Abstract
IMPORTANCE
At least 13 medication-associated diethylene glycol (DEG) mass poisonings have occurred since 1937. To our knowledge, this is the first longitudinal study characterizing long-term health outcomes among survivors beyond the acute poisoning period.
OBJECTIVE
To characterize renal and neurologic outcomes among survivors of a 2006 DEG mass-poisoning event in Panama for 2 years after exposure.
DESIGN, SETTING, AND PARTICIPANTS
This prospective longitudinal study used descriptive statistics and mixed-effects repeated-measures analysis to evaluate DEG-poisoned survivors at 4 consecutive 6-month intervals (0, 6, 12, and 18 months). Case patients included outbreak survivors with a history of (1) ingestion of DEG-contaminated medication, (2) hospitalization for DEG poisoning, and (3) an unexplained serum creatinine level of 1.5 mg/dL or higher (to convert to micromoles per liter, multiply by 88.4) during acute illness or unexplained exacerbation of preexisting end-stage renal disease.
MAIN OUTCOMES AND MEASURES
Demographics, mortality, dialysis dependence, renal function, neurologic signs and symptoms, and nerve conduction studies.
RESULTS
Of the 32 patients enrolled, 5 (15.6%) died and 1 was lost to follow-up, leaving 26 patients at 18 months. Three (9.4%) missed 1 or more evaluations. The median age was 62 years (range, 15–88 years), and 59.4% were female. Three (9.4%) patients had preexisting renal failure. Enrollment evaluations occurred at a median of 108 days (range, 65–154 days) after acute illness. The median serum creatinine level for the 22 patients who were not dialysis dependent at time 0 was 5.9 mg/dL (range, 1.8–17.1 mg/dL) during acute illness and 1.8 mg/dL (range, 0.9–5.9 mg/dL) at time 0. Among non–dialysis-dependent patients, there were no significant differences in the log of serum creatinine or estimated glomerular filtration rate over time. The number of patients with subjective generalized weakness declined significantly over time (P < .001). A similar finding was observed for any sensory loss (P = .05). The most common deficits at enrollment were bilateral lower extremity numbness in 13 patients (40.6%) and peripheral facial nerve motor deficits in 7 (21.9%). All patients with neurologic deficits at enrollment demonstrated improvement in motor function over time. Among 28 patients (90.3%) with abnormal nerve conduction study findings at enrollment, 10 (35.7%) had motor axonal involvement, the most common primary abnormality.
CONCLUSIONS AND RELEVANCE
Neurologic findings of survivors tended to improve over time. Renal function generally improved among non–dialysis-dependent patients between acute illness and the first evaluation with little variability thereafter. No evidence of delayed-onset neurologic or renal disease was observed.
Diethylene glycol (DEG) can be nephrotoxic and neurotoxic if ingested. Poisoning is characterized by acute kidney injury, neurologic impairment, and high mortality.1–3 Nerve conduction studies (NCS) of DEG-poisoned individuals have been conflicting, with some revealing markedly reduced motor amplitudes and others showing cadaveric nerve root demyelination.4–6 Renal biopsy specimens from individuals with DEG-induced acute kidney injury have demonstrated proximal tubule damage.1 The DEG metabolites, 2-hydroxyethoxyacetic acid and diglycolic acid, play prominent roles in toxicity.7–9 Since its formulation in the early 1900s, there have been at least 13 mass-poisoning incidents from DEG-contaminated pharmaceuticals throughout the world.10 Unfortunately, the long-term health outcomes among acute poisoning survivors are unknown.
From July 1, 2006, through October 10, 2006, a DEG-contaminated cough syrup was distributed throughout Panama. By January 2007, thousands had been exposed, with more than 100 deaths attributed to the syrup.11 At the request of the Panamanian Ministry of Health, we conducted a prospective longitudinal study of health outcomes among initially hospitalized outbreak survivors for 2 years after exposure.
Methods
This study was approved by the Gorgas Institute Review Board in Panama City, Panama, and the Centers for Disease Control and Prevention Institutional Review Board in Atlanta, Georgia. Written informed consent was obtained from all study participants.
