Abstract
Background and Aims
Esophageal squamous cell neoplasia (ESCN) has high mortality due to late detection. In high risk regions such as China, screening is performed by Lugol's chromoendoscopy (LCE). LCE has low specificity resulting in unnecessary tissue biopsy with subsequent increase in procedure cost and risk. The purpose of this study is to evaluate the accuracy of a novel, low-cost high resolution microendoscope (HRME) as an adjunct to LCE.
Methods
In this prospective trial, 147 consecutive high-risk patients were enrolled from two US and two Chinese tertiary centers. Three expert and four novice endoscopists performed white light endoscopy followed by LCE and HRME. All optical images were compared to gold standard of histopathology.
Results
Using a per biopsy analysis, sensitivity of LCE vs. LCE + HRME was 96% vs. 91% (p=0.0832), specificity 48% vs. 88% (p<0.001), PPV 22% vs 45% (p<0.0001), NPV 98% vs. 98% (p=0.3551), and overall accuracy 57% vs. 90% (p<0.001). Using a per patient analysis, sensitivity of LCE vs. LCE + HRME was 100% vs. 95% (p=0.16), specificity 29% vs. 79% (p<0.001), PPV 32% vs. 60%, 100% vs. 98%, and accuracy 47% vs. 83% (p<0.001). With use of HRME, 136 biopsies (60%; 95% CI: 53-66%) could have been spared, and 55 patients (48%; 95% CI: 38-57%) spared any biopsy.
Conclusion
In this trial, HRME improved the accuracy of LCE for ESCN screening and surveillance. HRME may be a cost-effective “optical” biopsy adjunct to LCE, potentially reducing unnecessary biopsy and facilitating real-time decision-making in globally underserved regions; ClinicalTrials.gov, NCT 01384708.
Keywords: Esophageal neoplasm, endoscopy, early detection of cancer
INTRODUCTION
Esophageal squamous cell neoplasia (ESCN) is the sixth leading cause of cancer death worldwide with only one in five patients surviving more than three years because of diagnosis at an advanced stage.1,2,3 In the developing world, ESCN is more common than adenocarcinoma, with particularly high prevalence in northern China, Central Asia, and Iran.4 In northern China, age-standardized incidence rates exceed 100 per 100,000 per year, compared to the US which is about 1.4 per 100,000.4,5 This geographic variation likely stems from a multitude of factors including low socioeconomic status, diet, nutritional deficiencies, and thermal injury.3 In high-risk regions, endoscopic screening has been implemented, albeit inconsistently and with limited success. The reasons for these shortcomings are multifactorial and include lack of funding, infrastructure and trained personnel.6 Given these constraints, a low-cost, portable and accurate method of diagnosing ESCN would be invaluable in improving early detection in resource-limited settings.
Early diagnosis of ESCN offers significant opportunity to improve outcomes. When detected at an early stage (severe dysplasia or intramucosal carcinoma), endoscopic treatment can be performed by endoscopic mucosal resection or radiofrequency ablation with dramatically improved morbidity and survival rates of >90%.7,8,9,10 Unfortunately, intramucosal neoplasia can appear as small erosions, flat mucosal lesions, or normal mucosa making it difficult to visualize on routine high-definition white light endoscopy (HD-WLE) even for advanced endoscopists.11 Currently, endoscopic screening and surveillance is performed in high-risk patients using Lugol's chromoendoscopy (LCE).12,13,14 Although LCE increases the sensitivity of HD-WLE to > 95%, specificity remains poor (< 65%) as inflammation and benign mucosal changes mimic neoplasia, resulting in unnecessary biopsies (LCE positive, pathology negative) and increased cost.13,15,16 Repeat procedures are always necessary after LCE if treatment is warranted, therefore increasing time and cost, as well as possible patient loss to follow up.
Advanced endoscopic imaging solutions have been developed but widespread dissemination has been limited by high cost or limited accuracy. Confocal laser endomicroscopy (CLE) provides 1100x magnified, subcellular views of the esophageal epithelium and can differentiate ESCN from benign esophageal mucosa with high diagnostic accuracy (>95%).17,18,19 Current CLE platforms are expensive (>$150,000) and available in only a few tertiary care centers worldwide.17,18 Narrow band imaging, an image-enhancement technology, uses narrow bandwidth filters of different wavelengths to visualize the microvasculature including intrapapillary capillary loop patterns. Despite a high accuracy as high as 95%, similar to LCE, NBI is limited by poor specificity.20,21,22,23,24 Additionally, NBI is operator dependent, with experienced endoscopists outperforming novice (for example, sensitivity of 100% for experienced endoscopists vs 53% for novice in one study on a per-lesion basis).23
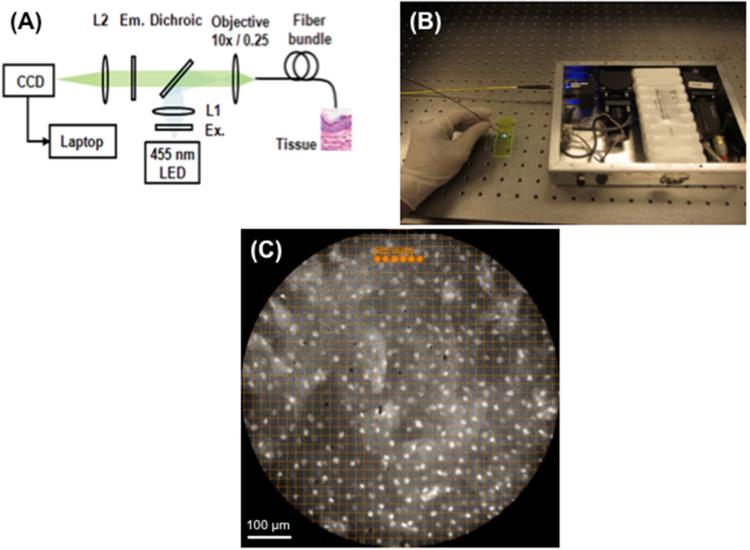
To address these issues, we developed a novel, low cost (< $3,500), portable, battery-operated, high-resolution microendoscope (HRME) that provides subcellular resolution images when used with a topical fluorescent agent.25,26,27,28,29,30,31 When inserted through the endoscope's accessory channel and placed in gentle contact with the mucosa, the one millimeter fiber-optic microendoscope provides clear delineation of cellular features, including nuclear size, crowding, and pleomorphism. Nuclear size can be quantified using image analysis software, assisting the endoscopist to rapidly differentiate benign epithelium from neoplasia in real-time (Figure 1).
