Abstract
Objective:
Two surveys (Harris and Rose surveys) were conducted to quantify the use of compounded hormone therapy (CHT; or bioidentical hormone therapy) among perimenopausal and postmenopausal women in the United States, to assess women's knowledge of CHT versus Food and Drug Administration (FDA)–approved hormone therapy, and to gather information on menopausal experience.
Methods:
The Harris survey was administered to 801 women aged 45 to 60 years who had experienced at least one menopausal symptom. The Rose survey was administered to 2,044 women aged 40 years or older who were ever users of hormone therapy. Women were queried about menopausal symptoms, hormone therapy use, and knowledge of CHT. Findings from the Rose survey were extrapolated using US Census Bureau data and prescription claims for FDA-approved hormone therapy to estimate the prevalence of CHT use.
Results:
According to extrapolations using Rose data, up to 2.5 million US women aged 40 years or older may use CHT annually, accounting for 28% to 68% of hormone therapy prescriptions. Harris data showed that 86% of women surveyed were unaware that CHT products are not FDA-approved. The Rose survey asked a subset of 1,771 women whether their hormone therapy had been personalized based on hormone levels; 21% (378) answered “yes” whereas 27% (476) did not know. In both surveys, most hormone therapy users stated that their physician had recommended the treatment.
Conclusions:
We estimate that 1 million to 2.5 million US women aged 40 years or older use CHT. The data suggest that many women are unaware that compounded hormones have not been evaluated or approved by the FDA. Providers have an educational opportunity to ensure that women considering hormone therapy understand the risks and benefits of inadequately regulated CHT.
Keywords: Compounded hormone therapy, Menopause, Bioidentical, Vasomotor symptoms, Hot flashes
Hormone therapy (HT) effectively treats moderate to severe vasomotor symptoms (VMS) and symptomatic vaginal atrophy and prevents postmenopausal osteoporosis in women transitioning through menopause.1 Although the use of commercially manufactured HT to treat menopausal symptoms has declined during the past 12 years in response to the now well-known safety findings of the Women's Health Initiative trials (primarily for women aged 60 y or older),2-8 use of custom compounded hormone therapy (CHT) seems to have increased.9-12 This seemingly paradoxical increase in CHT use suggests that postmenopausal women do not apply their concerns about the class effects of estrogens and progestogens identified in the Women's Health Initiative to CHT products.
Another driving force behind the use of CHT seems to be the absence of regulation governing product advertising, which allows purveyors of CHT to make unsubstantiated claims about its safety and efficacy.12,13 In addition, high-profile celebrities such as Oprah Winfrey and Suzanne Somers have promoted CHT to postmenopausal women.14,15 Physicians and pharmacists who stand to benefit economically from the sales of CHT may also have encouraged its use.13,16,17
Although the general consensus is that use of CHT has grown,9,11,18 prescriptions for CHT are not systematically tracked in the United States, and no one knows exactly how many women are managing their menopausal symptoms with compounded hormones. In a survey of 184 women visiting a physician at the Mayo Clinic Women's Health Clinic (Rochester, MN) about 8 years ago, Iftikhar et al19 found that 14% of respondents were current CHT users; this was twice the rate of prior CHT users. However, because of the survey's age and small nonrepresentative convenience sample, it cannot be assumed that these findings would apply to a broader population of postmenopausal women today.
Recently, two large Internet surveys of middle-aged or older US women were conducted—one conducted by Harris Interactive Inc (Harris) and one conducted by Rose Research LLC (Rose)—to measure the prevalence of HT and CHT use in the United States and to evaluate the extent to which perimenopausal and postmenopausal women recognize that CHT products are not approved by the US Food and Drug Administration (FDA). From the Rose survey, we extrapolated information on trends in HT use to US Census Bureau figures and used a report on HT prescriptions generated from Symphony Health's Pharmaceutical Audit Suite (PHAST) 2.0 database (Symphony Health, Horsham, PA) of US prescription information to estimate the amount of CHT used in the United States and the proportion of HT prescriptions that are compounded. We also examined survey data on menopausal experience, knowledge of and practices regarding HT, and treatment outcomes. We anticipated that the data would show a high rate of CHT use and limited knowledge regarding the regulatory status of CHT in a sample of reasonably representative perimenopausal and postmenopausal women.
METHODS
Survey design
The market research firms Harris and Rose each administered a population-based, cross-sectional Internet survey on menopause and HT to US women. The source populations were drawn from Web-based nonprobability consumer panels maintained by Harris and Global Market Insite (GMI). Members were recruited to their respective panels primarily through Internet recruitment drives (eg, advertisements and newsletters) and opted to join the panels. Privacy policies were fully disclosed to each member at registration, at which time they submitted a profile containing their E-mail address, name, home address, age, and other demographic information. An e-mail was sent to the e-mail address recorded at registration, with a link for panel applicants to confirm their desire to join the panel and to sign a confidentiality agreement.
The Harris survey was conducted between June 24 and July 10, 2013, and the Rose survey was conducted during three consecutive weeks in April 2014. The sample population was invited via e-mail to take the survey during the defined time frame. The e-mail provided an encrypted link to the survey, which is housed on a secure database and must be completed in a single sitting. Participation was voluntary, no medical procedures were conducted, and risk to participants was considered minimal. Although a written informed consent form was not formally obtained, respondents were advised—before they began the survey—that their opinions regarding medications or products that they might be taking for health were being sought, and they were assured that their answers and identifying information would remain confidential.
Responses were captured electronically and stored on a secure private server. Survey administrators applied several techniques to exclude duplicate or fraudulent responses. Respondents’ digital fingerprints were compared with their registered profile, and surveys completed in less than two fifths of the median time estimated for completion were excluded. Only aggregated deidentified data were provided for analyses. As a reward for completing the survey, panel members received sweepstakes entries and/or points redeemable for approximately US$10 in cash or merchandise.
Harris and GMI are members of The World Association for Opinion and Marketing Research and comply with the International Chamber of Commerce/World Association for Opinion and Marketing Research International Code on Market and Social Research. Harris also conforms to the American Association for Public Opinion Research Code of Professional Ethics and Practices, the Health Insurance Portability and Accountability Act, and other US privacy regulations and guidelines.
Sample and inclusion criteria
For the Harris poll, women aged 45 to 60 years who were currently going through menopause or had experienced menopause were eligible to participate. With the goal of accruing 800 completed surveys, Harris invited 10,781 women aged 45 to 60 years from its consumer panel to take a survey on an unspecified topic. Invitations were balanced by US Census Bureau statistics for age, race, geographic location, household income, and education levels; invitees were systematically sampled from among panel members matching each target demographic. Quotas were established according to the desired number of completers per demographic attribute, and additional invitations were sent in batches in blinded fashion until each quota was met. These steps were taken to help mitigate sample biases and to ensure reliable and projectable survey results.
For the Rose survey, women aged 40 years or older who (1) confirmed current or former use of an HT product from a list of generic and branded drugs approved by the FDA to treat menopausal symptoms or (2) identified themselves as ever users of any product for “hormone therapy replacement or supplement” were eligible to participate. Women who did not indicate current or prior use of an HT product on the list or who answered “no” when asked whether they had ever used any product for “hormone therapy replacement or supplement” were excluded. In all, 90,120 women aged 40 years or older who belonged to the GMI consumer panel were invited, with the intent of obtaining 2,000 completed surveys. As with the Harris poll, the Rose survey used systematic sampling of panel members to ensure that the number of women invited per demographic attribute comported with US Census Bureau figures. Additional responses were sent in waves until response quotas for all demographic categories were satisfied.
