Abstract
Ovarian cancer is the deadliest of all gynecologic cancers. Recent evidence demonstrates an association between enzymatic activity altering single nucleotide polymorphisms (SNP) with human cancer susceptibility. We sought to evaluate the association of SNPs in key oxidant and antioxidant enzymes with increased risk and survival in epithelial ovarian cancer. Individuals (n = 143) recruited were divided into controls, (n = 94): healthy volunteers, (n = 18), high-risk BRCA1/2 negative (n = 53), high-risk BRCA1/2 positive (n = 23) and ovarian cancer cases (n = 49). DNA was subjected to TaqMan SNP genotype analysis for selected oxidant and antioxidant enzymes. Of the seven selected SNP studied, no association with ovarian cancer risk (Pearson Chi-square) was found. However, a catalase SNP was identified as a predictor of ovarian cancer survival by the Cox regression model. The presence of this SNP was associated with a higher likelihood of death (hazard ratio (HR) of 3.68 (95% confidence interval (CI): 1.149–11.836)) for ovarian cancer patients. Kaplan-Meier survival analysis demonstrated a significant median overall survival difference (108 versus 60 months, p<0.05) for those without the catalase SNP as compared to those with the SNP. Additionally, age at diagnosis greater than the median was found to be a significant predictor of death (HR of 2.78 (95% CI: 1.022–7.578)). This study indicates a strong association with the catalase SNP and survival of ovarian cancer patients, and thus may serve as a prognosticator.
Introduction
Epithelial ovarian cancer (EOC) accounts for 85 to 90% of all cancers of the ovaries, fallopian tubes and primary peritoneum; and displays various histologies such as serous, mucinous, or endometrioid [1]. Ovarian cancer is the deadliest of all gynecologic cancers with an estimated 22,980 new cases and 14,270 deaths expected in 2014 in the US alone [1,2]. Typically, treatment of ovarian cancer is performed with either cytoreductive surgery (CRS) followed by platinum/taxane combination chemotherapy or neoadjuvant chemotherapy with interval CRS [3,4]. Generally, a 50–80% complete clinical response can be achieved in patients with advanced disease. Unfortunately, most treated patients will relapse within 18 months with chemoresistant disease [5]. While the chances of long-term patient survival are significantly increased when the cancer is detected at its early stage, to date, there is no reliable method available for early detection of this disease [5].
Epidemiologic studies have clearly established the role of family history as an important risk factor for both breast and ovarian cancers [6]. Mutations in BRCA are currently utilized to evaluate risk for breast and ovarian cancer, however, this method is not ideal because the mutations are so rare (1 out of 500 individuals), leading to a small overall impact on mortality rate [7]. Genomic variations between individuals have been increasingly used in the practice of medicine [8–12]. A single nucleotide polymorphism (SNP) occurs because of point mutations that are selectively maintained in populations and are distributed throughout the human genome at an estimated overall frequency of at least one in every 1000 base pairs [13]. Non-synonymous SNPs substitute encoded amino acids in proteins, and are more likely to alter the structure, function, and interaction of the protein [14]. Recent evidence demonstrates an association between enzymatic activity altering SNPs in oxidative DNA repair genes and antioxidant genes with human cancer susceptibility [15]. Additionally, a pro-oxidant state has been implicated in the pathogenesis of several malignancies, including ovarian cancer [16,17]. The current study is based on the fact that certain SNPs present in key oxidants and antioxidants enzymes result in altered enzymatic activity, as well as our previously published work delineating the role of oxidative stress in ovarian cancer. The goal of this study was to determine whether specific SNP in key oxidant and antioxidant enzymes are associated with the increased risk as well as overall survival of ovarian cancer patients.
Materials and Methods
Study design
We performed a case-control study comparing female subjects with and without EOC and determined whether there is an association with several selected SNPs in established redox genes. Eligible women were 19 to 80 years of age and were previously recruited through the Karmanos Cancer Institute’s Genetic Registry (KCIGR), Detroit, MI. Research activities and method of consent were conducted with the approval of Wayne State University Institutional Review Board (IRB#024199MP2F(5R)). Informed written consent forms were utilized and permission was granted for the collection of blood samples and for access to medical records for all subjects.
Patient Population
Recruited individuals (n = 143) were divided into controls (94), healthy volunteers (n = 18), high-risk BRCA1/2 negative (n = 53), high-risk BRCA1/2 positive (n = 23) and ovarian cancer cases (n = 49). Controls were selected primarily from research subjects, considered high-risk for breast and ovarian cancers, without ovarian cancer that underwent genetic screening for BRCA1/2 carrier status. Of note, the criteria used for screening included personal history of breast and ovarian cancers, family histories of breast and ovarian cancers, and BRCA1/2 mutations. Additionally, healthy volunteers were also recruited as controls from the metropolitan Detroit area with no such histories. Cases were selected based on histopathology-confirmed primary diagnosis of EOC. All participants, except healthy volunteers, had previously undergone BRCA1/2 testing and the results were made available to us.
