Abstract
Dermal exposure to sensitizing metals is a serious occupational and public health problem. The usual approach to dermal exposure assessment is to process samples by chemical methods that use reactants to digest the metal particles and quantify the mass. In the case of dermal exposure assessment, these reactants are not representative of the skin surface film liquids and hence, may overestimate bioaccessibility. We hypothesize that the amount and form of sensitizer on a sample that leaches in a biological fluid, as can be estimated using artificial sweat, may be a more relevant metric for assessing health risks. Beryllium metal (Be), nickel metal (Ni), and chromium carbide (Cr3C2) particles were characterized and masses of sensitizing ions were measured using established reactant-assisted digestion procedures and extraction in artificial sweat under physiologically relevant conditions. Chromium ions released into artificial sweat were speciated to understand valence states. The ratios of the fraction of metal dissolved in artificial sweat relative to that dissolved by chemical-specific reactants were 1/2 (Be), 1/108 (Ni), and 1/2500 (Cr). The divalent Be and Ni cations were stable in artificial sweat over time (did not precipitate) whereas hexavalent chromium [Cr(vi)] ions decayed over time. Further analysis using speciated isotope dilution mass spectrometry revealed that the decay of Cr(vi) was accompanied by the formation of Cr(iii) in the sweat model. Use of reactant-assisted analytical chemistry to quantify amounts of metal sensitizers on samples could overestimate biologically relevant exposure. In addition to mass, the valence state also influences penetration through the outer stratum corneum of the skin and is an important consideration when assessing exposure to complex sensitizers such as Cr which have multiple valence states with differing penetration efficiencies.
Introduction
Each day human skin comes into contact with metals, some of which have the capacity to cause allergic sensitization. Allergic contact dermatitis (ACD) is a serious problem for workers who handle materials that release soluble ions of sensitizing metals and to the general public that encounters and uses products made of these metal compounds or metal ion releasing substances. ACD is a life-long disease with no known cure, so once sensitized only avoidance of further exposure can prevent elicitation.1-3 The cost in terms of lost productivity and treatment is estimated to be $1 billion per year in the United States and workers with ACD may have to change jobs to avoid exposure.1,2
To induce sensitization, metal ions need to penetrate through the outer stratum corneum (SC) barrier layer of the skin and reach the underlying viable epidermis. It is generally believed that to be immunologically reactive, metal ions must bind to macromolecules such as proteins to form a hapten complex. Antigen presenting cells display this hapten comple on their cell surfaces and when the hapten is recognized as foreign by naïve T-lymphocyte cells, these cells undergo differentiation to form hapten-specific effector and memory helper T-cells (i.e., a person becomes sensitized). Upon repeated contact with the offending metal, at exposure levels that result in sufficient metal ion release and SC penetration, memory T-cells are recruited to the site of skin contact and elicit an inflammatory reaction. 2,4-6
The usual approach to dermal exposure assessment is to collect a sample that (assumingly) represents the total mass contaminants on skin, digest the substrate using reactants that have been chosen for their distinct reactivity with specific components in a sample and quantity the ‘total’ or ‘soluble’ mass of contaminants. A major shortcoming of this approach is that samples are quantified without regard for bioaccessibility in skin surface film liquids. To protect from the risk of metal-induced ACD, exposure assessors seek to measure the biologically relevant fraction of a sensitizer that contacts the skin (Fig. 1). Ideally, this exposure metric would be predictive of the bioaccessible fraction (the amount of material that dissolves in a biological fluid and is available for penetration through the SC to the underlying biologically active epidermis) and, in conjunction with permeation data, permit calculation of bioavailability (dose). Traditional sample collection techniques which remove or intercept contaminants are likely not representative of the biologically relevant fraction of a metal sensitizer that is in contact with skin surface fluids. Following sample collection, the usual approach is to process samples by chemically valid methods that use reactants such as acids, oxidizers, or bases to digest (fully dissolve) the metal particles and quantify the ‘total’ (soluble plus insoluble) or just the soluble mass of contaminants by atomic spectroscopy. These digestions are carried out using reactants that have been chosen for their distinct reactivity with specific metal components in a sample.7 However, in the case of dermal exposure assessment, the reactants are not representative of the skin surface film liquids (SSFLs). In reality, upon contact with the skin surface, metal particles are immersed in SSFLs which consist mainly of sweat (electrolytes, amino acids, nitrogenous substances, etc.) and sebum lipids with pH in the range 4.2 to 6.1.8 Sebum lipids have a negligible effect on metal dissolution9 and it is the sweat component of skin film liquids that influences the rate of metal dissolution to water-soluble ions (bioaccessibility) and influences the form (valence state and/or complexation) of allergenic metals.10,11 Several factors influence subsequent penetration of metal ions across the SC barrier and ultimately bioavailability; however, the scope of this research is limited to sample analysis issues relevant to bioaccessibility.
Fig. 1.
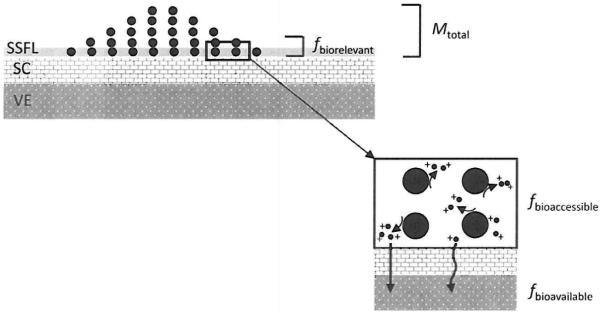
A paradigm for measurement of skin exposure to metal sensitizers. Particles [denoted as ●] deposit on the outer stratum corneum (SC) at some mass loading (Mtotal). Depending upon the loading, all or just a fraction of these particles will contact the skin surface film liquids (SSFLs). This fraction of particles in contact with SSFLs represents the biorelevant fraction (fbiorelevant). i.e., the material available to react with skin liquids. A portion of fbiorelevant will undergo dissolution (oxidation or reduction) to form bioaccessible (fbioaccessible) water soluble metal ions [denoted as ●+]. Depending upon the properties of the metal ions (formation of complexes. etc.) and the barrier properties of the SC some portion will penetrate through or around corneocyte cells and reach the underlying viable epidermis (VE) and interact with the immune system (fbioavailable).