Participants were identified using data provided by the Panamanian Ministry of Health and 2 large Panama City public hospitals. Case patients met all the following criteria: (1) cough syrup ingestion after July 1, 2006; (2) DEG poisoning diagnosis with hospitalization; and (3) an unexplained serum creatinine level (SCr) of more than 1.5 mg/dL (to convert to micromoles per liter, multiply by 88.4) during acute illness or unexplained exacerbation of existing end-stage renal disease (ESRD). Persons with preexisting chronic neurologic disorders were excluded. In the 3 situations in which an evaluation was missed during the study and symptoms and clinical findings were unchanged immediately before and after the missed evaluation, we assigned a value to the missing evaluation to be consistent with these findings.
We reviewed hospital medical records for information on demographics, exposure, medical history, acute illness, and outcome data. Evaluations were performed at Hospital Santo Tomas in Panama City, Panama, in January 2007 (time 0; enrollment), June 2007 (6 months), January 2008 (12 months), and July 2008 (18 months). Evaluations consisted of a questionnaire administered in Spanish, SCr testing, neurologic examination by a US board-certified neurologist (J.J.S.), and NCS testing. The same neurologist, NCS technician, and NCS machine were used at each evaluation (Nicolet Viking; Nicolet Biomedical).
We calculated estimated glomerular filtration rates (eGFRs) as milliliters per minute per 1.73 square meters according to established clinical practice guidelines.12 An eGFR of less than 60 or an SCr level of more than 1.5 mg/dL was considered abnormal. Dialysis-dependent patients at the time of sample collection were removed from the SCr and eGFR analyses for that evaluation period. Nerve conduction studies consisted of motor and sensory studies (bilateral median, ulnar, peroneal, and posterior tibial nerves), F-wave assessments, and sural sensory studies. These were deferred if patients refused or had 2 consecutive normal findings on examination. The NCS findings were categorized as normal, equivocal, primarily axonal, primarily demyelinating, or mixed axonal and demyelinating. For any patient who died during the study, we obtained cause of death from the medical examiner report.
Descriptive analyses and generalized linear mixed models to account for repeated-measures analyses were performed with SAS, version 9.3 for Windows (SAS Institute). Continuous variables are expressed as a median (with range), and categorical variables are expressed as a percentage. Mixed-effects repeated-measures analyses to test for differences over time were conducted using SAS PROC MIXED for 2 continuous variables (SCr and eGFR) and SAS PROC NLMIXED for the clinically relevant dichotomous variables (dialysis dependence, generalized weakness, normal findings on neurologic examination, any motor deficits, and any sensory loss).
Results
Participant Enrollment, Demographics, and Acute Illness History
Thirty-four persons were enrolled; 2 did not meet inclusion criteria due to undocumented elevated SCr and were excluded. Among the 32 remaining patients, the median age was 62 years (range, 15–88 years), and 28 (87.5%) had at least 1 preexisting medical condition before DEG exposure, including hypertension (23 [71.9%]), type 2 diabetes mellitus (12 [37.5%]), ESRD (3 [9.4%]), and alcoholism (1 [3.1%]) (Table 1). The median duration of acute illness hospitalization was 23 days (range, 2–111 days), and 18 (58.1%) were admitted to the intensive care unit. Of the 29 patients (90.6%) with no ESRD history, 22 (75.9%) had SCr levels of more than 4.5 mg/dL during their acute poisoning, and 8 (27.6%) were dialysis dependent at hospital discharge. The median SCr level among the 29 patients without a history of ESRD during their acute illness was 6.7 mg/dL (range, 1.8–17.1 mg/dL). The median SCr level among the 22 patients who were not dialysis dependent at the first follow-up evaluation (time 0 or January 2007) was 5.9 mg/dL (range, 1.8–17.1 mg/dL) during their acute illness.
Table 1.