Samples used for this study were collected from participants recruited between 1999 and 2012. DNA samples were utilized to determine the presence of polymorphisms in the genes described in Table 1. The SNPs were chosen based on previously reported associations with several cancers [18–26]. Of the 143 subjects, 49 (34.3%) had a primary diagnosis of ovarian cancer while 94 (65.7%) without cancer served as controls. For the ovarian cancer cohort: 13 (26.5%) were BRCA1/2 positive as compared to 34 (69.4%) BRCA1/2 negative; 2 (4.1%) cases were missing. The data is normally distributed with the age of enrollment ranged from 18 to 90 with a mean of 52 ± 15 and a median age of 52. The age at diagnosis ranged from 23 to 77, with a mean of 52 ± 11 and a median age of 52. The racial distribution was 88.8% (Caucasian), 8.4% (African-American) and 2.8% (Other). Personal and family histories of breast cancer, ovarian cancer, other cancers, and BRCA1/2 were quantified. The frequencies of the presence of the SNP (heterozygous plus homozygous mutant) compared to homozygous wild type were determined for each gene studied.
Table 1. Characteristics of single nucleotide polymorphisms examined for genotyping.
| Gene (RS) | SNP | NCBI MAF | Chromosomal location | Known AA Switch | Effect on activity |
|---|---|---|---|---|---|
| CAT (rs1001179) | C-262T | 0.123 | 11p13 | Unknown | Decrease |
| CYBA (rs4673) | C242T | 0.303 | 16q24.3 | Tyr to His | Increase |
| GPX1 (rs3448) | C-1040T | 0.176 | 3p21.31 | Unknown | Unknown |
| GSR (rs1002149) | G201T | 0.191 | 8p12 | Unknown | Unknown |
| MnSOD (rs4880) | T47C | 0.371 | 6q25.3 | Ala to Val | Decrease |
| MPO (rs2243828) | T-764C | 0.23 | 17q22 | Unknown | Decrease |
| NOS2 (rs2297518) | C2087T | 0.173 | 17q11.2 | Ser to Leu | Increase |
AA; amino acid, Ala; alanine, CAT; catalase, CYBA; NAD(P)H oxidase subunit (NOX4), GSR; glutathione reductase, GPX; glutathione peroxidase, His; histidine, Leu; leucine, MAF; minor allele frequency, MnSOD; manganese superoxide dismutase, MPO; myeloperoxidase, NCBI; National Center for Biotechnology Information, NOS2; nitric oxide synthase, Ser; serine, SNP; single nucleotide polymorphism, Tyr; tyrosine, Val; valine.
Purification of DNA and the TaqMan SNP Genotyping Assay for SNPs
DNA, from blood samples, was isolated by the Applied Genomics Technology Center (AGTC, Detroit, MI). DNA was extracted with QIAamp DNA mini kit per the manufacturer’s protocol (Qiagen, Valencia, CA) [27]. The TaqMan SNP Genotyping Assay Sets (Applied Biosystems, Carlsbad, CA) (NCBI dbSNP genome build 37, MAF source 1000 genomes) were used to genotype selected SNPs described in Table 1. The AGTC performed this assay and analysis was done utilizing the QuantStudio 12K Flex Real-Time PCR System (Applied Biosystems).
Statistical analysis
Data were analyzed using SPSS (IBM, Armonk, New York) for Mac V.22. The variables selected for the analyses include genotypes, age at diagnosis, and age at enrolment, personal and family histories of breast, ovarian, and BRCA1/2 mutations, in addition to other malignancies. Using the median age at diagnosis/enrolment as a cut point, we dichotomized the “age at diagnosis” variable. The “race” variable was categorized as: Caucasian, African-American or Other. We consolidated the following tumor and clinical variables into binary categorical schemes: International Federation of Gynecology and Obstetrics (FIGO) stages into early (IIA-IIIB) and advanced (IIIC-IV); FIGO grades (G1/2) and (G3); histology (serous and other). For all the genes studied, the “genotype” variable was dichotomized using the following scheme: homozygous wild type versus homozygous mutant plus heterozygous mutant. To compare cases to controls on the selected demographic, clinical, and genotypic characteristics, we performed Pearson Chi-square analysis. The recurrence rate was determined as the percentage of patients that have gone into remission, but the disease has returned months or years later, based on physical examination, radiological studies and serum CA-125 levels.
Cox regression and Kaplan-Meier analyses of variables as a predictor of overall survival
To study the impact of the SNPs on overall survival, Cox regression analyses were performed using the above-listed variables and classification schemes, using the likelihood ratio forward stepwise method. Several method simulations were performed such as: forced entry (ENTER), forward LR (likelihood ratio), etc. The forward LR was chosen for the final analysis. This method is a stringent model that selects the strongest predictors of the outcome to be included in the final model. Table 2 includes the strongest predictors kept by the model as well as variables rejected by the model. All patients received the standard of care after tumor board discussion. Details on treatment characteristics were not available. Additionally, Kaplan-Meier survival curves were generated for the variables selected by the model. We conducted all analyses at p-value < 0.05 for statistical significance.
Table 2. Cox regression analysis for selected SNPs in key oxidants and antioxidants genes in ovarian cancer.
| Variables in the Equation | ||||
| 95% CI for HR | ||||
| Significance | HR | Lower | Upper | |
| Age at Diagnosis > Mean | .045* | 2.782* | 1.022 | 7.578 |
| CAT (CT+TT) | .028* | 3.688* | 1.149 | 11.836 |
| Variables Analyzed by Cox Regression but Rejected by the Model. | ||||
| Score | Significance | |||
| Race (Caucasian) | .580 | .446 | ||
| Stage (III-IV) | .708 | .400 | ||
| Grade (High) | .708 | .400 | ||
| GSR (CT+TT) | .411 | .522 | ||
| GPX (CT+TT) | .000 | .988 | ||
| MnSOD (CT+TT) | 1.020 | .312 | ||
| NOS2 (CT+TT) | 2.084 | .149 | ||
| CYBA (CT+TT) | 1.229 | .268 | ||
| MPO (CT+TT) | .178 | .673 | ||
| Histology (Serous) | 1.016 | .314 | ||
Adjusted Hazard Ratio (HR) for the variables included in the model.
