Abstract
Background & Aims
Chemotherapy of patients with inactive hepatitis B virus (HBV) infection can lead to viral reactivation and flares of hepatitis. We investigated the proportions of patients screened for HBV infection before chemotherapy over time and outcomes of screened patients.
Methods
In a retrospective study, we collected data from a pharmacy database on patients who underwent cytotoxic chemotherapy for solid or hematologic malignancies at the Mayo Clinic in Rochester, Minnesota, from January 1, 2006 through September 30, 2011. Laboratory data were collected from electronic medical records. Screening was identified based on tests for HB surface antigen, for any reason at any time before chemotherapy.
Results
Of 8005 patients undergoing chemotherapy, 1279 (16%) were screened for HBV infection before chemotherapy, including 668/1805 patients with hematologic malignancies (37%). The proportion of patients screened for HBV increased from 14.3% in 2006–2008 to 17.7% in 2009–2011 (P<.01). This trend was attributed mostly to an increase the proportion of patients with hematologic malignancies, from 32.7% in 2006–2008 to 40.6% in 2009–2011 (P<.01). Of 13 patients who tested positive for HBV, 5 did not receive prophylactic antiviral therapy; HBV infection was reactivated in 2 of these patients. None the 8 patients who received an antiviral agent before chemotherapy experienced HBV reactivation. Of 58 unscreened patients who had increases in levels of alanine aminotransferase (>300 U/L), only 1 appeared to have an undiagnosed HBV infection.
Conclusion
Only a small percentage of patients receiving chemotherapy are screened for HBV infection. However, a larger proportion was screened during 2009–2011 than 2006–2008, especially of patients with hematologic malignancies. Strategies are needed to ensure that patients receiving chemotherapy are protected from the consequences of undiagnosed HBV infection.
Keywords: Universal screening, Abnormal liver enzyme, Preemptive antiviral, Risk factor
Introduction
Reactivation of hepatitis B virus (HBV) may occur in patients with inactive HBV infection when they become immunosuppressed. With widespread application in clinical practice of potential immunosuppressive agents, such as immune-modifying biological and cytotoxic chemotherapy, there is a concern that the number of patients at risk of reactivation of HBV is increasing. HBV reactivation is clinically significant, not only because it represents a potentially serious, sometimes fatal, condition, but also because it may limit the ability for the clinician to treat the underlying condition, such as cancer. HBV reactivation is best prevented with preemptive antiviral therapy, which has been shown to be more effective than reactive therapy given in response to hepatitis flare.1, 2
In recognition of the these data, the Centers for Disease Control and Prevention (CDC) and the American Association for the Study of Liver Diseases (AASLD) recommend HBV screening of all patients before immunosuppressive therapy. However, not all government authorities or professional societies are in support of the HBV screening recommendation. For example, the American Society of Clinical Oncology (ASCO) makes HBV screening optional in that the physician should take into consideration the degree of immunosuppression and the risk in the individual patient before making a decision for HBV screening. Opponents of universal HBV screening in this setting point to insufficient evidence to determine the net benefits and harms of routine screening for chronic HBV infection as well as cost-effectiveness.3–7
These conflicting guidelines have been a source of confusion for health care providers in chemotherapy practice. Anecdotally, cases of HBV reactivation are not uncommonly encountered in Hepatology practices. Ideally, all patients with HBV infection are identified and prophylaxed prior to initiation of chemotherapy; the lack of consensus may be for cost-effectiveness of screening all patients regardless of the prevalence of HBV infection and the risk of serious HBV reactivation. The aims of this study are (1) to describe HBV screening practices in patients receiving chemotherapy at a large US academic medical center; (2) to identify factors associated with screening; and (3) to determine the outcomes in patients who underwent HBV screening.