Demographic, Health, and Hospital Course Data Among 32 Survivors of a DEG Mass-Poisoning Event on Initial Follow-up Evaluation After Recovery of Acute Illnessa
| Characteristic | Value |
|---|---|
| Age at enrollment, median (range), y | 62 (15–88) |
| Female sex | 19 (59.4) |
| Self-reported race | |
| Black | 5 (15.6) |
| White | 11 (34.4) |
| Mestizo or other | 16 (50.0) |
| Medical history before DEG exposure (self-reported or documented) | |
| Type 2 diabetes mellitus | 12 (37.5) |
| Hypertension | 23 (71.9) |
| Heart disease | 4 (12.5) |
| Alcoholism | 1 (0.3) |
| End-stage renal disease | 3 (9.4) |
| Any renal conditionb | 6 (18.7) |
| No. of days between date of acute hospital admission and date of enrollment, median (range)c | 108 (65–154) |
| Admitted to ICU during acute illness | 18 (58.1) |
| Duration of hospital stay during acute illness, median (range), d | 23 (2–111) |
| SCr level during acute illness, median (range), mg/dL | 6.9 (1.8–17.1) |
| 1.5–3.0 | 10 (31.3) |
| 3.1–4.5 | 0 |
| 4.6–6.0 | 3 (9.4) |
| >6.0 | 19 (59.4) |
| Newly dialysis dependent at time of discharge from hospitald | 8 (25.0) |
Abbreviations: DEG, diethylene glycol; ICU, intensive care unit; SCr, serum creatinine.
SI conversion factor: To convert serum creatinine to micromoles per liter, multiply by 88.4.
Values are presented as number (percentage) unless otherwise indicated.
Includes renal cysts, lupus nephritis, and end-stage renal disease.
For 1 participant with a long-term hospitalization before DEG exposure, date of acute admission was replaced by date of ingestion of the contaminated cough syrup.
Data missing on 7 participants.
Findings at Enrollment
The median interval between acute illness and enrollment was 108 days (range, 65–154 days). Ten patients (31.3%) were dialysis dependent at enrollment (Table 2); 3 (30.0%) had pre-exposure ESRD, 5 (50.0%) were newly dialysis dependent after acute illness resolution at hospital discharge, and 2 (20.0%) had been discharged without dialysis. The median SCr level among the 22 non–dialysis-dependent patients was 1.8 mg/dL (range, 0.9–5.9 mg/dL), and 5 (22.7%) had an SCr level of 1.5 mg/dL or less. Almost all non–dialysis-dependent patients at the first evaluation had SCr levels less than 5.9 mg/dL (21 [95.5%]), and most had SCr levels less than 3.0 mg/dL (18 [81.8%]). None of the non–dialysis-dependent patients had a normal eGFR (≥90 mL/min/1.73 m2), 12 (54.5%) had mild to moderately decreased rates (30–89 mL/min/1.73 m2), 8 (36.4%) had severely decreased rates (15–29 mL/min/1.73 m2), and 2 (9.1%) had kidney failure (<15 mL/min/1.73 m2).
Table 2.
