Abstract
Background
Parenteral nutrition associated liver disease (PNALD) is a serious condition affecting many children with short bowel syndrome. The aim of this study was to longitudinally assess serum alanine aminotransferase (ALT), a marker for hepatocyte injury, in enterally fed children with PNALD.
Methods
Retrospective chart review of 31 patients treated from 1999 to 2006 by the Center for Advanced Intestinal Rehabilitation at Children's Hospital Boston. Inclusion criteria included: PN duration > 3 months with subsequent tolerance of full enteral nutrition, and evidence of PN associated liver injury. Time to normalize ALT and direct bilirubin was estimated using Kaplan-Meier and Cox proportional-hazards methods.
Results
Mean age PN cessation was 6 months (range 2-14 months). Median PN duration was 18 weeks (IQR = 13, 33 weeks) and median follow-up was 24 weeks (IQR = 14, 48 weeks). After transition to full enteral nutrition, 74% of children normalized direct bilirubin, whereas only 50% normalized ALT. Kaplan-Meier median time to direct bilirubin and ALT normalization were 13 weeks and 35 weeks respectively (P=0.001).
Conclusion
Children with PNALD who have achieved PN independence have persistent ALT elevation despite normal direct bilirubin levels. This implies that hepatic injury may be ongoing beyond the time of bilirubin normalization in this cohort of patients.
Keywords: parenteral nutrition, alanine aminotransferase, direct bilirubin, intestinal failure, short bowel syndrome
INTRODUCTION
Parenteral nutrition (PN) is a life-saving intervention in children with short bowel syndrome1. Unfortunately, up to 65% of these children experience some liver dysfunction, which may manifest histologically as cholestasis or cirrhosis, and/or biochemically as hyperbilirubinemia and elevated serum aminotransferases2,3. Parenteral nutrition-associated liver disease (PNALD) progresses to end-stage liver failure in 15% to 50% of patients4,5 and together with sepsis is the most common cause of death in patients with short-bowel syndrome6-8.
Once PN-associated liver disease develops, treatment options are limited. By far the most successful strategy for reversing PNALD is a transition to full enteral feeding9,10 with most patients normalizing direct bilirubin in three to four months after discontinuing PN11. Intravenous Ω-3 fatty acid supplementation has also shown promise in the reversal of PN-associated hyperbilirubinemia within a similar time frame12.
The aim of this study was to assess the pattern of serum alanine aminotransferase (ALT) normalization in a cohort of patients with short bowel syndrome and PNALD who were successfully rehabilitated to full enteral nutrition. Specifically, we evaluated the time to normalization of serum ALT versus normalization of direct bilirubin. We chose to focus on ALT because it is primarily concentrated in the liver, while aspartate aminotransferase and alkaline phosphatase are found in multiple tissues13,14, making ALT a potentially more sensitive marker for ongoing hepatocyte injury15.
METHODS
Study Population
After obtaining approval from the Institutional Review Board at Children's Hospital Boston (protocol #M06010049), data was retrospectively collected from the medical records of all eligible patients who attended the Center for Advanced Intestinal Rehabilitation (CAIR) Clinic, a multidisciplinary intestinal rehabilitation program, between May 1999 and January 2006. Inclusion criteria included: age 0-18 years, severe short bowel syndrome as defined by history of parenteral nutrition (PN) > 90 days, subsequent tolerance of full enteral nutrition (100% of nutrient and energy requirement via the gastrointestinal tract for > 3 weeks), and follow-up in CAIR Clinic after reaching full enteral nutrition. Exclusion criteria included: hemodynamic instability, renal failure, suspected congenital obstruction of the hepatobiliary system (e.g.: biliary atresia or choledochal cyst), and diagnosis of hepatitis, alpha-1-antitrypsin deficiency, copper deficiency or HIV infection.
