Abstract
Quantitative real-time PCR (RT-qPCR), a sensitive technique for quantifying gene expression, depends on the stability of the reference gene(s) used for data normalization. Several studies examining the selection of reference genes have been performed in ornamental plants but none in sweet osmanthus (Osmanthus fragrans Lour.). Based on transcriptomic sequencing data from O. fragrans buds at four developmental stages, six reference genes (OfACT, OfEF1α, OfIDH, OfRAN1, OfTUB, and OfUBC2) with stable expression (0.5 to 2 fold change in expression levels between any two developmental stages), as well as the commonly used reference gene Of18S, were selected as candidates for gene expression normalization in the RT-qPCR analysis of O. fragrans. For the normalization of RT-qPCR with two dyes, SYBR Green and EvaGreen, the expressional stability of seven candidate reference genes in 43 O. fragrans samples was analyzed using geNorm, NormFinder and BestKeeper. For RT-qPCR using SYBR Green, OfRAN1 and OfUBC2 were the optimal reference genes for all samples and different cultivars, OfACT and OfEF1α were suitable for different floral developmental stages, and OfACT was the optimal reference gene for different temperature treatments. The geometric mean values of the optimal reference gene pairs for the normalization of RT-qPCR are recommended to be used for all samples, different cultivars and different floral developmental stages in O. fragrans. For RT-qPCR using EvaGreen, OfUBC2 was the optimal reference gene for all samples and different cultivars, and OfACT was the optimal reference gene for different floral developmental stages and different temperature treatments. As the worst reference gene, Of18S should not be used as a reference gene in O. fragrans in the future. Our results provide a reference gene application guideline for O. fragrans gene expression characterization using RT-qPCR.
Introduction
As one of ten Chinese traditional flowers, Osmanthus fragrans Lour. is particularly appreciated in China for its aesthetic value, unique scent and cultural significance. O. fragrans cultivars have been divided into 4 groups, Asiaticus, Albus, Luteus, and Aurantiacus [1], according to their ornamental traits (flower color and flowering characteristics). Cultivars in the Asiaticus group flower not just in autumn, whereas cultivars in the other three groups only flower in autumn. These three groups differ substantially in petal color. Cultivars in the Albus group typically have butter-yellow flowers (Royal Horticultural Society Color Chart, RHSCC value of 1 to 8), those in the Luteus group typically show golden yellow flowers (RHSCC value of 9 to 20), and the Aurantiacus Group is characterized by orange/orange-red flowers (RHSCC value of 21 to 30). Previous studies have demonstrated that the petal coloration of O. fragrans is directly affected by carotenoid composition and content [2,3]. Hence, understanding the expression patterns of carotenoid-related genes in O. fragrans will help characterize the diverse carotenoid coloration in the flower petals of different O. fragrans cultivars. Moreover, petal color in this species is sensitive to ambient temperature, but how temperature regulates the petal coloration of O. fragrans remains unknown. Therefore, investigating the expression of carotenoid-related genes under different temperature conditions will help gain insight into the regulatory mechanisms of environmental factors in O. fragrans petal coloration.
There are several biological techniques for detecting the expression levels of genes, such as semi-quantitative PCR (semi-PCR), northern blotting, RNase protection assays (RPAs), gene chips, RNA sequencing and quantitative real-time RT-PCR (RT-qPCR). Of these techniques, RT-qPCR is presently regarded as the most reliable method because of its sensitivity, accuracy and high throughput [4–6]. Ideal reference genes (previously known as “housekeeping genes”) are needed as internal controls for normalization in RT-qPCR to quantify the expression level of a target gene. Generally involved in basic cellular processes, traditional reference genes, such as actin (ACT), beta-tubulin (TUB), elongation factor 1-alpha (EF1a), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ubiquitin (UBQ) and 18S ribosomal RNA (18S) have been widely used as internal controls for gene expression analysis in many plants [7–11]. However, several studies have shown that these traditional reference genes have a smaller variance of expression but are not stably expressed in all experimental conditions, revealing that no reference gene is universally stable [12–14]. Therefore, the identification and validation of potential reference genes in specific experimental conditions is necessary for target gene quantification.
Studies of optimal reference gene selection for gene expression normalization have been conducted in many ornamental plants, such as petunia [15], tree peony [16], Chrysanthemum lavandulifolium [17], cineraria [18], rose [19] and Prunus mume [10], in addition to model plants and important crops [20–23]. Nevertheless, little information, if any, is available concerning the selection of reference genes in O. fragrans. To date, Of18S is the most widely used reference gene in the semi-PCR and RT-qPCR analyses of O. fragrans [2,3,8]. However, some studies have demonstrated that the 18S gene performs poorly as a reference gene for RT-qPCR analyses in Salvia miltiorrhiza [24], tree peony [16], Chinese cabbage [11], and watermelon [25]. There is some doubt as to whether Of18S is a suitable reference gene in O. fragrans for gene expression normalization in different cultivars, in different floral development stages or under different temperature treatments. The use of inappropriate reference genes can result in inaccurate measurement of the expression levels of carotenoid-related genes, which may lead to incorrect conclusions with respect to diversified carotenoid coloration in different O. fragrans cultivars. Thus, the systematic exploration and validation of the most stable reference genes is important and requisite in O. fragrans.
In this study, in addition to Of18S, the commonly used reference gene in O. fragrans, six candidate reference genes with little variation in expression level in four bud transcriptomes were selected for further study: ACT, EF1α, NADP-isocitrate dehydrogenase (IDH), GTP-binding protein (RAN1), TUB and Ubiquitin-conjugating enzyme E2 (UBC2). We then compared the performance of these seven candidate reference genes in different cultivars, different floral developmental stages and under different temperature treatments using RT-qPCR with two dyes, SYBR Green and EvaGreen. Three algorithms, geNorm [26], NormFinder [27] and BestKeeper [28], were used to determine the most suitable reference gene(s) for the normalization of gene expression in O. fragrans.
