Abstract
Background
The Prague C & M Criteria, developed for the endoscopic grading of Barrett's esophagus (BE), (C=circumferential length, M=maximal length) were previously validated among a panel of 30 expert endoscopists with a special interest in BE. Its performance among gastroenterology (GI) trainees is unknown.
Objective
To test inter-observer agreement among GI trainees for the Prague C & M criteria, identification of the gastroesophageal junction (GEJ) and the diaphragmatic hiatus.
Design
A prospective study.
Setting
Two tertiary-referral centers.
Patients and Interventions
Standardized endoscopic videos were used.
Main Outcome Measurements
Inter-observer agreement
Results
18 high quality videos (normal esophagus, short and long lengths of BE: equally distributed) were independently evaluated by 18 GI trainees [Year 1 (5), Year 2 (6), Year 3 (7)] after administration of a formal teaching module by an expert endoscopist. Overall intraclass correlation coefficients (ICC) for assessment of the C & M extent of the endoscopic BE segment above the GEJ were 0.94 (0.89-0.98) and 0.96 (0.94-0.98), respectively. The overall ICC for GEJ and diaphragmatic hiatus location recognition were 0.92 (0.86-0.96) and 0.90 (0.82-0.95) respectively. The year of training did not affect inter-observer agreement.
Limitations
The use of videos for endoscopic evaluation.
Conclusion
After standardized teaching, the Prague C&M criteria have high overall validity among gastroenterology trainees irrespective of the level of training for endoscopic evaluation of visualized BE lengths as well as key endoscopic landmarks.
Background
Barrett's Esophagus (BE) is a premalignant condition defined by the transformation of normal esophageal squamous epithelium to columnar epithelium containing goblet cells, known as intestinal metaplasia (1). BE is detected in approximately 5-15% of patients with chronic gastroesophageal reflux disease (GERD), however, the exact prevalence of BE in the general population is unknown (2). Recent estimates suggest approximately 0.4-1.6% of adults in the Western population may harbor this condition (3). BE is the most important identifiable risk factor for progression to esophageal adenocarcinoma (EAC); one of the fastest rising incidence cancers with a dismal 5-year survival rate of approximately 10-15% (4-9). Consequently, patients with BE are routinely enrolled in surveillance programs to detect dysplasia with the intent of identifying early stage disease and improving overall survival.
The initial step in diagnosing BE is the accurate endoscopic recognition of columnar lined esophagus (CLE). An accurate endoscopic evaluation of BE is critical as precise assessment of the length of intestinal metaplasia affects prognosis and influences the extent of biopsies for histologic sampling. Several studies have evaluated the risk of dysplasia and cancer in relation to the length of BE (9-11). A recent multicenter project suggested a 21% increase in the risk of high grade dysplasia (HGD) and EAC for every 1 cm increase in BE length and demonstrated BE length as a significant predictor of progression to HGD/EAC in patients with non-dysplastic Barrett's esophagus (11). Traditionally, there has not been a validated method for the endoscopic detection and classification of BE thus leading to a significant amount of variation in describing endoscopic findings related to BE (12,13). The Prague C&M Criteria was developed to standardize the endoscopic grading of BE as opposed to the use of subjective terms such as “long”, “short”, or “ultra-short” (14). A universally accepted standardized endoscopic grading system for BE is of vital importance to facilitate accurate recognition and optimal treatment.