Mortality Outcomes and Laboratory Findings Among 32 Survivors of Diethylene Glycol Poisoning After Recovery From Acute Illness (Time 0) and 3 Subsequent Serial Evaluations Over 18 Monthsa
| Characteristic | Time 0 | Months |
P Value |
||
|---|---|---|---|---|---|
| 6 | 12 | 18 | |||
| No. of participants evaluated | 32 | 29 | 28 | 26 | |
| Persons lost to follow-up | 0 | 0 | 1 | ||
| Deathsb | 3 | 1 | 1 | ||
| Dialysis dependencec,d | 10 (31.3) | 11 (37.9) | 11 (39.3) | 9 (34.6) | .85 |
| No dialysis dependence | 22 (68.7) | 18 (62.1) | 17 (60.7) | 17 (65.4) | |
| SCr, median (range), mg/dLd,e | 1.8 (0.9–5.9) | 1.5 (0.7–4.0) | 1.5 (0.8–4.8) | 1.6 (0.7–5.2) | .21 |
| <1.5 | 5 (22.7) | 9 (50.0) | 7 (41.2) | 7 (41.2) | |
| 1.5–3.0 | 13 (59.1) | 8 (44.4) | 8 (47.0) | 8 (47.1) | |
| 3.1–4.5 | 3 (13.6) | 1 (5.6) | 1 (5.9) | 0 | |
| 4.6–6.0 | 1 (4.5) | 0 | 1 (5.9) | 2 (11.8) | |
| eGFR, median (range), mL/min/1.73 m2d,e | 32.6 (7.0–75.9) | 37.6 (11.5–81.6) | 39.6 (9.4–80.9) | 40.9 (9.8–80.9) | .11 |
| G1 (≥90) | 0 | 0 | 0 | 0 | |
| G2 (60–89) | 2 (9.1) | 3 (16.7) | 4 (23.5) | 4 (23.5) | |
| G3a (45–59) | 3 (13.6) | 4 (22.2) | 2 (11.8) | 3 (17.7) | |
| G3b (30–44) | 7 (31.8) | 6 (33.3) | 7 (41.2) | 3 (17.7) | |
| G4 (15–29) | 8 (36.4) | 4 (22.2) | 3 (17.7) | 5 (29.4) | |
| G5 (<15) | 2 (9.1) | 1 (5.6) | 1 (5.9) | 2 (11.8) | |
Abbreviations: eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
SI conversion factor: To convert serum creatinine to micromoles per liter, multiply by 88.4.
Values are presented as number (percentage) unless otherwise indicated.
The causes of death included pneumonia, myocardial infarction, pericarditis, and cardiomyopathy (1 for each): cause of death for 1 patient could not be identified.
Includes 3 patients who were dialysis dependent before diethylene glycol exposure.
Repeated-measures analysis performed.
The calculations for SCr and eGFR exclude patients with dialysis dependence during that period.
The primary neurologic symptom at enrollment was generalized weakness (28 [87.5%]). Five patients (15.6%) reported facial weakness resulting in difficulty with speech, eating, or eye closure. On examination, 23 patients (71.9%) demonstrated at least 1 abnormal neurologic finding, including bilateral lower extremity sensory loss (13 [40.6%]), bilateral upper extremity motor deficits (4 [12.5%]), bilateral lower extremity motor deficits (3 [9.4%]), and both upper and lower extremity motor deficits (2 [6.3%]). Among the 7 patients (21.9%) with peripheral facial muscle motor deficits, 2 also had facial sensory loss in a trigeminal distribution. One patient had isolated trigeminal sensory loss only.
Findings on NCS were abnormal in 28 patients (90.3%; 1 patient was missing data). The most common findings were mild equivocal abnormalities, including absent sural sensory responses or mild slowing of motor conduction velocities (13 [46.4%]). Ten patients (35.7%) demonstrated moderate to severe motor axonal neuropathy with diminished motor amplitudes and normal conduction velocities. The remaining 5 patients showed either mixed axonal and demyelinating features (4 [14.3%]) or predominantly demyelinating features alone (1 [3.6%]).
Follow-up Evaluations (6–18Months)
Twenty-three of the 32 patients (71.9%) enrolled at time 0 participated in all 4 evaluations; 5 (15.6%) died, 2 (6.3%) missed the 12-month evaluation, 1 (3.1%) was lost to follow-up at 18 months, and 1 (3.1%) was unable to be examined at 6 months but had questionnaire and laboratory data available for inclusion (Table 2). The median age among deceased patients was 64 years (range, 48–74 years); pneumonia and cardiac conditions were reported causes of death in the 4 autopsy reports available. Three of the 5 deaths (60.0%) occurred within 10 months of hospital discharge.