Figure 1.
(A) Device configuration. (B) The device is battery operated and easily fits in a briefcase. (C) To facilitate objective, real-time assessment of nuclear size and spacing, a grid with 19.4 μm spacing was superimposed on the display monitor and 15.1 μm diameter dots were placed; this image is normal esophageal mucosa.
The aims of this international, multicenter trial were to evaluate the accuracy and efficiency of this novel approach in the hands of both expert and novice endoscopists performing ESCN screening and surveillance. Specifically we compared (1) the performance characteristics of LCE vs. LCE + HRME in the hands of both expert and novice microendoscopists and (2) the number of LCE positive biopsies saved and patients saved any biopsy with use of HRME. Our goal is to see if HRME can enhance the current gold standard of LCE by increasing specificity and decreasing unnecessary biopsies.
METHODS
Study design and patient selection
This prospective, single-arm study was approved by the Institutional Review Boards at the Icahn School of Medicine at Mount Sinai, New York, NY (GCO# 10-0982/GCO# 10-0443); Rice University, Houston, TX ; MD Anderson Cancer Center, Houston, TX (2010-0234); First University Hospital, Changchun, China; and Cancer Institute and Hospital, the Chinese Academy of Medical Sciences (CICAMS), Beijing, China. The two Chinese sites are both areas of high incidence of ESCN. One hundred and forty-seven consecutive patients previously scheduled for upper endoscopy with LCE were enrolled. Screening subjects were from high-risk provinces (northern China) or had a history of oropharyngeal cancer (United States). Surveillance subjects had a history of esophageal dysplasia or suspected, unlocalized neoplasia (China and United States). All subjects were ≥18 years of age and required to sign informed consent and complete a follow-up telephone interview 2-5 days post-procedure. Study exclusion criteria included known cancer or a nodule or ulcer >2 cm (a clinical scenario in which there would have been no role for endoscopic therapy, so microendoscopy would not have changed management), allergy to the fluorescent agent proflavine or to Lugol's iodine solution, active gastrointestinal bleeding, and contraindication to endoscopy (patients who could not undergo endoscopy or sedation for standard medical reasons, such as early pregnancy or significant cardiopulmonary disease/hemodynamic instability, or those who could not undergo biopsies, such as presence of varices).
High resolution microendoscopy (HRME)
HRME methodology has been described previously.25,26,30 Briefly, the HRME is a battery-powered, portable, fluorescence microscope, with a one millimeter diameter flexible fiber-optic probe. The commercially available fiber-bundle probe (Sumitomo, Fujikura, and Schott) contains 30,000 optical fibers with approximately 4 μm core spacing and imaging diameters of 330 μm, 720 μm, and 1400 μm and is inserted through the endoscopic biopsy channel. When placed in gentle contact with mucosa, histopathology-like images are obtained in real time, at a subcellular resolution. Excitation light from a 455 nm light-emitting diode is delivered through the fiber bundle. The fluorescent emission from proflavine (with peak absorption and emission wavelengths of 455 nm and 515 nm, respectively) is collected through the fiber bundle and imaged onto a digital camera. The probe provides a 720 μm diameter field of view and 4.5 μm spatial resolution. It can be disinfected by soaking in glutaraldehyde solution and can be used 60-75 times before the probe's fiber surface needs repolishing. Repolishing takes approximately 10-15 minutes and can be done repeatedly without affecting probe performance or longevity.
Image analysis interpretation software
To facilitate objective, real-time interpretation, image analysis software was used to identify morphologic features that could classify HRME images as neoplastic (high grade dysplasia, cancer) or benign (normal, inflammation, low grade dysplasia). A two-class linear discriminant algorithm was developed and evaluated using 375 images of esophageal tissue obtained in a pilot trial.31 The diagnostic value of the following features were analyzed: nuclear size, nuclear-tocytoplasmic ratio, nearest internuclear distance, nuclear eccentricity, nuclear solidity, and the major axis of the ellipse best approximating each nucleus. The single best performing feature for differentiating neoplastic from non-neoplastic tissue was mean nuclear area, with neoplastic samples showing mean nuclear area greater than 15.1 μm.31 Based on these findings, we developed tools to assist endoscopists performing HRME imaging with objective, real-time visual assessment of nuclear size and spacing throughout the trial. HRME images were displayed in real time with two visual assists superimposed: 1) 15.1 μm dots were placed on the top of each image frame and 2) a grid with 19.4 μm spacing was overlaid on each image frame to aid endoscopists in identifying whether the majority of nuclei in the image exceeded the 15.1 um diameter threshold.