* p<0.05, degrees of freedom = 1 for all analyses. CYBA; NAD(P)H oxidase subunit (NOX4), GPX; glutathione peroxidase, GSR; glutathione reductase, MnSOD; manganese superoxide dismutase, MPO; myeloperoxidase, NOS2; inducible nitric oxide synthase. For this analysis, several “Method”simulations were performed such as: forced entry (ENTER), forward LR (likelihood ratio), etc. The forward LR was chosen for the final analysis. Table 4 includes the strongest predictors kept by the model as well as those rejected by the model. The Cox regression model generated the scores in the table. The P-values are noted in the column significance; *p<0.05, is considered statistically significant.
Results
We performed side-by-side comparison between ovarian cancer cases and controls using Pearson chi-square analysis. Racial distribution was statistically similar between the groups (p>0.05). As expected, “personal or family history of ovarian cancer”, “personal or family history of other cancers” and “advanced age” were significantly different between the groups and are known ovarian cancer risk factors (p<0.05, Table 3). Comparative analyses for manganese superoxide dismutase (MnSOD, rs4880), NAD(P)H oxidase (CYBA, rs4673), glutathione peroxidase (GPX1, rs3448), inducible nitric oxide synthase (NOS2, rs2297518), myeloperoxidase (MPO, rs2243828), glutathione reductase (GSR, rs1002149), and catalase (CAT, rs1001179) did not find a significant difference between the cases and controls (Table 3). Out of the 49 ovarian cancer cases, 38 (77.5%) were further analyzed by the Cox regression method, and 11 (22.5%) were dropped due to missing data. The majority of the cases were serous histology, advanced stage, and high-grade tumors (Table 4). The recurrence rate was found to be 60.5%.
Table 3. Comparison of cases and controls based on demographic, personal or family history of cancer, and genotypic characteristics.
| Controls (%) | Ovarian Cancer (%) | P-value (Pearson Chi-square, 2-tailed) | |
|---|---|---|---|
| Age at enrolment (n = 125) | n (76) | n (49) | <0.001* |
| < Median | 51 (67.1) | 8 (16.3) | |
| > Median | 25 (32.9) | 41 (83.7) | |
| Race (n = 143) | n (94) | n (49) | |
| Caucasian | 81 (86.2) | 46 (93.9) | |
| African-American | 10 (10.6) | 2 (4.1) | |
| Other | 3 (3.2) | 1 (2.0) | |
| Personal / Family History of Cancer (Yes) | n (94) | n (49) | |
| Breast (n = 105) | 67 (71.3) | 38 (77.6) | .480 |
| Ovarian (n = 81) | 33 (35.1) | 48 (98.0) | <0.001* |
| BRCA1/2 (n = 23) | 18 (19.1) | 5 (10.2) | .168 |
| Other Cancer (n = 69) | 39 (44.3) | 30 (62.5) | .043* |
| SNP (Yes) | |||
| NOS2 (n = 49) | 34 (37.4) | 15 (30.6) | .424 |
| CYBA (n = 92) | 57 (60.6) | 35 (71.4) | .201 |
| MPO (n = 56) | 36 (39.1) | 20 (41.7) | .771 |
| GSR (n = 36) | 27 (30.3) | 9 (19.6) | .180 |
| GPX (n = 61) | 40 (43.5) | 21 (42.9) | .943 |
| CAT (n = 49) | 30 (31.9) | 19 (38.8) | .412 |
| MnSOD (n = 103) | 66 (72.5) | 37 (75.5) | .703 |
*p< 0.05, CAT; catalase, CYBA; NAD(P)H oxidase subunit (NOX4), GPX; glutathione peroxidase, GSR; glutathione reductase, MnSOD; manganese superoxide dismutase, MPO; myeloperoxidase, NOS2; inducible nitric oxide synthase.
Table 4. Stage, grade and pathologic characteristics of the cancer cases.