Materials and Methods
Plant materials
The O. fragrans orange-red-flowered cultivar ‘Yanhong Gui’ was used for sample collection from different floral development stages and different temperature treatments. Plants were potted and grown in the resource nursery of Zhejiang Agriculture and Forestry University in Lin’an, Zhejiang Province, China. During bud development (from 9 Aug 2014 to 23 Sep 2014), bud samples of O. fragrans ‘Yanhong Gui’ were collected weekly, and floral samples (from 23 Sep Aug 2014 to 25 Sep 2014) were collected daily during flower opening. In total, 10 samples during bud development and flower opening constituted the experimental samples of different floral development stages. To collect samples under different temperature treatments, O. fragrans ‘Yanhong Gui’ plants with globular-shaped buds were treated at 12, 15, 19 and 32°C. To monitor floral development, flower petal samples under four temperature treatments were collected at four developmental stages (linggeng, half opening, full opening and initial senescence), as well as petal samples before the treatments, generating 17 petal samples under different temperature treatments. Additionally, petal samples were collected from the fully opened flowers of 16 cultivars, including ‘Hangzhou Huang’, ‘Jinqiu Gui’ and ‘Yuanban Jingui’ from the Luteus group; ‘Xiaoye Sugui’ and ‘Yu Linglong’ from the Albus group; ‘Chenghong Dangui’, ‘Mantiao Hong’, ‘Wuyi Dangui’, ‘Yanhong Gui’, ‘Yingye Dangui’, ‘Zhusha Dangui’ and ‘Zhuangyuan Hong’ from the Aurantiacus group; and ‘Chenghuang Siji Gui’, ‘Ri Xiang Gui’, ‘Tian Nv San Hua’ and ‘Tian Xiang Tai Ge’ from the Asiaticus group. In total, 43 experimental samples comprised 10 at various stages of floral development, 17 exposed to various temperature treatments, and 16 from different cultivars. All samples were immediately frozen in liquid nitrogen after collection and stored at -80°C until use.
RNA extraction, quality control and cDNA synthesis
Total RNA from all samples was extracted using the RNAprep Pure Plant Kit (Tiangen, China). All RNA samples were adjusted to the same concentration after measuring the RNA concentration on a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). The quality of the RNA was further verified using 1.5% (w/v) agarose gel electrophoresis and ethidium bromide staining. The first strand cDNA was synthesized using 1 μg of total RNA with the Reverse Transcriptase M-MLV (Takara, Japan) according to the manufacturer’s protocol.
Selection of candidate reference genes
In our preliminary study, four normalized cDNA libraries from O. fragrans ‘Yanhong Gui’ buds at four developmental stages were constructed and sequenced using the Illumina HiSeq2000 platform (unpublished data). A total of 184,860 unigenes were identified, and the relative expression levels of these unigenes were analyzed at four developmental stages. The level of gene expression was determined by calculating the number of unambiguous tags for each gene and then normalizing this to the number of transcripts per million tags (TPM). The difference in gene expression between the samples was determined using the TPM value. Six reference genes (OfACT, OfEF1α, OfIDH, OfRAN1, OfTUB, and OfUBC2) exhibiting stable expression (0.5–2 fold change in expression level) at four developmental stages (Table 1), as well as Of18S, the commonly used reference gene in O. fragrans [8], were selected as candidates for gene expression normalization in the quantitative real-time PCR analysis of O. fragrans.
Table 1. Expression of the candidate reference genes in the transcriptomic sequencing data.
| Gene name | Function | Expression (TPM) | Fold change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S2/S1 | S3/S2 | S3/S1 | S4/S3 | S4/S2 | S4/S1 | ||
| OfACT | Actin | 31.897 | 28.050 | 32.974 | 31.683 | 0.879 | 1.176 | 1.034 | 0.961 | 1.130 | 0.993 |
| OfEF1α | Elongation factor-1α | 99.761 | 73.916 | 87.638 | 74.726 | 0.741 | 1.186 | 0.878 | 0.853 | 1.011 | 0.749 |
| OfIDH | NADP-isocitrate dehydrogenase | 10.117 | 8.567 | 12.444 | 7.237 | 0.847 | 1.453 | 1.230 | 0.582 | 0.845 | 0.715 |
| OfRAN1 | GTP-binding protein RAN1 | 0.051 | 0.046 | 0.031 | 0.047 | 0.902 | 0.674 | 0.608 | 1.516 | 1.022 | 0.922 |
| OfTUB | Beta-tubulin | 0.984 | 1.013 | 1.007 | 1.029 | 1.029 | 0.994 | 1.023 | 1.022 | 1.016 | 1.046 |
| OfUBC2 | Ubiquitin-conjugating enzyme E2 | 1.159 | 1.534 | 0.829 | 1.384 | 1.324 | 0.540 | 0.715 | 1.669 | 0.902 | 1.194 |
PCR primer design and test of amplification efficiency
Besides Of18S, primers for other six candidate reference genes were designed using Primer Premier 5 with amplicon lengths of 75–143 bp (Table 2). Of18S expression analysis was performed with primers that have been widely used previously [2,8]. A gene specificity test for all primer sets was performed using RT-qPCR as previously described [29]. The efficiency of each primer set was evaluated by producing a standard curve using serial dilutions of a cDNA mixture from four developmental buds.
Table 2. Reference gene primer sequences and amplicon characteristics using SYBR Green or EvaGreen.
| Gene name | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) | Amplicon length (bp) | SYBR Green | EvaGreen | ||
|---|---|---|---|---|---|---|---|
| PCR efficiency (%) | Regression coefficient (R2) | PCR efficiency (%) | Regression coefficient (R2) | ||||
| OfACT | CCCAAGGCAAACAGAGAAAAAAT | ACCCCATCACCAGAATCAAGAA | 143 | 109.6 | 0.9984 | 102.2 | 0.9992 |
| OfEF1α | CGTTTGCCACTTCAGGATGTCTA | GTACCAGGTTTCAGGACTCCAGTTT | 89 | 97.7 | 0.9974 | 101.8 | 0.9972 |
| OfIDH | CTTGAAGCAGATGTGGAAGAGTC | CTTTGTCCATCCTGGGACCAGTC | 118 | 101.8 | 0.9952 | 94.6 | 0.9978 |
| OfRAN1 | AGAACCGACAGGTGAAGGCAA | TGGCAAGGTACAGAAAGGGCT | 117 | 100.4 | 0.9903 | 94.5 | 1.0000 |
| OfTUB | AGAAGGGATGGATGGAATGGA | GTCTTCTTCGTCCTCGGCAGT | 106 | 103.8 | 0.9981 | 97.8 | 0.9955 |
| OfUBC2 | TGTTGACAAAACCGATGGAAGGA | GTGGAGTGTGGAGGATAAGGGTG | 75 | 97.7 | 0.9948 | 92.8 | 0.9969 |
| Of18S | AGCCTGAGAAACGGCTACCAC | ATACGCTATTGGAGCTGGAA | 208 | 104.7 | 0.9926 | 106.0 | 1.0000 |
RT-qPCR
RT-qPCR was performed on an ABI 7300 real-time PCR instrument (AppliedBiosystems, Foster City, CA) using SYBR Green or EvaGreen dyes to detect dsDNA synthesis. For the RT-qPCR with SYBR Green, the reaction mixture (20 μL total volume) contained 10 μL of SYBR Premix Ex Taq II (TaKaRa, Japan), 0.8 μL of each primer (10 μM), 2 μL of diluted cDNA (~ 50 ng), 0.4 μL of 50× ROX Reference Dye and 6 μL of ddH2O. The PCR program was performed as follows: an initial denaturation at 95°C for 30 s, 40 cycles of 95°C for 5 s and 60°C for 31 s, and a melting curve analysis with a temperature ramp from 60°C to 95°C. For the RT-qPCR with EvaGreen, the reaction mixture (20 μL total volume) contained 10 μL of 2× HRM Analysis PreMix (with EvaGreen) (Tiangen, China), 0.6 μL of each primer (10 μM), 2 μL of diluted cDNA (~ 50 ng), 0.4 μL of 50× ROX Reference Dye and 6.4 μL of ddH2O. The PCR program was performed as follows: an initial denaturation at 95°C for 2 min, 40 cycles of 95°C for 5 s and 60°C for 30 s, and a melting curve analysis with a temperature ramp from 60°C to 95°C. No-template controls for each primer set were included in every reaction, and the real-time RT-PCR was performed in triplicate.
