Abstract
Background
Previous diffusion tensor imaging (DTI) studies have shown white matter compromise in children and adults with autism spectrum disorder (ASD), which may relate to reduced connectivity and impaired function of distributed networks. However, tract-specific evidence remains limited in ASD. We applied tract-based spatial statistics (TBSS) for an unbiased whole-brain quantitative estimation of the fractional anisotropy (FA), mean diffusion (MD) and axial and radial diffusion of the white matter tracts in children and adolescents with ASD.
Methods
DTI was performed in 26 ASD and 24 typically developing (TD) participants, aged 9–20 years. Groups were matched for age and IQ. Each participant’s aligned FA, MD and axial and radial diffusion data were projected onto the mean FA skeleton representing the centers of all tracts and the resulting data fed into voxelwise group statistics.
Results
TBSS revealed decreased FA, and increased MD and radial diffusion in the ASD group compared to the TD group in the corpus callosum, anterior and posterior limbs of the internal capsule, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, cingulum, anterior thalamic radiation, and corticospinal tract. No single site with inverse effects (increased FA, reduced MD or radial diffusion in the ASD group) was detected. In clusters of significant group difference, age was positively correlated with FA and negatively correlated with MD and radial diffusion in the TD, but not the ASD group.
Conclusions
Our findings reveal white matter compromise affecting numerous tracts in children and adolescents with ASD. Slightly varying patterns of diffusion abnormalities detected for some tracts may suggest tract-specific patterns of white matter abnormalities associated with ASD. Age-dependent effects further show that maturational changes (increasing FA, decreasing MD and radial diffusion with age) are diminished in ASD from school-age childhood into young adulthood.
Keywords: Diffusion tensor imaging, autism spectrum disorder, brain connectivity, fractional anisotropy, mean diffusion, axial diffusion, radial diffusion
Autism spectrum disorder (ASD) is characterized by sociocommunicative, cognitive, and sensorimotor impairments whose underlying neuropathology is not fully understood. Structural magnetic resonance imaging (MRI) findings have shown abnormal patterns of white matter growth in ASD. Courchesne et al. (2001) reported atypically increased cerebral white matter volume in boys with autism below the age of 5 years, whereas in adolescent boys cerebral white matter volume was reduced. Abnormal early growth patterns in ASD likely relate to atypical interregional connectivity later in life (Belmonte et al., 2004; Kana et al., 2008; Kleinhans et al., 2008; Koshino et al., 2005; Müller, 2007; Rippon et al., 2007; Villalobos et al., 2005). Deficiency in interhemispheric information transfer (Nyden et al., 2004) and interhemispheric functional underconnectivity have also been reported (Just et al., 2007). Impaired connectivity may be related to social and cognitive symptoms seen in ASD (Dawson et al., 1998; Hill, 2004).
Compared with conventional anatomical MRI, DTI provides additional information about aberrant white matter microstructure. DTI indices (fractional anisotropy [FA], mean diffusion [MD], and axial and radial diffusion) are markers of pathological and developmental changes in axonal density and size, myelination, and organizational coherence of fibers within a voxel (Basser, 1995; Basser and Pierpaoli, 1996; Song et al., 2005).
In a first small-sample DTI study of ASD, reduced FA was reported in brain regions related to social cognition, such as the ventromedial prefrontal, anterior cingulate, and temporoparietal regions (Barnea-Goraly et al., 2004). Several DTI studies have subsequently reported reduced FA in children and adults with ASD in corpus callosum (Alexander et al., 2007; Keller et al., 2007), internal capsule (Brito et al., 2009; Cheung et al., 2009; Keller et al., 2007), frontal (Sundaram et al., 2008), and temporal (Lee et al., 2007) regions. Contrary to these studies, increased FA in frontal lobe, right cingulate gyrus, bilateral insula, right superior temporal gyrus and bilateral middle cerebellar peduncle was reported in one recent study of children and adolescents with ASD (Cheung et al., 2009).
Several autism DTI studies (Alexander et al., 2007; Catani et al., 2008; Lee et al., 2007) have reported region-of-interest (ROI) analyses, which rely on a priori hypotheses regarding regional impairment and are limited to selection of a few among numerous white matter tracts. Other studies have implemented voxelwise whole brain approaches (Barnea-Goraly et al., 2004; Cheung et al., 2009; Ke et al., 2009; Keller et al., 2007). While these studies are not limited to a priori selection of ROIs, they suffer from topological variability of the brain, which can cause misregistration of white matter tracts across participants. Spatial smoothing applied to compensate for such misregistration can also affect DTI indices (Jones et al., 2005).