| Tumor Characteristics | Number (%) |
|---|---|
| Stage (n = 38) | |
| IA-IIIB | 10 (26.3) |
| IIIC-IV | 28 (73.7) |
| Total | 38 (100) |
| Grade (n = 38) | |
| G1/2 | 6 (15.8) |
| G3 | 32 (84.2) |
| Total | 38 (100) |
| Histology (n = 38) | |
| Serous | 34 (89.5) |
| Clear Cell | 1 (2.6) |
| Endometrioid | 1 (2.6) |
| Total | 38 (100) |
The CAT SNP is a predictor of shorter survival
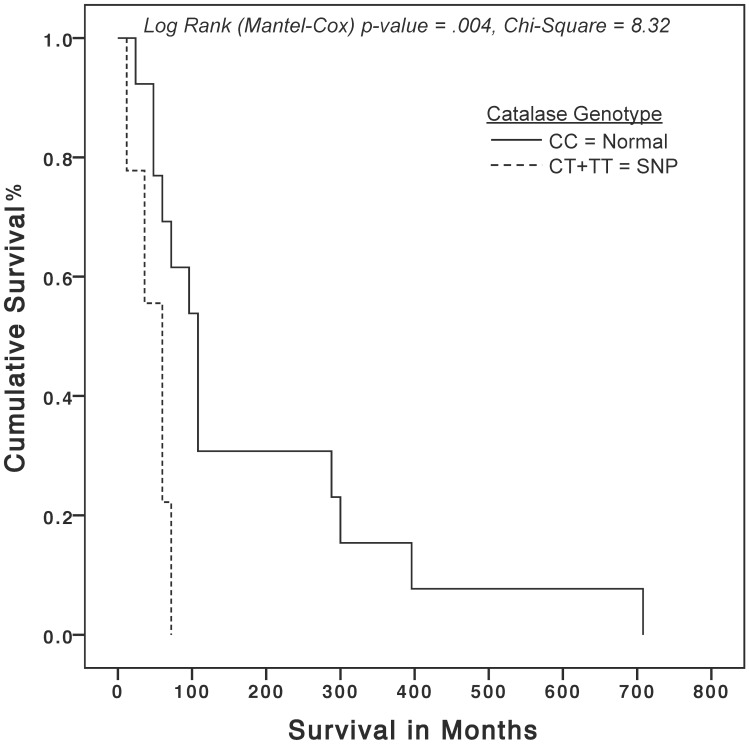
At the time of these analyses, there were 26 deaths (18.2%) and 117 (81.8%) subjects alive. Among the SNPs examined, only CAT (rs1001179) was identified as a predictor of shorter survival by the Cox regression model with a hazard ratio (HR) of 3.68 (95% CI: 1.149–11.836, p = 0.028) (Table 4A). As expected, “age at diagnosis” greater than the median (52) was found to be a significant predictor of death with an HR of 2.78 (95% CI: 1.022–7.578, p = 0.045) (Table 4). The variables selected for the analyses, but rejected by the model are listed in Table 4. Kaplan-Meier (K-M) survival analysis factored by CAT genotype, which used 84.6% of the deaths, demonstrated a statistically significant median overall survival difference (108 [95% CI: 79–137] versus 60 [95% CI: 40–80] months, p<0.05) and a mean overall survival difference (182 [95% CI: 75.5–288] versus 47 months [95% CI: 31–60], p<0.05) for subjects with the normal genotype as compared to the CAT SNP genotype (Fig 1).
Fig 1. Kaplan-Meier overall survival curves for in ovarian cancer utilizing a specific catalase SNP.
The solid curve represents cases with (CC) homozygous wild-type genotype as compared to the dashed curve, which represents cases with homozygous mutant plus heterozygous mutant (CT+TT) genotypes. The X-axis represents patient survival in months; the Y-axis represents cumulative survival percentage. Chi-square p-value 0<0.05 is considered statistically significant.
Discussion
A large body of evidence suggests that ovarian cancer patients have decreased levels of circulating antioxidants and higher levels of oxidative stress [16,17,28–32]. We have reported the existence of a persistent pro-oxidant state in EOC that included increased expression of key pro-oxidant enzymes such as inducible nitric oxide synthase (iNOS), NAD(P)H oxidase, and MPO [16,32,33]. Interestingly, the expression of MPO in EOC cells and tissues came as a surprise as it is an oxidant-generating enzyme typically found in cells of myeloid origin [34]. We have also determined that MPO can produce the nitrosonium cation (NO+) utilizing NO produced by iNOS. This is important because NO+ causes s-nitrosylation of caspase-3, and inhibition of its activity, resulting in a decrease in apoptosis [32]. This mechanism further explains the observation that EOC cells manifest significantly decreased apoptosis and increased survival [32,33,35,36]. Interestingly, the evaluation of mutations in the various redox enzymes in the form of SNPs is an active area of scientific research [37–45]. Genetic polymorphisms are known to be associated with cancer susceptibility and can be determined by studying functional polymorphisms in genes that control the levels of cellular reactive oxygen species and oxidative damage, including SNPs for genes involved in carcinogen metabolism (detoxification and/or activation), antioxidants, and DNA repair pathways [46]. For example, germline mutations in BRCA1 or BRCA2 are associated with ovarian cancer at a rate of only 20–40%, suggesting the presence of other unidentified mutations in other genes as an etiology [14,47,48]. Additional genetic variations, many of which have been identified in recent genome-wide association studies (GWAS), have been hypothesized to act as low to moderate penetrant alleles, which contribute to ovarian cancer risk, as well as other diseases [14,49]. In support of this, recent studies have also associated genetic polymorphisms in genes involved in suppression of tumorigenicity as well as those involved in cell cycle with ovarian cancer [50,51].