Reference gene expressional stability determination
The expression levels of the seven tested reference genes in all samples were determined using cycle threshold values (Ct). geNorm (version 3.5), NormFinder (version 0.953) and BestKeeper (version 1) [26–28] were used to analyze the expressional stability of the seven candidate reference genes in O. fragrans.
The geNorm software is a visual basic application (VBA) for determining the most stable reference genes from a set of tested genes by gene expressional stability measure (M). Stepwise exclusion of the gene with the highest M value allows the ranking of the tested genes according to their expression stabilities. Additionally, the pairwise variation (PV) between the sequential normalization factors was calculated to determine the optimal number of reference genes [26]. The NormFinder software used an ANOVA-based model to estimate intra- and inter-group variation and ranked the reference genes according to the stability of their expression patterns in a given sample set under certain experimental conditions [27]. BestKeeper was used to perform numerous pairwise correlation analyses using raw Ct values of each gene and assess reference gene expressional stability using the standard deviation (SD) and the coefficient of variance (CV) of the Ct values [28].
Results
Performance of the primers for each reference gene
Six reference genes, OfACT, OfEF1α, OfIDH, OfRAN1, OfTUB, and OfUBC2, were selected as candidate reference genes because they exhibited stable expression (0.5–2 fold change in expression level) during O. fragrans bud development (Table 1). In addition, the commonly used O. fragrans reference gene, Of18S was also selected as a candidate for gene expression normalization in the quantitative real-time PCR analysis of O. fragrans. A melting curve analysis of each primer set was performed using RT-qPCR after 40 cycles of amplification. The presence of a single peak indicated that the expected amplicons were amplified with SYBR Green and EvaGreen. The results of the agarose gel electrophoresis demonstrated that all seven primer pairs amplified a single band of the expected size from various cDNA templates. Using SYBR Green, the correlation coefficients (R2) ranged from 0.9903 to 0.9984, and PCR amplification efficiencies between 97.7 and 109.6% were obtained from the standard curves generated using a ten-fold serial dilution of cDNA. Using EvaGreen, R2 ranged in value from 0.9955 to 1.0000, and PCR amplification efficiencies ranged from 92.8 to 106.0%. These results indicated that each primer set was suitable for gene expression analysis with RT-qPCR using either SYBR Green or EvaGreen.
Reference gene expression levels
The seven candidate reference genes exhibited relatively wide ranges of Ct values using SYBR Green, from 10.09 to 28.37 in 43 tested sample pools, and the mean values of the seven genes were between 14.27 and 25.05 (Fig 1A). The mean values of these seven genes using EvaGreen were between 12.52 and 24.10 (Fig 1), which were generally lower than those using SYBR Green. Using either SYBR Green or EvaGreen, the least abundant transcripts were OfUBC2 and OfTUB with the highest mean Ct values, whereas Of18S exhibited the highest expression level with the lowest Cq value of all the samples. In addition, each candidate gene exhibited a specific Ct value variation tendency under the applied conditions. Using SYBR Green, OfRAN1 exhibited stable gene expression (below 6 cycles), whereas Of18S had obvious expression variation (above 8 cycles) as shown in Fig 1A. Similarly, OfRAN1 also exhibited stable gene expression (below 5 cycles), whereas Of18S had obvious expression variation (above 9 cycles) using EvaGreen (Fig 1B).
Fig 1. Expression profiles of seven candidate reference genes from 43 samples using SYBR Green (A) or EvaGreen (B).
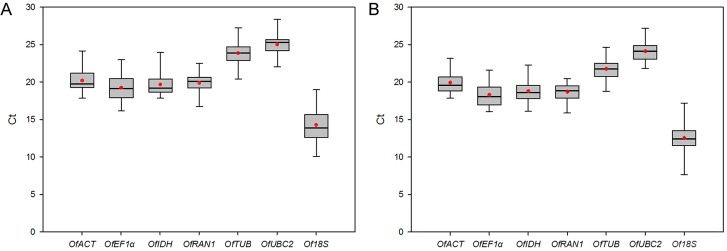
The expression data are displayed as Ct values for each reference gene in all samples. The red point is the mean, and the line across the box is the median. The boxes indicate the 25/75 percentiles. The whisker caps indicate the minimum and maximum values.
