Abstract
Background
Reports indicate that blacks have lower survival following the diagnosis of a poor prognosis cancer, compared with whites. We explored the extent to which this disparity is attributable to the underuse of surgery.
Study Design
Using the SEER-Medicare database we identified 57,364 patients, ages ≥65, with a new diagnosis of non-metastatic liver, lung, pancreatic and esophageal cancer, from 2000-2005. We evaluated racial differences in resection rates after adjustment for patient, tumor and hospital characteristics, using hierarchical logistic regression. Cox proportional hazards regression was used to assess racial differences in survival, after adjusting for patient, tumor and hospital characteristics, and receipt of surgery.
Results
Compared with whites, blacks were less likely to undergo surgery for liver (adjusted OR [aOR], 0.49, 95%CI 0.29-0.83), lung (aOR 0.62 95%CI 0.56-0.69), pancreas (aOR 0.53, 95%CI 0.41-0.70) and esophagus cancers (aOR 0.64, 95%CI 0.42-0.99). Hospitals varied in their surgery rates among patients with potentially resectable disease. However, resection rates were consistently lower for blacks, regardless of the resection rate of the treating hospital. Although there were no racial differences in overall survival with liver and esophageal cancer, blacks experienced poorer survival for lung (adjusted HR [aHR]1.05, 95%CI 1.00-1.10) and pancreas cancer (aHR 1.15, 95%CI 1.03-1.30). In both instances, there were no residual racial disparities in overall survival after further adjusting for use of surgery.
Conclusions
Blacks are less likely to undergo surgery following the diagnosis of a poor prognosis cancer. Our findings suggest that surgery is an important predictor of overall mortality, and that efforts to reduce racial disparities will require stakeholders to gain a better understanding of why elderly blacks are less likely to get to the operating room.
Introduction
Compared with white patients, blacks have substantially lower survival rates following the diagnosis of a poor prognosis cancer. With esophageal cancer, for example, 5-year overall survival in blacks is 11.2% compared to 17.7% for whites.1 Similar disparities have been noted with liver, lung and pancreatic cancer.2-6 Factors underlying racial disparities are no doubt multifactorial, and include patient characteristics associated with worse health outcomes independent of cancer, including lower socioeconomic status and higher prevalence of comorbidities.7,8 Blacks may also have poorer survival because they tend to present with disease of a more advanced stage at the time of diagnosis, possibly related in part to racial differences in access to health care.2
It is plausible that blacks have persistently worse outcomes compared with whites because they are less likely to undergo cancer-directed surgery. For most poor prognosis cancers, chemotherapy and radiation have limited effectiveness, and surgical resection remains the only potential curative therapy, and the treatment most likely to prolong life. Although previous studies have suggested that blacks are substantially less likely to undergo surgery for some cancer types, this issue has not been carefully studied among patients with poor prognosis cancers.3,4,9-13 Moreover, there is little understanding of the extent to which lower surgery rates in blacks may reflect the fact that blacks receive care at different hospitals, which may have lower overall rates of surgery.14,15 Finally, there have been no comprehensive studies assessing the extent to which the comparative underuse of surgery by race explains differences in survival.
In this context, we performed a retrospective cohort study using national SEER-Medicare data to evaluate racial differences in resection rates, among patients ages 65 and older. We then assessed the extent to which the underuse of cancer-directed surgery explains racial disparities in overall survival.
Methods
Database and subjects
This study is based on the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The SEER-Medicare linked database contains clinical, demographic, and medical claims data on patients age 65 years or older. The SEER program of the National Cancer Institute is a prospective cancer-specific database that contains patient demographics and information related to tumor stage, grade, location and surgical treatment. The Medicare claims data includes information about patient demographics, Medicare enrollment dates, health maintenance organization membership, and fee for- service claims for inpatient and outpatient services provided to patients, including surgical care.