Data Collection and Follow-up
31 eligible patients were identified and data was abstracted from this group. All available serum liver function tests before and after transitioning to full enteral nutrition were recorded. Patients were followed from PN cessation until normalization of both serum ALT (≤ 30 U/L) and direct bilirubin (≤ 0.4 mg/dL), or until lost to follow-up (no recorded LFTs for > 6 months).
Statistical Methods
Demographic and clinical characteristics of patients who had normal and abnormal serum ALT at time of PN cessation were compared. If no ALT level was available at the date of PN cessation, the closest date prior to cessation of PN was used. Numerical variables were described using means with standard deviations or medians with interquartile ranges (IQR), and categorical variables were described using proportions. Numerical variables across groups were compared using appropriate parametric and non-parametric tests.
When analyzing time to normalization of serum ALT or direct bilirubin, only participants with abnormal ALT or direct bilirubin at the time of PN cessation were included. McNemar's test was used to evaluate the difference between the proportion of patients who normalized ALT versus direct bilirubin, and the Wilcoxon rank-sum test was utilized to assess the difference between times to normalize the two tests. The median times to normalization of serum ALT and direct bilirubin were determined by Kaplan-Meier analysis and based on the Aalen estimator in separate Cox-proportional hazard models. The trajectories of ALT and direct bilirubin over time were plotted over biweekly medians beginning at time of cessation of PN. Adjustments for potential confounding factors (gestational age, primary diagnosis, bowel length, presence of ileocecal valve, duration of PN and age at PN cessation, sepsis, and peak liver function tests at PN cessation) were considered in this analysis.
All analyses were performed in SAS 9.1 (SAS Institute Inc., Cary, N.C.) and S-plus 8.0 (Insightful, Seattle, WA). Statistical tests were considered significant if P ≤ 0.05.
RESULTS
Population Description
We analyzed data from 31 patients (mean age 6 months, range 2 to 14 months) with short bowel syndrome and suspected PNALD. The most common etiology of short bowel syndrome was necrotizing enterocolitis (41.9% of patients), followed by volvulus (25.8 % of patients). The majority of patients (59.3%) had an intact ileocecal valve. Table 1 shows demographic and clinical characteristics of all patients. Patients were followed for a median of 24 weeks (IQR 14, 48 weeks) after transition to full enteral nutrition. Of the thirty-one patients, 24 had abnormal ALT (ALT > 30 U/L) and 19 patients had abnormal direct bilirubin (> 0.4 mg/dL) at time of cessation of PN.
Table 1.
Characteristics of 31 infants with short bowel syndrome
| Characteristics | Population (N = 31) |
|---|---|
| Male gender, n (%) | 18 (60%) |