Throughout this report, women who responded to the invitation are referred to as “respondents.” Those respondents who were deemed eligible after answering all screening questions and who completed the remainder of the survey are referred to as “completers.”
Survey instrument
Questionnaires were developed by TherapeuticsMD in conjunction with survey administrators. Before the Rose survey was fielded, it was tested for face validity among a sample of 100 women and adjusted as needed. Each of the finalized surveys took about 15 minutes to complete. Most questions were multiple choice and allowed participants to select only one answer. Answer choices were rotated randomly for each participant to minimize potential bias, and a response was required before proceeding to the next question.
Harris respondents were asked whether they had ever experienced menopausal symptoms and whether they were currently experiencing them. Both Harris and Rose completers were asked about age at menopausal symptom onset, whether they had ever experienced specific menopausal symptoms, and severity of any symptoms. Completers in both surveys were also asked about HT use (type, where obtained, duration, and effectiveness) and specifically about CHT use. Because CHT is described in different ways, pertinent questions attempted to define CHT using terminology associated with how this treatment is prescribed. Harris completers were given a list of treatments of menopause symptoms and asked to indicate any treatment they had tried, including “bioidentical hormone replacement therapy (HRT) from a specialty pharmacy (personalized specifically for you by a special compounding pharmacy—local or mail order/Internet).” Ever users in the Rose survey were asked, “Was your prescription for hormone therapy specifically formulated, personalized, or compounded specifically for you based on your hormone levels?” This question was added to the survey after 273 of the eventual completers had already taken the survey to permit the collection of more complete information on CHT use. Only Harris completers were asked, “Do you believe that bioidentical hormone therapies compounded at a specialty pharmacy are FDA-approved?”
Data analyses
Weighting by US Census Bureau statistics was applied to the final pool of Harris respondents to ensure that demographic attributes of age, race, and geographic distribution were proportional with the general population of US women. The Harris survey also weighted results to the US population by age, income level, educational status, and race, which did not materially affect outcomes. The pool of Rose respondents yielded a population of completers well-matched to the general population on target demographic variables and did not require weighting. Both surveys were slightly underweighted for race (80%-84% of completers were white). In analyzing data for eligible completers, we considered only women who answered 100% of the survey questions posed; partial interviews were excluded.
For the Rose survey, responses were analyzed for the entire pool of completers and stratified by age (40-44, 45-49, 50-54, 55-59, 60-64, 65-69, 70-74, 75-79, and ≥80 y). Subset analyses were conducted for women aged 50 to 64 years, which is the age group more likely to experience menopausal symptoms. In addition, responses were stratified by region, income level, ethnicity, current or former use, and hysterectomy or menopause status (perimenopausal and postmenopausal vs premenopausal).
There is no consensus for calculating response rates or estimating sampling error for a sample drawn from a nonprobability panel.20 As recommended by the American Association for Public Opinion Research20 in accordance with terms defined in the International Organization for Standardization report ISO 26362:2009, we reported the participation rate and cooperation rate, calculated as follows:
 |
We also calculated the qualification rate using the following formula:
 |
To estimate US trends in CHT use and annual spending on CHT, we used Rose survey information on rate of current HT use, number and cost of HT products used, and duration of use; the 2012 census estimates; and a summary report of HT prescriptions filled by US women aged 18 years or older. The report was generated using PHAST 2.0 (Symphony Health), a database of prescription information collected weekly from retail, mail order, and specialty pharmacies across the United States. (Calculations are detailed in “Results.”)
RESULTS
Response rates and demographic information
The Harris survey invited 10,781 women aged 45 to 60 years, via e-mail, to participate in an online survey between June 24 and July 10, 2013 (Fig. 1). A total of 1,099 women responded (participation rate, 10%). Of these women, 855 had experienced or were experiencing menopausal symptoms and were thus eligible (qualification rate, 78%). The cooperation rate was 94%, with 801 of 855 eligible women completing the survey.
FIG. 1.
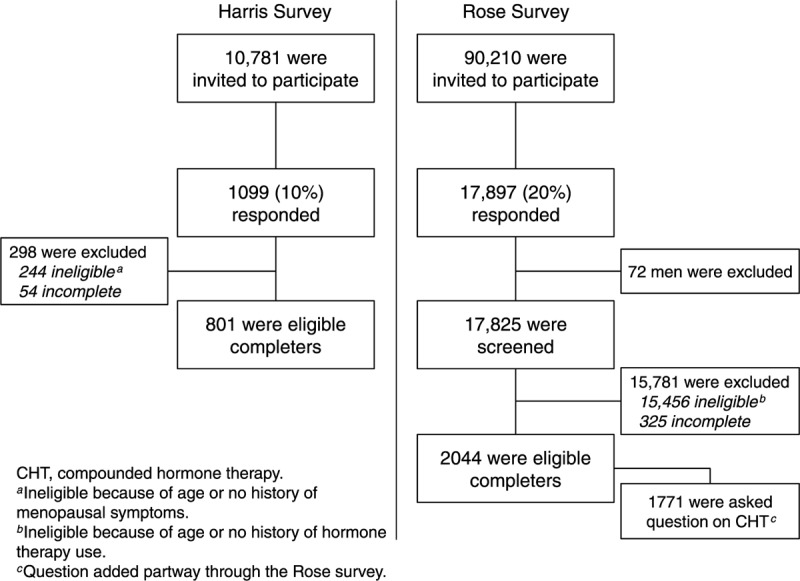
Survey sample and disposition for the Harris and Rose surveys.
An invitation to take the Rose survey was e-mailed to 90,210 individuals during a 3-week period in April 2014, and 17,897 invitees responded (participation rate, 20%). Seventy-two respondents were men and were excluded. Screening of the remaining 17,825 Rose respondents identified 2,369 women aged 40 years or older who were currently using or had previously used HT and were eligible to complete the survey (qualification rate, 13%). The cooperation rate was 86%, with 2,044 of 2,369 eligible women completing the survey. Subset analysis in the Rose survey included 855 respondents aged 50 to 64 years, of whom 839 completed the survey and 714 were asked about CHT use. The cooperation rates among eligible women were very high (94% for the Harris survey and 86% for the Rose survey), with the number of dropoffs in each survey too low to draw meaningful conclusions about differences between eligible completers and noncompleters.
Most completers in the Harris and Rose surveys were white, had some postsecondary education or vocational training, and had public or private healthcare coverage (Table 1). Approximately three quarters of completers in each survey were postmenopausal, having indicated that their last menstrual cycle occurred more than 12 months earlier. We considered the remaining Harris completers perimenopausal because all had experienced menopausal symptoms. In the Rose survey, 5% of completers stated that they had just started menopause, and 7% stated that they had been going through menopause for less than 1 year; these women were considered perimenopausal. Another 15% stated that they had yet to go through menopause and were categorized as nonmenopausal. Among Rose completers aged 50 to 64 years, 84% were postmenopausal, 13% were perimenopausal, and 4% had yet to go through menopause.
TABLE 1.