Training in the interpretation of HRME for ESCN
Seven endoscopists (three experienced, four novice) performed HD-WLE with LCE (the current gold standard for screening) followed by HRME. Experienced endoscopists had performed >50 microendoscopy exams and novice endoscopists had no prior experience using HRME. Prior to patient enrollment, all seven endoscopists viewed a training slideshow describing the various features of the HRME ESCN classification system,32 including still HRME images and six HRME videos. Endoscopists were instructed to classify tissue as neoplastic if the majority of nuclei in the image exceeded the 15.1 um reference marker. After viewing the training set, each endoscopist was shown a test set of 40 new HRME movies. Endoscopists were blinded to the HD-WLE and LCE images and histopathology. An accuracy of ≥80% on this initial test was achieved by each endoscopist prior to trial participation and patient enrollment.
Performing endoscopy with HRME
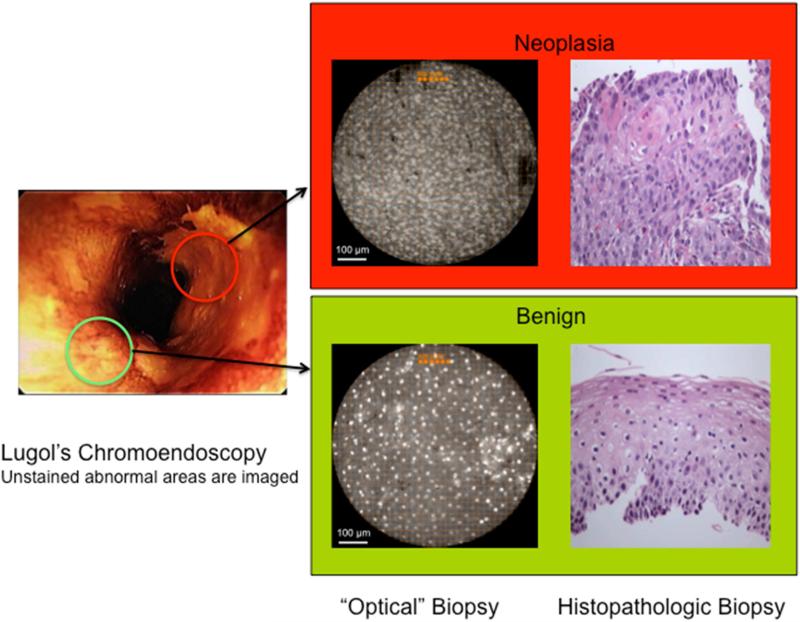
HD-WLE using LCE dye-stain (12 g iodine + 24 g potassium iodide in 1000 ml water) was performed with an Olympus 180 endoscope, using only the white light mode, in each patient. Per standard of care, LCE voiding lesions were identified and targeted for one to two biopsies based on size. LCE voiding lesions ranged in size from 2 mm to 2 cm (less than 2 mm lesions are typically not biopsied). While LCE voiding (abnormal) areas were targeted for imaging and biopsy, it should be noted that residual LCE dye did not interfere with HRME imaging quality, in part due to the bright fluorescence of the proflavine dye. Prior to biopsy of each LCE voiding lesion, HRME imaging was performed of the entire lesion. For HRME imaging, an average of 4.77 mL (1-10 mL) topical proflavine hemisulfate 0.01% (w/v) was sprayed on each LCE-voiding area (total amount was based on size/number of LCE lesions). Proflavine was used under an Investigational New Drug (IND) application from the Food and Drug Administration (IND #102,217). After proflavine application, the HRME probe was inserted through the endoscope biopsy channel and placed on mucosa to evaluate each targeted area (< 30 seconds). For each optical site, digital videos were acquired at 12 frames per second for a total of three seconds and recorded using a foot pedal controlled by the endoscopist. A typical 5-7 mm LCE-voiding area was imaged with 5-7 still images. At each imaged site, the endoscopist's impression (neoplastic, non-neoplastic) was recorded during the procedure for each modality (LCE, HRME), which was used to determine the performance characteristics of HRME (Figure 2). To ensure accurate biopsy correlation, all imaged areas prior to biopsy were marked with a dimple created by pressing the probe against the mucosa. This target was used for biopsy correlation.
Figure 2.
Lugol's unstained areas are imaged with HRME and an ‘optical biopsy’ is obtained with the corresponding tissue biopsy (original magnification 100x) of the area. Only one of the two Lugol's “abnormal” areas was neoplastic (upper panel).
The entire HRME process added an additional four to six minutes to the standard endoscopic evaluation. To determine both the device and LCE's performance characteristics and sensitivity, 25% of the total biopsies obtained were from LCE-avid (normal) areas. All specimens were processed and sectioned both in a standardized manner.
Reference Standard – Pathology
All slides received a local site (standard of care) pathology diagnosis in addition to a centralized diagnosis by a single, expert gastrointestinal pathologist (AP), who was blinded to the endoscopic, HRME, and local pathology results. For the purposes of this study, the final diagnosis was a binary classification of either “non-neoplasia” (normal, inflammation, low-grade dysplasia) or “neoplasia” (high-grade dysplasia, ESCN).33,34,35,36 Biospies diagnosed as moderate dysplasia were re-classified as low-grade or high-grade dysplasia depending on whether architectural and cytological abnormalities were confined to the lower half of the squamous epithelium or also involved the upper half, and this re-classification was made by a consensus review of two pathologists (AP, SD).