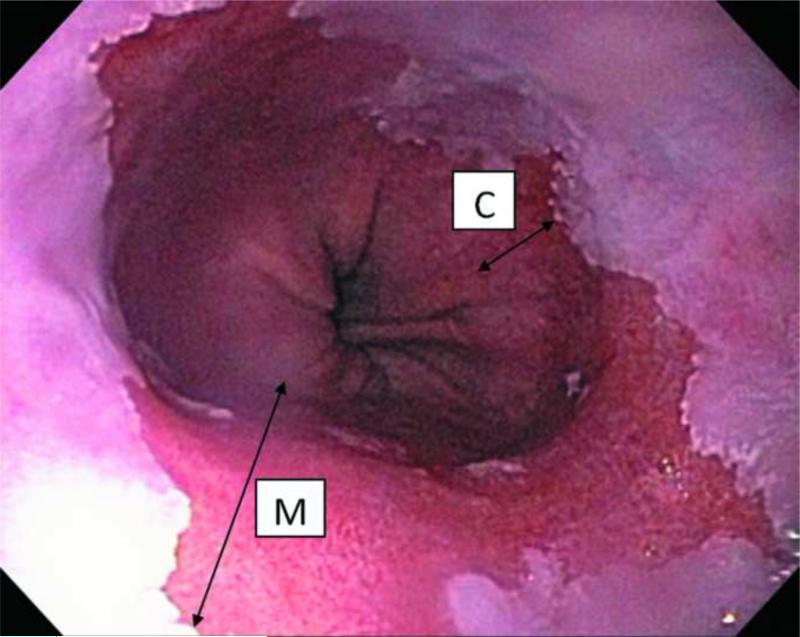
To address the need for a standardized approach to endoscopic grading, a subgroup of the International Working Group for the Classification of Oesophagitis (IWGCO) with a special interest in BE developed the Prague C&M Criteria which uses the “C” value as the “circumferential extent” and the “M” value as “maximal extent” of BE above the gastroesophageal junction (GEJ) in centimeters (Fig. 1). The criteria were initially validated by 29 expert endoscopists with a special interest in BE from 14 countries using a standard scoring form to grade 29 video sequences (15). This yielded high intraclass correlation coefficient (ICC) values of 0.94 (95%CI 0.91-0.97) and 0.93 (95%CI 0.89-0.96) for the C & M extent of the endoscopic BE segment above the GEJ, respectively. Near equally high ICC values of 0.88 (95%CI 0.82- 0.93) and 0.85 (95%CI 0.78-0.91) were seen for critically important endoscopic landmarks such as the GEJ and diaphragmatic hiatus, respectively (15). Although these agreements were impressive, results may have been biased as the assessments were performed by expert endoscopists with a special interest in BE. The performance of the Prague C&M criteria among less experienced endoscopists in GI fellowship training has not been examined.
Figure 1.
Endoscopic view of Barrett's esophagus showing C: extent of circumferential metaplasia and M: maximal extent of metaplasia.
The aim of this study was to determine whether the high inter-observer agreement seen with experts using the Prague C&M criteria could be reproduced among gastroenterology (GI) trainees after standardized teaching.
Methods
Setting
The study was conducted at two separate tertiary care teaching institutions (The University of Kansas School of Medicine and Washington University School of Medicine) involving GI trainees at all levels of training.
Material/Training
A one hour teaching session using an instructional DVD provided by the IWGCO was conducted by the senior author (P.S.), who was involved in the development of the Prague C&M criteria. The assessors (GI trainees) were orientated to the Prague C&M criteria, which included its application as well as review of recognition of endoscopic landmarks including the squamo-columnar junction, top of the gastric folds (GEJ), and the diaphragmatic pinch. Given that the recognition of the GEJ is crucial to the endoscopic detection of BE, in this study, the proximal extent of the gastric folds was selected to represent the GEJ (16). Instructions were also provided regarding the measurement of contiguous segments of BE only, with exclusion of islands of squamous and columnar mucosa from the assessment. In the teaching videos, landmarks were highlighted by the use of freeze-framing and superimposition of lines and arrows edited onto the image. An open discussion was permitted and adequate opportunity was provided to all assessors to have their questions answered.
Inter-observer agreement was assessed with the use of 18 video sequences of normal esophagus as well as suspected long and short segment BE used during the initial study validating the Prague Criteria among experts. A strict protocol was used in the initial making of the videos including monitoring and recording, in a standardized manner, the depth of endoscope insertion as judged by the centimeter markings at the bite block. The assistant's documentation of endoscope insertion depth was audio recorded directly onto the videotape during image acquisition. Endoscopic images were gathered at each centimeter of depth of insertion with the endoscope being maintained at each level for enough time to display all findings. During the withdrawal phase of the procedure, the endoscopist then moved the endoscope gradually, with the assistant saying “moving” as this was done and also providing the depth of insertion to which the endoscope had been moved. Air insufflation was maintained to provide good visualization of endoscopic landmarks and mucosa but insufficient to efface the gastric mucosal folds. All videos were recorded in a standardized manner using standard white light endoscopy, at a gradual pull back rate, with no indicators of endoscopic landmarks relevant to judging the presence and extent of BE from different endoscope manufacturers (Olympus, Fujinon, or Pentax). The endoscope insertion depth was highlighted in numeric display in centimeters at the top left-hand corner of the video image. The reviewers were then asked to view the videos and record their findings on an assessment score sheet for analysis (Fig. 2).