Demographic characteristics of Harris (N = 801; 73%) and Rose (N = 2,044; 11%) completers
| Characteristics | Harris survey | Rose survey |
| Age group (Harris/Rose) | ||
| 40–44 y | NA | 278 (14) |
| 45–50/45–49 y | 208 (26) | 286 (14) |
| 51–55/50–54 y | 312 (39) | 307 (15) |
| 56-59/55-59 y | 280 (35) | 287 (14) |
| 60-64 y | NA | 245 (12) |
| 65-69 y | NA | 186 (9) |
| 70-74 y | NA | 145 (7) |
| 75-79 y | NA | 125 (6) |
| ≥80 y | NA | 185 (9) |
| Race/ethnicity | ||
| White | 641 (80) | 1,726 (84) |
| Black | 72 (9) | 136 (7) |
| Hispanic | 72 (9) | 99 (5) |
| Othera | 16 (2) | 83 (4) |
| Highest level of education | ||
| Less than or some high school | NA | 46 (2) |
| Completed high school | 264 (33) | 372 (18) |
| Vocational training | NA | 94 (5) |
| Some college (no degree) | NA | 485 (24) |
| Associate's degree | NA | 282 (14) |
| Bachelor's degree | 449 (56) | 434 (21) |
| Some graduate school | NA | 99 (5) |
| Graduate degree | 88 (11) | 232 (11) |
| Household income for 2012 | ||
| US$<25,000 | 128 (16) | 305 (15) |
| US$25,000-49,999 | 168 (21) | 599 (29) |
| US$50,000-74,999 | 144 (18) | 434 (21) |
| US$75,000-99,000 | 120 (15) | 244 (12) |
| ≥US$100,000 | 216 (27) | 335 (16) |
| Declined to answer | NA | 127 (6) |
| Healthcare coverage | ||
| PPO/HMO | 489 (61) | 792 (39) |
| Traditional insurance | 56 (7) | 239 (12) |
| Medicare/Medicaid | 88 (11) | 752 (37) |
| Other/unknown | 56 (7) | 165 (8) |
| No coverage | 104 (13) | 96 (5) |
| Prior hysterectomy | 160 (20) | 927 (45) |
| Menopause status | ||
| Menopausalb | 601 (75) | 1,594 (78) |
| Perimenopausal | 200 (25)c | 259 (12)d |
| Nonmenopausal | NA | 301 (15) |
Data are presented as n (%).
NA, not available; PPO, preferred provider organization; HMO, health maintenance organization.
aFor the Harris survey, “other” includes Asian American and other. For the Rose survey, “other” includes Asian American, Pacific Islander, Native American/Alaskan Native, mixed race, and other.
b“Menopausal” refers to women who reported that their last menstrual cycle was more than 1 year ago.
cBecause eligibility for the Harris survey required at least one menopausal symptom, women who stated that they had not ceased menstruating for 1 year or longer were assumed to be perimenopausal.
dIn the Rose survey, “perimenopausal” refers to women who stated that they had been going through menopause for less than 1 year and included some women who stated that they had stopped menstruating more than 1 year ago.
Prevalence, cost, and knowledge of CHT
We used a four-step process to estimate the prevalence of CHT use among US women. In step 1, we calculated the estimated number of US women aged 40 years or older who are currently using menopausal HT (compounded and FDA-approved). First, we determined the rate of current HT use for Rose respondents per age range: 40 to 44, 45 to 49, 50 to 54, 55 to 59, 60 to 69, 70 to 74, 75 to 79, and 80 years or older. Next, we multiplied the current use rate for each age range by the total number of same-age US women per US Census Bureau population estimates for 2012 (Table 2).21 This shows that approximately 3.7 million US women aged 40 years or older currently use HT.
TABLE 2.
Current rate of HT use among Rose respondents extrapolated to an age-matched population of US women
| Age range (y) | Number of US women21 | Current HT use among Rose respondents, by age (%) | Estimated number of US women using HT |
| 40-44 | 10,569,227 | 7 | 739,846 |
| 45-49 | 10,962,854 | 7 | 767,400 |
| 50-54 | 11,499,014 | 5 | 574,951 |
| 55-59 | 10,704,108 | 3 | 321,123 |
| 60-64 | 9,279,200 | 5 | 463,960 |
| 65-69 | 7,370,497 | 3 | 221,115 |
| 70-74 | 5,412,023 | 3 | 162,361 |
| 75-79 | 4,198,131 | 3 | 125,944 |
| ≥80 | 7,349,650 | 5 | 367,483 |
| Total | 77,344,704 | – | 3,744,183 |
HT, hormone therapy.
In step 2, we calculated the number of HT prescriptions (compounded and FDA-approved) dispensed annually. We multiplied the estimated 3.7 million current HT users by the mean number of HT products taken per month in the Rose survey (1.7) by the estimated duration of use, which we assumed to range from 9 to 12 months. This suggests that 57 million to 75 million prescriptions for HT are filled annually (Fig. 2).
FIG. 2.
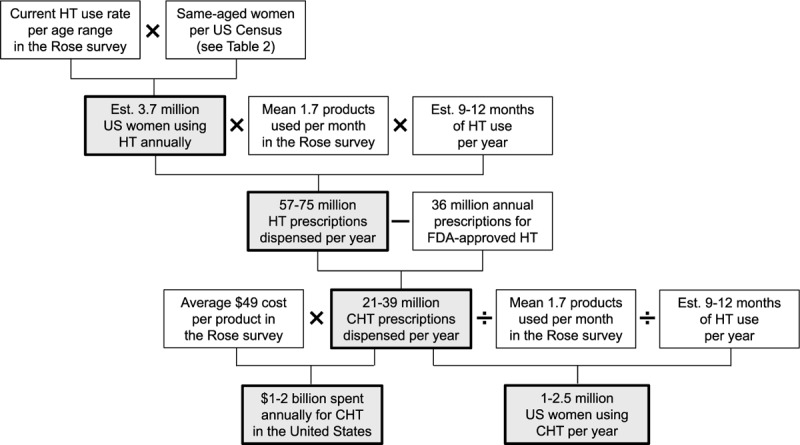
Extrapolation of Rose survey data to quantify the annual amount and cost of compounded hormone therapy (CHT) in the United States. HT, hormone therapy; Est., estimated; FDA, Food and Drug Administration.
In step 3, we estimated the number of CHT prescriptions dispensed annually. According to PHAST 2.0 prescription data, approximately 36 million prescriptions for FDA-approved HT were filled in 2012; a small percentage may have been filled for men and for women younger than 40 years (<1%).6 Subtracting the 36 million annual prescriptions of FDA-approved HT from the 57 million to 75 million annual prescriptions for all HT indicates that 21 million to 39 million prescriptions for CHT may be filled annually, accounting for 28% to 68% of HT use.
In step 4, we determined the number of US women aged 40 years or older using CHT annually by dividing the number of CHT prescriptions filled annually by the mean 1.7 HT products taken per month in the Rose survey (1.7) by assumed duration of use (9-12 mo). This suggests that 1 million to 2.5 million women may use CHT annually.
To calculate the estimated amount spent on CHT annually, we multiplied the number of CHT prescriptions filled annually by the average price of US$49 that Rose completers reported paying out of pocket for HT. Results show that between US$1 billion and US$2 billion may be spent on CHT each year in the United States.