Statistical analysis
Sample size calculations to provide adequate power were done for the primary outcome of specificity. In particular, a sample size of n = 120 patients with no evidence of biopsy-confirmed neoplasia would provide 88% power to detect a difference in specificities of 63% for LCE and of 75% for LCE + HRME. This calculation is based on a comparison of paired samples; in this study the pairing occurs as a result of each subject receiving an examination by each of the two modalities.37 The overall recruitment goal was based on an estimate that approximately one-third of all subjects with a history of neoplasia would have at least one positive biopsy. nQuery Advisor v7.0 was used for this calculation.
The parameters of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for each method with the patient as unit of observation (per patient analyses) were estimated using observed proportions; the corresponding 95% confidence intervals are exact binomial intervals.38 The comparison of each of these parameters estimated using the standard method (LCE) to the same parameter estimated using the new proposed method (LCE + HRME) was carried out using a log-binomial model that took into account the correlations that arose when the same patient was assessed by each of the two methods.39 Comparison of these parameters between novice and expert endoscopists was done also using a log-binomial model. For the per biopsy analyses, log-binomial models were used to account for the correlations that arose from there being multiple specimens from the same patient as well as correlations that arose from using both methods on the same patient. These models produced estimates of the relevant parameters, the associated confidence intervals, and p-values. To test whether the number of biopsies required (or number of patients requiring a biopsy) using LCE + HRME results could have been significantly reduced below the number required based on LCE alone, a chi-square test was performed on the binomial proportion defined by the number of biopsies (or, patients with biopsy) classified as positive by LCE but negative by HRME divided by the total number of biopsies (patients with biopsy) performed. This chi-square test tested the null hypothesis that the above proportion was zero, which would be the case if HRME provided no useful information to eliminate false positive LCE readings.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Patient characteristics
From August 2010 to August 2013, a total of 147 patients were enrolled: thirty from Mount Sinai, 7 from MD Anderson, 31 from First University Hospital, and 79 from CICAMS. The prevalence of neoplasia (neoplasia positive patients/total number of patients) was 43% in the US and 19% in China. This difference was likely due to the fact that more of the US patients were surveillance endoscopies, whereas routine screening was done in those provinces of China. In the US, one experienced and one novice microendoscopist scoped 37 patients. In China, two experienced and 3 novice microendoscopists scoped 110 patients. Of the 147 patients, 87 (59%) were male, 112 (76%) were Chinese, 26 (18%) were Caucasian. Median age was 60 (range 30-81), and the indication for the procedure was initial screening in 42 (29%) and surveillance in 105 (71%). In the screening group, 2% of the patients had neoplasia compared to 34% in the surveillance group. Endoscopic characteristics are shown in Table 1. Nodularity and ulcers were all less than 2 centimeters, and no case with obvious localized carcinoma was included. Of note, there were no complications or side effects from proflavine.
Table 1.
Clinical features of patients
| Age, median years (range) | 60 (31-80) | |
| Gender, n (%) | Male | 87 (59%) |
| Female | 60 (41%) | |
| Ethnicity, n (%) | Caucasian | 26 (18%) |
| Chinese | 112 (76%) | |
| African American | 3 (2%) | |
| Hispanic | 6 (4%) | |
| Indication for procedure, n (%) | Screening | 42 (29%) |
| Surveillance | 105 (71%) | |
| Macroscopic appearance, n (%) | Nodularity | 11 (7%) |
| Ulceration | 7 (5%) | |
| Neither | 128 (87%) | |
| Both | 1 (1%) | |
| Final pathologic diagnosis | Neoplasia | 37 (25%) |
| Benign | 110 (75%) | |
| Sites, n (%) | First University | 31 (21%) |
| Mount Sinai | 30 (20%) | |
| MD Anderson | 7 (5%) | |
| CICAMS | 79 (54%) |
Performance Characteristics
Per Biopsy Analysis
A total of 386 biopsies were taken from 147 patients. There were 159 biopsies taken from LCE normal (stained) mucosa to evaluate the false negative rate. Sensitivity of LCE compared to LCE + HRME was 96% vs. 91% (p=0.083), specificity 48% vs. 88% (p<0.001), PPV 22% vs. 45% (p<0.0001), NPV 98% vs. 98% (p=0.355), and overall accuracy 57% vs. 90% (p<0.001). This data and accompanying confidence intervals are shown in Table 2. Thus, the addition of HRME significantly increased the specificity and overall accuracy of LCE.
Table 2.
Per biopsy and per patient analysis of LCE versus LCE + HRME
| Per Biopsy Analysis n=386 biopsies | Per Patient Analysis n=147 patients | |||
|---|---|---|---|---|
| LCE | LCE + HRME | LCE | LCE + HRME | |
| Sensitivity | 97% [90-100] | 90% [81-99] | 100%[91-100] | 95% [82-99] |
| Specificity | 48% [43-53] | 88%1 [84-92] | 29% [21-39] | 79%1 [70-86] |
| PPV | 22% [16-31%] | 45%1 [33-61] | 32% [24-41] | 60% [47-73] |
| NPV | 98% [96-100] | 97% [96-99] | 100%[89-100] | 98% [92-99] |
| Accuracy | 57% [52-62] | 90%1 [86-93] | 47% [39-55] | 83%1 [76-89] |
p<0.0001
Per Patient Analysis
For each patient, there was a range of zero to six LCE voiding lesions. Sensitivity of LCE compared to LCE + HRME was 100% vs. 95% (p=0.157), specificity 29% vs. 79% (p<0.001), PPV 32% vs. 60%, NPV 100% vs. 98%, and accuracy 47% vs. 83% (p<0.001) (Table 2). On a per patient basis, there was a statistically significant improvement in specificity and overall accuracy without significant change in sensitivity. First University Hospital had 5 patients that were HRME positive, pathology positive; Mount Sinai had 16; MD Anderson had zero; and CICAMS had 14.