Figure 2.
Case report form (CRF) used by assessors for analysis of endoscopic videos
Video Evaluation using the Prague C & M Criteria
Reviewers (GI trainees) were required to identify the location of critical endoscopic landmarks in each video and record their locations in centimeters. This location was determined to be the centimeter marking just before when the observed landmark comes into full-view on endoscopic withdrawal. The C value is the circumferential extent of BE whereas the M value represents the maximal length at the proximal margin of the longest tongue-like segment of BE above the GEJ. The assessors documented the location of the diaphragmatic hiatus, top of the gastric folds which signifies the GEJ, the “C value” and “M value” of the BE as well as the video quality on a case report form (CRF) (Fig. 1). The quality of videos was rated by all the trainees as part of their assessment of the videos.
Statistical analysis
Inter-observer agreement for the assessment of the C & M extent of the endoscopic BE segment above the GEJ as well as other endoscopic landmarks were expressed as intraclass correlation coefficients (ICCs) with 95 percent confidence intervals (CIs). ICC is a general measurement of agreement of consensus from a group of two or more raters. An ICC of 1 indicates perfect agreement, with lower values commonly being interpreted as follow: >0.8 excellent, 0.8-0.6 good, 0.6-0.4 fair, and <0.4 poor. It is important to be aware that there are different statistical forms of ICC, the use of which is dependent on the study design and the rating system being assessed. In this study, we used a random effects two- way analysis of variance (ANOVA) model because we designed the study to be representative of a random sample of endoscopists each of whom rated the same random set of endoscopy videos. This design was chosen so the results would be generalizable to other similar raters undertaking clinical endoscopic assessment of patients with similar metaplastic lesions. The unit of analysis for these models was individual endoscopist ratings because clinical assessments, and use of the Prague C&M Criteria, are usually conducted by a single endoscopist, as opposed to a group of raters who could provide a mean rating. ICCs and 95% CIs were estimated using the icc23 command in STATA 11 (StataCorp LP, College Station, TX). In addition to estimating ICCs for all endoscopists together, we stratified our analyses by geographic location, training level (year 1, 2, and 3), and BE length (<1 cm and ≥1 cm). To ensure that the sample size was adequate for estimation, 18 assessors were included from the two centers. A minimally acceptable level of agreement (p0) was set at 0.6, and a rho value (p1) of ≥ 0.8 was expected for a 1 cm difference in observations between assessors. 18 videos were evaluated in order to detect this difference at α= 0.05 and β=0.2 per the methods proposed by Walter et al. (17)
Results
A panel of 18 gastroenterology trainees were recruited for the study. Nine GI fellows [Year 1 (n=3), Year 2 (n=3), Year 3 (n=3)] from the University of Kansas School of Medicine and nine GI fellows [Year 1 (n=2), Year 2 (n=3), Year 3 (n=4)] from Washington University School of Medicine participated in the study, totaling 18 GI fellows [Year 1 (n=5), Year 2 (n=6), Year (n=7) between the two centers. The ratings on the video quality were as follows: 30.2% were rated excellent, 48.5% good, 18.2% fair, and 3.1% poor. None of the videos were rated as “not scorable.”
Prague C & M Criteria inter-observer agreement
The overall ICC values for the assessment of the C & M extent of the endoscopic BE segment above the GEJ were 0.94 (95%CI 0.89-0.98) for the “C” extent and 0.96 (95%CI 0.94-0.98) for the “M” extent (Table 1). Institutional ICC values for the assessment of the C & M extent of the endoscopic BE segment above the GEJ for the University of Kansas School of Medicine and Washington University School of Medicine in Saint Louis were 0.93 (95%CI 0.86-0.97) and 0.97 (95%CI 0.95-0.99) for the “C” extent, respectively and 0.96 (95%CI 0.92-0.98) and 0.98 (95%CI 0.96-0.99) for the “M” extent, respectively (Table 1). Furthermore, the ICC values among the different levels of training for “C” and “M” extent were 0.94 (95%CI 0.87-0.98) and 0.96 (95%CI 0.91-0.98) for first year, respectively, 0.96 (95%CI 0.92- 0.99) and 0.97 (95%CI 0.95-0.99) for second year, respectively and 0.94 (95%CI 0.88-0.98) and 0.97 (95%CI 0.94-0.99) for third year, respectively (Table 2). These ICCs represent excellent levels of agreement with no significant difference found between the two centers for either the C or M grading and regardless of the year of endoscopic training.