Reported prevalence of CHT use
Two percent (16 of 801) of Harris completers affirmed that they had used CHT. Assuming that the 16 CHT users belong to the subset of HT ever users (n = 123), CHT use would account for 13% of the total HT used by Harris completers. Twenty-one percent (378 of 1,771) of Rose completers reported current or prior use of personalized CHT, with no difference in the rate of CHT use observed among the subset of women aged 50 to 64 years (153 of 714). Rates of CHT use were higher in the younger age groups and lower in the older age groups compared with the overall population of Rose completers (Fig. 3). Rose completers taking CHT were more than twice as likely as women using conventional HT to obtain it through their physician's office (15% vs 6%, respectively), although women in both groups were most likely to obtain their HT products from a local pharmacy (59% vs 54%, respectively; Fig. 4).
FIG. 3.
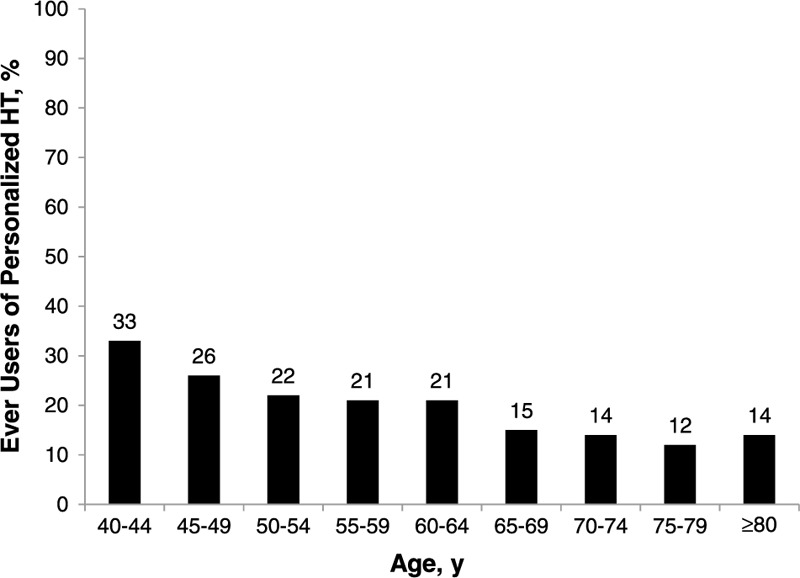
Use of personalized hormone therapy (HT) among Rose completers, by age (n = 378).
FIG. 4.
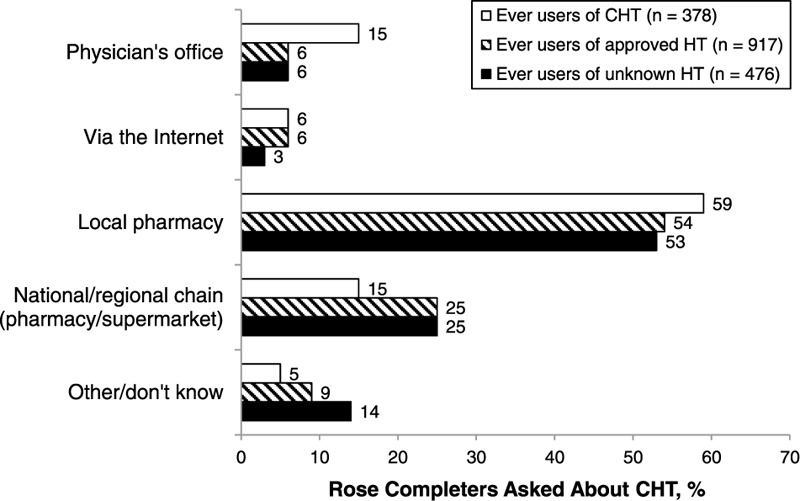
Sources of hormone therapy (HT) products as self-reported by women belonging to the subset of Rose ever users questioned about compounded hormone therapy (CHT) use (n = 1,771).
Knowledge of CHT
All Harris completers (N = 801) were asked, “Do you believe that bioidentical hormone therapies compounded at a specialty pharmacy are FDA-approved?” Only 14% correctly answered “no,” whereas 10% answered “yes” and 76% stated that they were not sure (Fig. 5). When Rose completers (N = 2,044) were asked whether their HT had been personalized or compounded for them, 27% of the women stated that they did not know.
FIG. 5.
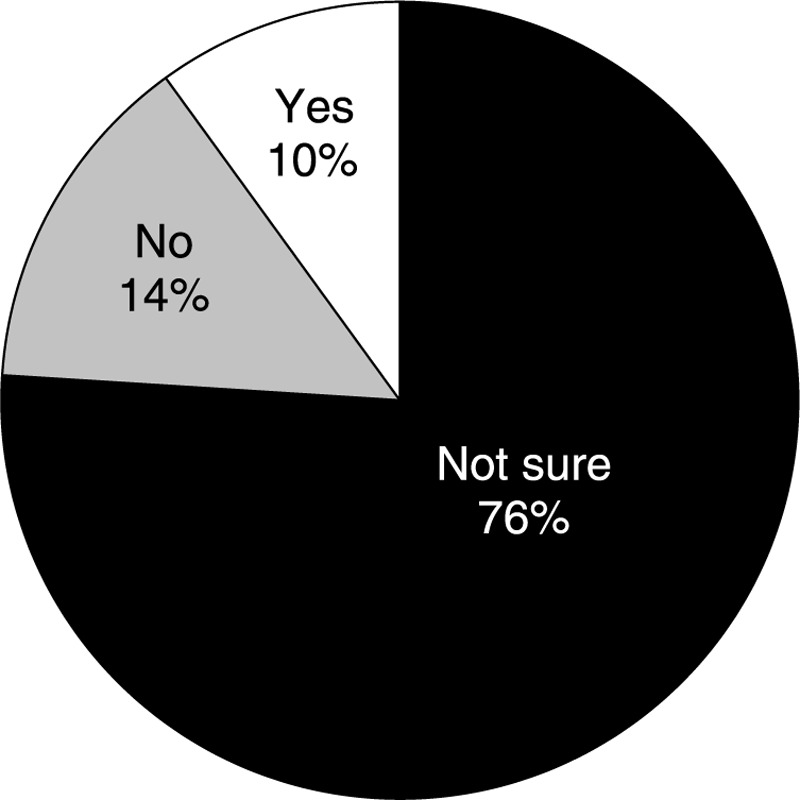
Responses of Harris completers (N = 801) to the question, “Do you believe that bioidentical hormone therapies compounded at a specialty pharmacy are FDA-approved?”
General findings on menopause symptoms and treatment
Any HT use
In the Harris survey, 15% of completers were ever users of HT, 6% were current users of HT, and 9% were prior users of HT. Patterns of HT use among Rose respondents (n = 17,825) were similar, with 13% reporting ever use of HT. Approximately 5% of Rose respondents were current HT users, suggesting that 8% were prior users. In the subset of Rose respondents aged 50 to 64 years, 4% were current users and 6% were prior users. The mean duration of HT use among ever users in the Harris survey was 50 months (Fig. 6A). The Rose survey expressed duration of use as a range. Based on the midpoint for each range, the approximate median use for Rose completers was 28 months (Fig. 6B).
FIG. 6.