Biopsies Saved
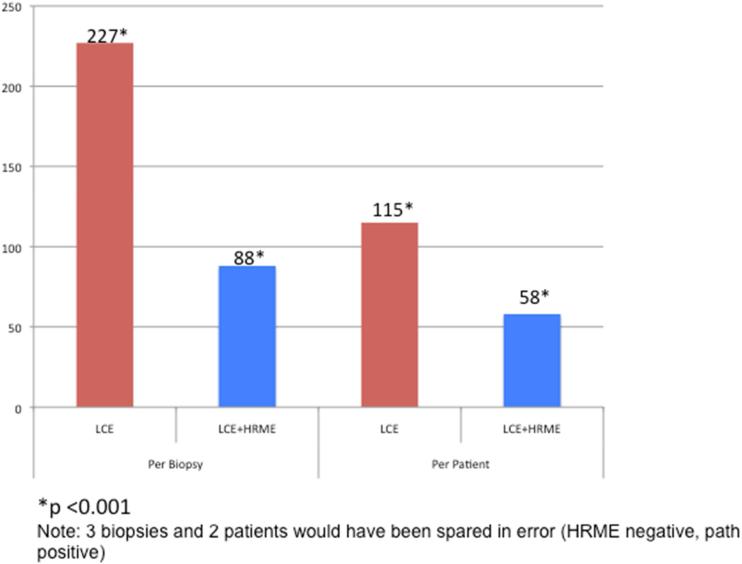
There were a total of 227 LCE positive biopsies and only 88 LCE + HRME positive biopsies; thus, 139 biopsies could have been spared (61%; 95% CI: 55-68%) with use of HRME (Figure 3). Only 3 biopsies (1%; 95% CI 0.3-4%) would have been spared in error (negative on HRME, positive on pathology). There were a total of 115 LCE positive patients and only 58 LCE + HRME positive patients; thus, 57 patients could have been spared (50%; 95% CI: 40-59%) with use of HRME (Figure 3). Only 2 patients (2%; 95% CI 0.2-6%) would have been spared in error (negative on HRME, positive on pathology). The addition of HRME to LCE would have theoretically decreased the average number of biopsies obtained per patient (2.63 LCE vs. 1.57 LCE + HRME) by allowing targeted tissue sampling.
Figure 3.
Comparison of total number of mucosal biopsies obtained and total number of patients requiring a biopsy with LCE versus LCE + HRME
Novice vs. experienced endoscopists: biopsy and patient based analyses
Using a per biopsy analysis, accuracy of LCE + HRME for novice vs. experienced endoscopists was 88% vs. 90%, with comparable sensitivity 89% vs. 92%, specificity 87% vs. 89%, PPV 44% vs. 67%, and NPV 99% vs. 98%. Using a per patient analysis, accuracy of LCE + HRME for the novice vs. experienced endoscopists was 79% vs. 89%, sensitivity 88% vs. 100%, specificity 77% vs. 83%, PPV 47% vs. 75%, and NPV 96% vs. 100% (Table 3). There was no significant difference between novice and experts in accuracy, sensitivity, specificity or NPV.
Table 3.
Per biopsy and per patient analysis of experienced versus novice endoscopists using LCE + HRME
| Per Biopsy Analysis n=386 biopsies | Per Patient Analysis n=147 patients | |||
|---|---|---|---|---|
| Novice | Experts | Novice | Experts | |
| Sensitivity | 90% [77-100] | 90% [79-100] | 88% [62-98] | 100%[84-100] |
| Specificity | 88% [83-94] | 89% [83-95] | 77% [66-86] | 83% [67-93] |
| PPV | 40% [27-58] | 55% [40-77] | 47% [28-66] | 75% [55-89] |
| NPV | 98% [96-99] | 97% [94-99] | 96% [88-99] | 100%[89-100] |
| Accuracy | 88% [83-94] | 90% [85-95] | 79% [69-87] | 89% [78-95] |
DISCUSSION
With global cancer rates rising, there is an increasing need for robust, cost-effective diagnostic technology that can be used in low-resource areas by less experienced clinical providers. Given the cost and performance limitation of currently available techniques, an accurate, easily interpretable imaging modality for diagnosing ESCN in real-time would be invaluable. Such a technology could reduce the number of unnecessary biopsies, enable immediate endoscopic therapy of neoplasia, and decrease repeat procedures and loss of patients to follow up.
This is the first prospective, in vivo study assessing the accuracy and biopsy efficiency of low-cost HRME as an adjunct to Lugol's screening for ESCN. Indeed, the role of such an optical biopsy technology is to complement and enhance red-flag imaging (LCE) by improving accuracy and efficiency. In this international, multi-center trial, HRME imaging significantly improved specificity and accuracy of the LCE gold-standard. On a per biopsy basis, specificity and accuracy improved from 48% to 88% and 57% to 90%, respectively. On a per patient basis, specificity and accuracy improved from 29% to 79% and 47% to 83%, respectively, without a statistically significant reduction in sensitivity. In this study, we enrolled consecutive subjects undergoing screening (no prior history of neoplasia) or surveillance (previously diagnosed low or moderate grade dysplasia or for suspected but as of yet undiagnosed and unlocalized high grade dysplasia or cancer). We did not include subjects with nodules or ulcers > 2 cm in size where there would have been no role for endoscopic therapy and thus HRME results would not have had a clinical impact. Of all the indeterminate and smaller lesions (ulcers and nodules < 2 cm) that were imaged with the HRME, 42% were neoplastic (high grade dysplasia, cancer) and 58% were benign (normal, inflammation, low grade dysplasia). Thus, there clearly was still a role for the HRME in making a diagnosis in these indeterminate lesions.