For this study, we sought to evaluate the association of specific SNPs in key oxidant and antioxidant enzymes with increased risk and overall survival of ovarian cancer. The analysis of the patient population revealed that the average age at diagnosis and racial distribution of those diagnosed with ovarian cancer were consistent with known risk factors for ovarian cancer, specifically, women of North American decent and those over 50 years old. Currently we demonstrated that there is no association between the selected SNPs and risk of developing ovarian cancer (Table 2). It is important to emphasize the fact that although the selected SNPs for this study were not found to be associated with ovarian cancer risk, additional change of function SNPs for these enzymes exist and should be explored further. Of the SNPs studied when examining survival, we found the CAT SNP (rs1001179) to be a significant predictor of death when present in ovarian cancer patients as illustrated by the Cox regression and K-M survival analyses (Table 3 and Fig 1). Specifically, ovarian cancer patients with the CAT SNP died significantly sooner than those without it (Fig 1). The CAT SNP (rs1001179) is found in the promoter region of the CAT gene, substituting allele C with T at position -262 in the 5’ region of chromosome 11 and is correlated with decreased enzyme activity level [52]. Catalase is a very important and ubiquitous enzyme involved in the degradation of two molecules of hydrogen peroxide (H2O2) to water and oxygen. The current findings are consistent with several other studies, which linked this specific SNP with risk, response to adjuvant treatment and survival of cancer patients [18,19,24,53]. Specifically, low serum CAT levels were associated with adverse prognosis for ovarian cancer [21]. Our data provides a possible explanation for low serum CAT levels, which may be a result of a CAT SNP that lowers enzymatic activity. Moreover, mechanistic studies have identified H2O2, a result of oxidative stress, enhance angiogenesis and tumor invasiveness through several pathways including: hypoxia inducible factor 1-alpha, p38 MAPK and snail [54,55]. It appears that the final common pathway culminates to epidermal growth factor (EGF)-induced down-regulation of epithelial cadherin expression that can be inhibited by exogenous CAT [54]. Epithelial-cadherin is a cell-cell adhesion glycoprotein encoded by the CDH1 gene in humans, which has been characterized as a tumor suppressor [56,57]. Its loss of function is correlated with several solid tumors including ovarian and thought to contribute to tumor progression and metastasis [58].
It is important to emphasize that the lack of association between the selected SNPs in this study with ovarian cancer risk does not definitively answer this important question because additional change-of-function SNPs for these enzymes exist and should be explored further. Recent genetic studies have linked MPO to lung and ovarian cancers by demonstrating a striking correlation between the relative risk for development of the disease and the incidence of functionally distinct MPO polymorphisms [59]. Additionally, a SNP in NAD(P)H oxidase (rs4673) has been associated with increased risk of ovarian cancer [60]. In breast cancer, the presence of the CAT SNP (rs1001179), was shown to confer increased risk [24]. We have selected numerous additional SNPs based on their effect on enzyme activities or association with cancer. Several SNPs in NOS2 have been associated with gastric, esophageal, skin and urogenital cancers [20,22]. Also, SNPs in MnSOD, GPX1, GPX4, CAT were found to be associated with prostate cancer [24].
Other studies have found a SNP in MnSOD (rs4880) and a SNP in MPO (rs2333227) to be associated with increased risk for ovarian cancer [34]. The MPO SNP we have analyzed in this study is in 100% concordance with SNP rs2333227 [61]. Thus, in addition to examining other changes of functional SNPs, increasing the size of our cohort may be sufficient to reach statistical significance in several of the SNPs chosen for this study. The strength of our study includes the comprehensive nature of redox genes studied and the translational aspect of our approach by assessing simultaneously clinical and genotypic characteristics of the population. We believe the fact that our control cohort is heterogeneous represents strength, because it includes patients considered at high risk for BRCA1/2 mutation and those without any established risk factors for ovarian cancer, reflecting the baseline risk group (general population). Interestingly, patients who tested negative for BRCA1/2 mutations, as well as, those with family history of BRCA1/2 mutations but also tested negative for BRCA1/2 should be considered at a higher risk profile than the general population. More importantly, to our knowledge, we are the first to report an association between the presence of this specific CAT SNP and ovarian cancer survival. The study has several limitations such as small sample size, the retrospective nature inherent to case-control studies, and the geographic restriction of the population. In our patient population, the recurrence rate was found to be 60.5%; however, the exact date of recurrence was not established making the computation of progression-free survival (PFS) impossible. We acknowledge that the determination of PFS would have strengthened our findings as PFS has often been used as a primary endpoint or a surrogate to overall survival in clinical trials [62–67].
It is now evident that oxidative stress plays a major role in the pathogenesis of cancer including ovarian cancer, however the exact mechanisms remain to be clarified. In this preliminary study we were able to show that a specific CAT SNP is associated with poor survival in ovarian cancer patients. Further studies examining other SNPs in key oxidants and antioxidant enzymes with higher number of patients will be needed to establish this link. SNPs in these enzymes may serve as potential markers for ovarian cancer, which are urgently needed. Our study indicates a strong association with the CAT SNP and survival of ovarian cancer patients, and thus may serve as a prognosticator.