| Gestational age (weeks), mean +/− SD | 31.3 +/− 5.1 |
| Residual small bowel length (cm), mean +/− SD | 86.8 +/− 54.5 |
| Presence of ileocecal valve, n (%) | 16 (59%) |
| Duration of PN (days), median (IQR) | 124 (93, 232) |
| Peak ALT prior to cessation of PN (U/L), median (IQR) | 157 (116, 239) |
| Peak Direct Bilirubin prior to cessation of PN (mg/dL), median (IQR) | 5.3 (3.4, 11.2) |
SD = standard deviation, IQR = interquartile range
Normalization of ALT vs. Normalization of Direct Bilirubin
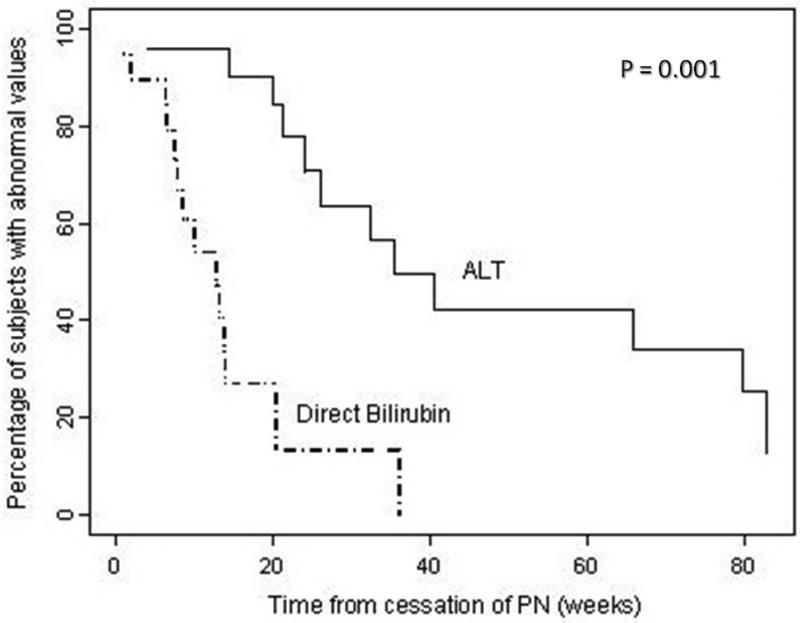
Twelve of twenty-four patients with abnormal ALT (50%) and five of nineteen patients with abnormal direct bilirubin (26%) at the time of cessation of PN failed to normalize by last follow-up. Among the 15 subjects with both abnormal ALT and direct bilirubin at cessation of PN, 7 patients normalized ALT and 11 patients normalized direct bilirubin during follow-up. After reaching full enteral nutrition, direct bilirubin normalized more quickly than ALT (median 13 weeks with 95% CI = 8, 14 weeks for direct bilirubin versus median 35 weeks with 95% CI = 24, 80 weeks for ALT), a statistically significant difference (P = 0.001). Using Kaplan-Meier analysis, 42.4% of patients have abnormal ALT one year after stopping PN, while all patients normalized direct bilirubin (Figure 1). Of the seven patients who normalized both ALT and direct bilirubin during the study period, direct bilirubin normalized in a median of 8 weeks (IQR = 6, 10 weeks), while ALT normalized in a median of 41 weeks (IQR = 24, 80 weeks).
Figure 1.
Kaplan Meier curves showing percentage of subjects with abnormal ALT (solid line) and direct bilirubin (dashed line) after cessation of PN.
In Cox-proportional hazards models, gestational age was the only predictor that was significantly associated with time to normalization of direct bilirubin (P < 0.005). Patients in the 25th percentile for gestational age (28 weeks) normalized direct bilirubin in a median of 28 weeks while patients in the 75th percentile for gestational age (35 weeks) took a median of 8 weeks to normalize direct bilirubin. Bowel length, presence of ileocecal valve, primary diagnosis, duration of PN, age at cessation of PN, sepsis while on PN, and level of liver function tests at cessation of PN were not significantly associated with the time to normalization of ALT or direct bilirubin and did not appreciably affect the time or hazard estimates.
Comparison of Mean Trajectory of ALT and Direct Bilirubin over Time
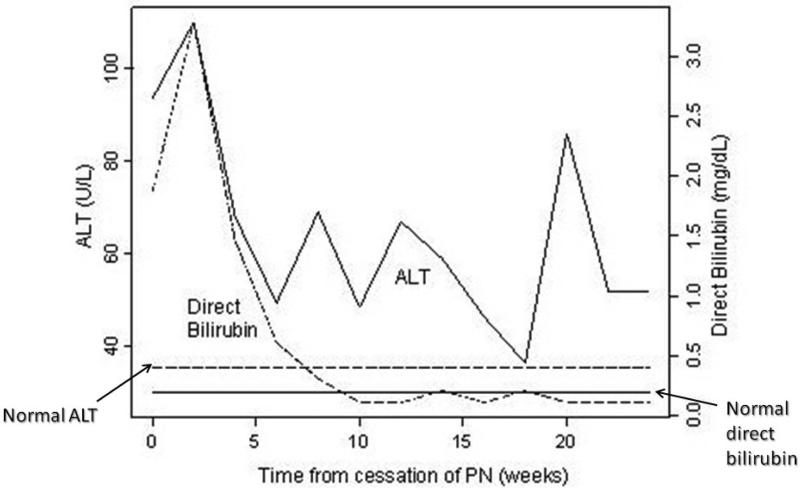
Once full enteral tolerance was reached, direct bilirubin steadily declined to normal range. ALT levels decreased overall, but were variable over time and remained elevated at the end of the study (Figure 2). Median peak ALT after cessation of PN was 143 U/L (IQR = 83, 182 U/L), and median peak direct bilirubin after cessation of PN was 2.7 mg/dL (IQR = 0.1, 6.1 mg/dL).