Methods
Data Sources
After an approval was obtained from the Institutional Review Board of the Mayo Clinic, an electronic pharmacy database was queried to identify a cohort of patients who underwent cytotoxic chemotherapy as an outpatient for the first time at Mayo Clinic, Rochester between January 1, 2006 and September 30, 2011. Pharmaceutical agents or antibodies that may suppress the immune system such as high dose steroids were included, whereas antibodies that do not affect immune function such as Herceptin, as well as anti-androgen and anti-estrogen drugs were excluded. Investigational drugs and any agents not approved by the US Food and Drug Administration were excluded.
From the pharmacy database, patients of all ages were identified. Demographic data such as age, sex, race and ethnicity were obtained from the institutional registration file. Information about the underlying malignancy was available in a clinical database in the Division of Medical Oncology. We categorized the diagnoses as solid or hematologic malignancies, the latter including acute leukemia, chronic leukemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, multiple myeloma, and non-specific hematologic cancer.
To assess whether there could have been patients with undiagnosed HBV experiencing a hepatitis flare, we reviewed the medical records of the patients with the highest peak ALT (300 U/L or greater).
Screening and Testing for HBV and Reactivation
During the study period, there was no institutional protocol on HBV screening for oncology patients and it was entirely under the discretion of the physician whether to order HBV testing prior to initiation of chemotherapy. Testing for HBsAg for any reason at any time prior to the initiation of chemotherapy was considered screening, whereas patients tested for the first time after chemotherapy was begun were separately identified. HBV serology results as well as other laboratory data were obtained electronically from the Department of Laboratory Medicine and Pathology. During the study period, HBsAg testing at Mayo Clinic, Rochester was performed using a chemiluminescence assay from Ortho-Clinical Diagnostic (Rochester, NY). HBV DNA in the serum was detected by real-time polymerase chain reaction. During the study period, several commercial assays were used with the lowest limit of detection being 6 – 1,000 IU/mL. Since HBV DNA levels were not systematically followed during chemotherapy in patients with HBV infection, we defined ‘HBV reactivation’ by ALT≥100IU/L or total bilirubin≥2.5mg/dL accompanied by HBV>1,000 IU/mL.8, 9
Statistical Analysis
The primary goal of the analysis was to determine the prevalence of HBV screening among patients undergoing chemotherapy. For descriptive analyses, the χ2 and t-tests were used to compare characteristics between patients who were screened and those who were not. The Cochran Armitage test was used to detect trends in screening by year. To identify factors associated with screening, the logistic regression analysis was used. Candidate predictors included age, sex, cancer type, race/ethnicity, and abnormalities of AST or ALT. Uni- and multivariable analyses were conducted to identify independent factors associated with HBV screening. Statistical analyses were performed using SAS software version 9.3 (SAS institute Inc., Cary, NC).
Results
During the study period, 8,005 patients initiated chemotherapy, 23% (n= 1,805) of whom had hematologic malignancies. The median of age was 61 years (interquartile range=51.4–70.0) and men accounted for 50.8% (n=4,064). In Table 1, screening for HBV prior to chemotherapy occurred in 1,279 (16%) patients, including 668 (37%) with hematologic malignancies. The median interval between HBV testing and initiation of chemotherapy was approximately 6 months (0.46 years, interquartile range (IQR): 0.06–4.10). In addition, 523 patients were tested after the chemotherapy was begun, with the time gap being 0.47 years (IQR: 0.23–1.31). Thus, HBV testing before or after initiation of chemotherapy occurred in 23% (n=1,802).
Table 1.