We used the appropriate Patient Entitlement and Diagnosis Summary File (PEDSF) to identify black and white patients aged 65 years and older with a new diagnosis of non-metastatic (Any T, Any N, MO)16-19 liver, lung, pancreatic and esophageal cancer from 2000-2005, with follow-up through 2008. The PEDSF contains one record per person for individuals in the SEER database who have been matched with the Medicare enrollment records. We excluded patients enrolled in managed care plans, diagnosed with stage 0 (in situ) cancer and patients diagnosed with cancer at autopsy. 20
Race and rates of surgical resection
We explored the relationship between race and surgical resection for each one of the four cancer types, accounting for patient factors, tumor factors and characteristics associated with the treating facility. The primary exposure variable, race, was identified using the Medicare database, which has an accuracy of over 95% for black and white race designations.21 The primary outcome, cancer-directed surgery within six months of diagnosis, was defined as a procedure with potentially curative intent (Appendix A). Procedures performed for diagnostic purposes, palliation and management of complications were not considered cancer-directed surgery. The covariates used for risk adjustment included patient factors (including age [65-69, 70-74, 75-79, ≥79 years], gender, comorbid conditions [0, 1, ≥2], marital status [married, single, other], socioeconomic status [median zip code level income]), tumor specific factors (including SEER stage [local or regional], size [<2.0 cm, 2.0-2.9 cm, 3.0-3.9 cm, 4.0-4.9 cm, ≥5 cm]), and hospital characteristics (including teaching status and operative volume [high, medium, low]). Comorbid conditions were quantified using the Charlson comorbidity index.
We conducted a patient level analysis to examine the association between race and use of surgery. Hierarchical logistic regression was used to determine the odds ratio (OR) of surgical resection associated with race, while sequentially adjusting for patient, tumor and hospital characteristics. To account for the clustering of patients within hospitals, we included a hospital-specific random intercept in the logistic regression model. In addition to assessing the general relationship between race and the use of surgery, we examined the effect of hospital operative volume on race-related differences in resection rates. Hospitals were divided into cancer procedure-specific terciles (low, medium and high) according to the overall number of liver, lung, pancreatic, and esophageal cancer cases with curative intent performed in that facility from 2000-2005. Risk-adjusted resection rates were examined by race for each tercile.
Surgical resection and racial disparities in survival
We then examined the extent to which race-related differences in the use of surgery may explain previously documented differences in mortality. Univariate analysis of overall survival was performed using Kaplan-Meier estimates, and the log-rank test was used to examine racial differences in overall survival. Cox proportional hazards regression was used to assess the effect of race on overall survival, after sequentially adjusting for patient, tumor and hospital characteristics. The analysis was adjusted for clustering of patients within hospitals using robust sandwich estimates for the standard error. 22
All models represent complete case analyses. All analyses were performed using SAS [9.1] (SAS institute, Cary, NC) software. P<0.05 was considered statistically significant, and all tests were two-sided. The Institutional Review Board of the University of Michigan approved the study protocol.
Results
Patient characteristics
There were 2,698 liver cancer patients, 44,832 lung cancer patients, 6,060 pancreatic cancer patients and 3,774 esophageal cancer patients in the cohort, all ages 65 years and older. Black patients were slightly younger at the time of diagnosis compared with whites for all four cancers (p<0.05 for all cancers). Overall, the median income was significantly lower for black patients than whites (p<0.001 for all cancers). Black patients were also more likely to be single, separated or divorced for all four cancers (p<0.001 for all cancers). Blacks with lung cancer were more likely to present with more advanced disease compared with whites (p<0.001), but that was not the case among patients with pancreatic, liver or esophageal cancer. Black patients diagnosed with lung, pancreatic and esophageal cancer were less likely to undergo treatment at high volume centers, compared with their white counterparts (Table 1).
Table 1.
Patient, tumor and hospital characteristics. Based on SEER-Medicare 2000-2005, with follow up through 2008.