The results of this trial demonstrate that the use of HRME, in conjunction with LCE, can significantly increase the efficiency of screening and surveillance endoscopy for ESCN, compared to LCE alone. Fifty-five patients (48%) would have been saved any biopsy, and 136 biopsies (60%) taken with LCE could have been prevented with the addition of HRME. Given that pathology costs frequently exceed endoscopy costs, this reduction in tissue sampling may offer a significant cost-saving advantage as well as prevent delays in diagnosis and treatment.
Interestingly, there was no significant difference in accuracy between novice and experienced endoscopists (90% vs. 88%) in the per biopsy analysis. This ability of novice endoscopists to quickly and effectively learn HRME imaging of ESCN contrasts with the results of a similar study of CLE imaging of Barrett's esophagus, which showed considerably lower accuracy in the hands of novice (84%) vs. experienced (96%) endoscopists.40 There are no formal studies of the accuracy of novice and experienced endoscopists using CLE to image ESCN. Over half of the procedures in our study were performed in rural hospitals in northern China by endoscopists with no prior microendoscopy experience. This is significantly different from most CLE trials, which have been performed in tertiary centers, and from most NBI trials, which have shown a significant difference in the performance of novice and experienced endoscopists due to the significant learning curve associated with the interpretation of specific vascular and mucosal changes.23 CLE relies on interpretation of complex glandular, cellular and vascular patterns which may result in a steeper learning curve.41 It is likely that the use of objective, quantifiable image-analysis criteria and software size guides accounted for the rapid learning curve and consistent performance of all endoscopists, both novice and expert.
There are no formal studies comparing HRME to CLE. The CLE systems are commercially available instruments with the advantage of extensive industrial design, testing, and support from established medical device companies. The HRME described in this trial has been developed in an academic setting and not commercially available. The goal of this system is not to compete with CLE but provide a lower-cost, portable alternative in low-resource areas with less experienced providers. Comparing the performance attributes of the two systems, the field of view is slightly superior for HRME compared to CLE (HRME: 0.41 mm3, probe-based CLE 0.05 to 0.28 mm3, CLE 0.23 mm3); the lateral resolution of HRME is slight inferior to that of CLE (HRME: 4.4 μm, probe-based CLE: 1.0 and 3.5 μm, CLE 0.7 μm); and HRME and CLE have similar frame rates (12 frames/second for both HRME and probe-based CLE, 1.2 frames/second for CLE).
Our results combining HRME and LCE are close to that of CLE for the detection of ESCN. CLE has been shown to have 95% accuracy while HRME had 90% accuracy in this trial.17,20,22,24 While there are no guidelines for squamous neoplasia, current guidelines developed by the American Society of Gastrointestinal Endoscopy for esophageal adenocarcinoma suggest that a threshold sensitivity ≥ 90%, specificity ≥ 80%, and NPV ≥ 98% are needed for an imaging technology to replace standard biopsy protocol.40 The only imaging modality currently meeting these benchmarks is CLE.40 Our results reveal sensitivity, specificity and NPV of 95%, 79%, and 98% (per patient). Again, while there are no such guidelines for ESCN, these results are promising.
Our study has several strengths. It was performed prospectively, in real-time in low- and high-resource clinical settings by both novice and experienced endoscopists. Pathologic interpretation was standardized by the use of a single, expert gastrointestinal pathologist. Because the HRME device is affordable, portable and battery-powered, it was easily used in both low- and high-resource settings, suggesting the potential for it to be implemented successfully in low-resource settings. Additionally, the use of a simple software overlay to assist in visual interpretation of HRME images by providing a semi-quantitative assessment of nuclear size increases the likelihood of successful technology dissemination, aiding less experienced clinicians.
Several limitations exist as well. First, HRME is, at present, not commercially available and proflavine is an investigational drug. Second, there were three lesions that were positive on biopsy that were incorrectly interpreted as negative on HRME, which accounts for the imperfect sensitivity of HRME. Two of these biopsies had only focal dysplasia (one with a small area of moderate dysplasia in low grade dysplasia) and one was originally moderate dysplasia prior to the re-review by the pathologists. In the focal dysplasia, there was one small area of moderate dysplasia in low grade dysplasia. In moderate dysplasia, surface changes are not pronounced requiring deeper imaging to differentiate moderate from low grade dysplasia. HRME only images superficially (10 μm),42 and the deeper epithelial changes will not be appreciated. This is a limitation of the technology, which may explain the false negative interpretation. A third limitation of HRME, because of the probe size and the need for the probe to contact the entire surface of the lesion, is that large lesions can take up to 2-3 minutes to image, which may be laborious. Our group is developing a new mosaicking software to “stitch” HRME images of larger surface areas together in a smoother fashion. This will enhance the ease and accuracy of imaging by allowing larger areas to be scanned more quickly.