In the present study, we utilized a comparatively novel tract-based spatial statistics (TBSS) approach (Smith et al., 2006) to investigate white matter fiber tract specific changes in children with ASD compared to typically developing (TD) children. TBSS provides the ability to spatially localize group differences in DTI data in an observer-independent and hypothesis-free fashion. TBSS uses nonlinear registration and takes advantage of the spatial determinants of major white matter tracts, thus minimizing registration error and bias and eliminating the need for arbitrary smoothing. The data-driven approach of TBSS is particularly useful in a disorder like ASD, for which regional patterns of brain abnormalities are not fully determined.
Our study aimed to examine whether expected white matter compromise in children and adolescents with ASD would localize to a limited number of tracts or would be distributed across many cortico-cortical as well as subcortico-cortical tracts.
Methods
Participants
Twenty-six children with ASD (15 with autistic disorder, 11 with Asperger’s disorder; 25 males, 1 female) and twenty-four TD children (23 males, 1 female), matched for age, and verbal and nonverbal IQ were included. Clinical diagnoses were confirmed by an expert clinical psychologist using the Autism Diagnostic Interview–Revised (ADI-R) (Rutter et al., 2003) and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2001). Children with associated medical conditions were excluded. One ASD participant was taking a Selective Serotonin Reuptake Inhibitor and a stimulant, one a stimulant and a Serotonin and Norepinephrine Reuptake Inhibitor and seven ASD participants were on prescribed psychoactive medications. Medication information was unavailable for five ASD participants.
TD children had no reported personal or family history of autism or any other neurological or psychiatric conditions. Independent-sample t-tests confirmed that ASD and TD groups were matched on age, t(48)=0.3, p=.76, verbal IQ, t(48)=0.8, p=.38 and nonverbal IQ, t(48)=0.4, p=.71, as determined using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) (WASI; Table 1).
Table 1.
Demographic data.
| ASD (n = 26) | TD (n = 24) | |
|---|---|---|
| Mean (sem) Range |
Mean (sem) Range |
|
| Age (years) | 12.8 (0.6) 9–20 |
13.0 (0.6) 9–19 |
| Verbal IQ | 104.3 (3.4) 71–147 |
108.2 (2.6) 74–130 |
| Nonverbal IQ | 108.8 (3.3) 69–140 |
110.3 (2.5) 85–129 |
| Handedness | 23 right, 3 left | 22 right, 2 left |
The research protocol was approved by the Institutional Review Boards of the University of California, San Diego and San Diego State University. Written informed assent and consent was obtained from all participants at the time of their visit.
DTI Scanning Procedure
DTI scans were performed on a 3T whole-body GE MR system (Signa Excite HD; GE Healthcare, Milwaukee, USA) at the Center for Functional Magnetic Resonance Imaging, University of California, San Diego. A standard 8-channel head coil was used to acquire all images. Head movement was minimized using foam pillows around participants’ heads.
Single-shot echo-planar diffusion weighted imaging was performed with the following parameters: repetition time (TR) = 10000 ms, echo time (TE) = 99.4 ms, field of view (FOV) = 240 mm, slice thickness = 5mm, slice gap = 0, number of slices = 27 (axial), matrix size = 128×128. Two degrees of diffusion weighting were used: b = 0 and 2000 s/mm2. Data were acquired in 15 non-linear directions with four repetitions. A pair of field maps with two different echo times and with identical DTI parameters were also acquired.
DTI Data Analysis
Distortions due to magnetic field inhomogeneities were corrected using field maps derived from the phase difference image obtained from a pair of images with different echo times. The phase difference image between the two images is proportional to variations in the local magnetic field. Custom software using methods described by Jezzard and Balaban (Jezzard and Balaban, 1995) was used for field map correction. One child in each ASD and TD group was excluded due to image artifacts associated with excessive motion (>2mm). No significant group differences were detected for translational motion (0.65±0.09mm [mean±SEM] ASD and 0.69±0.08mm TD; p = 0.7) or rotational motion (0.015±0.002 rad ASD and 0.011±0.003 rad TD; p = 0.4) as determined by the root mean square of the translational and rotational motion in three cardinal directions. DTI data were processed using the diffusion toolbox of FSL (Smith et al., 2004) version 4.1.0 to generate three eigenvalues from which two tensor-derived rotational invariants (FA, MD), axial diffusion (λ1) and radial diffusion (λ2+λ3/2) measurements were derived. To measure anisotropy, we calculated FA, as previously defined (Basser et al., 1994), which was scaled to a range from 0 (equal diffusion in all directions) to 1 (diffusion in only one direction).