Patient characteristics (Total N=8,005)
| Screened (n=1279) | Not-screened (n=6726) | p-value (screened vs. not- screened) |
||
|---|---|---|---|---|
| Tested after chemotherapy (n=523) |
Not-tested (n=6203) |
|||
| Age (Mean±SD years) | 58.8±13.5 | 57.3±13.3 | 60.7±13.8 | <0.01 |
| Male gender (%) | 703 (55%) | 296 (57%) | 3065 (49%) | <0.01 |
| Hematologic cancer (%) | 668 (52%) | 306 (59%) | 831 (13%) | <0.01 |
| Race | ||||
| White (%) | 1163 (90.9%) | 488 (93.3%) | 5670 (91.4%) | (Reference) |
| Black (%) | 20 (1.6%) | 5 (1.0%) | 61 (1.0%) | 0.14 |
| Asian (%) | 17 (1.3%) | 4 (0.8%) | 67 (1.1%) | 0.76 |
| Others (%) | 79 (6.2%) | 26 (5.0%) | 405 (6.5%) | 0.13 |
| AST (Mean ± SD U/L) | 42.4±49.9 (n=1257)* | 210.1±996.8 (n=519)** | 58.1±119.8** | <0.01 |
| ALT (Mean ± SD U/L) | 55.4±75.7 (n=952)* | 170.0±547.7 (n=417)** | 61.3±124.3** | <0.01 |
at initiation of chemo;
peak after chemo was begun
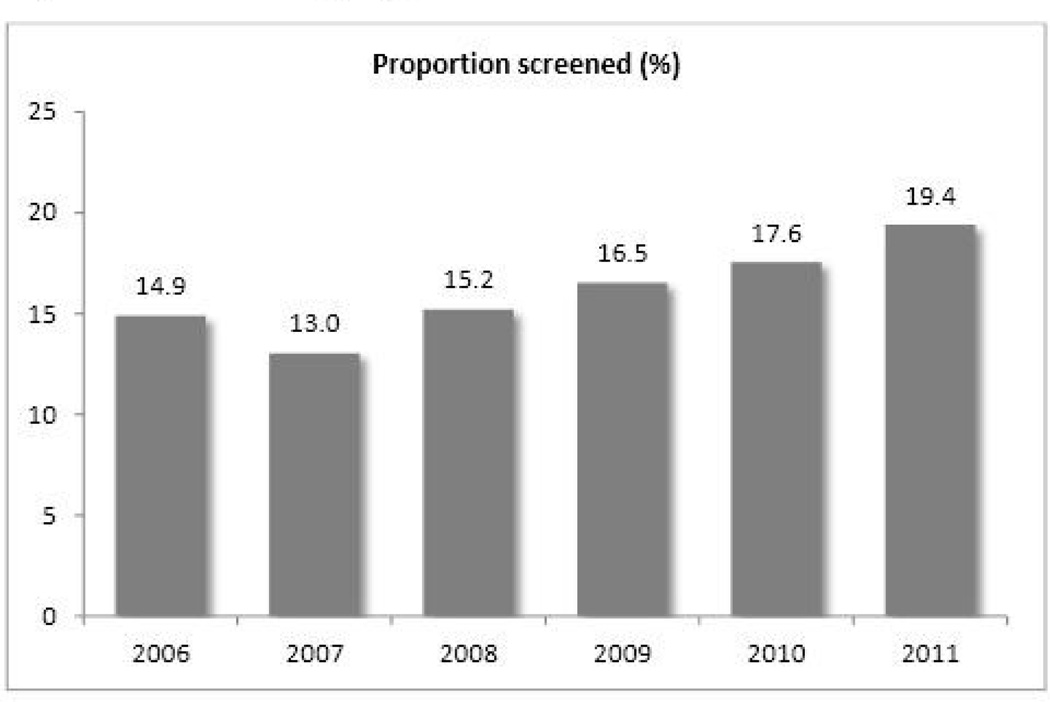
Patients that had screening were younger and more likely to be male. There was no significant difference in the screening rate by race. Serum AST and ALT at the initiation of chemotherapy were higher in screened patients (AST (U/L) 42.4±49.9 versus 31.3±34.3, p<0.01, and ALT (U/L) 55.4±75.7 versus 39.8±53.6, p<0.01, respectively). Of those who did not have screening, the peak AST and ALT during chemotherapy were higher in patients who were tested for HBV eventually. Figure 1 displays the proportion of patients who underwent screening, which increased from 14.3% in 2006–2008 to 17.7% in 2009–2011 (p<0.01). This trend was mostly attributable to an increase among patients with hematologic malignancies (32.7% vs. 40.6%, p<0.01), while there was no demonstrable change in screening among patients with solid tumors (9.7% vs. 10.1%, p=0.63) (Table 2). Otherwise, the increase was pervasive regardless of the age, gender or race, although the trend in non-white patients was less clear, in part due to the small number. Screening increased significantly among patients with normal aminotransferase, whereas the trend was less noticeable in patients who already had abnormal aminotransferase.