Table 1.
Intraclass Correlation Coefficients for C&M Extent and Endoscopic Landmarks Overall and Stratified by Geographic Location
| Parameters | Overall (n=18) | Kansas University (n=9) | Washington University (n=9) |
|---|---|---|---|
| Circumferential extent of Barrett's segment (C value) | 0.94 (0.89-0.98) | 0.93 (0.86-0.97) | 0.97 (0.95-0.99) |
| Maximum extent of Barrett's segment (M value) | 0.96 (0.94-0.98) | 0.96 (0.92-0.98) | 0.98 (0.96-0.99) |
| Location of GEJ | 0.92 (0.86-0.96) | 0.90 (0.81-0.96) | 0.96 (0.93-0.98) |
| Location of the diaphragmatic hiatus | 0.90 (0.82-0.95) | 0.90 (0.83-0.96) | 0.90 (0.83-0.96) |
Table 2.
Intraclass Correlation Coefficients of the C&M Extent and Endoscopic Landmarks Stratified by Year of Endoscopic Training
| Parameters | Year of Endoscopic Training | ||
|---|---|---|---|
| Year 1 (n=5) | Year 2 (n=6) | Year 3 (n=7) | |
| Circumferential extent of Barrett's segment (C value) | 0.94 (0.87-0.98) | 0.96 (0.92-0.99) | 0.94 (0.88-0.98) |
| Maximum extent of Barrett's segment (M value) | 0.96 (0.91-0.98) | 0.97 (0.95-0.99) | 0.97 (0.94-0.99) |
| Location of GEJ | 0.88 (0.78-0.95) | 0.95 (0.91-0.98) | 0.92 (0.84-0.97) |
| Location of the diaphragmatic hiatus | 0.88 (0.79-0.95) | 0.88 (0.78-0.94) | 0.91 (0.84-0.96) |
Assessors were able to agree on the presence of endoscopic BE ≥1 cm in length with excellent agreement [ICC 0.95 (95%CI 0.90-1.01)]. However, the recognition of endoscopic BE <1 cm in length had poor agreement [ICC 0.15 (95%CI 0.00-0.48)].
Identification of endoscopic landmarks
During the study the proximal extent of the gastric folds was selected to represent the GEJ. The overall ICC values for the recognition of the GEJ and diaphragmatic hiatus were 0.92 (95%CI 0.86-0.96) and 0.90 (95%CI 0.82-0.95), respectively (Table 1). Institutional ICC values for recognizing the location of the GEJ for the University of Kansas School of Medicine and Washington University School of Medicine were 0.90 (95%CI 0.81-0.96) and 0.96 (95%CI 0.93-0.98), respectively and 0.90 (95%CI 0.83-0.96) and 0.90 (95%CI 0.83-0.96), respectively (Table 1). The ICC values among the different levels of training for identification of the GEJ and diaphragmatic hiatus was 0.88 (95%CI 0.78-0.95) and 0.88 (95%CI 0.79-0.95) for first years, respectively, 0.95 (95%CI 0.91-0.98) and 0.88 (95%CI 0.78-0.94) for second years, respectively and 0.92 (95%CI 0.84-0.97) and 0.91 (95%CI 0.84-0.96) for third years, respectively (Table 2). These ICCs represent excellent levels of agreement with no significant difference found between the two centers for identifying both the GEJ and diaphragmatic hiatus, regardless of the year of endoscopic training.
Discussion
Traditionally, there has been a significant amount of variation in the endoscopic description and classification of BE (12,13). The Prague C&M Criteria were developed to standardize the endoscopic grading of BE rather than using subjective nomenclature (14). The prevalent use of a standardized and practical criteria would allow for the accurate endoscopic evaluation of BE potentially leading to improved clinical management including risk stratification and treatment as well as for research purposes. The initial study performed with expert endoscopists with a special interest in BE revealed excellent inter-observer ICCs of 0.94, 0.93, 0.88, and 0.85 for the C value, M value, proximal extent of the gastric folds and diaphragmatic hiatus, respectively (15). This excellent inter-observer agreement has since been reproduced in an Asian multinational study of 34 endoscopists (18). The Prague C&M Criteria has since been validated as a method for endoscopic grading of BE, however, this is among expert endoscopists and its applicability and agreement among GI trainees with limited endoscopic experience is unknown.