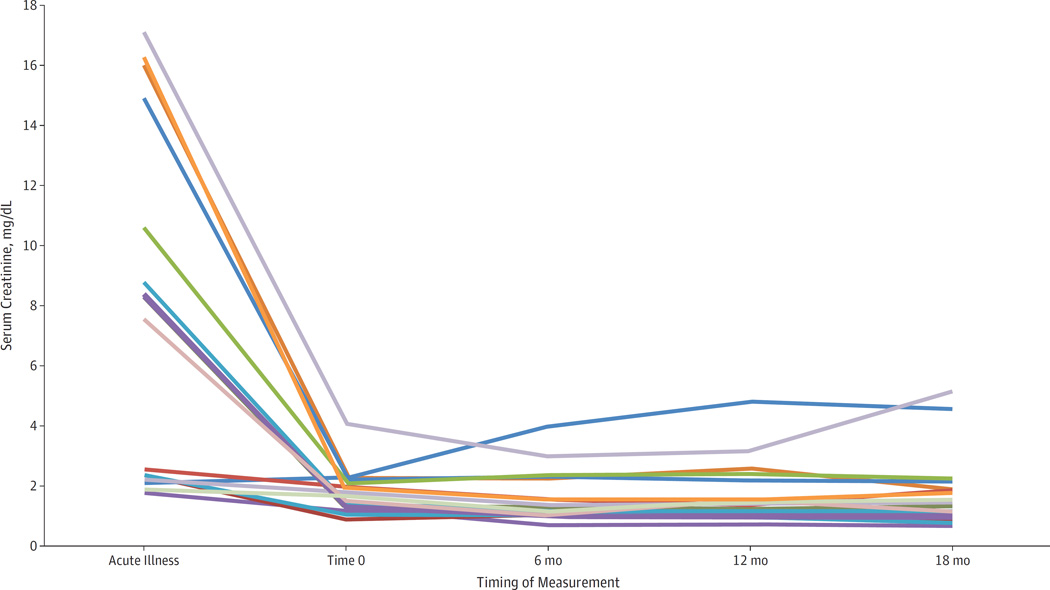
Most patients showed little variability in SCr during the study (Figure). Dialysis-dependent patients at enrollment generally remained so over time (9 [90.0%]), although 1 became dialysis free by 18 months. Among the 5 patients with normal SCr levels at enrollment, 4 (80.0%) had normal levels through 18 months and 1 died before the 12-month evaluation due to nonrenal causes. Seven of the 13 non–dialysis-dependent patients (53.8%) with a mildly elevated SCr level at enrollment (1.5–3.0 mg/dL)maintained a mildly elevated level at 18 months; 3 (23.1%) had a normal SCr level, 1 (7.7%) had a moderately elevated SCr level of 4.6 mg/dL, 1 (7.7%) was lost to follow-up, and 1 (7.7%) became dialysis dependent at 6 months and later died. Of the 4 non–dialysis-dependent patients with a moderately elevated SCr level (>3.0 mg/dL) at enrollment, 3 (75.0%) had died at 18 months and 1 maintained an elevated SCr level (5.2 mg/dL). Although none of the patients demonstrated normal eGFR during the study, the proportion with mildly decreased function (60–89 mL/min/1.73 m2) increased from 9.1% at time 0 to 23.5% at 18 months.
Figure.
Trends in Serum Creatinine Measurements Among 16 Survivors of a Diethylene Glycol Mass-Poisoning Event During Acute Illness (July–October 2006) and at 4 Serial Follow-up Evaluations (January 2007–July 2008)
The proportion of patients reporting subjective neurologic symptoms (general, upper extremity, lower extremity, and facial weakness) decreased over time (Table 3). The largest decline occurred during the first 12 months. Of note, 1patientwith severe lower extremity and facial motor deficits reported complete resolution by 18 months. Although 8 patients (30.8%) continued to experience subjective weakness at the 18-month evaluation, all reported subjective improvement in routine activities such as speaking, walking, writing, and holding objects.
Table 3.