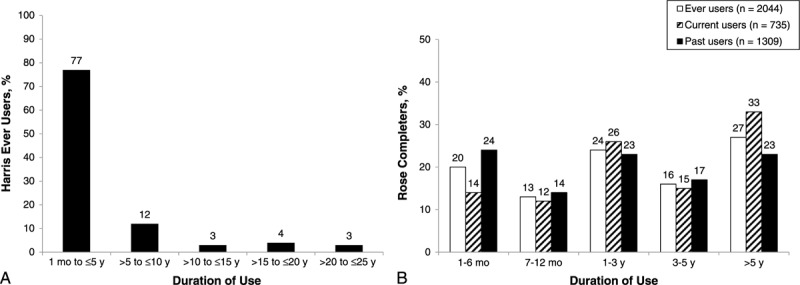
Duration of hormone therapy use in (A) Harris ever users (n = 123) and (B) Rose completers, by history of hormone therapy use.
Both surveys showed that HT was generally effective in relieving menopausal symptoms. Approximately 89% of ever users in the Harris survey stated that HT provided moderate or significant relief; 83% of ever users in the Rose survey stated that HT was extremely effective, very effective, or somewhat effective.
Most ever users in the Harris and Rose surveys stated that their physician had recommended HT (91% and 63%, respectively). Rose completers were more likely to have received a prescription for HT from an ob-gyn than from a general practitioner or other types of physician (57% vs 33% vs 8%, respectively).
Use of nonhormonal treatments
Harris completers were asked about various nonhormonal treatments. Responses showed that 42% of Harris completers had tried lifestyle changes (eg, exercise, diet, and stress reduction), 26% had tried natural therapies and dietary supplements (eg, black cohosh, ginseng, and phytoestrogens), and 10% had tried nonhormonal prescription medications such as gabapentin and antidepressants. More than one third of Harris completers never received any treatment of menopausal symptoms. Most women who had used nonhormonal treatments stated that the treatments provided moderate to significant relief, including 78% of women who made lifestyle changes, 66% of women who tried natural therapies and dietary supplements, and 82% of women who used nonhormonal prescriptions.
History of menopausal symptoms
Seventy-eight percent of Harris respondents reported that they had experienced or were experiencing menopausal symptoms. Almost half (47%) of the women who had experienced menopausal symptoms were symptomatic at the time the survey was taken. Rose completers were not asked directly whether they had ever had menopausal symptoms, but the survey's use of adaptive questioning allowed us to determine that 15% had no history of symptoms. In both surveys, the mean age at menopausal symptom onset was 47 years.
Harris and Rose completers were asked about specific menopausal symptoms and asked to rate symptoms they had experienced as mild, moderate, or severe. All completers in the Harris survey had experienced menopausal symptoms (a requisite for eligibility): 91% of women had a history of hot flashes (of whom 67% described hot flashes as moderate to severe), 65% had vaginal dryness, 85% had sleep disturbance, 48% had urinary complaints, and 48% had sexual dysfunction (Fig. 7A).
FIG. 7.
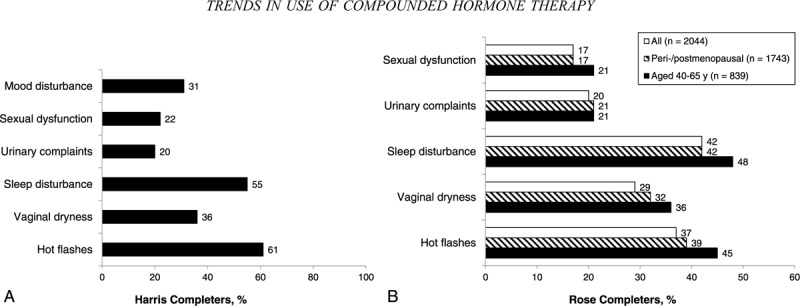
Percentages of (A) Harris completers (N = 801) who experienced moderate to severe menopausal symptoms and (B) Rose completers who experienced moderate to severe symptoms (overall, stratified by perimenopause/postmenopause status, and among women aged 40-65 y).
When Rose completers (including the 15% who reported that they have not yet entered the menopausal transition) were questioned about specific menopausal symptoms, 59% of women acknowledged a history of hot flashes (of whom 62% indicated hot flashes to be moderate to severe), 49% had experienced vaginal dryness, 66% had sleep disturbance, 44% had urinary complaints, and 28% had sexual dysfunction (Fig. 7B). In the subset of 1,743 Rose completers considered perimenopausal and postmenopausal, 61% reported experiencing hot flashes (of whom 64% considered hot flashes to be moderate to severe), 51% had vaginal dryness, 65% had sleep disturbance, 45% had urinary complaints, and 29% had sexual dysfunction. The experience of menopausal symptoms among all Rose completers aged 50 to 64 years approached that of the overall population in the Harris survey, with similar rates of hot flashes (71%), vaginal dryness (59%), sleep disturbance (73%), urinary complaints (46%), and sexual dysfunction (35%).
Knowledge of and information on HT
Most Harris completers (63%) recalled consulting their physician about their menopausal symptoms and treatment options. Two thirds of women who spoke with a physician had also discussed the issues with a family member or friend (Fig. 8). Another 14% of completers stated that they had only talked to a family member or friend, and 23% never spoke with anyone about the topics.
FIG. 8.
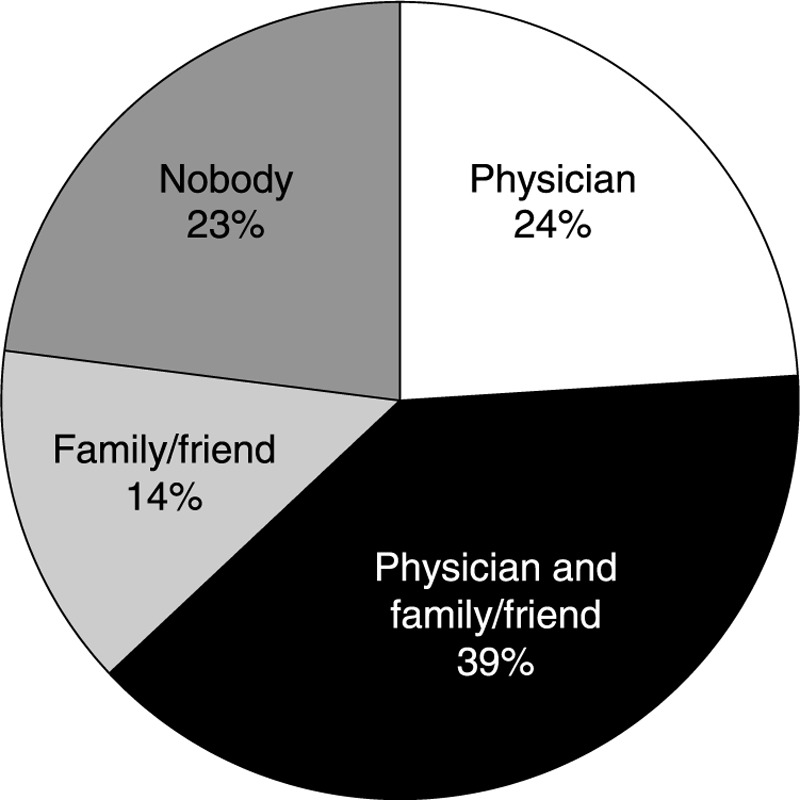
Parties with whom Harris completers (N = 801) reported discussing their menopausal symptoms and/or treatment options.