In this multicenter study involving 18 GI trainees who received a formal 1 hour interactive teaching module, the Prague C&M criteria demonstrated excellent inter-observer agreement with regards to the grading of endoscopic BE as well as the identification of the proximal extent of the gastric folds and diaphragmatic hiatus. These results are consistent with the findings of previous studies using the Prague C&M criteria among experts. To our knowledge, this is the first study to systematically evaluate the endoscopic grading of BE with the use of the Prague C&M criteria by inexperienced endoscopists. The excellent ICC values, regardless of the year of endoscopic training, illustrate the lack of a substantial learning curve and that the one hour teaching session was adequate to instruct trainees on the criteria. The absence of a learning curve and the excellent ICC values observed in this study among trainee endoscopists indicate that the Prague C&M criteria and its associated terminology were easy to interpret and demonstrate its applicability to the routine practice of BE evaluation and management.
A uniform endoscopic classification system is advantageous for clinical practice as it allows for accurate assessment of the length of BE which is crucial for surveillance with the accurate number and location of biopsy specimens. This is key given that BE requires histologic confirmation and it has been shown that the yield of intestinal metaplasia in BE is dependent upon the length of the segment, the use of endoscopy with advanced imaging modalities and number of biopsies (19-21).
Although this investigation demonstrated excellent inter-observer agreement; we acknowledge potential limitations. One limitation is the use of video clips for all evaluations rather than live endoscopy. Given the technical difficulty of simulating live assessment among 18 trainees, review of high quality, standardized endoscopic videos provided a suitable alternative. Additionally, the freeze- frame capability of the videos allowed the advantage of detailed review by the evaluators. The depth of endoscope insertion was judged by markings at the bite block and variable positioning of the bite block could create subtle differences in measurement. Another limitation is that the Prague C&M criteria describes only the length of the endoscopically presumed BE and not the actual surface area, which may be more critical with regards to prognosis/progression of neoplastic transformation (8,9,22,23). The use of advanced imaging modalities such as NBI in assisting the detection of BE was not assessed. Finally, the 1st year trainees were in the 2nd half of their clinical training, had performed approximately 200-300 upper endoscopies and all trainees were using the Prague criteria in clinical practice – this familiarity with the grading system may have accounted for the lack of differences seen among the trainees at different levels.
In conclusion, after standardized teaching, the Prague C&M criteria have high overall validity among gastroenterology trainees regardless of the level of training for the endoscopic evaluation of visualized BE lengths as well as key endoscopic landmarks. The Prague C&M criteria has been shown to be reliable in assessing the extent of endoscopic BE and can be performed after a short training exercise. In conclusion, these criteria can easily be adopted in clinical practice given their high overall agreement among expert and trainee endoscopists.