Lastly, our study did not blind endoscopists to the LCE's evaluation. This, however, was done by design as HRME is meant to be used in a complementary role to Lugol's. Indeed, HRME is meant as a second-pass, “confirmatory” technology, not a red-flag screening modality. The ultimate goal of such a device is to reduce biopsy costs.
In summary, this prospective, international trial demonstrates that the accuracy of HRME imaging is comparable to other advanced, more costly imaging modalities such as CLE. The use of HRME as an adjunct to LCE may decrease the number of biopsies. In future studies, HRME may also provide the opportunity to diagnose and treat ESCN in a single session, facilitating real-time decision making (biopsy, no biopsy, or treat neoplasia immediately). This may reduce cost, decrease loss of follow up, and prevent delays in diagnosis and treatment. A formal cost-effectiveness analysis and a randomized trial is planned to validate these findings.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the grants NCI R21 CA156704 and ClinicalTrials.gov, Number NCT 01384708.
Abbreviations
- ESCN
Esophageal sqaumous cell neoplasia
- HRME
high resolution microendocsopy
- LCE
Lugol's chromoendoscopy
- HD-WLE
high-definition white light endoscopy
- CLE
confocal laser endomicroscopy
Author contributions:
Marion-Anna Protano - Conception and design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work; final approval of the version to be published
Hong Xu - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Guiqi Wang - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Alexandros Polydorides - Acquisition of data for the work; drafting the work; final approval of the version to be published
Sanford M. Dawsey - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Junseng Cui - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Liyan Xue - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Fan Zhang - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Timothy Quang - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Mark C. Pierce - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Dongsuk Shin - Conception and design of work and acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Richard A. Schwarz - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Manoop S. Bhutani - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Michelle Lee - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Neil Parikh - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Chin Hur - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Weiran Xu - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Erin Moshier - Analysis and interpretation of data for the work; revising it critically for important intellectual content; final approval of the version to be published
James Godbold - Analysis and interpretation of data for the work; drafting the work; final approval of the version to be published
Josephine Mitcham - Acquisition of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Courtney Thomas - Acquisition, analysis, and interpretation of data for the work; revising it critically for important intellectual content; final approval of the version to be published
Rebecca R. Richards-Kortum - Conception and design of the work; securing funding; acquisition, analysis, and interpretation of data for the work; drafting the work; final approval of the version to be published
Sharmila Anadasabapathy - Conception and design of the work; securing funding; acquisition, analysis, and interpretation of data for the work; drafting the work; final approval of the version to be published
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Richard A. Schwarz holds a patent related to optical diagnostic technologies that have been licensed to Remicalm LLC. Chin Hur has a financial relationship outside the submitted work with Gilead Pharmaceuticals. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Umar SB, Fleischer DE. Esophageal cancer: epidemiology, pathogenesis, and prevention. Nat Clin Pract Gastroenterol Hepatol. 2008;5:517–526. doi: 10.1038/ncpgasthep1223. [DOI] [PubMed] [Google Scholar]
- 2.Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98–100. doi: 10.1002/ijc.11029. [DOI] [PubMed] [Google Scholar]
- 3.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography, and ethnicity. J of Gastroenterol Hepatol. 2009;24:729–735. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 4.Wei WQ, Abnet CC, Lu N, et al. Risk factors of oesophageal squamous dysplasia in adult inhabitants of a high-risk region of China. Gut. 2005;54:759–763. doi: 10.1136/gut.2004.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlander N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975-2011. http://seer.cancer.gov/csr/1975_2011, based on November 2013 SEER data submission, posted to SEER website, April 2014. [Google Scholar]
- 6.Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pech O, Gossner L, May A, et al. Endoscopic resection of superficial esophageal squamous-cell carcinomas: Western experience. Am J Gastroenterol. 2004;99:1226–1232. doi: 10.1111/j.1572-0241.2004.30628.x. [DOI] [PubMed] [Google Scholar]
- 8.Katada C, Muto M, Saito D, et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae: a multicenter, retrospective cohort study. Endoscopy. 2007;39:779–783. doi: 10.1055/s-2007-966761. [DOI] [PubMed] [Google Scholar]
- 9.Bergman JJ, Zhang YM, He S, et al. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc. 2011;74:1181–1190. doi: 10.1016/j.gie.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker V, Bajbouj M, Schmid RM, et al. Multimodal endoscopic therapy for multifocal intraepithelial neoplasia and superficial esophageal squamous cell carcinoma – a case series. Endoscopy. 2011;43:360–364. doi: 10.1055/s-0030-1256310. [DOI] [PubMed] [Google Scholar]
- 11.Sidorenko EI, Sharma P. High resolution chromoendoscopy in the esophagus. Gastrointest Endosc Clin N Am. 2004;14:436–451. doi: 10.1016/j.giec.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Reddymasu SC, Sharma P. Advances in endoscopic imaging of the esophagus. Gastroenterol Clin N Am. 2008;37:763–774. doi: 10.1016/j.gtc.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma in Linxian, China. Cancer. 1998;83:220–231. [PubMed] [Google Scholar]
