Abstract
Androgen Receptor (AR)-dependent transcription is a major driver of prostate tumor cell proliferation. Consequently, it is the target of several antitumor chemotherapeutic agents, including the AR antagonist MDV3100/enzalutamide. Recent studies have shown that a single AR mutation (F876L) converts MDV3100 action from an antagonist to an agonist. Here we describe the generation of a novel class of Selective Androgen Receptor Degraders (SARDs) to address this resistance mechanism. Molecules containing hydrophobic degrons linked to small molecule Androgen Receptor (AR) ligands induce AR degradation, reduce expression of AR target genes and inhibit proliferation in androgen-dependent prostate cancer cell lines. These results suggest that selective AR degradation may be an effective therapeutic prostate tumor strategy in the context of AR mutations that confer resistance to third generation AR antagonists.
Keywords: Antiproliferation, cancer, drug design, hormones, protein degradation
Targeted degradation represents an intriguing strategy to regulate the function of therapeutically relevant proteins (e.g., transcription factors and scaffolding proteins) not amenable to traditional small molecule approaches[1,2]. Moreover, targeted protein degradation could overcome resistance mechanisms that modulate the activity of small molecule drugs following target engagement. For instance, point mutants conferring agonist activity to antagonists limit efficacy of androgen-receptor (AR) antagonists for treatment of prostate cancer[3]. While deletion of the disease-causing protein offers a direct solution to this problem, strategies for doing so via genome editing or RNAi remain clinically challenging[4,5]. As an alternative, we have developed several approaches for post-translational targeting of specific proteins to the ubiquitin-proteasome system (UPS)[6–9]. For instance, we recently reported a strategy for post-translational protein degradation whereby a hydrophobic moiety appended to the surface of a target protein engages the cellular quality control machinery. This ‘hydrophobic tag’ may mimic a partially denatured protein folding state, leading to degradation by the UPS. We demonstrated the feasibility of this approach by covalently coupling hydrophobic tags to engineered dehalogenase HaloTag-2[10–12] fusion proteins. Recently, a similar approach was applied to degradation of E. coli DHFR by non-covalent appendage of a hydrophobic tag[13]. A key next step in the development of this nascent technology is to degrade clinically relevant target proteins with a small drug-like molecule. To this end, here we show that coupling a hydrophobic tag to an androgen receptor agonist converts it to a potent Selective Androgen Receptor Degrader (SARD) capable of inducing >50% of AR degradation (DC50) at 1 μM. Remarkably, this SARD retained anti-proliferative activity in cell lines resistant to current standard-of-care drugs for castration-resistant prostate cancer (CRPC).
The androgen receptor (AR)[14] is a ligand-dependent transcription factor that upon binding to the androgen dihydrotestosterone (DHT), undergoes a conformational change leading to homodimerization, nuclear translocation and upregulation of gene transcription. While vital for the normal development and maintenance of the prostate, AR-mediated gene expression remains an important driver throughout prostate cancer progression. Many therapeutic strategies focus on regulating AR activity. For example, androgen deprivation therapy[15] combined with AR antagonists (i.e., anti-androgens) such as bicalutamide[16] has been used as a first-line treatment for early stage prostate cancer for decades. While initially effective at suppressing tumor growth, this strategy in most cases leads to the progression of an AR-dependent yet androgen independent form of the disease (i.e., CRPC)[17], which is responsible for the vast majority of prostate cancer deaths. Moreover, in CRPC, the first-generation anti-androgen drugs, such as flutamide[18] and bicalutamide[19], can display AR agonist activity. While the mechanisms responsible for the progression to CRPC are not entirely known, it has become clear that an increased level of AR protein is present in the majority of CRPC and that agents targeting androgen synthesis and/or AR signaling, such as abiraterone and MDV3100/enzalutamide, respectively, demonstrate clinical benefit to CRPC patients[20–22].
