT AF: the mortality effects of left atrial appendage closure versus warfarin for stroke prophylaxis in AF. Paper presented at: Heart Rhythm Society 34th Annual Scientific Sessions; 2013 May 9; Denver, CO.
- 25.Alli O, Doshi S, Kar S et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013 Apr 30;61(17):1790–8. doi: 10.1016/j.jacc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 26.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011 Feb 1;123(4):417–24. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 27.Reddy VY, Möbius-Winkler S, Miller MA et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) J Am Coll Cardiol. 2013 Jun 25;61(25):2551–6. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Park JW, Bethencourt A, Sievert H et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011 Apr 1;77(5):700–6. doi: 10.1002/ccd.22764. [DOI] [PubMed] [Google Scholar]
- 29.Lam YY, Yip GW, Yu CM et al. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv. 2012 Apr 1;79(5):794–800. doi: 10.1002/ccd.23136. [DOI] [PubMed] [Google Scholar]
- 30.Meerkin D, Butnaru A, Dratva D, Bertrand OF, Tzivoni D. Early safety of the Amplatzer Cardiac Plug™ for left atrial appendage occlusion. Int J Cardiol. 2013 Oct 9;168(4):3920–5. doi: 10.1016/j.ijcard.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 31.Urena M, Rodés-Cabau J, Freixa X et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013 Jul 9;62(2):96–102. doi: 10.1016/j.jacc.2013.02.089. [DOI] [PubMed] [Google Scholar]
- 32.Guérios EE, Schmid M, Gloekler S et al. Left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation. Arq Bras Cardiol. 2012 Jun;98(6):528–36. doi: 10.1590/s0066-782x2012005000044. [DOI] [PubMed] [Google Scholar]
- 33.Streb W, Szymala M, Kukulski T et al. Percutaneous closure of the left atrial appendage using the Amplatzer Cardiac Plug in patients with atrial fibrillation: evaluation of safety and feasibility. Kardiol Pol. 2013;71(1):8–16. [PubMed] [Google Scholar]
- 34.Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: initial experience in a canine model. Circ Cardiovasc Interv. 2010 Jun 1;3(3):224–9. doi: 10.1161/CIRCINTERVENTIONS.109.914978. [DOI] [PubMed] [Google Scholar]
- 35.Bartus K, Bednarek J, Myc J et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm. 2011 Feb;8(2):188–93. doi: 10.1016/j.hrthm.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 36.Bartus K, Han FT, Bednarek J et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013 Jul 9;62(2):108–18. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 37.Massumi A, Chelu MG, Nazeri A et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol. 2013 Mar 15;111(6):869–73. doi: 10.1016/j.amjcard.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 38.Price MJ, Gibson DN, Yakubov SJ et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol. 2014 Aug 12;64(6):565–72. doi: 10.1016/j.jacc.2014.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller MA, Gangireddy SR, Doshi SK et al. Multicenter study on acute and long-term safety and efficacy of percutaneous left atrial appendage closure using an epicardial suture snaring device. Heart Rhythm. 2014 Nov;11(11):1853–9. doi: 10.1016/j.hrthm.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 40.Giedrimas E, Lin AC, Knight BP. Left atrial thrombus after appendage closure using LARIAT. Circ Arrhythm Electrophysiol. 2013 Aug;6(4):e52–3. doi: 10.1161/CIRCEP.113.000532. [DOI] [PubMed] [Google Scholar]
- 41.Briceno DF, Fernando RR, Laing ST. Left atrial appendage thrombus post LARIAT closure device. Heart Rhythm. 2014 Sep;11(9):1600–1. doi: 10.1016/j.hrthm.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 42.Baker MS, Paul Mounsey J, Gehi AK, Chung EH. Left atrial thrombus after appendage ligation with LARIAT. Heart Rhythm. 2014 Aug;11(8):1489. doi: 10.1016/j.hrthm.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Koranne KP, Fernando RR, Laing ST. Left atrial thrombus after complete left atrial appendage exclusion with LARIAT device. Catheter Cardiovasc Interv. 2015 Feb 1;85(2):E54–7. doi: 10.1002/ccd.25549. [DOI] [PubMed] [Google Scholar]
- 44.Truesdell AG, Patel CP, Maini BS. Late-occurring left atrial appendage thrombus after ligation using LARIAT. J Interv Card Electrophysiol. 2014 Oct;41(1):101. doi: 10.1007/s10840-014-9916-9. [DOI] [PubMed] [Google Scholar]
- 45.Lam YY, Yan BP, Doshi SK et al. Preclinical evaluation of a new left atrial appendage occluder (Lifetech LAmbre™ device) in a canine model. Int J Cardiol. 2013 Oct 9;168(4):3996–4001. doi: 10.1016/j.ijcard.2013.06.083. [DOI] [PubMed] [Google Scholar]
- 46.Lam YY. A new left atrial appendage occluder (Lifetech LAmbre Device) for stroke prevention in atrial fibrillation. Cardiovasc Revasc Med. 2013 May–Jun;14(3):134–6. doi: 10.1016/j.carrev.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013 Nov 28;369(22):2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 48.Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011 Sep 8;365(10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 49.Granger CB, Alexander JH, McMurray JJ et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011 Sep 15;365(11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 50.Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]