Figure 2.
Median ALT (solid line) and direct bilirubin (dashed line) following cessation of PN.
DISCUSSION
Parenteral nutrition-associated liver injury is a significant cause of morbidity and mortality in patients with short bowel syndrome2-8. The functional definitions of cholestasis and PN-associated liver disease are closely tied to direct bilirubin levels5,11,12,16. It is sometimes assumed that normalization of bilirubin indicates cessation of liver injury, but our study suggests that this may not be the case. ALT remains elevated long after normalization of direct bilirubin (median 35 weeks versus 13 weeks, P < 0.001), and 50% of patients failed to normalize ALT by the end of the study period. Kaplan-Meier analysis of time to normalization of direct bilirubin versus ALT highlight this difference: at one year after stopping PN, 42.4% of patients still have abnormal ALT while all patients normalized direct bilirubin (Figure 1). Although gestational age was inversely associated with time to normalize direct bilirubin, this study was unable to identify any predictors of time to ALT normalization.
We considered several possible explanations for this difference in time to normalize ALT versus direct bilirubin. Persistently elevated ALT in the face of normal bilirubin may indicate ongoing hepatocyte inflammation. Direct bilirubin declined steadily after cessation of PN. Serum ALT levels decreased over time, but were variable and median levels never fell to within normal range (Figure 2). We were able to confirm that spikes in ALT were not associated with episodes of sepsis. ALT may simply be a more sensitive marker for detecting liver injury than direct bilirubin. Whether a slower rate of clearance of ALT is a possible explanation for these findings seems unlikely, since direct bilirubin includes both conjugated bilirubin and bilirubin bound to albumin, which has a half-life of 21 days18,19. ALT has a half-life of only 47 hours, hence this cannot account for elevations persisting months after cessation of PN20. Another possible explanation for our findings relates to the variety of histopathologic features of PNALD2-3. Since both cholestasis and steatosis are described (as well as more advanced findings including fibrosis and cirrhosis), the persistence of ALT elevations might correlate with fixed hepatic fibrosis or cirrhosis in these patients, as opposed to more rapidly resolving cholestasis.
A limitation of this study is that no patients had liver biopsies performed after transitioning to full enteral nutrition. In our experience, ultrasound does not provide enough detail to supplant liver biopsy. Although we suspect that persistent ALT elevation in this cohort of patients represents ongoing inflammation or fixed injury that may not be detected by direct bilirubin, pathologic correlation is required. Other limitations of this study are the retrospective design and relatively small sample size.