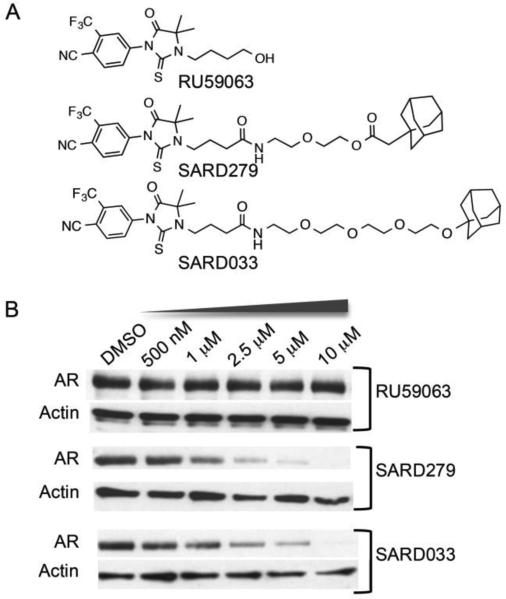
Hypothesizing that increased AR levels may drive the development of CRPC and considering the clinical success of the selective estrogen receptor degrader (SERD) fulvestrant[23] we sought to induce AR degradation via our hydrophobic tagging approach. To accomplish this, we designed a series of selective androgen receptor degraders (SARDs) based on the high affinity AR agonist RU59063[24] connected via a short PEG linker to an adamantyl group (Figure 1A), a hydrophobic degron shown to be effective in our previous work with Halotag fusion proteins.
Figure 1.
Figure 1. (A) Structures of Selective Androgen Receptor Degraders (SARDs) based on the androgen receptor agonist RU59063. (B) Immunoblot analyses of LNCaP human prostate tumor cells incubated with SARDS or parent ligand for 24 hours.
Gratifyingly, such heterodimeric molecules retained the ability to bind directly to the AR (Figure S1): competition radioligand binding assay using [3H]-R1881 showed that appending of the adamantyl group to RU59063 reduced affinity for the AR approximately 37-fold in the case of SARD279, and nearly 300-fold for SARD033. In accordance with their binding affinities, the synthesized SARDs induced AR degradation at sub-micromolar concentrations. For example, SARD279, in which the adamantyl moiety is coupled to RU59063 via a 8 atom ester linkage reduced AR protein levels by 50% at 1 μM (DC50) (Figure 1B), while no degradation was detected in cells treated with the parental AR ligand. SARD033, possessing an adamantyl moiety attached via a longer ether linkage induced AR degradation with a ~2 μM DC50 value (Figure 1B). SARD-mediated AR degradation requires direct interaction with AR since co-incubation with the competitive AR agonist RU59063 blocked the activity of SARD279 (not shown).
Predictably, target degradation by the SARDs is selective for the AR; the glucocorticoid receptor (GR), another steroid receptor not recognized by the parent ligand RU59063, is not degraded in LNCaP cells under conditions that result in near-complete degradation of the androgen receptor (Figure S2). Futhermore, consistent with our initial report on protein hydrophobic-tagging[10], degradation of the AR is dependent on the UPS – pretreatment/co-treatment of LNCaP cells with the proteasome-specific inhibitor, epoxomicin, prevents SARD-mediated degradation of the AR. To further explore the mechanism of SARD-mediated AR degradation, we investigated the possible involvement of Heat Shock Proteins (HSPs), given their known role in stabilizing misfolded proteins or targeting them for degradation by the UPS. We found that incubating cells with the potent Hsp90 inhibitor geldanamycin, at concentrations that did not affect AR levels, enhanced AR degradation at sub-DC50 concentrations of SARD279 (Figure S3). Immunoblotting revealed a geldanamycin-dependent increase in Hsp70 levels, consistent with the finding that Hsp90 inhibition leads to activation of Heat Shock Factor 1 (HSF1) and its target genes, including HSP70[25]. Hsp70 and the associated E3 ubiquitin ligase CHIP play a key role in targeting intractably misfolded proteins to the UPS[26], and we have shown that labeling HaloTag with hydrophobic tags leads to enhanced association with Hsp70[27]. Together, this suggests that the Hsp70/CHIP complex mediates SARD-induced AR degradation and that elevated Hsp70 levels underlie the increase in SARD279 activity in the context of Hsp90 inhibition.