Supporting Information
(PDF)
Acknowledgments
We would like to thank Dr. Michael S. Simon, Karmanos Cancer Institute, Detroit, MI for his invaluable efforts and the Genetic Counselors in Clinical Genetics. Patients are screened for eligibility at a weekly clinical conference and Dr. Simon or the Genetic Counselors introduce/explain the study to the eligible patients, consent them, and arrange for the blood draw. We would also like to thank Dr. Michael A. Tainsky and Nancy Levin, Karmanos Cancer Institute, Detroit, MI, for their support and for providing the DNA samples for this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Erickson BK, Conner MG, Landen CN Jr (2013) The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol 209: 409–414. 10.1016/j.ajog.2013.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63: 11–30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 3. Robella M, Vaira M, Marsanic P, Mellano A, Borsano A, Cinquegrana A, et al. (2014) Treatment of peritoneal carcinomatosis from ovarian cancer by surgical cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC). Minerva Chir 69: 27–35. [PubMed] [Google Scholar]
- 4. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA (2014) Ovarian cancer. Lancet 384: 1376–1388. 10.1016/S0140-6736(13)62146-7 [DOI] [PubMed] [Google Scholar]
- 5. Marcus CS, Maxwell GL, Darcy KM, Hamilton CA, McGuire WP (2014) Current Approaches and Challenges in Managing and Monitoring Treatment Response in Ovarian Cancer. J Cancer 5: 25–30. 10.7150/jca.7810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCI (2012) National Cancer Institute: PDQ Genetics of Breast and Ovarian Cancer. Available: http://wwwcancergov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional/page2.
- 7. Fasching PA, Gayther S, Pearce L, Schildkraut JM, Goode E, Thiel F, et al. (2009) Role of genetic polymorphisms and ovarian cancer susceptibility. Mol Oncol 3: 171–181. 10.1016/j.molonc.2009.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh M, Singh P, Juneja PK, Singh S, Kaur T (2011) SNP-SNP interactions within APOE gene influence plasma lipids in postmenopausal osteoporosis. Rheumatol Int 31: 421–423. 10.1007/s00296-010-1449-7 [DOI] [PubMed] [Google Scholar]
- 9. Hamosh A, King TM, Rosenstein BJ, Corey M, Levison H, Durie P, et al. (1992) Cystic fibrosis patients bearing both the common missense mutation Gly——Asp at codon 551 and the delta F508 mutation are clinically indistinguishable from delta F508 homozygotes, except for decreased risk of meconium ileus. Am J Hum Genet 51: 245–250. [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstein JA (2001) Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol 52: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee CR (2004) CYP2C9 genotype as a predictor of drug disposition in humans. Methods Find Exp Clin Pharmacol 26: 463–472. [PubMed] [Google Scholar]
- 12. Yanase K, Tsukahara S, Mitsuhashi J, Sugimoto Y (2006) Functional SNPs of the breast cancer resistance protein-therapeutic effects and inhibitor development. Cancer Lett 234: 73–80. [DOI] [PubMed] [Google Scholar]
- 13. Erichsen HC, Chanock SJ (2004) SNPs in cancer research and treatment. Br J Cancer 90: 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savas S, Schmidt S, Jarjanazi H, Ozcelik H (2006) Functional nsSNPs from carcinogenesis-related genes expressed in breast tissue: potential breast cancer risk alleles and their distribution across human populations. Hum Genomics 2: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klaunig JE, Kamendulis LM, Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38: 96–109. 10.1177/0192623309356453 [DOI] [PubMed] [Google Scholar]
- 16. Jiang Z, Fletcher NM, Ali-Fehmi R, Diamond MP, Abu-Soud HM, Munkarah AR, et al. (2011) Modulation of redox signaling promotes apoptosis in epithelial ovarian cancer cells. Gynecol Oncol 122: 418–423. 10.1016/j.ygyno.2011.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saed GM, Fletcher NM, Jiang ZL, Abu-Soud HM, Diamond MP (2011) Dichloroacetate induces apoptosis of epithelial ovarian cancer cells through a mechanism involving modulation of oxidative stress. Reprod Sci 18: 1253–1261. 10.1177/1933719111411731 [DOI] [PubMed] [Google Scholar]
- 18. Ahn J, Ambrosone CB, Kanetsky PA, Tian C, Lehman TA, Kropp S, et al. (2006) Polymorphisms in genes related to oxidative stress (CAT, MnSOD, MPO, and eNOS) and acute toxicities from radiation therapy following lumpectomy for breast cancer. Clin Cancer Res 12: 7063–7070. [DOI] [PubMed] [Google Scholar]
- 19. Ambrosone CB, Ahn J, Singh KK, Rezaishiraz H, Furberg H, Sweeney C, et al. (2005) Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res 65: 1105–1111. [PubMed] [Google Scholar]
- 20. Crawford A, Fassett RG, Geraghty DP, Kunde DA, Ball MJ, Robertson IK, et al. (2012) Relationships between single nucleotide polymorphisms of antioxidant enzymes and disease. Gene 501: 89–103. 10.1016/j.gene.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 21. Didziapetriene J, Bublevic J, Smailyte G, Kazbariene B, Stukas R (2014) Significance of blood serum catalase activity and malondialdehyde level for survival prognosis of ovarian cancer patients. Medicina (Kaunas) 50: 204–208. [DOI] [PubMed] [Google Scholar]