- 14.Hashimoto CL, Iriya ER, Baba, et al. Lugol's dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275–282. doi: 10.1111/j.1572-0241.2005.30189.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida Y, Goda K, Tajiri H, et al. Assessment of novel endoscopic techniques for visualizing superficial esophageal squamous cell carcinoma: autofluorescence and narrow-band imaging. Dis Esophagus. 2009;22:439–446. doi: 10.1111/j.1442-2050.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Freitag CPF, Barros SGS, Kruel CDP, et al. Esophageal dysplasia are detected by endoscopy with Lugol in patients at risk for squamous cell carcinoma in southern Brazil. Dis Esophagus. 1999;12:191–195. doi: 10.1046/j.1442-2050.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 17.Pech O, Rabenstein T, Manner H, et al. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma of the esophagus. Clin Gastroenterol Hepatol. 2008;6:89–94. doi: 10.1016/j.cgh.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Li QY, Yu T, et al. Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy. 2009;41:99–106. doi: 10.1055/s-0028-1119492. [DOI] [PubMed] [Google Scholar]
- 19.Deinert K, Kiesslich R, Vieth M, et al. In-vivo microvascular imaging of early squamous-cell cancer cancer of the esophagus by confocal laser microendoscopy. Endoscopy. 2007;39:366–368. doi: 10.1055/s-2007-966217. [DOI] [PubMed] [Google Scholar]
- 20.Muto M, Minashi K, Yano T, et al. Early detection of the superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Onc. 2010;28:1566–1572. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ide E, Carneiro FO, Frazao MS, et al. Endoscopic detection of early esophageal squamous cell carcinoma in patients with achalasia: narrow-band imaging versus Lugol's staining. J Oncol. 2013 doi: 10.1155/2013/736756. published online ahead of print July 9, 2013 doi:1155.nm736756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ide E, Maluf-Filho F, Chaves DM, et al. Narrow-band imaging without magnification for detecting early esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:4408–4413. doi: 10.3748/wjg.v17.i39.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishihara R, Takeuchi Y, Chatani R, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010;23:480–486. doi: 10.1111/j.1442-2050.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 24.Takenaka R, Kawahara Y, Okada H, et al. Narrow-band imaging provides reliable screening for esophageal malignancy in patients with head and neck cancers. Am J Gastroenterol. 2009;104:2942–2948. doi: 10.1038/ajg.2009.426. [DOI] [PubMed] [Google Scholar]
- 25.Pierce M, Dihua Y, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J Vis Exp. 2011;47:2306. doi: 10.3791/2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muldoon TJ, Pierce MC, Nida DL, et al. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007;15:16413–16423. doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muldoon TJ, Anandasabapathy S, Maru D, et al. High-resolution imaging in Barrett's esophagus: a novel, low-cost endoscopic microscope. Gastrointest Endosc. 2008;68:737–744. doi: 10.1016/j.gie.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muldoon TJ, Thekkek N, Roblyer D, et al. Evaluation of quantitative image analysis criteria for the high-resolution detection of neoplasia in Barrett's esophagus. J Biomed Opt. 2010;15:26–27. doi: 10.1117/1.3406386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vila PM, Polydorides AD, Protano MA, et al. Accuracy and inter-rater reliability for the diagnosis of Barrett's neoplasia among new and experienced users of a novel, low-cost, portable microendoscope.. Gastroenterology, presented at Digestive Disease Week; Chicago, Illinois. May 7-11, 2011. [Google Scholar]
- 30.Pierce MC, Vila PM, Polydorides AD, et al. Low-Cost endomicroscopy in the esophagus and colon. Am J Gastroenterol. 2011;106:1722–24. doi: 10.1038/ajg.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin D, Protano MA, Polydorides AD, et al. Quantitative analysis of high-resolution microendoscopic images for diagnosis of esophageal squamous cell carcinoma. Clin Gastroenterol Hepatol. 2014;13:272–9. e1. doi: 10.1016/j.cgh.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Protano MA, Xu H, Zhang F, et al. Low-cost microendoscopy for the diagnosis of esophageal squamous cell neoplasia in northern China: an evaluation of interobserver agreement and accuracy.. Gastroenterology, presented at Digestive Disease Week; Chicago, Illinois. May 7-11, 2011. [Google Scholar]
- 33.Bosman FT, Carneiro F, Hruban RH, et al. World Health Organization Classification of Tumours of the Digestive System. 4th ed. World Health Organization; Lyon, France: 2010. [Google Scholar]
- 34.Dawsey SM, Lewin KJ, Liu FS, et al. Esophageal morphology from Linxian, China: Squamous histologic findings in 754 patients. Cancer. 1994;73:2027–2037. doi: 10.1002/1097-0142(19940415)73:8<2027::aid-cncr2820730803>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Dawsey SM, Lewin KJ, Wang GQ, et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. Cancer. 1994;74:1686–1692. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Wang GQ, Abnet CC, Shen Q, et al. Precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–192. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miettinen OS. On the matched-pairs design in the case of all-or-none responses. Biometrics. 1986;24:339–352. [PubMed] [Google Scholar]
- 38.Blyth CR, Still HA. Binomial confidence intervals. Journal of the Am Stat Assoc. 1983;78:108–116. [Google Scholar]
- 39.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 40.Canto MI, Anandasabapathy S, Brugge W, et al. In vivo endomicroscopy improves detection of Barrett's esophagus-related neoplasia: a multicenter international randomized controlled trial. Gastrointest Endosc. 2014;79:211–221. doi: 10.1016/j.gie.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iguchi Y, Niwa Y, Miyahara R. Pilot study on confocal endomicroscopy for diagnosis of the depth of squamous cell esophageal cancer in vivo. J Gatroenterol Hepatol. 2009;24:1733–1739. doi: 10.1111/j.1440-1746.2009.05892.x. [DOI] [PubMed] [Google Scholar]
- 42.Koucky MH, Pierce MC. Axial response of high-resolution microendscopy in scattering media. Biomed Opt Express. 2013;4:2247–2256. doi: 10.1364/BOE.4.002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.