We used Tract-Based Spatial Statistics (TBSS (Smith et al., 2006)), included in the FSL suite (Smith et al., 2004) to carry out voxelwise statistical analyses for FA, MD and axial and radial diffusion in ASD and TD groups. All participants’ FA data were aligned into a common space using the nonlinear registration tool FNIRT, which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). A mean FA image was created and thinned to create a mean FA skeleton that represented the centers of all tracts common to both the ASD and TD groups. A lower FA threshold of 0.2 was used to prevent inclusion of non-skeleton voxels. Each subject’s aligned FA image was then projected onto the mean FA skeleton and the resulting data were fed into voxelwise cross-subject statistics, which were based on a non-parametric approach utilizing permutation test theory with a standard general linear model design matrix. Permutation testing (5000 permutations) was performed using the Randomise program, which applies Monte Carlo permutation testing to generate random permutations (Nichols and Holmes, 2002). Using the same nonlinear registration, skeleton and skeleton projection vectors derived from the FA images, MD, axial diffusion and radial diffusion data were equally projected onto the skeleton before voxelwise statistical analysis across subjects (Smith et al., 2007). A restrictive statistical threshold was used (cluster-based thresholding t > 3, p < 0.05, corrected for multiple comparisons). A nonparametric statistical test was selected since it does not require the assumption of Gaussian distribution for DTI indices. The John Hopkins University (JHU) white matter tractography atlas (Wakana et al., 2004) was used for tract labeling and in the case of overlap, voxel assignment was based on >50% chance of being on the labeled tract. Each tract was followed up to the end of the skeleton and its label was checked against the atlas throughout. All voxels in a given tract were included for the group analysis. Shapiro-Wilk tests carried out to test for normality showed that the diffusion data satisfied parametric assumptions and therefore, Pearson correlation analyses were performed to detect possible effects of age on DTI indices.
Results
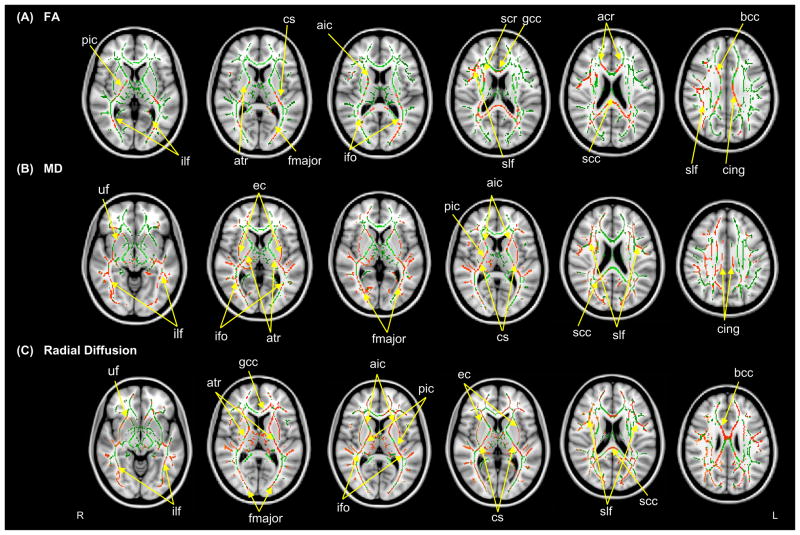
TBSS analysis revealed decreased FA in the ASD compared to the TD group in numerous white matter tracts (Table 2). FA reduction was robust in the anterior and posterior limbs and retrolenticular part of the internal capsule bilaterally, and body, genu and splenium of the corpus callosum. Major white matter fiber tracts with reduced FA in the ASD group were bilateral inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, corticospinal tract, cingulum, anterior thalamic radiation, anterior corona radiata, right superior corona radiate and forceps major (Figure 1). No tract was found to have significantly greater FA in ASD compared to TD group.
Table 2.