- 22. Janicka A, Szymanska-Pasternak J, Bober J (2013) [Polymorphisms in the oxidative stress-related genes and cancer risk]. Ann Acad Med Stetin 59: 18–28. [PubMed] [Google Scholar]
- 23. Li Y, Ambrosone CB, McCullough MJ, Ahn J, Stevens VL, Thun MJ, et al. (2009) Oxidative stress-related genotypes, fruit and vegetable consumption and breast cancer risk. Carcinogenesis 30: 777–784. 10.1093/carcin/bgp053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saadat M, Saadat S (2014) Genetic Polymorphism of CAT C-262 T and Susceptibility to Breast Cancer, a Case-Control Study and Meta-Analysis of the Literatures. Pathol Oncol Res. [DOI] [PubMed] [Google Scholar]
- 25. Seibold P, Hall P, Schoof N, Nevanlinna H, Heikkinen T, Benner A, et al. (2013) Polymorphisms in oxidative stress-related genes and mortality in breast cancer patients—potential differential effects by radiotherapy? Breast 22: 817–823. 10.1016/j.breast.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 26. Tsai SM, Wu SH, Hou MF, Chen YL, Ma H, Tsai LY (2012) Oxidative stress-related enzyme gene polymorphisms and susceptibility to breast cancer in non-smoking, non-alcohol-consuming Taiwanese women: a case-control study. Ann Clin Biochem 49: 152–158. 10.1258/acb.2011.011098 [DOI] [PubMed] [Google Scholar]
- 27. Kaplun L, Fridman AL, Chen W, Levin NK, Ahsan S, Petrucelli N, et al. (2012) Variants in the Signaling Protein TSAd are Associated with Susceptibility to Ovarian Cancer in BRCA1/2 Negative High Risk Families. Biomark Insights 7: 151–157. 10.4137/BMI.S10815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Senthil K, Aranganathan S, Nalini N (2004) Evidence of oxidative stress in the circulation of ovarian cancer patients. Clin Chim Acta 339: 27–32. [DOI] [PubMed] [Google Scholar]
- 29. Hileman EO, Liu J, Albitar M, Keating MJ, Huang P (2004) Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol 53: 209–219. [DOI] [PubMed] [Google Scholar]
- 30. Behrman HR, Kodaman PH, Preston SL, Gao S (2001) Oxidative stress and the ovary. J Soc Gynecol Investig 8: S40–42. [DOI] [PubMed] [Google Scholar]
- 31. Fletcher NM, Jiang Z, Ali-Fehmi R, Levin NK, Belotte J, Tainsky MA, et al. (2011) Myeloperoxidase and free iron levels: potential biomarkers for early detection and prognosis of ovarian cancer. Cancer Biomark 10: 267–275. 10.3233/CBM-2012-0255 [DOI] [PubMed] [Google Scholar]
- 32. Saed GM, Ali-Fehmi R, Jiang ZL, Fletcher NM, Diamond MP, Abu-Soud HM, et al. (2010) Myeloperoxidase serves as a redox switch that regulates apoptosis in epithelial ovarian cancer. Gynecol Oncol 116: 276–281. 10.1016/j.ygyno.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malone JM, Saed GM, Diamond MP, Sokol RJ, Munkarah AR (2006) The effects of the inhibition of inducible nitric oxide synthase on angiogenesis of epithelial ovarian cancer. Am J Obstet Gynecol 194: 1110–1116; discussion 1116–1118. [DOI] [PubMed] [Google Scholar]
- 34. Castillo-Tong DC, Pils D, Heinze G, Braicu I, Sehouli J, Reinthaller A, et al. (2013) Association of myeloperoxidase with ovarian cancer. Tumour Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abu-Soud HM, Hazen SL (2000) Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem 275: 37524–37532. [DOI] [PubMed] [Google Scholar]
- 36. Abu-Soud HM, Hazen SL (2000) Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem 275: 5425–5430. [DOI] [PubMed] [Google Scholar]
- 37. Becquemont L (2009) Pharmacogenomics of adverse drug reactions: practical applications and perspectives. Pharmacogenomics 10: 961–969. 10.2217/pgs.09.37 [DOI] [PubMed] [Google Scholar]
- 38. Ciccolini J, Gross E, Dahan L, Lacarelle B, Mercier C (2010) Routine dihydropyrimidine dehydrogenase testing for anticipating 5-fluorouracil-related severe toxicities: hype or hope? Clin Colorectal Cancer 9: 224–228. 10.3816/CCC.2010.n.033 [DOI] [PubMed] [Google Scholar]
- 39. Gomes AM, Winter S, Klein K, Turpeinen M, Schaeffeler E, Schwab M, et al. (2009) Pharmacogenomics of human liver cytochrome P450 oxidoreductase: multifactorial analysis and impact on microsomal drug oxidation. Pharmacogenomics 10: 579–599. 10.2217/pgs.09.7 [DOI] [PubMed] [Google Scholar]
- 40. Hart SN, Wang S, Nakamoto K, Wesselman C, Li Y, Zhong XB (2008) Genetic polymorphisms in cytochrome P450 oxidoreductase influence microsomal P450-catalyzed drug metabolism. Pharmacogenet Genomics 18: 11–24. [DOI] [PubMed] [Google Scholar]
- 41. Huser V, Cimino JJ (2013) Providing pharmacogenomics clinical decision support using whole genome sequencing data as input. AMIA Jt Summits Transl Sci Proc 2013: 81 [PubMed] [Google Scholar]
- 42. Lee SY, McLeod HL (2011) Pharmacogenetic tests in cancer chemotherapy: what physicians should know for clinical application. J Pathol 223: 15–27. 10.1002/path.2766 [DOI] [PubMed] [Google Scholar]
- 43. Rotimi CN, Jorde LB (2010) Ancestry and disease in the age of genomic medicine. N Engl J Med 363: 1551–1558. 10.1056/NEJMra0911564 [DOI] [PubMed] [Google Scholar]