Traditionally, bioaccessibility is assessed in vitro using artificial biological fluids with compositions that mimic humans and conditions of temperature and pH that match in vivo. The available literature supports the premise that reactant-assisted analytical chemistry methods can overestimate the sweat-extractable (bioaccessible) masses of metal sensitizers.12-15 We hypothesize that the amount and form of sensitizer on a sample that leaches in a biological fluid may be a more relevant metric for assessing health risks. A similar concept for sample analysis has been put forth for inhalation exposure assessment of nickel using artificial lung fluids.16 The focus of this study was to investigate the extraction of metal allergens under physiologically relevant conditions of the skin surface as an alternative to the usual reactant-assisted digestion procedures that are used currently. For this study, we used beryllium (Be), nickel (Ni), and chromium (Cr), all of which are industrially important metals and known skin sensitizers.1,2,17 Two of these metals (Be and Ni) release simple divalent cations whereas Cr was chosen as a more complex sensitizer which releases ions with multiple biologically relevant valence states. This study was undertaken to test the hypothesis that the amount and form of a metal sensitizer determined using reactant-assisted digestion procedures are greater than the amount that leaches into artificial sweat and to compare the effects of skin temperature and the type of metal sensitizer on bioaccessibility.
Materials and methods
A two-step approach was taken for these experiments. First the study powders were characterized to understand physico-chemical properties involved in dissolution. Next, the reactant-assisted digestion and sweat extraction of the metals were evaluated and compared to understand bioaccessibility.
Study powders
Bulk chromium carbide (Cr3C2) and Ni metal powders were sampled from containers of feedstock materials at a hard metal manufacturer.18 The Be metal powder was obtained from a primary production facility.19 Chemical properties of the bulk powders were characterized using Auger spectroscopy to determine the surface elemental composition (outer 50-75 Å layer) and powder X-ray diffraction (XRD) to identify crystalline constituents. Physical properties were characterized using transmission or scanning electron microscopy to evaluate the morphology and size; helium pycnometry to determine the density; and nitrogen gas adsorption to determine the surface area using the Brunauer, Emmett, and Teller (BET) method.
Reactant-assisted digestion procedures
The masses of reactant-digestable allergenic metals were determined from three replicate samples of each metal powder using appropriate analytical chemistry methods (Table 1). These methods represent common published methods used in occupational and environmental exposure assessment.
Table 1.
Comparison of analytical chemistry method treatment conditions used to digest metal study powders and human in vivo skin surface conditions for the determination of allergen mass
| Treatments |
||||
|---|---|---|---|---|
| Sensitizer | Protocol | Reactantsa | Thermal | Agitation |
| Be - soluble | Profumo et al.20 | 0.01 M HCl | Ambient temperature (20 °C) | Ultrasonic |
| Ni - soluble | Zatka et al.21 | 0.1 M Citrate | Ambient temperature (20 °C) | Orbital shaker |
| Cr - total | NIOSH 7303 | Cone. HNO3/HCl | Hot block (95 °C) | None |
| All (Be, Ni, Cr) | Sweat extraction | Artificial sweat | Skin (36 °C) | None |
HCl = hydrochloric acid (pH 2.0); citrate = ammonium citrate/citric acid buffer (pH 4.4); cone. HNO3/HCl = concentrated nitric acid/hydrochloric acid (pH < 1); artificial sweat (pH 5.3) from Harvey et al.22
Beryllium
Most published methods for analysis of beryllium are limited to the ‘total’ mass without regard to form; however, Profumo et al.20 developed a protocol that uses sequential sample digestions to distinguish between the masses of soluble and poorly soluble beryllium compounds. In this protocol, 0.01 M hydrochloric acid is used to dissolve the soluble beryllium fraction followed by increasingly more aggressive acid-assisted digestions to dissolve poorly soluble beryllium metal, oxide, and silicate compounds. Prior to our experimental studies, we performed a pilot study in which three samples of a mixture of beryllium sulfate tetrahydrate salt >99.99% (Sigma-Aldrich, St Louis, MO), beryllium metal powder,19 and beryllium oxide powder (NIST SRM® 1877: beryllium oxide powder) were accurately weighed to within 0.01 mg using a microbalance (Model AX-205, Mettler-Toledo, Switzerland) in a temperature- and humidity-controlled room. Ten milliliters of 0.01 M hydrochloric acid were added to each sample and the suspensions were subjected to ultrasonic agitation for 5 minutes at room temperature (20 °C), filtered, diluted to 20 mL using deionized water, and quantified for soluble Be using inductively coupled plasma-optical emission spectroscopy (ICP-OES). The recovery of the soluble (beryllium sulfate) fraction was 94.6 ± 3.7% and the total recovery (soluble sulfate plus poorly soluble metal and oxide) was 101.0 ± 3.7%. For the experimental study, approximately 2 mg of beryllium metal powder per sample was accurately weighed to within 0.01 mg in a controlled environment using a microbalance (Model AX-205, Mettler-Toledo), samples were digested as described above, and quantified for soluble Be by ICP-OES.
Nickel
The nickel metal powder was analyzed by a commercial laboratory (Concord Analytical Services Ltd, Concord, Canada) using the established Zatka sequential digestion procedure.21 In this protocol, ammonium citrate/citric acid buffer (pH 4.4) is used to dissolve the ‘water-soluble’ nickel fraction followed by increasingly more aggressive digestions to dissolve the sulfidic nickel, metallic nickel, and oxidic nickel groups in a sample. For the experimental study, samples of Ni metal powder, each approximately 10 mg, were accurately weighed using a microbalance in a temperature- and humidity-controlled room. Next, 30 mL of 0.1 M buffer (17 g ammonium citrate dibasic/5.2 g citric acid per 1 L water) was added to each sample and the suspensions were subjected to agitation using an orbital shaker for 60 minutes at room temperature (20 oc), passed through a filter with 0.45 μm pore size, diluted to 50 mL using deionized water, and quantified for soluble Ni using ICP-OES.