Before starting HT, only 22% of Rose completers considered themselves very familiar or extremely familiar with HT. Another 35% of Rose completers stated that they were somewhat familiar with HT, and the rest were not very familiar (26%) or not at all familiar (18%) with HT. Among women who were somewhat familiar to extremely familiar with HT before they consulted a physician (n = 1,147), 42% stated that their information came from friends and family. Other sources of information included brochures at the physician's office (28%), the Internet (14%), television (11%), and print publications (6%); almost one quarter (21%) were not sure where they had obtained their information (Fig. 9).
FIG. 9.
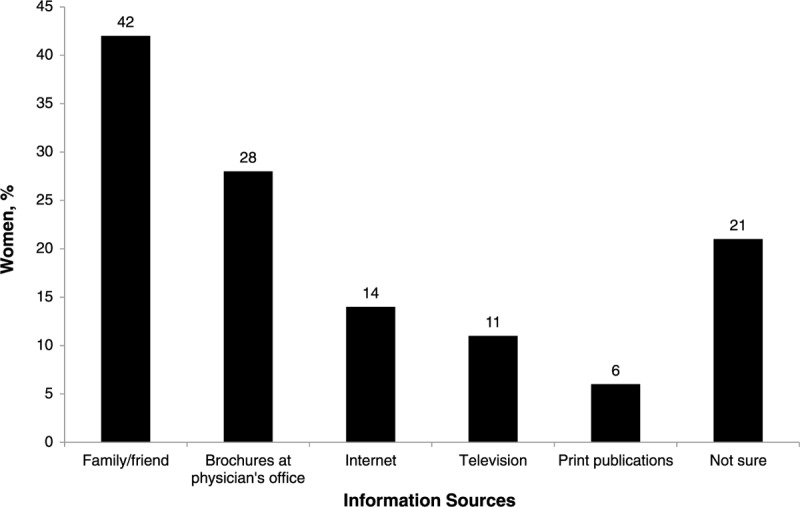
Sources of hormone therapy information among Rose completers who stated that they were already somewhat familiar to extremely familiar with hormone therapy even before they visited their physician to seek treatment (n = 1,147).
DISCUSSION
Our analyses and calculations conservatively estimate that between 1 million and 2.5 million US women aged 40 years or older are currently using CHT, accounting for approximately 28% to 68% of HT prescriptions filled annually and costing US$1 billion to US$2 billion each year. We believe that this is the first report in the literature to estimate the number of US women using CHT and the amount spent on CHT annually in the United States.
To support our extrapolated findings on CHT use, we searched the literature for recent studies reporting HT use among US women that might corroborate the 5% rate of current HT use in the Rose survey. Sprague et al4 reported that 4.7% (95% CI, 3.3-6.1) of US women aged 40 years or older who participated in the National Health and Nutrition Examination Survey (NHANES; n = 10,107) used oral HT (estrogen or progestin alone or combined) in 2009-2010. Steinkellner et al5 evaluated the 2009 pharmacy claims for approximately 10 million insured women aged 50 years or older and similarly estimated current use of oral estrogen-based or progestogen-based HT at 4.8%. The rate of current use climbed to 8.8% when all HT delivery methods were considered.5
Jewett et al8 interpolated data from NHANES to estimate that 1.8% of US women aged 40 to 84 years used combined oral estrogen-progestin therapy in 2010. They did not estimate the prevalence of oral estrogen-only or nonoral formulations.8 However, Sprague et al4 reported that 2.9% of women aged 40 years or older in NHANES used oral estrogen-only formulations in 2009-2010, and Steinkellner et al5 stated that 4.3% of women aged 50 years or older used nonoral HT in 2009. Taken together, the findings of these three studies—all of which applied a more limited definition of HT and two of which targeted a narrower age range than the Rose survey—suggest that the 5% rate of current HT use observed in the Rose survey may underestimate the extent of HT use in the United States.4,5,8
The Source Healthcare Analytics PHAST 2.0 prescription data report, which showed that 36 million annual prescriptions for FDA-approved HT were prescribed in 2012, agrees closely with a 2011 report by Stagnitti and Lefkowitz,6 which showed that adult US women filled 32 million prescriptions for FDA-approved HT in 2008. In addition, the average US$49 cost per product from the Rose survey that we used in calculating the prevalence of CHT use seems reasonably consistent with real-world pricing for these agents.19,22,23 Although the mean use of HT exceeded 1 year in the Rose and Harris surveys, we assumed minimal use of 9 months and maximal use of 12 months in estimating the annual prevalence of CHT to ensure that the range accounted for those women who are not fully adherent. According to a Mayo Clinic study, 54% of CHT users reported that they had used CHT longer than 1 year, suggesting that our estimate of 9 to 12 months of use is reasonable.19
The Rose and Harris surveys showed that at least three quarters of women experienced symptoms during the menopausal transition, with symptom onset in both surveys at about age 47 years. More than 91% of perimenopausal and postmenopausal women in the Harris poll had a history of VMS, which 67% self-judged as moderate to severe. These findings are consistent with other representative population-based studies of VMS during the menopausal transition, such as the Study of Women's Health Across the Nation, Melbourne Women's Midlife Health Project, and Penn Ovarian Aging Study, which reported VMS rates ranging from 40% to 97%.24-26
HT is indicated for and considered the most effective treatment of menopause-related moderate to severe VMS.1,27 It is also indicated for moderate to severe vulvovaginal atrophy that is unresponsive to vaginal moisturizers or lubricants.27 In relation to the high prevalence of symptoms in the Rose and Harris surveys, the rate of HT use in the surveys was low, consistent with the literature. For example, only 123 Harris completers had ever used HT, implying that more than half of women who developed moderate to severe hot flashes during the menopausal transition may not have received effective treatment.
Completers of the Harris and Rose surveys were asked about their experience with specific types of HT. The pool of responses revealed that some prescribing of HT is inconsistent with evidence-based practices.1,9,27,28 In the Rose survey, 25% of products taken by current HT users were hormone formulations that the FDA has not approved to treat menopausal symptoms, including testosterone-based regimens, hormone pellets, and vaginal and injected progestogens (alone or with estrogen). Among Rose completers who stated that they had undergone hysterectomy, 95 were currently using a progestogen-containing regimen. Furthermore, several current HT users in the Rose survey stated that they had undergone salivary testing to measure levels of sex steroid hormones. Despite the lack of evidence and national recommendations against using salivary testing to titrate HT,9,12,29,30 its use has increased in recent years, concurrent with the rising prevalence of CHT use.11
Our data support the general consensus that the CHT market is growing.9,11,18 Conversely, the market for FDA-approved HT shrank dramatically in the past decade or so.4-6,31 Some women who are experiencing menopausal symptoms may choose CHT over FDA-approved HT because of Internet-perpetrated myths that compounded hormones are more natural and thus safer than commercially manufactured ones.32 Findings from the Harris survey suggest that few women understand that CHT products are not FDA-approved, and the results of the Rose survey indicate that many women are unsure whether the HT they are taking is compounded. Other surveys have revealed that postmenopausal women have inaccurate information on CHT.19,33-37 The Mayo Clinic study by Iftikhar et al19 reported that 46% of women surveyed were familiar with CHT, and 67% of these women considered CHT safer than FDA-approved HT. Sayakhot et al36 administered a questionnaire on menopause to 114 Australian women aged 40 to 51 years who had breast cancer, which showed that 79% were confused about the risks and benefits of CHT. Pharmacy compounders in the United States have never been required to label CHT or to provide detailed prescribing information,38 which may contribute to US women's general lack of accurate knowledge of these products.