| Liver | Lung | Pancreas | Esophagus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Characteristicsŧ | Overall | White | Black | Overall | White | Black | Overall | White | Black | Overall | White | Black |
| 2,698 | 2,429 (90.0%) | 269 (10.0%) | 44,832 | 41,329 (92.2%) | 3,503 (7.8%) | 6,060 | 5,465 (90.2%) | 595 (9.8%) | 3,774 | 3,436 (91.0%) | 338 (9.0%) | |
|
| ||||||||||||
| Surgery | 397 (14.7%) | 373 (15.4%) | 24 (8.9%)** | 15,370 (34.3%) | 14,562 (35.2%) | 808 (23.1%)** | 1,785 (29.5%) | 1,672 (30.6%) | 113 (19.0%) ** | 737 (19.5%) | 698 (20.3%) | 39 (11.5%)** |
| Male | 1,725 (63.9%) | 1,569 (64.6%) | 156 (58.0%)* | 23,147 (51.6%) | 21,209 (51.3%) | 1,938 (55.3%)** | 2,637 (43.5%) | 2,409 (44.1%) | 228 (38.3%)** | 2,692 (71.3%) | 2,464 (71.7%) | 228 (67.5%) |
| Age, mean | 74.9 | 75.0 | 74.1* | 74.8 | 74.8 | 73.9** | 76.2 | 76.3 | 75.2** | 75.2 | 75.4 | 73.7 ** |
| Married | 1,576 (58.4%) | 1,479 (60.9%) | 97 (36.1%)** | 23,656 (52.8%) | 22,318 (54.0%) | 1,338 (38.2%)** | 3,256 (53.7%) | 3,042 (55.7%) | 214 (36.0%)** | 2,194 (58.1%) | 2,064 (60.1%) | 130 (38.5%)** |
| Comorbid Conditions | ||||||||||||
| 0 | 674 (25.0%) | 606 (24.9%) | 68 (25.3%) | 13,061 (29.1%) | 12,134 (29.4%) | 927 (26.5%)** | 2,235 (36.9%) | 2,065 (37.8%) | 170 (28.6%)** | 1,519 (40.2%) | 1,397 (40.7%) | 122 (36.1%) |
| 1 | 566 (21.0%) | 506 (20.8%) | 60 (22.3%) | 14,336 (32.0%) | 13,363 (32.3%) | 973 (27.8%) | 1,807 (29.8%) | 1,629 (29.8%) | 178 (29.9%) | 1,011 (26.8%) | 918 (26.7%) | 93 (27.5%) |
| ≥2 | 1,458 (54.0%) | 1,317 (54.2%) | 141 (52.4%) | 17,435 (38.9%) | 15,832 (38.3%) | 1,603 (45.8%) | 2,018 (33.3%) | 1,771 (32.4%) | 247 (41.5%) | 1,244 (33.0%) | 1,121 (32.6%) | 123 (36.4%) |
| Income, median | $47,414 | $48,795 | $34,556** | $47,340 | $48,412 | $34,662 ** | $49,577 | $51,131 | $35,325** | $48,767 | $50,216 | $33,960** |
|
| ||||||||||||
| Tumor size (cm) | ||||||||||||
| < 2.0 | 118 (4.4%) | 106 (4.4%) | 12 (4.5%) | 6,158 (13.7%) | 5,795 (14.0%) | 363 (10.4%)** | 357 (5.9%) | 334 (6.1%) | 23 (3.9%)* | 282 (7.5%) | 268 (7.8%) | 14 (4.1%) |
| 2.0 – 2.9 | 180 (6.7%) | 171 (7.0%) | 9 (3.3%) | 8,637 (19.3%) | 8,073 (19.5%) | 564 (16.1%) | 962 (15.9%) | 896 (16.4%) | 66 (11.1%) | 276 (7.3%) | 252 (7.3%) | 24 (7.1%) |
| 3.0 – 3.9 | 245 (9.1%) | 216 (8.9%) | 29 (10.8%) | 7,151 (16.0%) | 6,621 (16.0%) | 530 (15.1%) | 1,159 (19.1%) | 1,046 (19.1%) | 113 (19.0%) | 321 (8.5%) | 291 (8.5%) | 30 (8.9%) |
| 4.0 – 4.9 | 248 (9.2%) | 227 (9.3%) | 21 (7.8%) | 4,939 (11.0%) | 4,555 (11.0%) | 384 (11.0%) | 882 (14.6%) | 793 (14.5%) | 89 (15.0%) | 310 (8.2%) | 285 (8.3%) | 25 (7.4%) |
| ≥5.0 | 1,172 (43.4%) | 1,043 (42.9%) | 129 (48.0%) | 9,983 (22.3%) | 9,029 (21.8%) | 954 (27.2%) | 1,032 (17.0%) | 925 (16.9%) | 107 (18.0%) | 848 (22.5%) | 759 (22.1%) | 89 (26.3%) |
| Tumor SEER stage | ||||||||||||
| Local | 1,755 (65.0%) | 1,584 (65.2%) | 171 (63.6%) | 19,897 (44.4%) | 18,436 (44.6%) | 1,461 (41.7%)** | 1,668 (27.5%) | 1,427 (26.1%) | 184 (30.9%) ** | 1,909 (50.6%) | 1,749 (50.9%) | 160 (47.3%) |