Neurologic Features and Nerve Conduction Study Findings Among 32 Survivors of Diethylene Glycol Poisoning After Recovery From Acute Illness at Time 0 (Enrollment) and 6, 12, and 18 Monthsa
| Characteristic | Time 0 | Months |
P Value |
||
|---|---|---|---|---|---|
| 6 | 12 | 18 | |||
| No. of participants evaluated | 32 | 29b | 28 | 26 | |
| Self-reported symptomsc,d | |||||
| Generalized weaknesse | 28 (87.5) | 8 (27.6) | 11 (39.3) | 8 (30.8) | <.001 |
| Upper extremity weakness | 12 (37.5) | 3 (10.3) | 0 | 3 (12.5) | |
| Lower extremity weakness | 17 (53.1) | 8 (27.5) | 7 (26.9) | 5 (19.2) | |
| Facial weakness | 5 (15.6) | 2 (6.9) | 2 (7.1) | 2 (7.7) | |
| Neurologic examination findingsd | |||||
| Normale | 9 (28.1) | 8 (28.6) | 7 (26.9) | 6 (23.1) | .79 |
| Abnormal | 23 (71.9) | 20 (71.4) | 19 (73.1) | 20 (76.9) | |
| Motor deficits (any)e | 10 (31.3) | 8 (27.6) | 8 (28.6) | 9 (34.6) | .79 |
| Facial | 7 (21.9) | 6 (20.7) | 7 (25.0) | 6 (23.1) | |
| Bilateral upper extremities | 4 (12.5) | 2 (7.1) | 0 | 0 | |
| Bilateral lower extremities | 3 (9.4) | 2 (6.9) | 2 (7.1) | 1 (3.8) | |
| Sensory loss (any)e | 16 (50.0) | 11 (37.9) | 6 (21.4) | 8 (30.8) | .05 |
| Facial | 3 (9.4) | 2 (6.9) | 1 (3.6) | 3 (11.5) | |
| Bilateral upper extremities | 2 (6.3) | 1 (3.5) | 0 | 0 | |
| Bilateral lower extremities | 13 (40.6) | 11 (37.9) | 5 (17.9) | 5 (19.2) | |
| Nerve conduction study findingsd | |||||
| Normale | 3 (9.7) | 3 (11.1) | 2 (8.3) | 2 (8.3) | .81 |
| Abnormal | 28 (90.3) | 24 (88.9) | 22 (91.7) | 22 (91.7) | |
| Equivocal findings | 13 (46.4) | 12 (50.0) | 10 (45.5) | 10 (45.5) | |
| Predominantly demyelinating | 1 (3.6) | 1 (4.2) | 1 (4.6) | 1 (4.6) | |
| Predominantly axonal | 10 (35.7) | 6 (25.0) | 5 (22.70) | 5 (22.7) | |
| Mixed axonal and demyelinating | 4 (14.3) | 5 (20.8) | 6 (27.3) | 6 (27.3) | |
Values are presented as number (percentage) unless otherwise indicated.
One patient was unavailable for physical examination in July 2007 and not included in the neurologic examination findings for that period.
Although not systematically assessed, many patients anecdotally described subjective blurry vision and hearing loss.
Percentages reflect missing data.
Repeated-measures analysis performed.
Of the 10 patients with motor deficiencies on clinical examination at enrollment, 7 (70.0%) continued to have deficits at 18 months (5with facial motor deficits and 2with bilateral or unilateral lower extremity motor deficits). Of the 9 participants (28.1%) with normal examination findings at enrollment, none subsequently developed deficits. While the proportion of patients with abnormal neurologic examination findings remained stable over time, objective functional improvement in both motor and sensory deficits was observed. In fact, all patients with neurologic deficits at enrollment demonstrated improvement in motor function over time. This clinical improvement was accompanied by concomitant improvement or resolution of electrodiagnostic parameters.
Repeated-Measures Analysis
Among non–dialysis-dependent patients, neither the natural log of SCr nor eGFR values demonstrated significant differences over time. Among all participants, dialysis dependence (yes or no), neurologic examination results (abnormal or normal), nerve conduction study results (abnormal or normal), and any motor deficits (yes or no) did not differ significantly across periods. Any sensory loss (yes or no) significantly decreased over time (P = .05). Generalized weakness (yes or no) also declined significantly over time (P < .001).