Most recognized women's health organizations in the United States, including the American Association of Clinical Endocrinologists, the American College of Obstetricians and Gynecologists, the American Society for Reproductive Medicine, The Endocrine Society, The North American Menopause Society, the US Preventive Services Task Force, and the Women's Health Practice and Research Network of the American College of Clinical Pharmacy, have position statements on HT that warn about the lack of scientific evidence supporting the efficacy and safety of CHT.1,9,28,39-41 International organizations such as the International Menopause Society, the Australian Menopause Society, and the British Menopause Society have issued similar guidance.42-44 Both The North American Menopause Society and the American College of Obstetricians and Gynecologists recommend that physicians who are considering prescribing CHT first determine whether an appropriate FDA-approved alternative is available.1,9,11 Some medical and legal experts have expressed concern that physicians who prescribe compounded drugs for reasons other than medical need may be held liable if a woman for whom they prescribed CHT experiences harm from the treatment.45,46
Strengths and limitations of the surveys
The Rose and Harris surveys have several limitations. Although the sample for each survey was matched to the US population of adult women by age, race, income, education, and region, the survey design limits the generalizability of the data to the population at large. Respondents were accrued from a nonprobability opt-in panel of registered survey takers rather than from a randomized sample of the general population.
Participation rates for both surveys were low but consistent with rates commonly reported for online surveys.20 Participation has dropped sharply across all survey methods in the past decade; for example, the Pew Research Center47 reported that the response rate for telephone surveys declined from 36% in 1997 to 9% in 2012. The link between low participation in online surveys and nonresponse bias is unclear, with some data showing no relationship.20,48 The qualification rate for the Rose survey was numerically low owing to the restrictive eligibility criteria (current and past HT users) and is not a limitation of the survey.
Women received an incentive only if they completed the survey, which may have influenced how they answered screening questions. In addition, all respondents had Internet access, and differences may exist between Internet users and nonusers. The women who took the survey may differ from women in the general population in other meaningful ways that the survey was not designed to capture. The subset of HT users in the Harris study was small, which further limits our ability to extrapolate these data to the general population. The self-reported nature of the questionnaire introduces the potential for recall bias, although self-reporting of HT use has generally been found to be concordant with prescription reimbursement data.49-51
The wording of the survey questions may have affected responses. It is not clear to what extent women interpreted terms such as “bioidentical,” “natural,” “individualized,” or “compounded” to mean CHT. In addition, we did not ask questions about some demographic characteristics or personal behaviors that have been associated with how women experience menopausal symptoms.
Despite these limitations, as a result of the surveys’ use of large sample sizes and standard methods for reducing nonresponse bias (such as purposive systematic sampling, quotas, and weighting), we believe that they have yielded helpful information on menopausal experience and the prevalence of CHT use among US women. The Rose survey, which was central to our extrapolations, accrued more than 17,000 women whose age-based composition mirrored that of the US population. The relative agreement between our observations on the overall use of HT among postmenopausal women and the results of other studies increases confidence in our findings. Data on HT use from other published peer-reviewed surveys and studies suggest that our conclusions may even underestimate the magnitude of CHT use in the United States. Given the substantial use of CHT by US women, larger studies determining what women know about these products could be useful in developing strategies for ensuring that providers have the necessary tools to educate women about CHT.
CONCLUSIONS
As many as 1 million to 2.5 million US women may be taking CHT. Many women seem not to distinguish between CHT products and FDA-approved medications and may be unaware of the inadequacy of medical evidence supporting CHT use or unaware of reported concerns with the quality and safety of CHT. Our data suggest that women turn to their physician for advice when considering HT for menopausal symptoms. Providers can play a pivotal role in helping women understand the different levels of evidence supporting the efficacy, safety, and quality of CHT versus FDA-approved HT and the differences in FDA regulation and monitoring between compounded and approved drugs.
Acknowledgments
We wish to acknowledge Mitchell Krassan of TherapeuticsMD for assisting with data analyses and Christin Melton, ELS, CMPP, of Precise Publications LLC, for providing medical writing support.
Footnotes
Funding/support: TherapeuticsMD supported the conduct and analysis of the surveys and funded medical writing support (provided by Christin Melton, ELS, CMPP, of Precise Publications LLC).
Financial disclosure/conflicts of interest: J.V.P. has received grants and research support (paid to the University of Virginia) from TherapeuticsMD Inc, Bionova Inc, and EndoCeutics Inc, and has served as a consultant (fees paid to the University of Virginia) for Pfizer Inc, Noven Pharmaceuticals Inc, TherapeuticsMD Inc, and Shionogi Inc. N.S. has received investigator-initiated grant support from Bayer Inc (paid to the University of Colorado School of Medicine) and has stock options in MenoGenix.
REFERENCES
- 1.The North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause 2012; 19:257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002; 288:321–333. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013; 310:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999-2010. Obstet Gynecol 2012; 120:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women's Health Initiative. Menopause 2012; 19:616–621. [DOI] [PubMed] [Google Scholar]
- 6.Stagnitti M, Lefkowitz D. Trends in Hormone Replacement Therapy Drug Utilization and Expenditures for Adult Women in the US Civilian Noninstitutionalized Population, 2001-2008. 2011; Rockville, MD: Agency for Healthcare Research and Quality, 1-9. [Google Scholar]
- 7.Jungheim ES, Colditz GA. Short-term use of unopposed estrogen: a balance of inferred risks and benefits. JAMA 2011; 305:1354–1355. [DOI] [PubMed] [Google Scholar]
- 8.Jewett PI, Gangnon RE, Trentham-Dietz A, et al. Trends of postmenopausal estrogen plus progestin prevalence in the United States between 1970 and 2010. Obstet Gynecol 2014; 124:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Committee on Gynecologic Practice and the American Society for Reproductive Medicine Practice Committee. Committee opinion no. 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol 2012; 120:411–415. [DOI] [PubMed] [Google Scholar]
- 10.MacLennan AH, Gill TK, Broadbent JL, et al. Continuing decline in hormone therapy use: population trends over 17 years. Climacteric 2009; 12:122–130. [DOI] [PubMed] [Google Scholar]
- 11.Pinkerton JV. Bioidentical” hormones. What you (and your patient) need to know. OBG Manag 2009; 21:42–52. [Google Scholar]
- 12.Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause 2004; 11:356–367. [DOI] [PubMed] [Google Scholar]