geNorm analysis
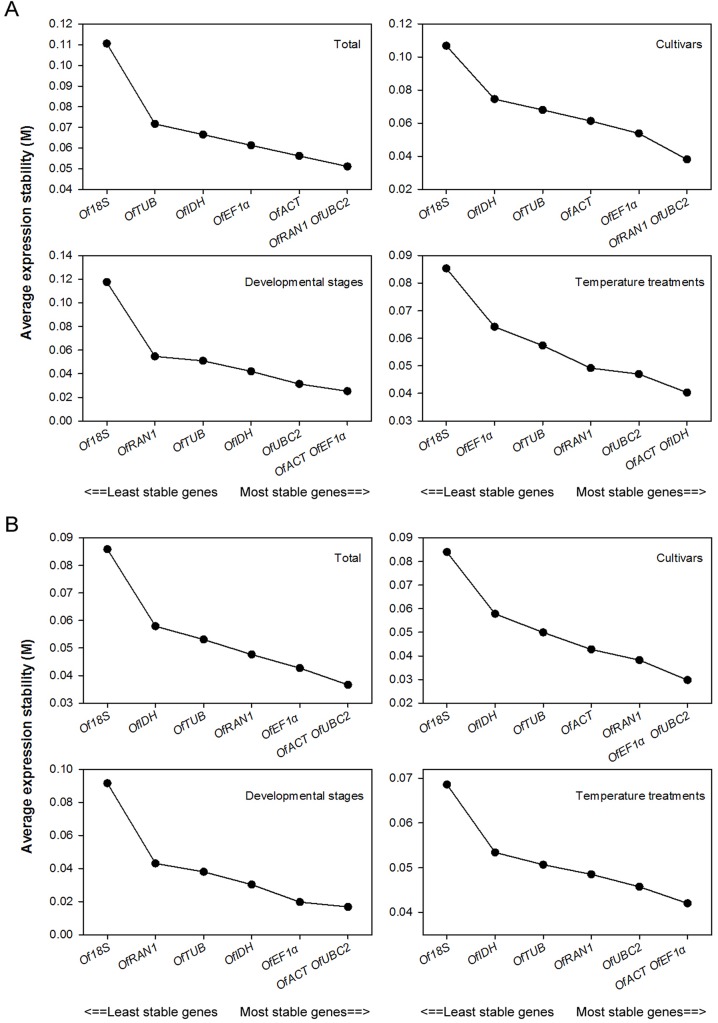
Before analyzing gene expressional stability, 43 samples were divided into four sample sets: cultivars (16 samples), developmental stages (10 samples), temperature treatments (17 samples), and total (43 samples). We first used geNorm to analyze the expressional stability of the seven candidate reference genes in all the samples and ranked them according to the gene stability index (M): the genes with the lowest M values have the most stable expression (Fig 2). An M value below a threshold of 1.5 is recommended to identify reference genes with stable expression [26]. In this study, the M values of all the reference genes in the four sample sets were much lower than 1.5 (Fig 2).
Fig 2. Expressional stability values (M) of seven candidate reference genes in four sample sets using SYBR Green (A) or EvaGreen (B) generated by the geNorm software.
Average expressional stability values (M) following stepwise exclusion of the least stable gene across all experimental sets. The least stable genes are on the left, and the most stable genes are on the right.
For all 43 samples, OfRAN1 and OfUBC2 were the most stably expressed genes with the lowest M value of 0.051 using SYBR Green (Fig 2A); OfACT and OfUBC2 were the most stably expressed genes with the lowest M value using EvaGreen (Fig 2B). Similarly, OfRAN1 and OfUBC2 were also the most stably expressed genes in different cultivars using SYBR Green (Fig 2A). Using EvaGreen, OfEF1α and OfUBC2 were the most stably expressed genes in different cultivars (Fig 2B). For different floral developmental stages, OfACT and OfEF1α were the most stable with an M value of 0.025 using SYBR Green, whereas OfACT and OfUBC2 were the most stable using EvaGreen (Fig 2). For different temperature treatments, OfACT and OfIDH were the most stable reference genes using SYBR Green, and OfACT and OfEF1α were the most stable using EvaGreen (Fig 2). Using either SYBR Green or EvaGreen, Of18S was the least stable gene with the highest M value in all four sample sets (Fig 2).
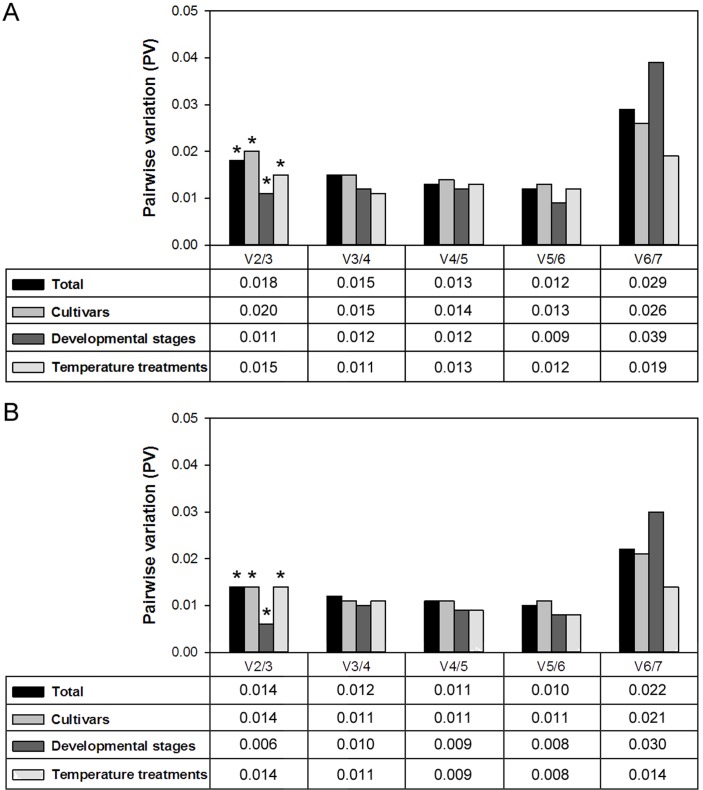
The pairwise variation (PV) was also calculated to determine the optimal number of genes required for normalization. The Vn/Vn+1 value was lower than 0.15 for all four sample sets using either SYBR Green or EvaGreen (Fig 3), indicating that adding an extra gene to obtain a reliable normalization factor was not necessary in our study. Therefore, two reference genes were necessary and sufficient for gene expression normalization in all sets of samples; i.e., using SYBR Green, the combination of OfRAN1 and OfUBC2 was appropriate for all samples and different cultivars, the combination of OfACT and OfEF1α was appropriate for different floral developmental stages, and the combination of OfACT and OfIDH was appropriate for different temperature treatments. Using EvaGreen, the combination of OfACT and OfUBC2 was appropriate for all samples and different floral developmental stages, the combination of OfEF1α and OfUBC2 was appropriate for different cultivars, and the combination of OfACT and OfEF1αwas appropriate for different temperature treatments.
Fig 3. Pairwise variation (PV) analysis of seven candidate genes in four sample sets using SYBR Green (A) or EvaGreen (B).
Asterisk indicates the optimal number of reference genes for four sample sets.
NormFinder analysis
Expression stability was then re-analyzed using the program NormFinder, which is based on a variance estimation approach [27] and ranks the genes according to their stability under a given set of experimental conditions. Using SYBR Green, the ranking generated by this approach was similar to that determined by geNorm because the four most stable genes and the three least stable genes in all sample sets ranked by NormFinder (Table 3) were the same as those generated by geNorm (Fig 2A). According to the results ranked by NormFinder, OfUBC2 was the most stable gene for all samples and different cultivars (Table 3). OfACT and OfEF1α were still the most stable genes for different floral developmental stages, whereas OfACT was ranked the highest for different temperature treatments using SYBR Green (Table 3). Generally, the rankings using EvaGreen were similar to those using SYBR Green (Table 3). Using EvaGreen, OfUBC2 was the most stable gene for all samples and different cultivars, and OfACT was the most stable gene for different floral developmental stages and different temperature treatments. In addition, using either SYBR Green or EvaGreen, Of18S was ranked the lowest in all sample sets (Table 3) by NormFinder, which was in agreement with the ranking of Of18S calculated by geNorm.