Chromium
The total (soluble plus insoluble) chromium content of particles was determined by a commercial laboratory (Wisconsin Occupational Health Laboratory, Madison, WI) using a method based on the NIOSH Manual of Analytical Methods 7303. For this study, samples of dried Cr3C2 powder, each approximately 0.05 g, were digested under reflux in a hot-block (95 °C) for 30 minutes with an equal mixture of concentrated nitric and hydrochloric acids (pH < 1), diluted to a final volume of 50 mL using deionized water, and quantified for Cr using ICP-OES.
Sweat extraction (bioaccessibility) procedure
For comparison with the reactant-assisted digestion protocols, the samples of the powders were extracted using an artificial sweat model under physiologically relevant conditions (Table 1). The model is an aqueous solution of 61 different constituents at concentrations that mimic human sweat and was prepared as described by Harvey et al. 22 Among constituents, the highest concentrations were sodium (30.7 mM), chloride (23.2 mM), lactic acid (14.0 mM), urea (10.0 mM), potassium (6.1 mM), and ammonium (5.3 mM). For healthy adults, the skin pH is generally in the range of 4.2 to 6.1,8 with a median of pH 5.3;23 for this study, the sweat solution was adjusted to pH 5.3 by addition of sodium hydroxide.
Initially, dissolution in artificial sweat was evaluated for triplicate samples of each metal powder under physiological skin surface conditions (pH 5.3, 36 °C) at 7 time points over approximately 12 hours to elucidate solubility kinetics (exact durations are shown in Fig. 3). The duration was selected as a typical 8 hour work shift plus additional time to account for take-home exposure which is a common occurrence in the ‘real-world.’ In our experiences with dermal exposure assessments in facilities that lacked skin protection and hygiene programs, take-home contamination was evident and metals were still present on workers’ skin at the start of their shift the following day.24 For each study material, masses equivalent to 0.05 g of metal were accurately weighed to 0.01 mg using a microbalance (Model AX-205, Mettler-Toledo) housed in a temperature- and humidity-controlled room. The chosen mass value exceeds dermal loadings that we have observed previously24 but the high mass value was needed to exceed analytical detection limits. The powders were sealed between two filters in a plastic ring assembly and immersed in 0.050 L of artificial sweat to extract soluble metals using the well-established static dissolution technique.25 The volume of artificial sweat was calculated from the average whole body sweat output of an adult (1.08 L h–1)26 weighted by the fraction of total body area for two hands (5%)27 for a period of one hour. Ideally, it would have been better to match the sweat volume used in our test to that excreted for a 12 hour period. Realistically, contaminant deposition is dynamic, not instantaneous, and throughout a work shift material is loaded onto the skin and removed from the skin (washed off from sweat, brushed off from contact with surfaces, etc.). Sweat is continuously excreted and the rate may vary depending upon the degree of exertion, etc. Hence, there are insufficient data to design an experimental system that captures these dynamics, so for our purposes, we used a fixed mass of contaminants and volume of sweat. The sweat extracts were stabilized by adding 2 mL of 25% nitric acid to each sample and frozen at –10 °C until analysis. The dissolved metal content of the sweat solutions was quantified using ICP-OES.
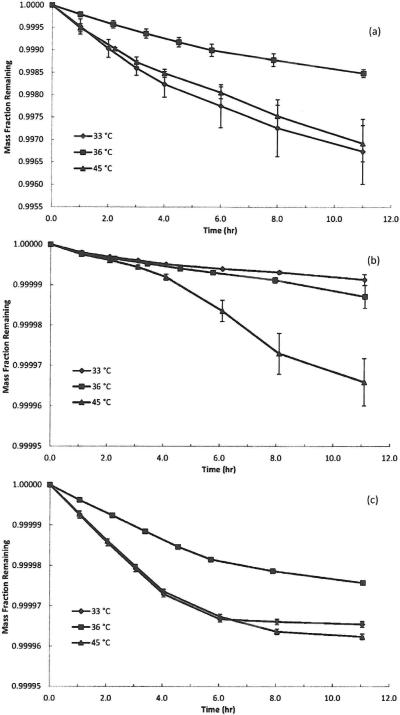
Fig. 3.
Plot of the mass fraction of the material remaining versus time for three sweat extraction temperatures (33, 36, and 45 °C) for (a) beryllium. (b) nickel. and (c) chromium. Dissolution of beryllium and chromi um was biphasic and consisted of an initial rapid phase followed by a slower long-term phase. Dissolution of nickel was linear over time.
Next, we investigated the influence of artificial sweat temperature on dissolution of the metal particles. Skin surface temperature varies depending upon the anatomical site, activity level, etc. Generally, appendages such as fingers and toes tend to be a few degrees cooler than the rest of the body. During exercise the skin surface temperature increases to promote evaporation. Hence, we also investigated dissolution at 33 and 45 °C to span a range of skin temperatures that may be encountered in vivo and quantified the dissolved metal content of the sweat extracts using the same procedures outlined above.
The chemical stability of each allergenic metal ion in artificial sweat was evaluated using beryllium sulfate tetrahydrate (Sigma-Aldrich), nickel sulfate hexahydrate >98% (Acros Organics, Geel, Belgium), and a high-purity ammonium dichromate ICP solution standard (Absolute Standards, Hamden, CT) by a commercial laboratory (Wisconsin Occupational Health Laboratory). For each soluble salt, a known amount of metal was fortified in artificial sweat and samples were subsequently collected at regular time intervals to measure the concentration. For soluble Ni and Be ions each sample was diluted in a 5% hydrochloric acid/5% nitric acid solution just prior to analysis using ICP-OES. For soluble Cr(vi) ions, samples were diluted in water and immediately analyzed using ion chromatography (IC) with an ultraviolet-visible detector. Additionally, for total chromium in artificial sweat, samples were collected upon fortification with ammonium dichromate salt (t = 0) and at the end of the experiment (t = 420 minutes). Each sample was diluted with 5% hydrochloric acid/5% nitric acid solution and analyzed for total chromium using ICP-OES.