TBSS findings for the ASD group and gross characterization and functional role of major white matter fiber tracts
| Tract | Findings for ASD group
|
Gross characterization | ||
|---|---|---|---|---|
| FA | MD | Radial Diffusion | ||
|
|
||||
| Inferior longitudinal fasciculus | ↓ | ↑ | ↑ | Connects visual cortex in the occipital lobe to the temporal pole |
| Inferior fronto-occipital fasciculus | ↓ | ↑ | ↑ | Connects frontal lobe to occipital and temporal lobes |
| Superior longitudinal fasciculus | ↓ | ↑ | ↑ | Connects frontal lobe to temporal and parietal lobes |
| Cingulum | ↓ | ↑ | ↑ | Connects the limbic lobe with neocortex |
| Genu of corpus callosum | ↓ | n.s. | ↑ | Provides interhemispheric connections between frontal regions |
| Body of corpus callosum | ↓ | n.s. | ↑ | Provides interhemispheric connections between parietal regions |
| Splenium of corpus callosum | ↓ | ↑ | ↑ | Provides interhemispheric connections between posterior parietal and occipital regions |
| Anterior limb of internal capsule | ↓ | ↑ | ↑ | Contain ascending and descending axons |
| Posterior limb of internal capsule | ↓ | ↑ | ↑ | |
| External capsule | n.s. | ↑ | ↑ | Connects ventral association areas to the putamen and tail of the caudate nucleus |
| Corticospinal tract | ↓ | ↑ | ↑ | Contains motor axons |
| Uncinate fasciculus | n.s. | ↑ | ↑ | Connects parts of the limbic system such as hippocampus and amygdala in the temporal lobe with the orbitofrontal cortex |
| Anterior thalamic radiation | ↓ | ↑ | ↑ | Connects dorsomedial and anterior thalamic nuclei with the prefrontal cortex |
(n.s. not significant).
Figure 1.
Tract-based spatial statistics (TBSS) revealed regions of reduced FA (A), increased MD (B) and radial diffusion (C) in children with ASD compared to the TD group. Red color symbolizes significant voxels at p<.05 (corrected for multiple comparisons at cluster level). Mean skeleton of detected fiber tracts is overlaid in green on standard T1-weighted anatomical image). Abbreviations are: ilf: Inferior longitudinal fasciculus, ifo: Inferior fronto-occipital fasciculus, slf: Superior longitudinal fasciculus, cs: Corticospinal tract, cing: Cingulum, bcc: Body of corpus callosum, gcc: Genu of corpus callosum, scc: Splenium of corpus callosum, aic: Anterior internal capsule, pic: Posterior internal capsule, ec: External capsule, fmajor: Forceps major, acr: Anterior corona radiate, scr: Superior corona radiate, atr: Anterior Thalamic Radiation, uf: Uncinate fasciculus.
Increased MD and radial diffusion in ASD group compared to TD group were seen in the splenium of the corpus callosum, bilateral inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, corticospinal tract, cingulum, anterior thalamic radiation, anterior corona radiata, right superior corona radiate, forceps major, uncinate fasciculus, anterior and posterior limbs of internal capsule and external capsule. Increased radial diffusion was found in the genu and anterior body of the corpus callosum but not for MD (Figure 1). No tract was shown to have significantly reduced MD and radial diffusion in ASD compared to TD group. Also, no significant group differences were detected for axial diffusion.