Despite these limitations, our study shows a statistically significant pattern of ALT elevation following normalization of direct bilirubin in short bowel syndrome patients with suspected PNALD. Further studies, including correlation with liver biopsies, are necessary to identify the cause and clinical significance of this persistent ALT elevation. A better understanding of PN-associated liver injury resolution may lead to more effective therapies for those patients who have reached enteral autonomy yet have evidence of ongoing liver dysfunction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wilmore DW, Dudrick SJ. Growth and development of an infant receiving all nutrients exclusively by vein. JAMA. 1968;203:860–864. [PubMed] [Google Scholar]
- 2.Freund HR. Abnormalities of liver function and hepatic damage associated with total parenteral nutrition. Nutrition. 1991;7(1):1–5. discussion 5-6. [PubMed] [Google Scholar]
- 3.Suita S, Masumoto K, Yamanouchi T, et al. Complications in neonates with short bowel syndrome and long-term parenteral nutrition. J Parenteral Enteral Nutr. 1999;23(5 Suppl):S106–9. doi: 10.1177/014860719902300526. [DOI] [PubMed] [Google Scholar]
- 4.Buchman AL. Complications of long-term home total parenteral nutrition: their identification, prevention and treatment. Dig Dis Sci. 2001;46(1):1–18. doi: 10.1023/a:1005628121546. [DOI] [PubMed] [Google Scholar]
- 5.Cavicchi M, Beau P, Crenn P. Prevalence of Liver Disease and Contributing Factors in Patients Receiving Home Parenteral Nutrition for Permanent Intestinal Failure. Ann Int Med. 2000;132(7):525–532. doi: 10.7326/0003-4819-132-7-200004040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Goulet OJ, Revillon Y, Jan D, et al. Neonatal short bowel syndrome. J Pediatr. 1991;119(1(Pt 1)):18–23. doi: 10.1016/s0022-3476(05)81032-7. [DOI] [PubMed] [Google Scholar]
- 7.Weber T, Tracy T, Connors RH. Short-bowel syndrome in children. Arch Surg. 1991;126:841–846. doi: 10.1001/archsurg.1991.01410310051007. [DOI] [PubMed] [Google Scholar]
- 8.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology. 1997;113(5):1767–78. doi: 10.1053/gast.1997.v113.pm9352883. [DOI] [PubMed] [Google Scholar]
- 9.Dahms BB. Serial liver biopsies in parenteral nutrition-associated cholestasis of early infancy. Gastroenterology. 1981;81(1):136 –44. [PubMed] [Google Scholar]
- 10.Zamir O, Nussbaum MS, Bhadra S, et al. Effect of enteral feeding on hepatic steatosis induced by total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1994;18(1):20–25. doi: 10.1177/014860719401800120. [DOI] [PubMed] [Google Scholar]
- 11.Javid PJ, Collier S, Richardson D, et al. The role of enteral nutrition in the reversal of parenteral nutrition-associated liver dysfunction in infants. J Pediatr Surg. 2005;40:1015–1018. doi: 10.1016/j.jpedsurg.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Gura KM, Lee S, Valim C, et al. Safety and Efficacy of a Fish-Oil–Based Fat Emulsion in the Treatment of Parenteral Nutrition–Associated Liver Disease. Pediatrics. 2008;121(3):e678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 13.D'Agata ID, Balistreri WF. Evaluation of Liver Disease in the Pediatric Patient. Pediatr Rev. 1999;20(11):376–90. [PubMed] [Google Scholar]
- 14.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342(17):1266–71. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 15.Ozer J, Ratner M, Shaw M. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Andorsky DJ, Lund DP, Lillehei CW, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J. Pediatr. 2001;139(1):27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 17.Chan S, McCowen KC, Bistrian BR, et al. Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home total parenteral nutrition. Surgery. 1999;126(1):28–34. doi: 10.1067/msy.1999.98925. [DOI] [PubMed] [Google Scholar]
- 18.Fevery J, Blanckaert N. What can we learn from analysis of serum bilirubin? J. Hepatology. 1986;2(1):113–21. doi: 10.1016/s0168-8278(86)80014-9. [DOI] [PubMed] [Google Scholar]
- 19.Price CP, Alberti KGMM. Biochemical Assessment of Liver Function. In: Wright R, Alberti KGGM, Karran S, Millward-Sadler GH, editors. Liver and Biliary Disease-Pathophysiology, Diagnosis, Management. WB Saunders; London: 1979. pp. 381–416. [Google Scholar]
- 20.Dufour DR, Lott JA, Nolte FS, et al. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46(12):2027–49. [PubMed] [Google Scholar]