Table 3. Expressional stability analysis of seven candidate reference genes using NormFinder in four sample sets with SYBR Green or EvaGreen.
| Rank | Total | Cultivars | Developmental stages | Temperature treatments | ||||
|---|---|---|---|---|---|---|---|---|
| Gene name | Stability value | Gene name | Stability value | Gene name | Stability value | Gene name | Stability value | |
| SYBR Green | ||||||||
| 1 | OfUBC2 | 0.010 | OfUBC2 | 0.013 | OfACT、OfEF1α | 0.009 | OfACT | 0.014 |
| 2 | OfACT | 0.013 | OfEF1α | 0.023 | OfUBC2 | 0.011 | OfUBC2 | 0.020 |
| 3 | OfRAN1 | 0.016 | OfRAN1 | 0.029 | OfIDH | 0.040 | OfRAN1 | 0.023 |
| 4 | OfEF1α | 0.025 | OfACT | 0.045 | OfRAN1 | 0.043 | OfIDH | 0.032 |
| 5 | OfIDH | 0.031 | OfTUB | 0.047 | OfTUB | 0.052 | OfEF1α | 0.043 |
| 6 | OfTUB | 0.036 | OfIDH | 0.056 | Of18S | 0.189 | OfTUB | 0.047 |
| 7 | Of18S | 0.059 | Of18S | 0.125 | —— | —— | Of18S | 0.090 |
| EvaGreen | ||||||||
| 1 | OfUBC2 | 0.013 | OfUBC2 | 0.007 | OfACT | 0.008 | OfACT | 0.016 |
| 2 | OfACT | 0.016 | OfEF1α | 0.018 | OfUBC2 | 0.008 | OfRAN1 | 0.024 |
| 3 | OfEF1α | 0.022 | OfACT | 0.026 | OfEF1α | 0.009 | OfUBC2 | 0.025 |
| 4 | OfRAN1 | 0.029 | OfRAN1 | 0.031 | OfIDH | 0.049 | OfEF1α | 0.026 |
| 5 | OfTUB | 0.038 | OfTUB | 0.036 | OfTUB | 0.050 | OfTUB | 0.030 |
| 6 | OfIDH | 0.043 | OfIDH | 0.044 | OfRAN1 | 0.056 | OfIDH | 0.035 |
| 7 | Of18S | 0.104 | Of18S | 0.099 | Of18S | 0.230 | Of18S | 0.069 |
BestKeeper analysis
BestKeeper, another popular analysis method, was also applied for reference gene expression analysis in this study. Genes with an SD greater than 1 are considered inconsistent; reference genes exhibiting the lowest SD are the most stable genes. Therefore, OfRAN1 was the most stable gene for all samples and different cultivars using SYBR Green (Table 4), which is in agreement with the results ranked by geNorm but differs from those determined by NormFinder. Using EvaGreen, the rankings generated by BestKeeper were similar to those using SYBR Green for all samples and different cultivars (Table 4). However, for different floral developmental stages and different temperature treatments, the rankings using EvaGreen differed greatly from those using SYBR Green (Table 4). Using SYBR Green, OfIDH was the most stable gene for different floral developmental stages, whereas OfRAN1 was ranked the highest for different temperature treatments (Table 4). However, using EvaGreen, OfEF1α was ranked the highest for different floral developmental stages, and OfTUB was the most stable gene for different temperature treatments (Table 4). For different floral developmental stages and different temperature treatments using either SYBR Green or EvaGreen, the rankings generated by BestKeeper were quite different from the results determined by geNorm and NormFinder.
Table 4. Expressional stability analysis of seven candidate reference genes using BestKeeper in four sample sets with SYBR Green or EvaGreen.
| Rank | Total | Cultivars | Developmental stages | Temperature treatments | ||||
|---|---|---|---|---|---|---|---|---|
| Gene name | Stability value | Gene name | Stability value | Gene name | Stability value | Gene name | Stability value | |
| SYBR Green | ||||||||
| 1 | OfRAN1 | 0.906 | OfRAN1 | 0.548 | OfIDH | 0.383 | OfRAN1 | 0.594 |
| 2 | OfTUB | 1.100 | OfUBC2 | 0.931 | OfACT | 0.536 | OfUBC2 | 0.648 |
| 3 | OfUBC2 | 1.122 | OfTUB | 1.011 | OfEF1α | 0.550 | OfACT | 0.805 |
| 4 | OfIDH | 1.125 | OfEF1α | 1.121 | OfUBC2 | 0.654 | OfIDH | 0.888 |
| 5 | OfACT | 1.176 | Of18S | 1.394 | OfRAN1 | 0.857 | OfTUB | 0.914 |
| 6 | OfEF1α | 1.396 | OfACT | 1.418 | OfTUB | 0.872 | Of18S | 0.972 |
| 7 | Of18S | 1.766 | OfIDH | 1.563 | Of18S | 2.071 | OfEF1α | 1.225 |
| EvaGreen | ||||||||
| 1 | OfRAN1 | 0.86 | OfRAN1 | 0.59 | OfEF1α | 0.21 | OfTUB | 0.67 |
| 2 | OfTUB | 1.00 | OfUBC2 | 0.99 | OfACT | 0.34 | OfUBC2 | 0.72 |
| 3 | OfUBC2 | 1.03 | OfEF1α | 1.02 | OfIDH | 0.43 | OfRAN1 | 0.79 |
| 4 | OfACT | 1.08 | OfTUB | 1.19 | OfUBC2 | 0.48 | OfACT | 0.83 |
| 5 | OfIDH | 1.21 | OfACT | 1.28 | OfTUB | 0.56 | Of18S | 0.93 |
| 6 | OfEF1α | 1.21 | Of18S | 1.48 | OfRAN1 | 0.64 | OfEF1α | 1.04 |
| 7 | Of18S | 1.50 | OfIDH | 1.66 | Of18S | 2.03 | OfIDH | 1.14 |
With respect to the worst reference gene, Of18S was ranked the lowest for all samples and different floral developmental stages using either SYBR Green or EvaGreen (Table 4), which was consistent with the rankings calculated by geNorm and NormFinder. However, BestKeeper indicated that Of18S was not the worst reference gene for different cultivars and different temperature treatments, which differs from the results calculated by geNorm and NormFinder (Table 4).