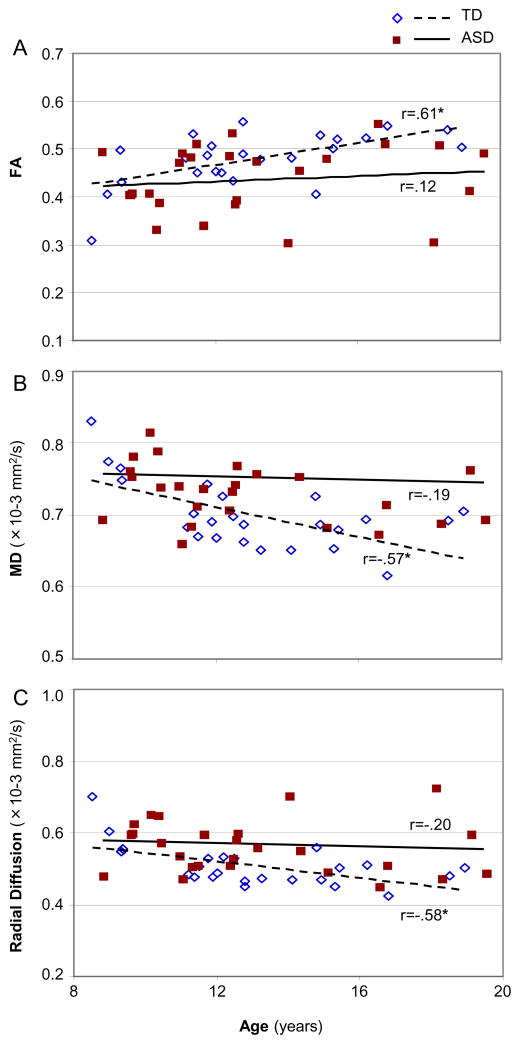
Pearson correlation analyses were performed to detect possible effects of age on FA, MD and radial diffusion. FA of the whole brain white matter skeleton was positively correlated with age in TD group (r=.61, p=.002) but only marginally in ASD group (r=.12, p=.55). MD and radial diffusion of the whole brain white matter skeleton were negatively correlated with age in TD group (MD: r=−.57, p=.003; radial diffusion: r=−.58, p=.003) but not in ASD group (MD: r=−.19, p=.32; radial diffusion: r=−.20, p=.33; Figure 2). When examining all clusters of significant group difference combined, age in the TD group was positively correlated with FA (r=.59, p=.002) and negatively correlated with MD and radial diffusion (MD: r= −.60, p=.002; radial diffusion: r=−.60, p=.002) whereas correlations were non-significant in the ASD group (FA: r=.31, p=.12; MD: r=−.21, p=.30; radial diffusion: r=−.09, p=.65). Although significant correlations with age were found for the TD group and not the ASD group in many of the analyses we carried out, a significant group by age interaction was found only for the MD of the whole brain (F=2.4, p=.04).
Figure 2.
Pearson correlation analyses between age and FA (A), MD (B) and radial diffusion (C) for all detected tracts in whole brain white matter skeleton in ASD and TD groups. (*, p<.05).
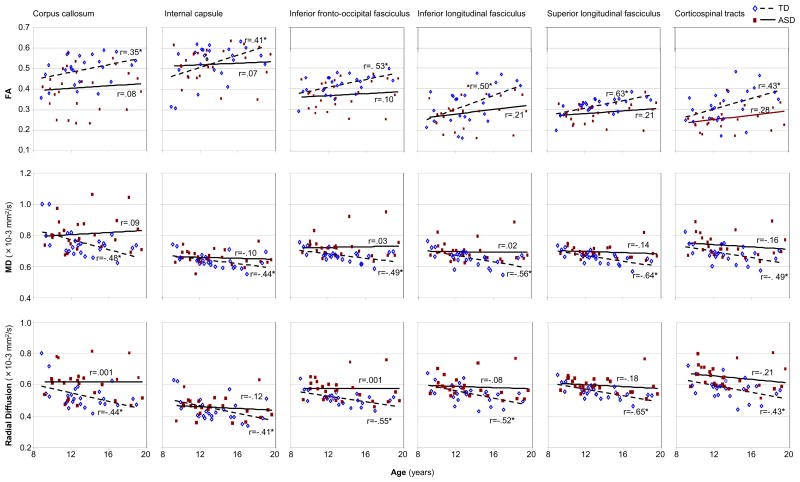
Post hoc analyses of age-related effects for individual white matter tracts were carried out to explore patterns of age correlation with DTI indices for individual tracts in which white matter abnormalities had been detected for the ASD group. Positive correlations between age and FA and negative correlation between age and MD and radial diffusion in the TD group, but not in the ASD group, were found for inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, corticospinal tract, cingulum and internal capsule. Age was marginally correlated with FA but significantly with MD of the corpus callosum in the TD, but not the ASD group. Although none of these relationships were significant after correction for multiple comparisons the consistency of this pattern across a number of major white tracts is apparent (Figure 3).
Figure 3.
Pearson correlation analyses between age and FA, MD and radial diffusion of white matter tracts in ASD and TD groups. (*, p<.05; uncorrected).
Discussion and Conclusion
In this study we used TBSS, an automated tract-based analysis to investigate microstructural changes in white matter fiber tracts in children with ASD. We found decreased FA and increased MD and radial diffusion in our ASD compared to our TD group in numerous white matter tracts, suggesting widespread white matter compromise in ASD.