| Regional | 943 (35.0%) | 845 (34.8%) | 98 (36.4%) | 24,935 (55.6%) | 22,893 (55.4%) | 2,042 (58.3%) | 1,611 (26.6%) | 4,038 (73.9%) | 411 (69.1%) | 1,865 (49.4%) | 1,687 (49.1%) | 178 (52.7%) |
|
| ||||||||||||
| NCI center | ||||||||||||
| Yes | 339 (12.6%) | 313 (12.9%) | 26 (9.7%) | 41,528 (92.6%) | 1,964 (4.8%) | 171 (4.9%) ** | 712 (11.7%) | 648 (11.9%) | 64 (10.8%) | 386 (10.2%) | 364 (10.6%) | 22 (6.5%) ** |
| Hospital volumeŧŧ | ||||||||||||
| Low | 1,077 (39.9%) | 972 (40.0%) | 105 (39.0%) | 15,024 (33.5%) | 13,503 (32.7%) | 1,521 (43.4%)** | 1,890 (31.2%) | 1,668 (30.5%) | 222 (37.3%)** | 1,368 (36.2%) | 1,226 (35.7%) | 142 (42.0%)** |
| Medium | 670 (24.8%) | 600 (24.7%) | 70 (26.0%) | 15,413 (34.4%) | 14,409 (34.9%) | 1,004 (28.7%) | 2,172 (35.8%) | 1,992 (36.5%) | 180 (30.3%) | 1,079 (28.6%) | 969 (28.2%) | 110 (32.5%) |
| High | 951 (35.2%) | 857 (35.3%) | 94 (34.9%) | 14,395 (32.1%) | 13,417 (32.5%) | 978 (27.9%) | 1,998 (33.0%) | 1,805 (33.0%) | 193 (32.4%) | 1,327 (35.2%) | 1,241 (36.1%) | 86 (25.4%) |
| Teaching hospital | ||||||||||||
| Yes | 1,741 (64.5%) | 1,544 (63.6%) | 197 (73.2%)** | 24522 (54.7%) | 22,246 (53.8%) | 2,276 (65.0%)** | 3,761 (62.1%) | 3,361 (61.5%) | 400 (67.2%)** | 2,261 (59.9%) | 2,042 (59.4%) | 219 (64.8%)** |
One overall test to compare blacks vs. whites for each characteristic. All patients are ages ≥ 65.
p<0.05
p<0.01
Terciles for hospital volume are procedure specific.
Race and use of cancer-directed surgery
Black patients were significantly less likely to undergo potentially curative resection than whites for liver (adjusted odds ratio [aOR] 0.49, 95% CI 0.29-0.83), lung (aOR 0.62, 95% CI 0.56-0.69), pancreatic (aOR 0.53, 95% CI 0.41-0.70) and esophageal (aOR 0.64, 95% CI 0.42-0.99) cancers. Sequentially adjusting for patient, tumor and hospital characteristics had no significant effect on racial disparities in the use of surgical resection for any of the four cancers (Table 2).
Table 2.
Use of cancer-directed surgery in black and white patients diagnosed with potentially resectable liver, lung, pancreatic and esophageal cancer. Based on SEER-Medicare 2000-2005, with follow up through 2008.
| Odds ratio (OR) of surgical resection associated with black vs. white race | ||||
|---|---|---|---|---|
|
| ||||
| Modelŧ | Liver OR (95%CI) | Lung OR (95%CI) | Pancreas OR (95%CI) | Esophagus OR (95%CI) |
| Unadjusted | 0.49 (0.31-0.79) | 0.59 (0.53-0.64) | 0.54 (0.42-0.68) | 0.69 (0.46-1.02) |
| Adjusted sequentially for: | ||||
| Patient factors* | 0.52 (0.31-0.86) | 0.62 (0.56-0.68) | 0.51 (0.40-0.66) | 0.62 (0.40-1.08) |
| Patient factors + tumor factors** | 0.52 (0.31-0.87) | 0.63 (0.57-0.70) | 0.55 (0.42-0.72) | 0.61 (0.40-0.94) |
| Patient factors + tumor factors + hospital characteristics*** | 0.49 (0.29-0.83) | 0.62 (0.56-0.69) | 0.53 (0.41-0.70) | 0.64 (0.42-0.99) |
Complete case analysis of 2,567 liver, 42,991 lung, 5,795 pancreas and 3,611 esophageal cancer patients.