Discussion
The short-term renal and neurologic effects of acute DEG poisoning have been well described; however, the long-term health outcomes among survivors are unknown. This gap limits physicians’ abilities to counsel DEG-poisoned patients on their prognosis and anticipate appropriate clinical interventions. It also hinders public health authorities directing resources during and after mass-poisoning events to support the long-term health care needs of a DEG-exposed population. Here, we demonstrate that renal function (as measured by SCr) improved within the first few months following DEG poisoning among survivors who did not develop dialysis dependence following resolution of their acute illness. Our models showed no significant differences in either SCr or eGFR values during the study period. However, these results are biased due to the deletion of dialysis-dependent cases at each time point and therefore cannot necessarily be generalized. Long-term renal complications after DEG-induced acute kidney injury were uncommon among persons who recovered renal function within 3 to 6 months after acute illness resolution. These observations are consistent with a single case series that observed 5 DEG-poisoned survivors for 26 months after exposure.5 In our study, survivors whose SCr returned to normal within 3 months after acute illness generally did not develop further evidence of nephrotoxicity for up to 2 years following exposure. Conversely, DEG-poisoned survivors who were dialysis dependent after acute illness resolution were unlikely to recover renal function later. Our findings also suggest that DEG-poisoned survivors with moderate SCr elevations or dialysis dependence after acute illness would benefit from close medical follow-up to prevent further health complications associated with ESRD.
The neurologic deficits we encountered among our patients were similar to those previously described in a few isolated case reports, including extremity motor deficits and severe bilateral peripheral facial motor deficits.1,5,13 Although not systematically assessed here, many patients incidentally described other symptoms previously associated with DEG poisoning, such as blurry vision and hearing loss.5 While neurologic symptoms persisted over time, improvement of function was evident and peaked in the first 6 to 8 months after acute illness. More important, patients with neurologic deficits at the beginning of our study did not experience recurrence, relapse, or worsening of their findings, and those without neurologic findings did not develop them subsequently. These findings greatly complement a small body of evidence5 suggesting that the long-term prognosis for recovery of neurologic function is promising, even after severe DEG-associated illness. Although the role of aggressive physical therapy was not assessed, we believe this would likely be of additional benefit.
Our results may not be generalizable since most of our patients were older adults with comorbidities, and we were unable to fully evaluate and exclude all other causes of renal insufficiency or neuropathy. In addition, all patients had access to tertiary-level care during their acute illness, which may have positively affected their recovery potential.
Conclusions
This study’s findings provide evidence for physicians and DEG survivors to anticipate the potential improvement in neurologic and renal sequelae following recovery from acute DEG poisoning. Public health authorities may assess the need for long-term medical resource planning (eg, hemodialysis machines) during a mass-poisoning event by determining the incidence and severity of renal impairment of exposed persons. Further studies are needed to understand the mechanism of DEG injury and identify interventions to maximize recovery after DEG poisoning since history suggests these outbreaks will continue to occur.
Acknowledgments
Additional Contributions: We gratefully acknowledge the tremendous help from all of our participants and support from the Panama Ministry of Health; Lauren Lewis, MD, MPH, Damon Del'Aglio, MD, and Bertram Ackermann, BS, Division of Environmental Hazards and Health Effects, National Centers for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia; Jorge Motta, MD, The Gorgas Memorial Institute, Panama City, Panama; Marisol Ng, MD, Donna Chen de Lee, MD, and Rosemary Jovane, Hospital Santo Tomás, Panama City, Panama; and Celia Gomez, MD, and Jose Manzanares, MD, Caja de Seguro Social, Panama City, Panama.
Footnotes
Author Contributions: Drs Schier and Kieszak had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Conklin, Sejvar, Victoria, Rodriguez, McGeehin, Schier.
Acquisition, analysis, or interpretation of data: Conklin, Sejvar, Kieszak, Sabogal, Sanchez, Flanders, Tulloch, Victoria, Rodriguez, Sosa, Schier.