Figure 1.
HBV screening by year
Table 2.
Trends in HBV Screening
| Era 1 | Era 2 | ||
|---|---|---|---|
| (2006–2008) | (2009–2011) | p-value | |
| Total | 586/4089 (14.3%) | 693/3916 (17.7%) | <0.01 |
| Age | |||
| <40 years | 59/362 (16.3%) | 68/294 (23.1%) | 0.03 |
| <70 years | 422/2741 (15.4%) | 467/2598 (18.0%) | 0.01 |
| >=70 years | 105/986 (10.7%) | 158/1024 (15.4%) | <0.01 |
| Sex | |||
| Male | 332/2084 (15.9%) | 371/1980 (18.7%) | 0.02 |
| Female | 254/2005 (12.7%) | 322/1936 (16.6%) | <0.01 |
| Cancer type | |||
| Hematologic | 270/825 (32.7%) | 398/980 (40.6%) | <0.01 |
| Solid | 316/3264 (9.7%) | 295/2936 (10.1%) | 0.63 |
| Race | |||
| White | 528/3689 (14.3%) | 635/3632 (17.5%) | <0.01 |
| Black | 11/46 (23.9%) | 9/40 (22.5%) | 0.88 |
| Asian | 3/37 (8.1%) | 14/51 (27.5%) | 0.02 |
| Others | 44/317 (13.9%) | 35/193 (18.1%) | 0.20 |
| AST or ALT* | |||
| Normal | 384/3235 (11.9%) | 509/3163 (16.1%) | <0.01 |
| Abnormal | 195/685 (28.5%) | 175/557 (31.4%) | 0.26 |
Reference Range of AST: <=48 (U/L) for male, <=43 (U/L) for female
*Reference Range of ALT:<=55 (U/L) for male, <=45 (U/L) for female
The increase in the screening rate was further explored with the logistic regression analysis. In univariate analysis, the following factors were associated with screening; age, sex, cancer type, AST, ALT, and the latter time period (2009–2011). In the multivariable model shown in Table 3, the latter time period was associated with a 26% increase in the odds for HBV screening (p<0.01). Other factors associated with screening included age (OR=0.99, p<0.01), abnormal AST or ALT (OR=3.21, p<0.01) and hematologic malignancy (OR=5.89, p<0.01).
Table 3.
Factors associated with HBV screening (multivariable analysis)
| Screened for HBV (HBsAg) | |||
|---|---|---|---|
| Predictor | OR | 95% CI | p-value |
| Age | 0.987 | 0.983–0.992 | <0.01 |
| Male | 1.059 | 0.929–1.207 | 0.39 |
| Hematologic versus solid | 5.891 | 5.146–6.743 | <0.01 |
| Abnormal AST or ALT* | 3.213 | 2.756–3.746 | <0.01 |
| Era 2 versus Era 1 | 1.261 | 1.107–1.436 | <0.01 |
Data were unavailable in 365.
There were 13 patients who were found to have HBV infection (1.0% among the tested), including 2 diagnosed after the initiation of chemotherapy. An antiviral agent was used in eight patients prior to chemotherapy, including 2 who had already been receiving antiviral therapy prior to consideration of the chemotherapy. None experienced HBV reactivation. Out of the remaining five patients, one (20%) experienced reactivation of HBV, which was successfully controlled with lamivudine. There was another patient with HBV DNA reactivation, who had far advanced hepatocellular carcinoma and died of the malignancy without evidence of a clinical flare. The remaining three patients were monitored without antiviral prophylaxis and none developed significant reactivation with one patient having an isolated spike of ALT of 156 (U/L).