Patient factors: age (65-69, 70-74, 75-79, ≥79 years), gender (male, female), comorbid conditions (0, 1, ≥2), marital status (married, single, other), socioeconomic status (median zip code level income).
Tumor factors: SEER stage (local, regional), size (<2.0 cm, 2.0-2.9 cm, 3.0-3.9 cm, 4.0-4.9 cm, ≥5 cm)
Hospital characteristics: operative volume (cancer-specific procedure volume), teaching status (yes, no)
As indicated in Figure 1, hospitals varied widely in their overall rates of resection among patients with potentially resectable cancer of each type. However, blacks were consistently less likely to undergo surgery at each type of hospital. For example, among hospitals with relatively low operative volume, 35% of blacks compared to 49% of whites underwent surgery for pancreatic cancer (p<0.001). Similarly, at hospitals with relatively high operative volume, 56% of blacks compared to 69% of whites underwent surgical resection for pancreatic cancer (p<0.001). Risk-adjusted cancer-specific resection rates for blacks were significantly lower across all terciles for all four cancers, compared with whites (Figure 1).
Figure 1.
Use of cancer-directed surgery in black and white patients with potentially resectable liver, lung, pancreatic and esophageal cancers, by hospital resection rates. Based on SEER-Medicare 2000-2005, with follow up through 2008.
* Complete case analysis of 2,567 liver, 42,991 lung, 5,795 pancreas and 3,611 esophageal cancer patients. Resection rates risk-adjusted for age (65-69, 70-74, 75-79, ≥79 years), gender (male, female), comorbid conditions (0, 1, ≥2), marital status (married, single, other), socioeconomic status (median zip code level income), SEER stage (local, regional) and tumor size (<2.0 cm, 2.0-2.9 cm, 3.0-3.9 cm, 4.0-4.9 cm, ≥5 cm).
Relationship between race and hazard ratio of mortality
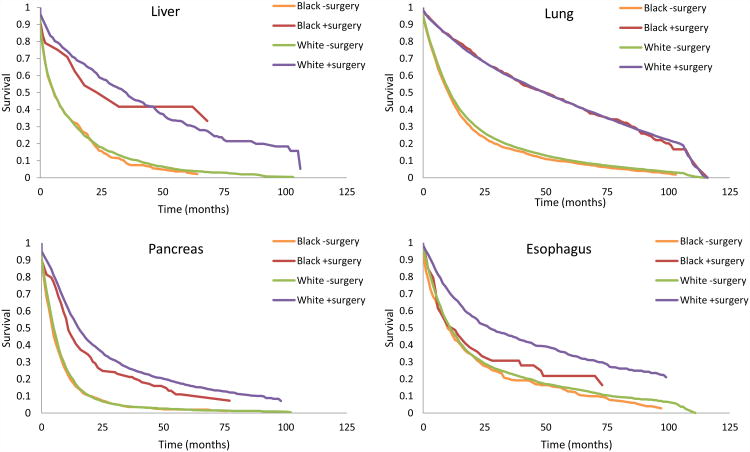
For patients with liver cancer and esophageal cancer, race was not associated with statistically significant differences in overall survival, after adjusting for patient characteristics (adjusted hazard ratio [aHR] 1.04, 95% CI 0.89-1.22 and aHR 1.12, 95% CI 0.98-1.28, respectively). However, among patients with lung cancer (aHR 1.05, 95% CI 1.00-1.10) and pancreatic cancer (aHR 1.15, 95% CI 1.03-1.30), race was a predictor of survival, despite adjusting for patient, tumor and hospital characteristics. After further accounting for race-related differences in the use of surgery, the disparity in survival was no longer apparent (Table 3). As illustrated in Figure 2, the receipt of cancer-directed surgery ameliorated racial differences in survival.
Table 3.