Acronyms
- BE
Barrett's esophagus
- GERD
Gastroesophageal reflux disease
- EAC
Esophageal adenocarcinoma
- CLE
Columnar lined esophagus
- HGD
High grade dysplasia
- IWGCO
International Working Group for the Classification of Oesophagitis
- GEJ
Gastroesophageal junction
- ICC
Intraclass correlation coefficient
- GI
Gastroenterology
- CRF
Case report form
- CI
Confidence intervals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma P. Clinical Practice. Barrett's esophagus. N Engl J Med. 2009;361(26):2548–56. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 2.Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett's esophagus in high-risk patients with chronic GERD. Gastrointest Endosc. 2005;61:226–31. doi: 10.1016/s0016-5107(04)02589-1. [DOI] [PubMed] [Google Scholar]
- 3.Pondugula K, Wani S, Sharma P. Barrett's esophagus and esophageal adenocarcinoma in adults: long-term GERD or something else? Curr Gastroenterol Rep. 2007;9(6):468–474. doi: 10.1007/s11894-007-0061-9. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastro-esophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340(11):825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 5.Solaymani-Dodaran M, Logan RFA, West J, et al. Risk of oesophageal cancer in Barrett's oesophagus and gastroesophageal reflux disease. Gut. 2004;53(8):1070–1074. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk GW. Barrett's esophagus. Gastroenterology. 2002;122(6):1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 7.Devesa SS, Blot WJ, Fraumeni JF., Jr. Changing patterns in incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 8.Iftikhar SY, James PD, Steele RJ, et al. Length of Barrett's oesophagus: an important factor in the development of dysplasia and adenocarcinoma. Gut. 1992;33:1155–1158. doi: 10.1136/gut.33.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menke-Pluymers MB, Hop WC, Dees J, et al. Risk factors for the development of an adenocarcinoma in columnar-lined (Barrett) esophagus. The Rotterdam Esophageal Tumor Study Group. Cancer. 1993;72:1155–1158. doi: 10.1002/1097-0142(19930815)72:4<1155::aid-cncr2820720404>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett's esophagus. Ann Intern Med. 2000;132(8):612–620. doi: 10.7326/0003-4819-132-8-200004180-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gaddam S, Young PE, Alsop BR, et al. Relationship between Barrett's Esophagus (BE) length and the risk of high grade dysplasia (HGD) and esophageal adenocarcinoma (EAC) in patients with non dysplastic Barrett's Esophagus results from a large multicenter cohort. Gastroenterology. 2011;140(5,suppl 1):S–81. [Google Scholar]
- 12.Dekel R, Wakelin DE, Wendel C, et al. Progression or regression of Barrett's esophagus—is it all in the eye of the beholder? Am J Gastroenterol. 2003;98:2612–2615. doi: 10.1111/j.1572-0241.2003.07680.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett's esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology. 1994:945–949. [PubMed] [Google Scholar]
- 14.Sharma P, Morales TG, Sampliner RE. Short segment Barrett's esophagus—the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol. 1998;93:1033–1036. doi: 10.1111/j.1572-0241.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C&M criteria. Gastroenterology. 2006;131(5):1392–9. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 16.McClave SA, Boyce HW, Jr, Gottfried MR. Early diagnosis of columnar-lined esophagus: a new endoscopic criterion. Gastrointest Endosc. 1987;33:413–416. doi: 10.1016/s0016-5107(87)71676-9. [DOI] [PubMed] [Google Scholar]
- 17.Shrout & Fleiss. Intraclass correlations: Uses in assessing rater reliability. Psychological Bullentin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 18.Visintainer PF, Orozco LC. ICC23: Stata module for calculations of intraclass correlation coefficient models 2 and 3 as described in Shrout and Fleiss. 1979 [Google Scholar]
- 19.Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101–110. doi: 10.1002/(sici)1097-0258(19980115)17:1<101::aid-sim727>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Lee YC, Cook MB, Bhatia S, et al. Interobserver reliability in the endoscopic diagnosis and grading of Barrett's esophagus: an Asian multinational study. Endoscopy. 2010;42:699–704. doi: 10.1055/s-0030-1255629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borovicka J, Fischer J, Neuweiler J, et al. Autofluorescense endoscopy in surveillance of Barrett's esophagus: a multicenter randomized trial on diagnostic efficacy. Endoscopy. 2006;38:867–872. doi: 10.1055/s-2006-944726. [DOI] [PubMed] [Google Scholar]
- 22.Kara MA, Peters FP, ten Kate FJW, et al. Endoscopic video autofluorescense imaging may improve the detection of early neoplasia in patients with Barrett's esophagus. Gastrointest Endosc. 2005;61:679–685. doi: 10.1016/s0016-5107(04)02577-5. [DOI] [PubMed] [Google Scholar]
- 23.Kara MA, Peters FP, Fockens P, et al. Endoscopic video autofluorescense imaging followed by narrow band imaging for detecting early neoplasia in Barrett's esophagus. Gastrointest Endosc. 2006;64:176–185. doi: 10.1016/j.gie.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton SR, Smith RR. The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett's esophagus. Am J Clin Pathol. 1987;87:301–312. doi: 10.1093/ajcp/87.3.301. [DOI] [PubMed] [Google Scholar]
- 25.Avidan B, Sonnenberg A, Schnell TG, et al. Hiatal hernia size, Barrett's length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97:1930–1936. doi: 10.1111/j.1572-0241.2002.05902.x. [DOI] [PubMed] [Google Scholar]