Overall analysis of the optimal and worst reference genes
The different software tools used to analyze the gene expressional stability in our study, generated different results and different statistical stability values for each gene. The inconsistencies between these three methods were expected because they are based on distinct statistical algorithms. Therefore, the optimal and worst reference genes for each sample set were generated with the aggregated results calculated by geNorm, NormFinder and BestKeeper in our study (Table 5). Using SYBR Green, OfRAN1 and OfUBC2 were the optimal reference genes for all samples and different cultivars, OfACT and OfEF1α were the optimal reference genes for different floral developmental stages, and OfACT was the optimal reference gene for different temperature treatments (Table 5). To reduce variation and improve normalization [26], the geometric mean of the optimal reference gene pairs is recommended to be used for the gene expression normalization of RT-qPCR using SYBR Green for all samples, different cultivars and different floral developmental stages in O. fragrans. Using EvaGreen, OfUBC2 was the optimal reference gene for all samples and different cultivars, and OfACT was the optimal reference gene for different floral developmental stages and different temperature treatments (Table 5). In addition, Of18S was the worst reference gene for each sample set using either SYBR Green or EvaGreen (Table 5).
Table 5. Optimal and worst reference genes in four sample sets using three methods.
| Sample sets | Optimal reference gene | Worst reference gene | ||||||
|---|---|---|---|---|---|---|---|---|
| geNorm | NormFinder | BestKeeper | Aggregated result | geNorm | NormFinder | BestKeeper | Aggregated result | |
| SYBR Green | ||||||||
| Total | OfRAN1, OfUBC2 | OfUBC2 | OfRAN1 | OfRAN1, OfUBC2 | Of18S | Of18S | Of18S | Of18S |
| Cultivars | OfRAN1, OfUBC2 | OfUBC2 | OfRAN1 | OfRAN1, OfUBC2 | Of18S | Of18S | OfIDH | Of18S |
| Developmental stages | OfACT, OfEF1α | OfACT, OfEF1α | OfIDH | OfACT, OfEF1α | Of18S | Of18S | Of18S | Of18S |
| Temperature treatments | OfACT, OfIDH | OfACT | OfRAN1 | OfACT | Of18S | Of18S | OfEF1α | Of18S |
| EvaGreen | ||||||||
| Total | OfACT, OfUBC2 | OfUBC2 | OfRAN1 | OfUBC2 | Of18S | Of18S | Of18S | Of18S |
| Cultivars | OfEF1α, OfUBC2 | OfUBC2 | OfRAN1 | OfUBC2 | Of18S | Of18S | OfIDH | Of18S |
| Developmental stages | OfACT, OfUBC2 | OfACT | OfEF1α | OfACT | Of18S | Of18S | Of18S | Of18S |
| Temperature treatments | OfACT, OfEF1α | OfACT | OfTUB | OfACT | Of18S | Of18S | OfIDH | Of18S |
Discussion
RT-qPCR has emerged as a powerful tool for gene expression analysis, particularly with respect to sensitivity and specificity [30]. Regardless of experimental conditions, the quantitative accuracy of RT-qPCR strongly depends on stably expressed reference genes. However, no one gene has a constant expression profile under all developmental or experimental conditions [30]. A systematic verification of the most suitable reference genes for specific experimental conditions is extremely important for gene expression studies using RT-qPCR in O. fragrans. Our study compared and analyzed the stability of seven candidate reference genes in four experimental sets, which is the first systematic study of reference gene expressional stability in O. fragrans. Out of the wide variety of commercially available fluorescent DNA dyes, SYBR Green remains the most widely used DNA dye for RT-qPCR applications despite numerous studies demonstrating that it inhibits PCR in a concentration-dependent manner and affects the DNA melting temperature [31–33]. The EvaGreen dye is marketed as a desirable alternative to SYBR Green because EvaGreen is less inhibitory to PCR and produces sharper peaks in melt curve analyses than SYBR Green [34,35]. Although the gene stability rankings generated by each method were not identical, the four most stable genes and the three least stable genes analyzed using geNorm, NormFinder and BestKeeper were identical in all sample sets. According to the aggregated results in our study (Table 5), the optimal reference gene for RT-qPCR using EvaGreen in each sample set was consistent with that for using SYBR Green because OfACT was the optimal reference gene for different temperature treatments using either SYBR Green or EvaGreen, and the optimal reference gene using EvaGreen for the other sample sets was one of the optimal reference genes using SYBR Green, suggesting that the usage of different dyes does not greatly affect the validation of the most stable reference gene.
RAN, an evolutionarily conserved small G-protein family protein, is essential for nuclear transport, nuclear assembly, mRNA processing, and cell cycle control [36–38]. RAN3, a homologue of the RAN gene from Antirrhinum majus, is commonly used as the reference gene for RT-qPCR in this species [39,40]. In our study, OfRAN1 was recommended as a suitable reference gene for all samples and different cultivars using SYBR Green, suggesting that OfRAN1 may be used as a reference gene for the normalization of target genes with RT-qPCR using SYBR Green among different O. fragrans cultivars and in complicated experimental sets. However, OfRAN1 performed poorly during floral development in O. fragrans because, using either SYBR Green or EvaGreen, the expressional stability of this gene was close to the bottom of the ranking order generated by the three statistical methods. In petunia, as validated using qBasePlus and geNorm, RAN1 was regarded as one of the most stably expressed genes during flower development [15], which was not consistent with the results in our study. The inconsistent expressional stability of RAN1 is probably related to different sampling. In addition to flower opening, samples during bud development were also included in our study; however, only samples from the four flower-opening stages were included in the petunia study [15].
OfUBC2 was a suitable reference gene for all samples and different cultivars using either SYBR Green or EvaGreen in this study. OfUBC2 was also stably expressed in different floral developmental stages and different temperature treatments, as shown by the geNorm, NormFinder and BestKeeper analyses. OfUBC2 is a homologue of the UBC gene, a classical traditional reference gene. UBC genes from other species also show stable expression in most experimental sets. For example, in hybrid roses, UBC was stably expressed in a whole dataset and in different tissues [41]. Moreover, in Platycladus orientalis, UBC was also top-ranked in all developmental stages and under all stress conditions [42]. In Arabidopsis thaliana, the UBC gene (at5g25760), is widely employed as the internal control for the normalization of target gene expression under cold treatment [43,44], and this gene has been validated as the only traditional reference gene out of 14 suitable Arabidopsis reference genes [44]. Interestingly, this gene was also stably expressed in the set tested and validated by Czechowski et al. [20]. However, UBC (at5g25760) is not always stably expressed in Arabidopsis; the expressional stability of this gene was low in Arabidopsis exposed to cadmium and copper treatments [45].