Out of the 6,203 patients who were never tested for HBsAg, 812 (13.1%) had abnormal ALT sometime within a year after chemotherapy was begun with a mean (± standard deviation) peak activity of 142.8 ± 206.0 U/L. The distribution of the peak ALT is shown in Figure 2. In an attempt to assess whether there could have been patients with undiagnosed HBV experiencing reactivation, we reviewed the medical records of the 58 patients with the highest peak ALT (300 U/L or greater). There were 10 patients with biliary obstruction and 13 with preceding abdominal/hepatobiliary procedures in whom the abnormal ALT was transient. Another 17 patients were in terminal or agonal circumstance without features of hepatic failure or a potential for undiagnosed HBV. A diagnosis of drug induced hepatotoxicity had been made in 12 - their ALT eventually normalized after discontinuation of the presumed offending agent, except in one patient (with a head and neck cancer) who died with abnormal ALT but without features of liver failure. Of the remaining six patients, two had known hepatitis C and were not tested for HBV while three were tested for markers other than HBsAg, namely anti-HBc and/or HBV DNA, all of which were negative. The last patient had unexplained ALT elevation and were not tested for HBV, making it possible that the patient may have had undiagnosed HBV. None of these 58 patients with the highest peak ALT had positive anti-HBc.
Discussion
In this study, we analyze a cohort of patients undergoing chemotherapy and assessed screening for HBV infection and the resultant outcome. The main findings include (1) the overall frequency of testing, be it for screening or diagnostic purpose, was low with less than a quarter of the patients being ever tested for HBV before or after initiation of chemotherapy; although screening did increase over time, mostly among patients with hematologic malignancies; (2) in addition the time period, several factors including younger age, abnormal aminotransferase and a diagnosis of hematologic malignancy were associated higher odds of being tested; (3) of patients with HBV infection, reactivation occurred in two out of five patients (40%) that did not receive prophylactic antiviral therapy; and (4) in patients who were not tested for HBV and experienced significant liver enzyme flare, the proportion of patients in whom undiagnosed HBV infection may have been responsible for the flare appeared small.
There remains a clear difference between guideline recommendations by the liver societies and those by the oncology community with regard to HBV screening prior to chemotherapy. The former strongly advocate universal screening whereas the latter advises clinical judgment depending on the risk. It is likely that there is an element of referral bias in experts’ opinions driving these recommendations. Gastroenterologists and hepatologists not uncommonly encounter chemotherapy patients suffering from aggressive reactivation of previously undiagnosed HBV infection. This is such a dramatic and regrettable event that even a single case makes a strong impression. In contrast, oncologists who prescribe chemotherapy to a large number of patients infrequently witness HBV reactivation. In general, the prevalence of HBV in most oncology practices remains low and, except in cases of stem cell transplantation or of patients receiving regimens containing rituximab or ofatumumab, the risk of reactivation in a given HBV carrier (as shown in our and others’ data) is also quite low.10
In light of these conflicting guideline recommendations, it is not surprising that HBV screening is not practiced uniformly in chemotherapy recipients. Our results are almost identical to what was reported by Hwang et al who found that only 17% of their new patients receiving chemotherapy underwent HBV screening.9 Surveys conducted among oncologists have shown that only a minority of oncologists (13–19%) report screening all patients receiving chemotherapy.11–13 Majority of oncologists responded that they screen select subgroups based on risk factors such as ethnicity or abnormal liver biochemistries. Our multivariable analysis identified age, abnormal liver enzymes and hematologic malignancies as markers for HBV screening – seemingly reflecting oncology guideline recommendations to base screening upon the clinician’s assessment of the risk of reactivation.