Finally, for chromium which has multiple biologically relevant valence states, a study was performed to evaluate the species of chromium compounds in artificial sweat (pH 5.3, 36 °C). For this experiment, the sweat solution was fortified with soluble Cr(vi) as the potassium dichromate salt at 2 or 10 μg mL–1 and samples were analyzed at 9 time points up to 420 minutes. At each time point, duplicate aliquots of artificial sweat were transferred to separate vials which contained an appropriate amount of isotopically enriched chromium speciation standard [53Cr(vi) or 50Cr(iii)] and EDTA solution for analysis by speciated isotope dilution mass spectrometry (SIDMS) to quantify both Cr(iii) and Cr(vi). The purpose of using EDTA as a sample diluent is to complex Cr(iiiIII) ions to stabilize them for analysis. Quantification of chromium species was performed using SIDMS with ion chromatography inductively coupled plasma dynamic reaction cell mass spectrometry (IC-ICP-DRC-MS). Prior to this experiment, the artificial sweat solution was analyzed using SIDMS to establish the background concentrations of chromium species from the trace contamination of reagents used to make the solution. The measured background concentration of Cr(iii) was 0.5 μg mL–1 and for Cr(vi) it was <0.005 μg mL–1. All fortifications, sample preparation, and analyses were performed by a commercial laboratory (Applied Speciation and Consulting, Bothell, WA).
Data analysis
All statistical analyses were performed using SAS® version 9.1 software (SAS Institute, Cary, NC). Summary statistics including mean and standard deviation were calculated for measurements with replicate samples. To investigate the influence of artificial sweat temperature on dissolution, analysis of variance (ANOVA) models were developed for the mass fractions dissolved and the half-times using the GLM procedure in SAS. Tukey's test option was specified for multiple comparisons. To investigate chromium speciation in artificial sweat regression models were developed using Microsoft Excel.
Results
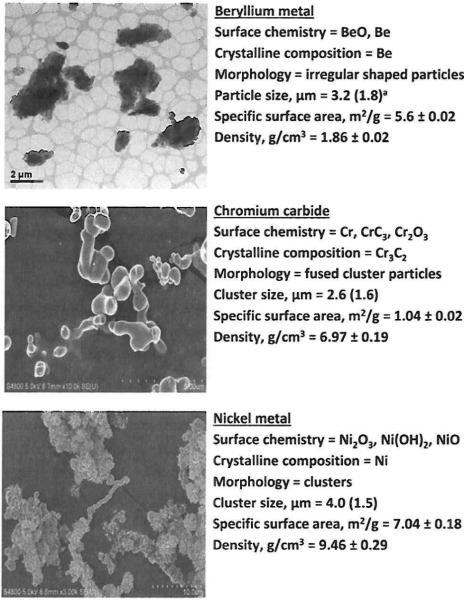
Fig. 2 summarizes the chemical and physical properties of the study powders. All powders had a thin layer of oxide and/or hydroxide compounds on their surfaces. The Be metal consisted of individual platelet-shaped particles whereas the other materials were clusters of smooth (Cr3C2) or irregular-shaped (Ni metal) primary particles. Geometric mean particle sizes were generally <4 μm. Among materials, values of specific surface area ranged from 1 to 7 m2 g–1. Measured density values were close to theoretically determined values for these compounds. Based on these data, all materials were high-purity crystalline powders.
Fig. 2.
Physicochemical properties of study powders. Note that the scale bar differs among images. a Geometric mean (geometric standard deviation).
Table 2 summarizes the cumulative percentage of each metal sensitizer dissolved using reactant-assisted digestion and sweat extraction (pH 5.3, 36 °C) procedures. The values were calculated as the cumulative mass of the solubilized metal ion from all time points in the kinetics study divided by the total mass of metal (not powder) in a sample. The ratios of amounts of the allergenic metal dissolved in artificial sweat relative to that dissolved by chemical-specific reactants were 1/2 (Be), 1/108 (Ni), and 1/2500 (Cr - total).
Table 2.
Amounts of metal sensitizers solubilized using reactant-assisted digestion protocols and artificial sweat extraction
| Sensitizer | Reactant digestiona (%) | Sweat extractionb (%) |
|---|---|---|
| Be | 0.29 ± 0.02 | 0.15 ± 0.01 |
| Ni | 0.14 ± 0.01 | 0.0013 ± 0.0003 |
| Cr - total | 6.08 ± 0.31 | 0.0024 ± 0.0000 |
Be = 0.01 M hydrochloric acid (pH 2.0, 20 °C); Ni = 0.1 M ammonium citrate/citric acid buffer (pH 4.4, 20 °C); Cr = concentrated nitric and hydrochloric acids (pH < 1, 95 °C).
Artificial sweat (pH 5.3, 36 °C) prepared as described by Harvey et al.22
As shown in Fig. 3, at all three sweat extraction temperatures evaluated (33, 36, and 45 °C), dissolution of Be and Cr was biphasic and consisted of an initial rapid phase followed by a slower long-term phase (which is consistent with previous reports28,29) whereas dissolution of Ni was linear over time. On a total mass basis, <0.35% of Be in the powder used for these tests dissolved in artificial sweat. Of this total amount that was solubilized, 0.2 to 0.3% dissolved in the initial rapid phase and the remaining 99.8 to 99.7% dissolved in the slower long-term phase. The dissolution half-time for the initial rapid phase was 4 to 6 hours (i.e., after 4 to 6 hours, only 0.1 to 0.15% of the material dissolved in this phase was remaining) and the dissolution half-time for the long-term phase was on the order of hundreds of days. For Ni, <0.003% of the total powder sample mass dissolved during our tests and in all cases, this release was linear with time; the calculated dissolution half-times were on the order of several hundred days. Among powders extracted in artificial sweat, only Ni exhibited a temperature-dependence. The amount of Ni that dissolved at 45 °C (0.003%) was significantly different from the amount dissolved at 33 °C (0.001%) and 36 °C (0.001%); p < 0.05. Note that regardless of temperature, the release in artificial sweat was still significantly lower than what was measured with the reactant-assisted (citrate-citric acid) procedure. On a total mass basis, <0.004% of Cr in the powder used for these tests had dissolved in artificial sweat. Of this total dissolved amount, 0.002 to 0.004% dissolved in the initial rapid phase and the remainder dissolved in the slower long-term phase. For the initial rapid phase, the dissolution half-times were 3 to 5 hours and for the slower long-term phase, the dissolution half-times were on the order of hundreds of days. In addition to highlighting the divergence between reactant-assisted and sweat extraction protocols, these data also indicate the persistence of these materials which necessitates hygienic practices such as hand washing or showering to remove these metals (powder and unabsorbed ions) from the contaminated skin.