Mortality in black and white patients diagnosed with lung, liver, pancreatic and esophageal cancer. Based on SEER-Medicare 2000-2005, with follow up through 2008.
| Hazard ratio (HR) of mortality associated with black vs. white race | ||||
|---|---|---|---|---|
|
| ||||
| Modelsŧ | Liver HR (95%CI) | Lung HR (95%CI) | Pancreas HR (95%CI) | Esophagus HR (95%CI) |
| Unadjusted | 1.11 (0.97-1.28) | 1.19 (1.14 - 1.25)ŧŧŧ | 1.23 (1.11-1.36)ŧŧŧ | 1.21 (1.07-1.37)ŧŧŧ |
| Adjusted sequentially for: | ||||
| Patient factors* | 1.04 (0.89-1.22) | 1.09 (1.04-1.14)ŧŧŧ | 1.17 (1.05-1.31)ŧŧŧ | 1.12 (0.98-1.28) |
| Patient factors + tumor factors** | 1.07 (0.92-1.24) | 1.04 (1.00-1.09) | 1.15 (1.03-1.28)ŧŧŧ | 1.08 (0.95-1.23) |
| Patient factors + tumor factors + hospital characteristics*** | 1.10 (0.95-1.27) | 1.05 (1.00-1.10)ŧŧ | 1.15 (1.03-1.30)ŧŧŧ | 1.05 (0.91-1.20) |
| Patient factors + tumor factors + hospital characteristics + surgery**** | 1.01 (0.88-1.17) | 0.98 (0.94-1.02) | 1.06 (0.94-1.19) | 1.02 (0.89-1.17) |
Complete case analysis of 2,567 liver, 42,991 lung, 5,795 pancreas and 3,611 esophageal cancer patients.
p<0.05
p <0.01
Patient factors: age (65-69, 70-74, 75-79, ≥79 years), gender (male, female), comorbid conditions (0, 1, ≥2), marital status (married, single, other), socioeconomic status (median zip code level income).
Tumor factors: SEER stage (local, regional), size (<2.0 cm, 2.0-2.9 cm, 3.0-3.9 cm, 4.0-4.9 cm, ≥5 cm)
Hospital characteristics: operative volume (cancer-specific procedure volume), teaching status (yes, no)
Receipt of cancer directed surgery within 6 months of diagnosis
Figure 2.
Unadjusted survival curves for black and white patients diagnosed with potentially resectable liver, lung, pancreatic and esophageal cancers, by use and non-use of cancer-directed surgery. Based on SEER-Medicare 2000-2005, with follow up through 2008.
Discussion
Consistent with previous reports, this study indicates that elderly black patients are substantially less likely to undergo cancer-directed surgery than whites for early stage liver, lung, pancreatic, and esophageal cancer. Moreover, our findings indicate that race-related differences in practice patterns are apparent within high and low volume hospitals. Finally, our findings suggest that surgery is an important predictor of mortality, and that the relative underuse of surgery is an important mechanism underlying worse overall survival in elderly blacks.
Previous studies have indicated that blacks with poor prognosis cancers are significantly less likely to undergo surgical resection, compared to whites.6,11,23-25 An analysis by Bach and colleagues identified that black patients with early stage lung cancer were half as likely to undergo cancer-directed surgery compared to whites.3 Utilizing the National Cancer Database, Bilimoria et al. revealed that pancreatectomy, the only curative treatment for localized pancreatic cancer, is underused in black patients with stage I disease.11 In separate studies of the SEER-Medicare database, Paulson et al. and Steyerberg et al. demonstrated that black patients with early stage esophageal cancer were substantially less likely to undergo surgery compared to whites.24,25 Mathur and colleagues documented similar findings in patients with early stage liver cancer.6
Mechanisms underlying differences in the use of cancer-directed surgery remain uncertain. We hypothesized that race-related differences in resection rates might be attributable to blacks receiving their care in resource-poor facilities with lower use of complex surgery, regardless of race. Previous studies, including a cross-sectional analysis by Bach and colleagues, have suggested that black and white patients seek care from different hospitals and physicians. Further, physicians treating a high proportion of black patients were more likely to report that they were unable to provide all of their patients with access to high quality care or access to high quality specialists, compared to those treating a low proportion of black patients.14 The findings of our study did not confirm our initial hypothesis, however. Black patients were substantially less likely to undergo surgery, regardless of whether they received treatment at hospitals with comparatively high or low rates of cancer-directed surgery.