- 44. Squassina A, Manchia M, Manolopoulos VG, Artac M, Lappa-Manakou C, Karkabouna S, et al. (2010) Realities and expectations of pharmacogenomics and personalized medicine: impact of translating genetic knowledge into clinical practice. Pharmacogenomics 11: 1149–1167. 10.2217/pgs.10.97 [DOI] [PubMed] [Google Scholar]
- 45. Wikoff WR, Frye RF, Zhu H, Gong Y, Boyle S, Churchill E, et al. (2013) Pharmacometabolomics reveals racial differences in response to atenolol treatment. PLoS One 8: e57639 10.1371/journal.pone.0057639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klaunig JE, Wang Z, Pu X, Zhou S (2011) Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol 254: 86–99. 10.1016/j.taap.2009.11.028 [DOI] [PubMed] [Google Scholar]
- 47. Prat J, Ribe A, Gallardo A (2005) Hereditary ovarian cancer. Hum Pathol 36: 861–870. [DOI] [PubMed] [Google Scholar]
- 48. Petrucelli N, Daly MB, Feldman GL (2010) Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 12: 245–259. 10.1097/GIM.0b013e3181d38f2f [DOI] [PubMed] [Google Scholar]
- 49. Ramus SJ, Vierkant RA, Johnatty SE, Pike MC, Van Den Berg DJ, Wu AH, et al. (2008) Consortium analysis of 7 candidate SNPs for ovarian cancer. Int J Cancer 123: 380–388. 10.1002/ijc.23448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goode EL, Fridley BL, Vierkant RA, Cunningham JM, Phelan CM, Anderson S, et al. (2009) Candidate gene analysis using imputed genotypes: cell cycle single-nucleotide polymorphisms and ovarian cancer risk. Cancer Epidemiol Biomarkers Prev 18: 935–944. 10.1158/1055-9965.EPI-08-0860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Notaridou M, Quaye L, Dafou D, Jones C, Song H, Hogdall E, et al. (2011) Common alleles in candidate susceptibility genes associated with risk and development of epithelial ovarian cancer. Int J Cancer 128: 2063–2074. 10.1002/ijc.25554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Forsberg L, Lyrenas L, de Faire U, Morgenstern R (2001) A common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic Biol Med 30: 500–505. [DOI] [PubMed] [Google Scholar]
- 53. Quick SK, Shields PG, Nie J, Platek ME, McCann SE, Hutson AD, et al. (2008) Effect modification by catalase genotype suggests a role for oxidative stress in the association of hormone replacement therapy with postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 17: 1082–1087. 10.1158/1055-9965.EPI-07-2755 [DOI] [PubMed] [Google Scholar]
- 54. Cheng JC, Klausen C, Leung PC (2010) Hydrogen peroxide mediates EGF-induced down-regulation of E-cadherin expression via p38 MAPK and snail in human ovarian cancer cells. Mol Endocrinol 24: 1569–1580. 10.1210/me.2010-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng JC, Klausen C, Leung PC (2013) Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett 329: 197–206. 10.1016/j.canlet.2012.10.029 [DOI] [PubMed] [Google Scholar]
- 56. Huntsman DG, Caldas C (1998) Assignment1 of the E-cadherin gene (CDH1) to chromosome 16q22.1 by radiation hybrid mapping. Cytogenet Cell Genet 83: 82–83. [DOI] [PubMed] [Google Scholar]
- 57. Semb H, Christofori G (1998) The tumor-suppressor function of E-cadherin. Am J Hum Genet 63: 1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wong AS, Gumbiner BM (2003) Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol 161: 1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dally H, Gassner K, Jager B, Schmezer P, Spiegelhalder B, Edler L, et al. (2002) Myeloperoxidase (MPO) genotype and lung cancer histologic types: the MPO -463 A allele is associated with reduced risk for small cell lung cancer in smokers. Int J Cancer 102: 530–535. [DOI] [PubMed] [Google Scholar]
- 60. Izakovicova Holla L, Kankova K, Znojil V (2009) Haplotype analysis of the NADPH oxidase p22 phox gene in patients with bronchial asthma. Int Arch Allergy Immunol 148: 73–80. 10.1159/000151508 [DOI] [PubMed] [Google Scholar]
- 61. He C, Tamimi RM, Hankinson SE, Hunter DJ, Han J (2009) A prospective study of genetic polymorphism in MPO, antioxidant status, and breast cancer risk. Breast Cancer Res Treat 113: 585–594. 10.1007/s10549-008-9962-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11: 570–579. [DOI] [PubMed] [Google Scholar]
- 63. Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47: 207–214. [DOI] [PubMed] [Google Scholar]
- 64. Gill S, Sargent D (2006) End points for adjuvant therapy trials: has the time come to accept disease-free survival as a surrogate end point for overall survival? Oncologist 11: 624–629. [DOI] [PubMed] [Google Scholar]
- 65. Stewart DJ (2012) Before we throw out progression-free survival as a valid end point. J Clin Oncol 30: 3426–3427. 10.1200/JCO.2012.44.1220 [DOI] [PubMed] [Google Scholar]
- 66. Booth CM, Eisenhauer EA (2012) Progression-free survival: meaningful or simply measurable? J Clin Oncol 30: 1030–1033. 10.1200/JCO.2011.38.7571 [DOI] [PubMed] [Google Scholar]
- 67. Broglio KR, Berry DA (2009) Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 101: 1642–1649. 10.1093/jnci/djp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.