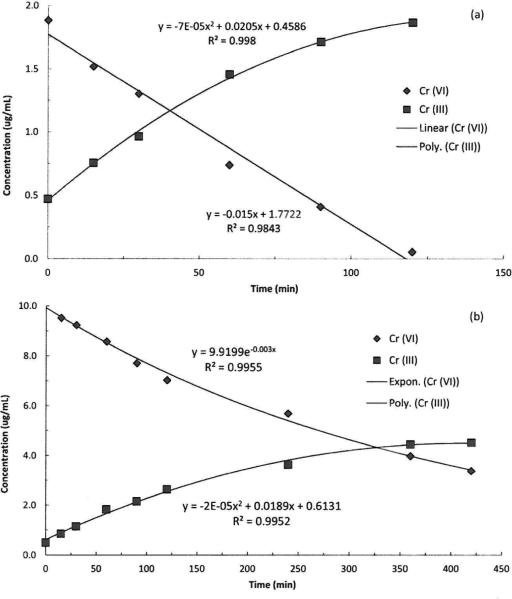
Fig. 4 plots the stability of each metal ion (added as its soluble salt) in artificial sweat (pH 5.3, 36 °C) over time. The concentration of soluble beryllium decreased during the first three hours to about 80% of the initial concentration and was stable thereafter. In contrast, the soluble Ni ion was stable in artificial sweat over the entire time period monitored. The concentration of soluble Cr(vi) began to decrease upon introduction to the artificial sweat and continued to decline until reaching the analytical limit of detection (5 μg per sample) at 7 hours; however, there was no change in the total soluble chromium concentration in the solution. Fig. 5 shows plots from the SIDMS analyses that illustrate that over time the concentration of Cr(vi) in the artificial sweat decreased with a concomitant appearance of Cr(iii). Note that the initial concentration of Cr(iii) was not zero because there was a background concentration of 0.5 μg mL–1 Cr(iii) due to impurities in the chemicals used to prepare the artificial sweat. As described in the Methods section, EDTA was used as a chelator to stabilize Cr(iii) and prevent precipitation; however, other elements can form complexes with EDTA and result in a quantitatively incomplete reaction between EDTA and Cr(iii). The artificial sweat model contained elevated concentrations of elements which competitively bind with EDTA so Cr(iii) results at longer time points, especially in the lower concentration experiment, could be biased low (for this reason the Cr(iii) data for the 2 μg mL–1 experiment are truncated at 120 minutes). Note that the Cr(vi) analyses would not be impacted by EDTA binding issues. At low Cr(vi) concentration, the rate of disappearance was linear up to 120 minutes and at higher concentration it followed an exponential decay. At both low and high Cr(vi) concentrations, the appearance of Cr(iii) was best described by a polynomial function. Note that the equilibrium of the artificial sweat system can support the existence of both molecules.
Fig. 4.
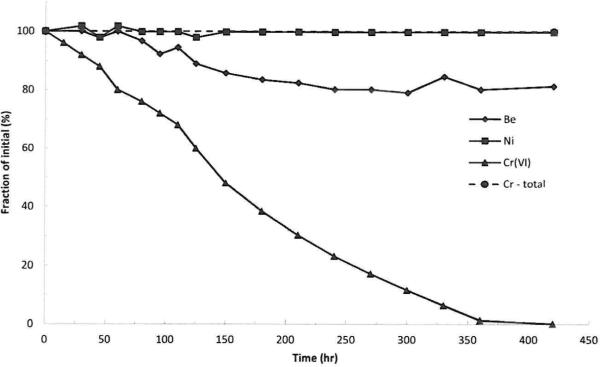
Plots of metal ion stability in artificial sweat (pH 5.3, 36 °C) with time.
Fig. 5.
Reduction kinetics of Cr(vi) to Cr(iii) in artificial sweat using SIDMS analysis at low (a) 2 μg mL–1 Cr(vi) and high (b) 10 μg mL–1 Cr(vi) concentrations.
Discussion
Consistent with our hypothesis, the masses of metal sensitizers dissolved using reactant-assisted digestion procedures were greater than those observed for artificial sweat at physiologically relevant temperatures. Our observations for Be, Ni, and Cr are consistent with previous reports that analytical chemistry methods overestimate the sweat-extractable masses of metal sensitizers.12-15 Importantly, the measured differences in dissolution were material-dependent. The greatest measured overestimation by a reactant-assisted digestion procedure was observed for Cr, followed by Ni, and the smallest difference was for Be. The observed dissolution of the metals is attributed to the thin oxide and/or hydroxide species formed on the surfaces of the study powders under ambient conditions (Fig. 2). For example, in the case of Be metal, the oxide species are loosely organized because they were not heat-treated to form regularly ordered crystalline species19 and hence susceptible to dissolution.30 In addition to surface reactivity with ambient oxygen, another parameter which influences dissolution of metals such as Ni is the presence of alloying compounds in the material. For example, nickel-copper alloys and nickel metal (which was used in our study) do not form effective passive layers on their surfaces whereas chromium and iron oxides passivate nickel alloy (e.g., stainless steel) surfaces and help minimize sweat-induced corrosion.31 In the case of Ni powder, according to Zatka et al. 21 nickel hydroxide is only partially leached during the citrate buffered digestion step, hence our results for reactant-assisted determination of soluble Ni may be biased low. Only Ni powder exhibited temperature-dependent dissolution with more mass dissolved at 45 °C compared to 33 and 36 °C. Similarly, Hemingway and Molokhian reported increased dissolution of Ni from the wire in artificial sweat over the range of 10 to 40 °C.