- 13.Complaint by the State of Tennessee vs HRC Medical Centers, Case No. 12C4047. Available at: http://www.tn.gov/attorneygeneral/cases/hrc/hrcamendedcomplaint.pdf April 15, 2013. Accessed June 30, 2014. [Google Scholar]
- 14.To: Oprah Winfrey; subject: hormones. What I know for sure. Available at: http://www.oprah.com/spirit/What-Oprah-Knows-for-Sure-About-Menopause-and-Hormones Accessed June 30, 2014. [Google Scholar]
- 15.Somers S. Bioidentical hormones and wellness. Available at: http://www.suzannesomers.com/pages/forever-health Accessed June 30, 2014. [Google Scholar]
- 16.Musgrave J. Not guilty: steroid doctor, pharmacist cleared on all counts. Palm Beach Post 2014. Available at: http://www.mypalmbeachpost.com/news/news/crime-law/doctor-and-pharmacist-acquitted-of-all-charges-in-/ndF4B Accessed June 30, 2014. [Google Scholar]
- 17.Roser MA. Ethical questions raised as doctors partner with pharmacies. Austin American-Statesman 2013. Available at: http://www.mystatesman.com/news/news/local/ethical-questions-raised-as-doctors-partner-with-p/nbndb Accessed June 14, 2014. [Google Scholar]
- 18.Bioidentical hormones: sound science of bad medicine? Senate hearing before the Special Committee on Aging, 110th Congress, First Session, 2007. [Google Scholar]
- 19.Iftikhar S, Shuster LT, Johnson RE, et al. Use of bioidentical compounded hormones for menopausal concerns: cross-sectional survey in an academic menopause center. J Womens Health (Larchmt) 2011; 20:559–565. [DOI] [PubMed] [Google Scholar]
- 20.Baker R, Blumberg S. Coupler MP; for the AAPOR Standards Committee Task Force. AAPOR report on online panels. Available at: http://www.aapor.org/AAPORKentico/AAPOR_Main/media/MainSiteFiles/AAPOROnlinePanelsTFReportFinalRevised1.pdf Accessed September 15, 2014. [Google Scholar]
- 21.US Census Bureau. Annual estimates if the resident population by single year of age and sex for the United States: April 1, 2010 to July 1, 2012. Available at: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=PEP_2012_PEPSYASEXN&prodType=table Accessed June 15, 2014. [Google Scholar]
- 22.Barenberg BJ, Smith T, Nihira MA. Compounded estradiol cream: a cost conscious alternative. J Okla State Med Assoc 2014; 107:155–156. [PubMed] [Google Scholar]
- 23.BodyLogicMD. If I decide to start on a bioidentical hormone therapy program, how much will it cost? Available at: http://www.bodylogicmd.com/faq/if-i-decide-to-start-on-a-bioidentical-hormone-therapy-program-how-much-will-it-cost 2014. Accessed August 5, 2014. [Google Scholar]
- 24.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women's Health Across the Nation. Am J Public Health 2006; 96:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guthrie JR, Dennerstein L, Taffe JR, et al. Hot flushes during the menopause transition: a longitudinal study in Australian-born women. Menopause 2005; 12:460–467. [DOI] [PubMed] [Google Scholar]
- 26.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause 2014; 21:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20:888–902. [DOI] [PubMed] [Google Scholar]
- 28.The Endocrine Society. The Endocrine Society position statement on bioidentical hormones. Available at: https://www.endocrine.org/∼/media/endosociety/Files/Advocacy%20and%20Outreach/Position%20Statements/All/BH_Position_Statement_final_10_25_06_w_Header.pdf. October 2006 Accessed April 11, 2014. [Google Scholar]
- 29.US Food and Drug Administration. Bio-identicals: sorting myths from facts. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm049311.htm December 7, 2013. Accessed April 14, 2014. [Google Scholar]
- 30.Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health 2007; 16:600–631. [DOI] [PubMed] [Google Scholar]
- 31.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol 2004; 104:1042–1050. [DOI] [PubMed] [Google Scholar]
- 32.Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol 2008; 20:400–407. [DOI] [PubMed] [Google Scholar]
- 33.Adams C, Cannell S. Women's beliefs about “natural” hormones and natural hormone replacement therapy. Menopause 2001; 8:433–440. [DOI] [PubMed] [Google Scholar]
- 34.Spark MJ, Willis J, Byrne G. Compounded progesterone: why is it acceptable to Australian women? Maturitas 2012; 73:318–324. [DOI] [PubMed] [Google Scholar]
- 35.Siyam T, Yuksel N. Beliefs about bioidentical hormone therapy: a cross-sectional survey of pharmacists. Maturitas 2013; 74:196–202. [DOI] [PubMed] [Google Scholar]
- 36.Sayakhot P, Vincent A, Teede H. Breast cancer and menopause: perceptions of diagnosis, menopausal therapies and health behaviors. Climacteric 2012; 15:59–67. [DOI] [PubMed] [Google Scholar]
- 37.Gibson-Helm M, Teede H, Vincent A. Symptoms, health behavior and understanding menopause therapy in women with premature menopause. Climacteric 2014; 17:1–8. [DOI] [PubMed] [Google Scholar]
- 38.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance: pharmacy compounding of human drug products under Section 503A of the Federal Food, Drug, and Cosmetic Act. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM377052.pdf July 2014. Accessed April 5, 2013. [Google Scholar]
- 39.Moyer VA. US Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:47–54. [DOI] [PubMed] [Google Scholar]
- 40.Cobin RH, Petak SM, Bledsoe MB. American Association of Clinical Endocrinologists (AACE) Reproductive Medicine Committee position statement on bioidentical hormones. Available at: https://www.aace.com/files/position-statements/aacebhstatement071507.pdf Accessed September 26, 2014. [Google Scholar]
- 41.McBane SE, Borgelt LM, Barnes KN, et al. Use of compounded bioidentical hormone therapy in menopausal women: an opinion statement of the Women's Health Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy 2014; 34:410–423. [DOI] [PubMed] [Google Scholar]
- 42.De Villiers TJ, Pines A, Panay N, et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013; 16:316–337. [DOI] [PubMed] [Google Scholar]
- 43.Australian Menopause Society. Bioidentical hormones for menopausal symptoms information sheet. Available at: http://www.menopause.org.au/images/stories/infosheets/docs/AMS_Bioidentical_Hormones_for_Menopausal_Symptoms.pdf Accessed September 26, 2014. [Google Scholar]
- 44.Panay N, Hamoda H, Arya R, et al. The 2013 British Menopause Society & Women's Health Concern recommendations on hormone replacement therapy. Menopause Int 2013; 19:59–68. [DOI] [PubMed] [Google Scholar]
- 45.Sellers S, Utian WH. Pharmacy compounding primer for physicians: prescriber beware. Drugs 2012; 72:2043–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenna KJ. Compounded sclerosing agents: risks and consequences. Vein 2008; 1:1. Available at: http://www.veindirectory.org/magazine/article/compounded_sclerosing_agents_risks_and_consequences Accessed August 11, 2014. [Google Scholar]
- 47.The Pew Research Center. Assessing the representativeness of public opinion surveys. Available at: http://www.people-press.org/files/legacy-pdf/Assessing%20the%20Representativeness%20of%20Public%20Opinion%20Surveys.pdf Accessed November 4, 2014. [Google Scholar]
- 48.Yeager DS, Krosnick JA, Chang L. Comparing the accuracy of RDD telephone surveys and Internet surveys conducted with probability and nonprobability samples. Public Opin Q 2011; 75:709–747. [Google Scholar]
- 49.Sandini L, Pentti K, Tuppurainen M, et al. Agreement of self-reported estrogen use with prescription data: an analysis of women from the Kuopio Osteoporosis Risk Factor and Prevention Study. Menopause 2008; 15:282–289. [DOI] [PubMed] [Google Scholar]
- 50.Banks E, Beral V, Cameron R, et al. Agreement between general practice prescription data and self-reported use of hormone replacement therapy and treatment for various illnesses. J Epidemiol Biostat 2001; 6:357–363. [DOI] [PubMed] [Google Scholar]
- 51.Lokkegaard E, Johnsen SP, Heitmann BL, et al. The validity of self-reported use of hormone replacement therapy among Danish nurses. Acta Obstet Gynecol Scand 2004; 83:476–481. [DOI] [PubMed] [Google Scholar]