It is encouraging that screening in hematology patients increased over time, a trend not seen in solid tumor patients. Nonetheless, our data also indicate that the risk based screening strategy broke down in many of our patients. For example, despite the increase in screening over time, more than 50% of the most recent hematology patients remained unscreened. Similarly, missed opportunities were seen in patients with unexplained abnormal aminotransferase activities or with demographic profile suggestive of high probability of chronic HBV infection. Clearly, race is an easily recognizable risk factor of HBV infection.13 Given the uniform consensus that most Asian Americans be tested for HBV, even in the absence of chemotherapy, our Asian patients should have been tested. Apparently, this is not unique to the setting of this study in which the proportion of Asian patients was low - in another large US study, Asian race was not associated with higher rate of screening, although in those who were tested, the prevalence was as high as 39%.9
Although the number was small, our patients with HBV infection who underwent chemotherapy without antiviral prophylaxis had a 40% risk of reactivation, whereas no patients receiving antiviral therapy did. The benefits of prophylactic therapy in patients with known HBV are well established.14 There have been at least three randomized controlled trials that demonstrated superiority of prophylactic therapy to initiating antiviral therapy in response to a hepatitis flare in both hematologic and solid tumor patients.1, 2, 15 While it is clear that intervention is effective in patients known to have HBV, the more difficult question has been whether universal screening to discover those patients is justified or cost-effective.6, 7
It was somewhat surprising that despite inadequate screening, there was little evidence undiagnosed HBV resulted in major clinical events in our practice. When records of patients with the most abnormal ALT were reviewed, very few had clinical scenarios that may have possibly been an episode of HBV flare. Potential explanations for the seemingly low impact of undiagnosed HBV in our cohort may include (1) that our practice is located in an area with a low prevalence of HBV infection; (2) that majority of our patients had solid tumors receiving low-risk chemotherapy; and (3) that our practice engage multidisciplinary teams of specialists, which provides opportunities for experienced clinicians to utilize focused diagnostic testing such that HBV testing was avoided in patients with a low pre-test probability.
One of the limitations of our study is linked to the main finding of the study that majority of chemotherapy recipients were not tested for HBV and, therefore, HBsAg data were missing in a large proportion of our patient sample. Clearly, it is possible that there may have been an unknown number of patients with HBV flare that were not identified in our chart review. Since most of the patients with high ALT activities had alternative explanation for the abnormality, we believe undiagnosed HBV flares may have been self-limited with low ALT. This is obviously a limitation of the retrospective nature of the study – there was no specific protocol to investigate all potential cases of HBV reactivation. However, data used to determine the screening rate and its trend were drawn from a database that prospectively tracked all chemotherapy recipients. The data to investigate predictive factors were also mostly complete. Finally, we make no claims about the generalizability of our data in other settings, in particular, practices enriched with patients with high prevalence of HBV infection.
Despite recommendations by CDC and liver societies that advocate screening all patients undergoing chemotherapy, the practical reality is that gastroenterologists have little influence in the daily clinical decision-making in chemotherapy recipients and that until practicing oncologists are convinced of the need, universal screening is unlikely to be adopted. It appears, however, screening is improving in the highest risk patients, namely those with hematologic malignancies or abnormal liver enzymes. Our data, despite its limitations, suggest that in our practice setting of low HBV prevalence, devastating HBV reactivation in undiagnosed patients was rare. We interpret them to suggest a strategy alternative to seemingly unrealistic insistence on universal screening, at least for low prevalence areas in the US and other developed countries, which may include (1) increasing screening efforts in patients with hematological malignancies, (2) enhancing the current risk based screening to target individuals at risk of HBV infection, particularly Asian/Pacific Islander and foreign-born patients as well as patients with abnormal liver biochemistry, (3) promoting awareness to implement timely therapy in patients with HBV infection. In addition, individualized management tools to estimate the probability HBV carriage and the risk of reactivation, taking into account complex comorbidity profiles often seen in chemotherapy recipients, may help optimize the screening and management strategies.
Table 4.