We next examined the effects of SARDS on AR-dependent gene expression in AR overexpressing LNCaP-AR cells, a validated cellular model of CRPC. Consistent with the documented AR agonist activity of RU59063 in CRPC, we observed increasing expression of a number of AR target genes such as FKBP52, PMPEA1 and NKX3.1 (Figure S4). Despite the agonist activity of RU59063, no such agonist activity was observed for SARD279- and SARD033-treated cells. Moreover, both SARDs antagonized gene expression induced by the synthetic androgen R1881. Importantly, with respect to this latter activity, both SARDS compared favorably to the recently FDA approved Selective Androgen Receptor Modulator (SARM) MDV3100/enzalutamide [21]. Given that both SARDs reduced expression of the AR target genes, we directly evaluated their pharmacological properties in androgen responsive element (ARE)-driven luciferase reporter assay in 293 cells. We determined that, despite their construction from an AR agonist, both SARDs blocked transactivation of the androgen receptor by the agonist R1881 (Figure S5). That the IC50 values of the SARDs in reporter assay -- 156 nM for SARD279 and 293 nM for SARD033 – were 10-fold lower than their DC50 values to degrade AR suggests that their ability to block expression of AR target genes may be multimodal: partly due to pharmacological antagonism of AR signaling and partly due to degradation of the AR itself.
We next asked whether SARD-induced AR degradation reduces proliferation of prostate cancer cells. We incubated androgen-dependent LnCAP cell with SARD279, SARD033, RU59063, or MDV3100 and measured cell numbers by vital dye staining and hemocytometry over the course of 9 days. In LNCaP cells, SARD279 and SARD033 both suppressed proliferation with similar efficacy to MDV3100 within 2 days (Figure 2). In contrast, proliferation of the AR-independent HEK293T and PC3 human prostate cancer cell lines was unaffected by SARD279, SARD033 or MDV3100 (Figure S6). The ability of these SARDs to specifically suppress AR-dependent proliferation argues that their activity results from targeted AR degradation rather than a non-specific cytotoxicity. Despite the initial efficacy of MDV3100 against AR-driven CRPC, within a year patients usually progress with an apparent reactivation of AR signaling, as exhibited by an increase in circulating PSA levels and radiographic tumor progression[28]. Numerous mechanisms have been proposed to explain this resistance, including intratumoral androgen synthesis that competes with MDV3100 binding as well as the F876L mutation in AR that enables the receptor to recognize MDV3100 as an agonist [3,29–31]. We sought to determine whether SARDs can circumvent resistance associated with these two mechanisms.
Figure 2.
SARDs block human prostate cancer cells (LNCaP) as well as MDV3100. LNCaP cell proliferation assay in the presence of indicated compounds at 3 μM. The assay was performed in 10% FBS, and viable cells were counted at each indicated day.
While MDV3100 is capable of antagonizing AR activity at castration levels of testosterone (less than 0.7 nM), studies have demonstrated that prostate cancer cells themselves upregulate de novo dihydrotestosterone (DHT) synthesisl[31,32]. In fact, 1 nM R1881 can abolish the antiproliferative activity of MDV3100 when assayed in cellular models of prostate cancer[21]. Hypothesizing that a SARD only needs to make a transient interaction with AR to enable its ubiquitination, we tested the activity of SARD279 and MDV3100 against proliferating LNCaP cells supplemented with 1 nM R1881. As expected, this level of R1881 blocked MDV3100 from antagonizing AR, whereas SARD279 exhibited antiproliferative activity (Figure 3). These data show that MDV3100 is ineffective in the presence of higher androgen levels whereas a SARD is still capable of degrading AR and inhibiting proliferation.
Figure 3.