Drafting of the manuscript: Conklin, Sejvar, Kieszak, Rodriguez, Schier.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Conklin, Kieszak, Flanders, Rodriguez.
Obtained funding: Conklin, Tulloch, Schier.
Administrative, technical, or material support: Conklin, Sejvar, Sabogal, Rodriguez, Sosa, McGeehin.
Study supervision: Conklin, Sejvar, Victoria, Rodriguez, Schier.
Conflict of Interest Disclosures: None reported.
Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Previous Presentation: Some of this content was presented orally at the 2011 Annual Meeting of the North American Congress of Clinical Toxicology; September 24, 2011;Washington, DC.
REFERENCES
- 1.Hasbani MJ, Sansing LH, Perrone J, Asbury AK, Bird SJ. Encephalopathy and peripheral neuropathy following diethylene glycol ingestion. Neurology. 2005;64(7):1273–1275. doi: 10.1212/01.WNL.0000156804.07265.1A. [DOI] [PubMed] [Google Scholar]
- 2.Scalzo AJ. Diethylene glycol toxicity revisited: the 1996 Haitian epidemic. J Toxicol Clin Toxicol. 1996;34(5):513–516. doi: 10.3109/15563659609028009. [DOI] [PubMed] [Google Scholar]
- 3.Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009;47(6):525–535. doi: 10.1080/15563650903086444. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari LA, Giannuzzi L. Clinical parameters, postmortem analysis and estimation of lethal dose in victims of a massive intoxication with diethylene glycol. Forensic Sci Int. 2005;153(1):45–51. doi: 10.1016/j.forsciint.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Alfred S, Coleman P, Harris D, Wigmore T, Stachowski E, Graudins A. Delayed neurologic sequelae resulting from epidemic diethylene glycol poisoning. Clin Toxicol (Phila) 2005;43(3):155–159. [PubMed] [Google Scholar]
- 6.Rollins YD, Filley CM, McNutt JT, Chahal S, Kleinschmidt-DeMasters BK. Fulminant ascending paralysis as a delayed sequela of diethylene glycol (Sterno) ingestion. Neurology. 2002;59(9):1460–1463. doi: 10.1212/01.wnl.0000032498.65787.8d. [DOI] [PubMed] [Google Scholar]
- 7.Landry GM, Martin S, McMartin KE. Diglycolic acid is the nephrotoxic metabolite in diethylene glycol poisoning inducing necrosis in human proximal tubule cells in vitro. Toxicol Sci. 2011;124(1):35–44. doi: 10.1093/toxsci/kfr204. [DOI] [PubMed] [Google Scholar]
- 8.Besenhofer LM, McLaren MC, Latimer B, et al. Role of tissue metabolite accumulation in the renal toxicity of diethylene glycol. Toxicol Sci. 2011;123(2):374–383. doi: 10.1093/toxsci/kfr197. [DOI] [PubMed] [Google Scholar]
- 9.Schier J, Hunt DR, Perala A, et al. Characterizing concentrations of diethylene glycol and suspected metabolites in human serum, urine, and cerebrospinal fluid samples from the Panama DEG mass poisoning. Clin Toxicol. 2013;51(10):923–929. doi: 10.3109/15563650.2013.850504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schier JG, Rubin CS, Miller D, Barr D, McGeehin MA. Medication-associated diethylene glycol mass poisoning: a review and discussion on the origin of contamination. J Public Health Policy. 2009;30(2):127–143. doi: 10.1057/jphp.2009.2. [DOI] [PubMed] [Google Scholar]
- 11.Rentz ED, Lewis L, Mujica OJ, et al. Outbreak of acute renal failure in Panama in 2006: a case-control study. Bull World Health Organ. 2008;86(10):749–756. doi: 10.2471/BLT.07.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Society of Nephrology. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–136. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 13.Geiling E, Cannon P. Pathologic effects of elixir of sulfanilamide (diethylene glycol) poisoning. JAMA. 1938;111(10):919–926. [Google Scholar]