It is well known that water soluble Be ions can penetrate the SC and induce sensitization or elicit inflammatory reactions in the skin.33-35 Nickel powder has been demonstrated to dissolve in artificial sweat and permeate intact skin in vitro.36,37 Lacy et al.38 applied nickel chloride to the skin of hamsters and reported that most of the Ni was retained in the skin for an extended period of time where it could lead to prolonged antigen processing and consequent immune responses in dermal tissue; urinary nickel levels indicated permeation through skin and systemic distribution in vivo. While the intact SC provides a rate-limiting barrier in permeation, the amount of allergen penetration can be markedly increased for damaged skin.39 Hence, in addition to the mass and form of the allergen, the barrier properties of the SCare also important determinants of exposure. Recently, investigators have begun to utilize measures of SC integrity as part of a comprehensive exposure assessment strategy for metal allergens.40,41
Chromium was chosen for the study because of its complex chemistry and the implication of the Cr(vi) and Cr(iii) valence states in the development of allergy.42,43 As noted above, to form a hapten complex, a metal ion must be able to penetrate through the SC and be able to form covalent bonds with proteins. With regard to penetration, valence states change the number of electrons in the outer shell and hence the ionic radius changes, which in turn affects the electrophilicity and protein reactivity of the ions. The electrophilic Cr(iii) ion exhibits a strong affinity for dermal tissues and creates stable complexes which slow the rate of diffusion and form reservoirs of the ions in skin.44 More Cr(iii) ions are needed to achieve the same degree of sensitization as Cr(vi) ions via topical application;45 however, if intradermal application is used to bypass the SC barrier, the same sensitivity is achieved.42 It has been reported that Cr(vi) readily penetrates the SC but does not form covalent bonds with organic substances.46 Gammelgaard et al.47 investigated the permeation of Cr(vi) and Cr(iii) through skin under occlusive conditions. This study reported that more ions crossed the SC and reached the epidermis and dermis layers of the skin following application of Cr(vi) compared to Cr(iii). The authors suggested that the increased permeation of Cr(vi) relative to Cr(iii) was not because the latter binds with skin proteins, rather they attributed the lower permeation rate to a greater skin barrier rejection of the positive Cr(iii) ions. Van Lierde et al.43 investigated the in vitro permeation of Cr through pig and human skin and observed that Cr(vi) was able to pass most easily through skin and that more was retained in the skin relative to Cr(iii). They also attributed the difference in permeation to the rejection of the positively charged Cr(iii) ions by the skin barrier. Larese-Filon et al.39 reported that similar masses of sweat-solubilized Cr (valence state not determined) penetrated through intact and damaged skin, but more of the solubilized Cr was retained in damaged skin. Based on this evidence from the literature, it can be concluded that bioavailability of Cr(vi) and Cr(iii) differs.
Several constituents present in human sweat may interact with chromium, including histamine, acetylcholine, uric acid, creatinine,43 ascorbic acid,48 lactic acid and glucose.44 Van Lierde et al.11,43 investigated the speciation of chromium ions released from leathers into simulated sweat (pH 5.5, 37 °C) and determined that methionine (7 mM) reduced Cr(vi) into Cr(iii), which in turn formed a soluble complex with lactic acid. In a system consisting of Cr(iii) and artificial sweat, they also reported the formation of a complex with lactic acid but no evidence of interactions with methionine. Little et al.48 proposed that the mechanism responsible for reduction of Cr(vi) is the reaction with thiol (–SH) functional groups to form thiol-esters followed by a series of reduction reactions to form Cr(iii). Mali et al.44 reported the evidence for reduction of Cr(vi) by measuring the disappearance of the dichromate ion in the presence of various water-soluble skin components - lactic acid was the most reactive yielding a 52% reduction of Cr(vi) with a much slower rate of reduction by glucose (0.02%). In our study, using an artificial sweat model that contained ascorbic acid (0.01 mM), uric acid (0.06 mM), creatinine (0.08 mM), glucose (0.2 mM), and lactic acid (14 mM) it was observed that Cr(vi) rapidly disappeared from artificial sweat over a period of about 400 minutes (Fig. 4). In a follow-on study using SIDMS, we determined that over time, in this artificial sweat model, as the concentration of Cr(vi) decreased there was a concomitant appearance of Cr(iii) (Fig. 5).
Reduction of Cr(vi) in our sweat model was linear (Fig. 5a) or exponential (Fig. 5b). To the best of our knowledge, this is the first time that the kinetics of Cr(vi) reduction in artificial sweat have been published and these data provide valuable insights for exposure assessment (Fig. 1). In the case of chromium compounds, once deposited on the skin, there are several competitive kinetic processes occurring simultaneously, including the (1) rate of particle dissolution, (2) rate of Cr(vi) reduction to Cr(iii) by sweat constituents, (3) rate of Cr(vi) and Cr(iii) ion penetration through the SC barrier, and (4) rate of reduction of Cr(vi) to Cr(iii) by skin proteins to yield the final hapten complex.42 The fraction of Cr that contacts the skin film liquids (fbiorelevant) will undergo dissolution to form water soluble ions and complexes. The skin surface composition and pH can influence dissolution (fbioacccssible) Larese-Filon et al.49 reported that the dissolution of Cr from suspensions of the metal powder in artificial sweat increased as pH decreased from 5.5 to 3.5. Various sweat constituents, including H+, can interact with Cr(vi) and reduce it to Cr(iii). Hence, once dissolved, pH can also influence the form of chromium ions. For example, Cr(iii) salts have poor solubility which decreases as sweat pH increases. Sweat pH can also influence penetration rates because Cr(vi) exists in the form of chromate [(CrO4)2–] ions (small ionic radius) at pH > 6 and in the form of dichromate [(Cr2O7)2–] or hydrogen chromate (HCrO4–) ions (larger ionic radius) at pH 2 to 6.50 Of these forms, the smaller chromate ion can more readily penetrate through the SC. As noted previously, the valence state affects Cr ion electrophilicity and protein reactivity which are important factors that influence penetration through the SC to the immunologically active viable epidermis and once inside the skin, the Cr(vi) is reduced to Cr(iii) by sulfhydryl groups (fbioavailable). It is currently unknown which of these four competitive kinetic processes is rate limiting in the development of ACD, although our data (Fig. 4 and 5) indicate that the rate of Cr(vi) reduction by sweat occurs very rapidly (on the order of hours).