SARD279 has more antiproliferative activity than MDV3100 in the presence of low androgen levels. LNCaP cells supplemented with 1 nM of the AR agonist R1881 were incubated for 7-days with MDV3100 or SARD279 after which cell proliferation was measured. The growth media contained 5% charcoal stripped FBS to eliminate exogenous androgens.
Another emerging mechanism of MDV3100 resistance is the F876L mutation in the AR, where the resistance is achieved by shifting AR responsiveness to MDV3100 and the like from antagonists to an agonists. Indeed, in LNCaP cells engineered to express the AR-F876L mutation (LNCaP/AR-F876L) MDV3100 functions as a robust agonist inducing the expression of PSA mRNA in the absence of androgens (Figure S7). Not surprisingly, eliminating AR with a SARD demonstrates no such activation. Consistent with the AR activity data, the proliferation of these cells can be induced with MDV3100 whereas SARD279 exhibits antiproliferative effects (Figure 4). Together, these data demonstrate that eliminating AR with a SARD can overcome resistance mechanisms associated with MDV3100.
Figure 4.
SARD-mediated antiproliferative activity in MDV3100-resistant cells. LNCaP/AR-F876L cells were incubated with indicated doses of MDV3100 or SARD279 for 7 days in the presence of 5% charcoal stripped FBS.
Here, we show that linking a hydrophobic tag-based degron to an AR agonist creates a novel class of anti-androgens (termed SARDs) that induce targeted AR degradation and override some resistance mechanisms to traditional prostate cancer drugs. Thus, SARDs may prove to be novel therapeutic approach to combat CRPC: since they remove AR, and thus block a key mitogenic pathway in prostate cancer cells.
This SARD approach is not expected to address drug resistant prostate cells expressing AR splice variant 7 lacking the ligand binding domain. However, our data suggest that in cells with an intact AR, if the residency time of an antagonist is insufficient – as is the case with MDV3100 in the milieu of increased androgens – SARD-mediated degradation of AR can be achieved with essentially the same AR binding ligand. The hydrophobic tagging strategy described here adds to the emerging paradigm of induced protein degradation as a therapeutic strategy9 and may prove useful for the manipulation of disease-relevant proteins, and related drug-resistant mutants, that have proven intractable to traditional small molecule approaches.
Supplementary Material
Acknowledgements
We are grateful to GlaxoSmithKline for continued support and helpful discussion. This work was supported by the NIH (T32GM067543, F32GM10052101, R01CA139818, R01AIO84140) and the DoD (W81XWH-12-1-0484). A.G.R. was a Leopoldina-Nationale Akademie der Wissenschaften Postdoctoral Fellow.
Footnotes
Conflict of Interest Statement: C.M.C consults for Canaan Partners and Arvinas, LLC. C.M.C. and K.R. have stock in Arvinas, LLC.
Contributor Information
Jeffrey L. Gustafson, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111
Taavi K. Neklesa, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111
Carly S. Cox, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111
Anke G. Roth, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111
Dennis L. Buckley, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111
Hyun Seop Tae, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111.
Thomas B. Sundberg, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111
D. Blake Stagg, Department of Pharmacology and Cancer Biology Duke University School of Medicine Durham, NC 277102.
John Hines, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111.
Donald P. McDonnell, Department of Pharmacology and Cancer Biology Duke University School of Medicine Durham, NC 277102
John D. Norris, Department of Pharmacology and Cancer Biology Duke University School of Medicine Durham, NC 277102
Craig M. Crews, Departments of Molecular, Cellular, and Developmental Biology, Chemistry, and Pharmacology Yale University New Haven, Connecticut, 065111.