Instead, racial disparities in the use of cancer-directed surgery primarily reflected black-white differences in the use of surgery within hospitals, regardless of operative volume. Although our study does not elucidate mechanisms underlying this difference, these disparate practice patterns may be driven by the physician-patient relationship. 11,26,27 Informed decision-making regarding a cancer operation requires a clear and comprehensive dialogue between doctors and patients that addresses the diagnosis, available treatments, focuses on risk/benefit tradeoffs, and alleviates fear. Though our study does not empirically address patient-physician encounters, previous studies explicitly investigating this subject have suggested that the quality of the patient-physician relationship significantly influences treatment decisions. Manfredi and colleagues identified that inadequate communication between black cancer patients and their care teams results in less effective decision-making and poorer perceived outcomes.26 It is plausible that deficiencies in the dynamics of physician-patient communication, the understanding of patient preferences, and overall patient trust are common to high and low volume hospitals, and may contribute to racial disparities in the use of cancer-directed surgery among elderly patients.20,27
It is also possible that racial differences in resection rates are related to unmeasured variations in comorbid conditions and illness severity. In a population based study, Olge and colleagues documented that black cancer patients were more likely to have pre-existing chronic illnesses compared with whites.28 Moreover, comorbid conditions are often associated with less aggressive cancer care.29 We cannot rule out the possibility of unmeasured differences not adequately captured in claims data. Unmeasured confounding is a frequent concern in analyses based on administrative data, including SEER-Medicare.
Our study has several other limitations. First, our findings do not directly inform the “optimal rate” of surgery in patients with these four cancers. In other words, our findings could indicate under-treatment in blacks or over-treatment of whites. As observed in our study, most elderly patients with these cancers succumb to their disease regardless of surgery. Thus, it is possible that black patients are prioritizing quality of life rather than life-prolonging measures due to cultural preferences and differences in values. However, this hypothesis is belied by previous studies indicating that blacks are less likely to utilize hospice services and are more likely to use costly life saving measures at the end of life. 30,31 Second, our analysis of SEER-Medicare data may not be generalizable to patients under the age of 65. It is plausible that racial disparities in the use of surgery and overall mortality are more pronounced in the general population and younger patients, as they are more likely to lack insurance and access to care, compared to Americans over the age of 65. Finally, this study does not inform how disparities in cancer care and outcomes vary across other US minority groups such as Hispanics, Asians and Native Americans due sample size.
Racial disparities in outcomes after diagnosis with poor prognosis cancer are widely recognized and considered an important public health and policy concern. The present study suggests that policy initiatives aimed at directing underserved elderly minority patients to high-volume surgeons or hospitals will not be sufficient to remedy lower rates of cancer-directed surgery, and associated poorer survival, among blacks with these highly fatal cancers. Efforts to reduce racial disparities among elderly patients and ultimately improve outcomes will require stakeholders at all levels to gain a better understanding of why blacks are less likely to get to the operating room, including insights about factors that influence patient-physician communication and trust.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number 2T32 CA009672-21 to SLR); and the Michigan Institute for Clinical and Health Research of the National Institutes of Health Clinical and Translational Science Awards program (grant number UL1RR024986 to SLR). This work was also supported by funding from the National Institute on Aging at the National Institutes of Health (grant numbers K05CA11557105 and K05CA1155710503S1 to JDB).
Contributor Information
Sha'Shonda L. Revels, Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor; Department of Surgery, University of Michigan, Ann Arbor.
Mousumi Banerjee, Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor; School of Public Health Department of Biostatistics, University of Michigan, Ann Arbor.
Huiying Yin, Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor;.
Christopher J. Sonnenday, Department of Surgery, University of Michigan, Ann Arbor; School of Public Health Department of Health Management & Policy, University of Michigan, Ann Arbor.
John D. Birkmeyer, Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor; Department of Surgery, University of Michigan, Ann Arbor.
References
- 1.Cancer of the Esophagus: 5-Year Relative and Period Survival (Percent) by Race, Sex, Diagnosis Year, Stage and Age. Surveillance, Epidemiology, and End Results (SEER) Program. Available from: http://seer.cancer.gov/csr/1975_2008/results_single/sect_08_table.08.pdf.