The traditional reference gene ACT is involved in basic cellular processes and has always been considered a potential reference gene in numerous species. In our study, OfACT was the optimal reference gene for different floral developmental stages. In many species, ACT has also been demonstrated to be stably expressed during the development of specific tissues. For example, ACT was stably expressed in the developmental series of soybean [46], during the flower development of petunia ‘V30’ [15], in different fruit developmental stages of Litchi chinensis [47] and in the different flower developmental stages of four different color lines in cineraria [18]. Moreover, ACT was also stably expressed under various abiotic stresses, including different temperatures, different hormones and wounding [19,42,48]. As in the present study, OfACT was also the optimal reference gene for different temperature treatments in O. fragrans.
For different floral developmental stages, OfEF-1a was the other optimal reference gene using SYBR Green and also performed well with EvaGreen. EF-1a is regarded as one of the most stable genes in the different fruit developmental stages of L. chinensis [47]. Moreover, EF-1a is also the most stably expressed gene in the six fruit developmental stages of Litsea cubeba [49] and in the whole dataset of the flower and leaf development of petunia ‘Mitchell’ [15].
The widely used reference gene Of18S had the most obvious expression variation in Ct values in the 43 tested samples (Fig 1). As expected, Of18S was ranked the last and regarded as the worst reference gene in all sample sets using either SYBR Green or EvaGreen. Moreover, Of18S exhibited a significantly higher expression level with the lowest Cq value of all genes tested, suggesting that this gene is unsuitable for the normalization of target genes with middle or low expression levels. Similarly, in Salvia miltiorrhiza [24], tree peony [16], Chinese cabbage [11], and watermelon [25], the 18S gene was also found to perform poorly as a reference gene.
Conclusions
In this study, we investigated the expressional stability of seven candidate reference genes for the normalization of RT-qPCR in different sample sets of O. fragrans using geNorm, NormFinder and BestKeeper. For RT-qPCR using SYBR Green, OfRAN1 and OfUBC2 were the optimal reference genes for all samples and different cultivars, OfACT and OfEF1α were the optimal reference genes for different floral developmental stages, and OfACT was the optimal reference gene for different temperature treatments. The geometric mean values of the optimal reference gene pairs are recommended to be used for all samples, different cultivars and different floral developmental stages. For RT-qPCR using EvaGreen, OfUBC2 was the optimal reference gene for all samples and different cultivars, and OfACT was the optimal reference gene for different floral developmental stages and different temperature treatments. The use of Of18S as a reference gene should be avoided in O. fragrans. To our knowledge, our study is the first systematic characterization of the expressional stability of reference genes in O. fragrans.
Acknowledgments
The authors would like to thank Yun Luo and Ting Lu for assistance with experimental samplings.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by the National Natural Science Foundation of China (Grant No. 31170656 and 31101571)(http://www.nsfc.gov.cn/), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ15C160004)(http://www.zjnsf.gov.cn/), Zhejiang Provincial Major Program of New Cultivar Breeding (Grant No. 2012C12909-9 and 2012C12909-19) and Open Foundation of Top Key Discipline of Forestry, Zhejiang Province (KF201321 and KF201324).
References
- 1. Xiang Q, Liu Y. An illustrated monograph of the sweet osmanthus variety in China Zhejiang Science & Technology Press; 2007. [Google Scholar]
- 2. Han Y, Wang X, Chen W, Dong M, Yuan W, Liu X, et al. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans . Tree Genet Genomes. 2014; 10: 329–338. [Google Scholar]
- 3. Han Y, Li L, Dong M, Yuan W, Shang F. cDNA cloning of the phytoene synthase (PSY) and expression analysis of PSY and carotenoid cleavage dioxygenase genes in Osmanthus fragrans . Biologia. 2013; 68: 258–263. [Google Scholar]
- 4. Bustin S. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002; 29: 23–39. [DOI] [PubMed] [Google Scholar]
- 5. Bustin S, Benes V, Nolan T, Pfaffl M. Quantitative real-time RT-PCR—a perspective. J Mol Endocrinol. 2005; 34: 597–601. [DOI] [PubMed] [Google Scholar]
- 6. Gachon C, Mingam A, Charrier B. Real-time PCR: what relevance to plant studies? J Exp Bot. 2004; 55: 1445–1454. [DOI] [PubMed] [Google Scholar]
- 7. Zhang C, Fu J, Wang Y, Gao S, Du D, Wu F, et al. Glucose supply improves petal coloration and anthocyanin biosynthesis in Paeonia suffruticosa ‘Luoyang Hong’cut flowers. Postharvest Biol Technol. 2015; 101: 73–81. [Google Scholar]
- 8. Baldermann S, Kato M, Kurosawa M, Kurobayashi Y, Fujita A, Fleischmann P, et al. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J Exp Bot. 2010; 61: 2967–2977. 10.1093/jxb/erq123 [DOI] [PubMed] [Google Scholar]
- 9. Wei LB, Miao HM, Zhao RH, Han XH, Zhang TD, Zhang H. Identification and testing of reference genes for Sesame gene expression analysis by quantitative real-time PCR. Planta. 2013; 237: 873–889. 10.1007/s00425-012-1805-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang T, Hao RJ, Pan HT, Cheng TR, Zhang QX. Selection of suitable reference genes for quantitative real-time polymerase chain reaction in Prunus mume during flowering stages and under different abiotic stress conditions. J Am Soc Hortic Sci. 2014; 139: 113–122. [Google Scholar]
- 11. Xu X, Yang Z, Sun X, Zhang L, Fang Z. Selection of reference genes for quantitative real-time PCR during flower bud development in CMS7311 of heading Chinese cabbage (Brassica rapa L. ssp. pekinensis). Acta Physiol Plant. 2014; 36: 809–814. [Google Scholar]
- 12. Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999; 75: 291–295. [DOI] [PubMed] [Google Scholar]
- 13. Thellin O, ElMoualij B, Heinen E, Zorzi W. A decade of improvements in quantification of gene expression and internal standard selection. Biotechnol Adv 2009; 27: 323–333. [DOI] [PubMed] [Google Scholar]
- 14. Guénin S, Mauriat M, Pelloux J, Van Wuytswinkel O, Bellini C, Gutierrez L. Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot. 2009; 60: 487–493. 10.1093/jxb/ern305 [DOI] [PubMed] [Google Scholar]
- 15. Mallona I, Lischewski S, Weiss J, Hause B, Egea-Cortines M. Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida . BMC Plant Biol. 2010; 10: 4 10.1186/1471-2229-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Dong L, Zhang C, Wang X. Reference gene selection for real-time quantitative PCR normalization in tree peony (Paeonia suffruticosa Andr.). J Agr Biotechnol. 2012; 20: 521–528. [Google Scholar]