References
- 1.Lin YH, Pratt MR. Chembiochem. 2014;15:805–809. doi: 10.1002/cbic.201400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crews CM. Chem. Biol. 2010;17:551–555. doi: 10.1016/j.chembiol.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, Chen Y, Greene GL, Shen Y, Sawyers CL. Elife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DH, Rossi JJ. Nat Rev Genet. 2007;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 5.Davidson BL, McCray PB. Nat Rev Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corson TW, Aberle N, Crews CM. ACS Chem. Biol. 2008;3:677–692. doi: 10.1021/cb8001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneekloth JS, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, Crews CM. J. Am. Chem. Soc. 2004;126:3748–54. doi: 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- 8.Schneekloth AR, Pucheault M, Tae HS, Crews CM. Bioorg Med Chem Lett. 2008;18:5904–5908. doi: 10.1016/j.bmcl.2008.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley DL, Crews CM. Angew. Chem. Int. Ed. Engl. 2014;53:2312–30. doi: 10.1002/anie.201307761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neklesa TK, Tae HS, Schneekloth AR, Stulberg MJ, Corson TW, Sundberg TB, Raina K, a Holley S, Crews CM. Nat. Chem. Biol. 2011;7:538–43. doi: 10.1038/nchembio.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tae HS, Sundberg TB, Neklesa TK, Noblin DJ, Gustafson JL, Roth AG, Raina K, Crews CM. Chembiochem. 2012;13:538–541. doi: 10.1002/cbic.201100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.V Los G, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, et al. ACS Chem. Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 13.Long MJC, Gollapalli DR, Hedstrom L. Chem. Biol. 2012;19:629–637. doi: 10.1016/j.chembiol.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinlein CA, Chang C. Endocr. Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 15.Huggins C, V Hodges C. Cancer Res. 1941;1:293–297. [Google Scholar]
- 16.Schellhammer PF. Expert Opin. Pharmacother. 2002;3:1313–1328. doi: 10.1517/14656566.3.9.1313. [DOI] [PubMed] [Google Scholar]
- 17.Feldman BJ, Feldman D. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Kelly WK. J. Clin. Oncol. 1993;11:1566–1572. doi: 10.1200/JCO.1993.11.8.1566. [DOI] [PubMed] [Google Scholar]
- 19.Small EJ, Carroll PR. Urology. 1994;43:408–410. doi: 10.1016/0090-4295(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto H, Rahman MM, Chang C. J. Cell. Biochem. 2004;91:3–12. doi: 10.1002/jcb.10757. [DOI] [PubMed] [Google Scholar]
- 21.Tran C, Ouk S, Clegg NJ, Chen Y, a Watson P, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, et al. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 23.Croxtall JD, McKeage K. Drugs. 2011;71:0–363. doi: 10.2165/11204810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Teutsch G, Goubet F, Battmann T, Bonfils A, Bouchoux F, Cerede E, Gofflo D, Gaillard-Kelly M, Philibert D, Steroid Biochem J. Mol. Biol. 1994;48:111–119. doi: 10.1016/0960-0760(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim HR, Kang HS, Kim HD. IUBMB Life. 1999;48:429–433. doi: 10.1080/713803536. [DOI] [PubMed] [Google Scholar]
- 26.McDonough H, Patterson C. Cell Stress Chaperones. 2003;8:303–308. doi: 10.1379/1466-1268(2003)008<0303:calbtc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neklesa TK, Noblin DJ, Kuzin A, Lew S, Seetharaman J, Acton TB, Kornhaber G, Xiao R, Montelione GT, Tong L, et al. ACS Chem Biol. 2013;8:2293–2300. doi: 10.1021/cb400569k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, Chen Y, Grillot K, Bischoff ED, Cai L, et al. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3948. DOI 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan A, Dong Y, Zhang H, Qi Y, Balk SP, Sartor O. Cancer Treat Rev. 2014;40:426–433. doi: 10.1016/j.ctrv.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, et al. Cancer Discov. 2013;3:1030–1043. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 31.Dillard PR, Lin MF, Khan SA. Mol Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishizaki F, Nishiyama T, Kawasaki T, Miyashiro Y, Hara N, Takizawa I, Naito M, Takahashi K. Sci Rep. 2013;3:1528. doi: 10.1038/srep01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.