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011 Jul-Aug;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999 Oct 14;341(16):1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002 Apr 24;287(16):2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection: a key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer. 2009 Sep 1;115(17):3979–3990. doi: 10.1002/cncr.24433. [DOI] [PubMed] [Google Scholar]
- 6.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010 Dec;145(12):1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer NJ, Gu N, Baser O, Morris AM, Birkmeyer JD. Socioeconomic status and surgical mortality in the elderly. Med Care. 2008 Sep;46(9):893–899. doi: 10.1097/MLR.0b013e31817925b0. [DOI] [PubMed] [Google Scholar]
- 8.Yang R, Cheung MC, Byrne MM, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010 May 15;116(10):2437–2447. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]
- 9.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010 Jul;211(1):105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 10.Mathur AK, Sonnenday CJ, Merion RM. Race and ethnicity in access to and outcomes of liver transplantation: a critical literature review. Am J Transplant. 2009 Dec;9(12):2662–2668. doi: 10.1111/j.1600-6143.2009.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007 Aug;246(2):173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merkow RP, Bilimoria KY, McCarter MD, et al. Effect of histologic subtype on treatment and outcomes for esophageal cancer in the United States. Cancer. 2012 Jul;118(13):3268–3276. doi: 10.1002/cncr.26608. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg. 2011 Oct;146(10):1128–1134. doi: 10.1001/archsurg.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004 Aug 5;351(6):575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 15.Virgo KS, Little AG, Fedewa SA, Chen AY, Flanders WD, Ward EM. Safety-net burden hospitals and likelihood of curative-intent surgery for non-small cell lung cancer. J Am Coll Surg. 2011 Nov;213(5):633–643. doi: 10.1016/j.jamcollsurg.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Cancer AJCo. Liver (Including Intrahepatic Bile Ducts) In: Greene FL, Compton CC, Fritz AG, Shah JP, Winchester DP, editors. AJCC Cancer Staging Atlas. Springer; New York: 2006. pp. 127–132. [Google Scholar]
- 17.Cancer AJCo. Lung. In: Greene FL, Compton CC, Fritz AG, Shah JP, Winchester DP, editors. AJCC Cancer Staging Atlas. Springer; New York: 2006. pp. 167–176. [Google Scholar]
- 18.Cancer AJCo. Exocrine Pancreas. In: Greene FL, Compton CC, Fritz AG, Shah JP, Winchester DP, editors. AJCC Cancer Staging Atlas. Springer; New York: 2006. pp. 155–163. [Google Scholar]
- 19.Cancer AJCo. Esophagus. In: Greene FL, Compton CC, Fritz AG, Shah JP, Winchester DP, editors. AJCC Cancer Staging Atlas. Springer; New York: 2006. pp. 77–88. [Google Scholar]
- 20.Morris AM, Billingsley KG, Hayanga AJ, Matthews B, Baldwin LM, Birkmeyer JD. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008 May 21;100(10):738–744. doi: 10.1093/jnci/djn396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arday SL, Arday DR, Monroe S, Zhang J. HCFA's racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000 Summer;21(4):107–116. [PMC free article] [PubMed] [Google Scholar]
- 22.Lin D, Wei L. The robust inference for the cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. [Google Scholar]
- 23.Greenstein AJ, Litle VR, Swanson SJ, et al. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Oncol. 2008 Mar;15(3):881–888. doi: 10.1245/s10434-007-9664-5. [DOI] [PubMed] [Google Scholar]
- 24.Paulson EC, Ra J, Armstrong K, Wirtalla C, Spitz F, Kelz RR. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008 Dec;143(12):1198–1203. doi: 10.1001/archsurg.143.12.1198. discussion 1203. [DOI] [PubMed] [Google Scholar]
- 25.Steyerberg EW, Earle CC, Neville BA, Weeks JC. Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2005 Jan 20;23(3):510–517. doi: 10.1200/JCO.2005.05.169. [DOI] [PubMed] [Google Scholar]
- 26.Manfredi C, Kaiser K, Matthews AK, Johnson TP. Are racial differences in patient-physician cancer communication and information explained by background, predisposing, and enabling factors? J Health Commun. 2010 Apr;15(3):272–292. doi: 10.1080/10810731003686598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004 Feb;19(2):101–110. doi: 10.1111/j.1525-1497.2004.30262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity. Cancer. 2000;88:653–663. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data. Med Care. 2002;40(supplement):IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 30.Unroe KT, Greiner MA, Hernandez AF, et al. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000-2007. Arch Intern Med. 2011 Feb 14;171(3):196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 31.Greiner KA, Perera S, Ahluwalia JS. Hospice usage by minorities in the last year of life: results from the National Mortality Followback Survey. J Am Geriatr Soc. 2003 Jul;51(7):970–978. doi: 10.1046/j.1365-2389.2003.51310.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.