As noted in the Results section, there was an initial concentration of Cr(iii) in the artificial sweat (0.5 μg mL–1) because of impurities in the chemicals used to prepare the formulation. Note that upon closer inspection of Fig. 5 there is an increasing mass balance discrepancy. For example, at t = 0, the mass concentration of Cr(vi) was 10.1 μg mL–1 and the concentration of Cr(iii) was 0.5 μg mL–1 for a total of 10.6 μg mL–1 (Fig. 4 and 5) but at t = 420 minutes the mass concentration of Cr(vi) was 3.4 μg mL–1 and the concentration of Cr(iii) was 4.5 μg mL–1 for a total of 7.9 μg mL–1 The encountered mass balance discrepancy is attributed to the performance of the EDTA chelation efficiency for the different Cr(iii) complexes which can be formed in the experimental system though it is possible that some of this discrepancy is because of isotopic interferences in the complex sweat model. The quantitation method for SIDMS uses isotopic ratios instead of external calibrations; therefore, differences in the chemical interactions of Cr(iii) complexes can result in differences in the isotopic ratios as well. This is an intrinsic limitation of SIDMS which emphasizes the importance of monitoring multiple valence states and molecular forms of the target metalloid compounds to better understand redox interactions. Other approaches are available which are designed to solubilize and stabilize Cr(iii) prior to quantitation; however, without the application of a complete digestion method the performance of any approach will continue to be operationally defined. A complete digestion method is not a viable option as the SIDMS method fails when an excessive amount of oxidation or reduction occurs. In the instance of this experiment Cr(vi) better represents the reduction capacity of the simulated sweat due to the method limitations. Note that the observed concentration discrepancy is not attributed to precipitation of chromium species because the total soluble chromium concentration is stable in this artificial sweat (Fig. 4).
This study builds upon a growing body of research that supports the premise that analytical methods for exposure assessment should consider physiologically relevant conditions. In the European Union, testing under the Nickel Directive (Directive 94/27/EC) which limits the release of the Ni ion from consumer products that come in contact with skin is performed in artificial sweat as defined in the protocol EN1811.51 Analytical chemistry methods can overestimate the sweat-extractable masses of metal sensitizers12-15 which is a major shortcoming of the usual sample analysis paradigm. This premise of physiologically relevant analysis conditions has also been advanced for inhalation exposure assessment.7,16,52,53 To protect from the risk of ACD induced by metals, exposure assessors seek to measure the biologically relevant fraction of a sensitizer that contacts the skin. Data presented in our study and by previous researchers indicate that the use of reactant-assisted analytical chemistry to quantify amounts of metal sensitizers on samples could overestimate biologically relevant exposure (Fig. 1). This observation has implications for risk assessment. For example, underestimation of risk can occur if exposures are overestimated (i.e., results from reactant-assisted analyses are equated with bioaccessibility) then used to construct an exposure-response relationship. In this scenario, the amount of exposure believed to be necessary to provoke an immune response would be falsely high (above the true safe level). On the other hand, overestimation of risk can occur if results from reactant-assisted analyses are equated with bioaccessibility and compared to a safe exposure level derived only from clinical data. Patch testing can be used to define a threshold level for elicitation of ACD in sensitized individuals.54,55 However, patch tests are performed using solutions of metal sensitizers so the ions are 100% bioaccessible. Our results (see Table 2) indicate that reactant-assisted analytical approaches overestimate the portion of exposure that is biorelevant. Hence, if reactant-assisted analyses are used to quantify the exposure, and the resultant values are compared to an elicitation threshold derived from patch test data, it is likely that the risk to workers will be overestimated. Hence, procedures based on artificial sweat extraction that provides biologically relevant estimates of exposure may ultimately lead to more protective risk-based decision making. Our study begins to address and point out many of the relevant factors that need to be considered in establishing standardized procedures for physiologically based sample analysis for dermal exposure assessment. Future needs to improve sample analysis procedures that provide information on the bioaccessible fraction include standardization of the sweat extraction parameters including artificial sweat composition, pH, and temperature(s) and sample contact time with artificial sweat (with consideration of sample stability). In the current study, temperature generally had a relatively minor effect on dissolution. In contrast, artificial sweat pH is known to have a significant effect on Be28 and Ni32 dissolution. Numerous studies have demonstrated varying effects of artificial sweat composition on dissolution. For example, sodium chloride10 and lactic acid56 can increase the dissolution of chromium and the amino acid methionine is important in the reduction of Cr(vi) to Cr(iii).11 Chloride ion increases dissolution of Ni31 whereas organic acids such as lactic acid32 and butyric and pyruvic acids57,58 have little influence on its dissolution.
Environmental impact.
There is a clear need to accurately measure exposures to metal sensitizers that contact the skin. The current approach is to process samples by chemical methods that use reactants (i.e., acids, oxidizers, or bases) to digest metal particles and quantify the mass. This approach may overestimate the bioaccessibility (the amount of material that dissolves in a biological fluid and is available for penetration). This study describes the comparison of established reactant-assisted digestion procedures to extraction in artificial sweat for three sensitizers (beryllium, nickel, and chromium). Errant estimation of bioaccessibility has implications for risk assessment. Underestimation of risk can occur if exposures are overestimated by reactant-assisted analyses and used to construct an exposure-response relationship. Conversely, overestimation of risk can occur when the results from reactant-assisted analyses are equated with bioaccessibility for comparison to an elicitation threshold derived from patch test data. Procedures based on artificial sweat extraction that provide bioaccessibility estimates and account for relevant factors such as the influence of the valence state may ultimately lead to more protective risk-assessments.