Potential causes of the high peak ALT (300 U/L or greater) in 58 patients
| Category | Number |
|---|---|
| Biliary obstruction | 10 |
| Postoperative | 13 |
| Terminal/Agonal | 17 |
| Drug-induced | 12 |
| Hepatitis C | 2 |
| Other | 4 |
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK-82843 and DK-92336)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution:
Chung. Wi: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript
Nicole M. Loo: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content
Joseph J. Larson: analysis and interpretation of data
Timothy J. Moynihan: critical revision of the manuscript for important intellectual content
Nageswar R. Madde: acquisition of data
Darryl C. Grendahl: acquisition of data, critical revision of the manuscript for important intellectual content
Steven R. Alberts: critical revision of the manuscript for important intellectual content
W. Ray Kim: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision
Disclosure: The authors disclose no conflict of interest
Reference
- 1.Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, Zhang HY, Lie A, Ngan R, Liang R. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, Lin TH, Hsiao HH, Young JH, Chang MC, Liao YM, Li CC, Wu HB, Tien HF, Chao TY, Liu TW, Cheng AL, Chen PJ. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 3.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2008;57:1–20. [PubMed] [Google Scholar]
- 4.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 5.Artz AS, Somerfield MR, Feld JJ, Giusti AF, Kramer BS, Sabichi AL, Zon RT, Wong SL. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3199–3202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 6.Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3270–3277. doi: 10.1200/JCO.2011.35.1635. [DOI] [PubMed] [Google Scholar]
- 7.Zurawska U, Hicks LK, Woo G, Bell CM, Krahn M, Chan KK, Feld JJ. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3167–3173. doi: 10.1200/JCO.2011.40.7510. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JPBA, Perrillo R. International survey on reactivation of hepatitis B induced by cancer chemotherapy; Abstract, AASLD Emergin Trends Conference 2013; 2013. [Google Scholar]
- 9.Hwang JP, Fisch MJ, Zhang H, Kallen MA, Routbort MJ, Lal LS, Vierling JM, Suarez-Almazor ME. Low rates of hepatitis B virus screening at the onset of chemotherapy. Journal of oncology practice / American Society of Clinical Oncology. 2012;8:e32–e39. doi: 10.1200/JOP.2011.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artz AS, Somerfield MR, Feld JJ, Giusti AF, Kramer BS, Sabichi AL, Zon R, Wong SL. Special Announcement, 2013 ( http://www.asco.org/qualityguidelines/asco-provisional-clinical-opinion-chronic-hepatitis-b-virus-infection-screening); original version: ASCO Provisional Clinical Opinion: Chronic Hepatitis B Virus Infection Screening in Patients Receiving Cytotoxic Chemotherapy for Treatment of Malignant Diseases. Journal of Clinical Oncology. 2010;28(19):3199–3202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 11.Tran TT, Rakoski MO, Martin P, Poordad F. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices. Alimentary pharmacology & therapeutics. 2010;31:240–246. doi: 10.1111/j.1365-2036.2009.04158.x. [DOI] [PubMed] [Google Scholar]
- 12.Khokhar OS, Farhadi A, McGrail L, Lewis JH. Oncologists and hepatitis B: a survey to determine current level of awareness and practice of antiviral prophylaxis to prevent reactivation. Chemotherapy. 2009;55:69–75. doi: 10.1159/000183731. [DOI] [PubMed] [Google Scholar]
- 13.Day FL, Link E, Thursky K, Rischin D. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. Journal of oncology practice / American Society of Clinical Oncology. 2011;7:141–147. doi: 10.1200/JOP.2010.000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo W, Chan HL. Hepatitis B virus reactivation associated with anti-neoplastic therapy. Journal of gastroenterology and hepatology. 2013;28:31–37. doi: 10.1111/j.1440-1746.2012.07280.x. [DOI] [PubMed] [Google Scholar]
- 15.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, Cho SH, Han JY, Lee YS. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]