- 17. Fu JX, Wang Y, Huang H, Zhang C, Dai SL. Reference gene selection for RT-qPCR analysis of Chrysanthemum lavandulifolium during its flowering stages. Mol Breeding. 2013; 31: 205–215. [Google Scholar]
- 18. Jin X, Fu J, Dai S, Sun Y, Hong Y. Reference gene selection for qPCR analysis in cineraria developing flowers. Sci Hortic-Amsterdam. 2013; 153: 64–70. [Google Scholar]
- 19. Meng Y, Li N, Tian J, Gao J, Zhang C. Identification and validation of reference genes for gene expression studies in postharvest rose flower (Rosa hybrida). Sci Hortic-Amsterdam. 2013; 158: 16–21. [Google Scholar]
- 20. Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis . Plant Physiol. 2005; 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu R, Fan C, Li H, Zhang Q, Fu Y-F. Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol Biol. 2009; 10: 93 10.1186/1471-2199-10-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reid KE, Olsson N, Schlosser J, Peng F, Lund ST. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006; 6: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Bioph Res Co. 2006; 345: 646–651. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Hou S, Cui G, Chen S, Wei J, Huang L. Characterization of reference genes for quantitative real-time PCR analysis in various tissues of Salvia miltiorrhiza . Mol Biol Rep. 2010; 37: 507–513. 10.1007/s11033-009-9703-3 [DOI] [PubMed] [Google Scholar]
- 25. Kong Q, Yuan J, Gao L, Zhao S, Jiang W, Huang Y, et al. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS One. 2014; 9: e90612 10.1371/journal.pone.0090612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002; 3: research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004; 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 28. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol lett. 2004; 26: 509–515. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Zhang C, Jia P, Wang X, Wang W, Dong L. Isolation and expression analysis of three EIN3-like genes in tree peony (Paeonia suffruticosa). Plant Cell Tiss Org. 2013; 112: 181–190. [Google Scholar]
- 30. Artico S, Nardeli SM, Brilhante O, Grossi-de-Sa MF, Alves-Ferreira M. Identification and evaluation of new reference genes in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC Plant Biol. 2010; 10: 49 10.1186/1471-2229-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ririe KM, Rasmussen RP, Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997; 245: 154–160. [DOI] [PubMed] [Google Scholar]
- 32. Giglio S, Monis PT, Saint CP. Demonstration of preferential binding of SYBR Green I to specific DNA fragments in real-time multiplex PCR. Nucleic Acids Res. 2003; 31: e136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nath K, Sarosy JW, Hahn J, Di Como CJ. Effects of ethidium bromide and SYBR Green I on different polymerase chain reaction systems. J Biochem Biophys Methods. 2000; 42: 15–29. [DOI] [PubMed] [Google Scholar]
- 34. Mao F, Leung WY, Xin X. Characterization of EvaGreen and the implication of its physicochemical properties for qPCR applications. BMC Biotechnol. 2007; 7: 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eischeid AC. SYTO dyes and EvaGreen outperform SYBR Green in real-time PCR. BMC Res Notes. 2011; 4: 263 10.1186/1756-0500-4-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ciciarello M, Mangiacasale R, Lavia P. Spatial control of mitosis by the GTPase Ran. Cell Mol Life Sci. 2007; 64: 1891–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fiore BD, Ciciarello M, Lavia P. Mitotic functions of the Ran GTPase network: the importance of being in the right place at the right time. Cell Cycle. 2004; 3: 303–311. [PubMed] [Google Scholar]
- 38. Xu PP, Cai WM. RAN1 is involved in plant cold resistance and development in rice (Oryza sativa). J Exp Bot. 2014; 65: 3277–3287. 10.1093/jxb/eru178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delgado-Benarroch L, Causier B, Weiss J, Egea-Cortines M. FORMOSA controls cell division and expansion during floral development in Antirrhinum majus . Planta. 2009; 229: 1219–1229. 10.1007/s00425-009-0910-x [DOI] [PubMed] [Google Scholar]
- 40. Bey M, Stüber K, Fellenberg K, Schwarz-Sommer Z, Sommer H, Saedler H, et al. Characterization of Antirrhinum petal development and identification of target genes of the class B MADS box gene DEFICIENS. Plant Cell. 2004; 16: 3197–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klie M, Debener T. Identification of superior reference genes for data normalisation of expression studies via quantitative PCR in hybrid roses (Rosa hybrida). BMC Res Notes. 2011; 4: 518 10.1186/1756-0500-4-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang E, Shi S, Liu J, Cheng T, Xue L, Yang X, et al. Selection of reference genes for quantitative gene expression studies in Platycladus orientalis (Cupressaceae) using real-time PCR. PLoS one. 2012; 7: e33278 10.1371/journal.pone.0033278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swiezewski S, Liu F, Magusin A, Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009; 462: 799–802. 10.1038/nature08618 [DOI] [PubMed] [Google Scholar]
- 44. Dekkers BJ, Willems L, Bassel GW, van Bolderen-Veldkamp RM, Ligterink W, Hilhorst HW, et al. Identification of reference genes for RT–qPCR expression analysis in Arabidopsis and tomato seeds. Plant Cell Physiol. 2012; 53: 28–37. 10.1093/pcp/pcr113 [DOI] [PubMed] [Google Scholar]
- 45. Remans T, Smeets K, Opdenakker K, Mathijsen D, Vangronsveld J, Cuypers A. Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta. 2008; 227: 1343–1349. 10.1007/s00425-008-0706-4 [DOI] [PubMed] [Google Scholar]
- 46. Jian B, Liu B, Bi Y, Hou W, Wu C, Han T. Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol Biol. 2008; 9: 59 10.1186/1471-2199-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhong HY, Chen JW, Li CQ, Chen L, Wu JY, Chen JY, et al. Selection of reliable reference genes for expression studies by reverse transcription quantitative real-time PCR in litchi under different experimental conditions. Plant Cell Rep. 2011; 30: 641–653. 10.1007/s00299-010-0992-8 [DOI] [PubMed] [Google Scholar]
- 48. Schmidt GW, Delaney SK. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics. 2010; 283: 233–241. 10.1007/s00438-010-0511-1 [DOI] [PubMed] [Google Scholar]
- 49. Lin L, Han X, Chen Y, Wu Q, Wang Y. Identification of appropriate reference genes for normalizing transcript expression by quantitative real-time PCR in Litsea cubeba . Mol Genet Genomics. 2013; 288: 727–737. 10.1007/s00438-013-0785-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.