Acknowledgements
Mention of a specific product or company does not constitute endorsement by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the NIOSH. The authors wish to thank Dr M. Keane at NIOSH and Dr F. Eloff at the NorthWest University, Potchefstroom, South Africa for critical review of this manuscript.
References
- 1.Forte G, Petrucci F, Bocca B. Inflammation Allergy: Drug Targets. 2008;7:145–162. doi: 10.2174/187152808785748146. [DOI] [PubMed] [Google Scholar]
- 2.Karlberg AT, Bergstrom MA, Borje A, Luthman K, Nilsson JL. Chern. Res. Toxicol. 2008;21:53–69. doi: 10.1021/tx7002239. [DOI] [PubMed] [Google Scholar]
- 3.Shelnutt SR, Goad P, Belsito DV. Grit. Rev. Toxicol. 2007;37:375–387. doi: 10.1080/10408440701266582. [DOI] [PubMed] [Google Scholar]
- 4.Arts JH, Mommers C, de Heer C. Grit. Rev. Toxicol. 2006;36:219–251. doi: 10.1080/10408440500534149. [DOI] [PubMed] [Google Scholar]
- 5.Aubin F. In: Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants. Agache P, Humbert P, editors. Springer-Verlag; Germany: 2004. pp. 583–590. [Google Scholar]
- 6.Toebak MJ, Gibbs S, Bruynzeel DP, Scheper RJ, Rustemeyer T. Contact Dermatitis. 2009;60:2–20. doi: 10.1111/j.1600-0536.2008.01443.x. [DOI] [PubMed] [Google Scholar]
- 7.Conard BR, Zelding N, Bradley GT. J. Environ. Manit. 2008;10:532–540. doi: 10.1039/b714884d. [DOI] [PubMed] [Google Scholar]
- 8.Agache P. In: Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants. Agache P, Humbert P, editors. Springer-Verlag; Germany: 2004. pp. 21–31. [Google Scholar]
- 9.Collins KJ. Br. J Ind. Med. 1957;14:191–197. doi: 10.1136/oem.14.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flint GN, Carter SV, Fairman B. Contact Dermatitis. 1998;39:315–316. doi: 10.1111/j.1600-0536.1998.tb05951.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Lierde V, Chery CC, Moens L, Vanhaecke F. Electrophoresis. 2005;26:1703–1711. doi: 10.1002/elps.200410221. [DOI] [PubMed] [Google Scholar]
- 12.Contado C, Pagnoni A. Sci. Total Environ. 2012;432:173–179. doi: 10.1016/j.scitotenv.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz SB, Finley BL. J. Toxicol. Environ. Health. 1993;40:585–599. doi: 10.1080/15287399309531820. [DOI] [PubMed] [Google Scholar]
- 14.Mutlu MM. J. Am. Leather Chern. Assoc. 2009;104:237–243. [Google Scholar]
- 15.Nygren O, Wahlberg JE. Analyst. 1998;123:935–937. doi: 10.1039/a707458a. [DOI] [PubMed] [Google Scholar]
- 16.Oller AR, Cappellini D, Henderson RG, Bates HK. J. Environ. Manit. 2009;11:823–829. doi: 10.1039/b820926j. [DOI] [PubMed] [Google Scholar]
- 17.Jerschow E, Hostynek JJ, Maibach HI. Food Chern. Toxicol. 2001;39:1095–1108. doi: 10.1016/s0278-6915(01)00059-x. [DOI] [PubMed] [Google Scholar]
- 18.Stefaniak AB, Day GA, Harvey CJ, Leonard SS, Schwegler-Berry DE, Chipera SJ, Sahakian NM, Chisholm WP. Ind. Health. 2007;45:793–803. doi: 10.2486/indhealth.45.793. [DOI] [PubMed] [Google Scholar]
- 19.Hoover MD, Castorina BT, Finch GL, Rothenberg SJ. Am. Ind. Hyg. Assoc. J. 1989;50:550–553. doi: 10.1080/15298668991375146. [DOI] [PubMed] [Google Scholar]
- 20.Profumo A, Spini G, Cucca L, Pesavento M. Talanta. 2002;57:929–934. doi: 10.1016/s0039-9140(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 21.Zatka V, Warner J, Maskery D. Environ. Sci. Technol. 1992;26:138–144. [Google Scholar]
- 22.Harvey CJ, LeBoufand RF, Stefaniak AB. Toxicol. in Vitro. 2010;24:1790–1796. doi: 10.1016/j.tiv.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Stefaniak AB, Harvey CJ. Toxicol. in Vitro. 2006;20:1265–1283. doi: 10.1016/j.tiv.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Day GA, Virji MA, Stefaniak AB. J Exposure Sci. Environ. Epidemiol. 2009;19:423–434. doi: 10.1038/jes.2008.33. [DOI] [PubMed] [Google Scholar]
- 25.Stefaniak AB, Guilmette RA, Day GA, Hoover MD, Breysse PN, Scripsick RC. Toxicol. in Vitro. 2005;19:123–134. doi: 10.1016/j.tiv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Agache P, Candas V. In: Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants. Agache P, Humbert P, editors. Springer-Verlag; Germany: 2004. pp. 302–309. [Google Scholar]
- 27.U. S. Environmental Protection Agency . Exposure Factors Handbook: 2011 Edition. Washington, D. C.: 2011. [Google Scholar]
- 28.Stefaniak AB, Virji MA, Day GA. Ann. Occup. Hyg. 2011;55:57–69. doi: 10.1093/annhyg/meq057. [DOI] [PubMed] [Google Scholar]
- 29.Wass U, Wahlberg JE. Contact Dermatitis. 1991;24:114–118. doi: 10.1111/j.1600-0536.1991.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 30.Finch GL, Mcwhinney JA, Eidson AF, Hoover MD, Rothenberg SJ. J. Aerosol Sci. 1988;19:333–342. [Google Scholar]
- 31.Morgan LG, Flint GN. In: Nickel and the Skin: Immunology and Toxicology. Maibach HI, Menne T, editors. CRC Press; Boca Raton, FL: 1989. pp. 46–54. [Google Scholar]