Each of the three affected diffusion measures is sensitive to complementary, but slightly different aspects of white matter compromise. While reduced FA and increased MD may reflect demyelination, axonal damage (Basser, 1995), or loss of white matter coherence (Basser and Pierpaoli, 1996; Werring et al., 2000a), increased radial diffusivity is more specifically considered a sensitive marker for demyelination (Song et al., 2005). No significant findings for axial diffusivity were found. This is consistent with previous ASD studies that included axial diffusivity measures, but failed to detect significant group differences (Alexander et al., 2007; Fletcher et al., 2010). Although axial diffusivity has been associated with axonal injury (Budde et al., 2009), significant differences in radial diffusivity without changes in axial diffusivity have been reported (Song et al., 2002) despite the fact that radial diffusivity is only about 30% of the magnitude of axial diffusivity (Song et al., 2003). Furthermore, the direction of the principal eigenvector associated with axial diffusivity may not always be preserved in pathological tissue and may not always be aligned with the underlying tissue architecture (i.e., may not reflect the predominant orientation of axons) (Field et al., 2004). Therefore, axial diffusivity may be a less sensitive marker of white matter abnormalities compared to other DTI indices.
Long-distance cortico-cortical white matter tracts including bilateral inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus showed reduced FA and increased MD and radial diffusion in ASD group. One previous study had also reported white matter compromise in ASD in inferior fronto-occipital fasciculus (Kumar et al., 2009). The inferior longitudinal fasciculus connects occipital lobe with parahippocampal gyrus and lateral temporal lobe and is thought to facilitate consolidation of visual memory as well as the attribution of emotional content in visual processing (Catani et al., 2003; Shinoura et al., 2007). Both the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus are visual pathways, specifically considered important for visual recognition and memory (Catani et al., 2002; Wakana et al., 2004). The superior longitudinal fasciculus connects frontal lobe to temporal and parietal lobes (Wakana et al., 2004) and is important for information exchange between Broca’s and Wernicke’s areas (Paus et al., 1999). White matter compromise in the superior longitudinal fasciculus, as observed in the present study, may be associated with impaired functional connectivity between frontal and parietal lobes in ASD (Just et al., 2007; Koshino et al., 2005; Monk et al., 2009). These findings suggest that atypical functional connectivity may be associated with impaired anatomical connectivity.
Related to our findings for the superior longitudinal fasciculus, Fletcher and colleagues (Fletcher et al., 2010) reported significantly increased MD and radial diffusion in the left arcuate fasciculus as well as atypical hemispheric asymmetry for these measures in adolescents with high-functioning autism, which the authors suggested may be related to language impairment in ASD. The arcuate fasciculus is a white matter fiber bundle that lies in the inferior portion of the superior longitudinal fasciculus, superior to the insula and extreme capsule, connecting cortical language regions such as Wernicke’s area in the posterior superior temporal gyrus, Broca’s area in the inferior frontal gyrus, and Geschwind’s area in the inferior parietal lobule (Catani et al., 2005; Fletcher et al., 2010).
In the corpus callosum, reduced FA and increased radial diffusion were observed in genu, body, and splenium, indicating global callosal impairment. Increased MD, however, was detected only in the splenium, which may suggest that white matter compromise may be slightly more pronounced in posterior callosal segments consistent with previous anatomical MRI findings (Alexander et al., 2007; Badaruddin et al., 2007; Egaas et al., 1995; Hardan et al., 2000; Vidal et al., 2006). Areas showing reduced FA in the ASD group included posterior midbody and isthmus of the corpus callosum, which likely contain interhemispheric connections for frontal and parietal regions (Schmahmann and Pandya, 2006). Reduced diffusion anisotropy in damaged white matter areas such as corpus callosum may indicate disorganization or maldevelopment of projectional, commissural and association fibers associated with impairments in functional domains that rely on effective interhemispheric information transfer (Ewing-Cobbs et al., 2006; Werring et al., 2000b) such as reading, calculation and working memory (Ewing-Cobbs et al., 2006).
We also found reduced FA, as well as increased MD and radial diffusivity in the cingulum bundle, which is the most prominent tract connecting limbic system and cerebral cortex, particularly the cingulate gyrus (Choi et al., 2009). The anterior cingulum is known to play an important role in executive control of attention, while the posterior cingulum has spatial attention and orienting functions (Hirono et al., 1998; Small et al., 2003). Deterioration in these regions may be related to executive dysfunction (Hill, 2004) and social attention impairments (Dawson et al., 2004) seen in ASD.
Cortico-subcortical white matter tracts were also found to be compromised. The posterior part of the internal capsule contains a number of white matter tracts, including posterior thalamic radiation, superior longitudinal fasciculus, inferior longitudinal fasciculus, and inferior fronto-occipital fasciculus (Schmahmann and Pandya, 2006). Abnormalities in the right posterior limb of the internal capsule, as observed here and in other studies (Brito et al., 2009; Keller et al., 2007), may be associated with motor and sensory deficits due to compromised fronto-thalamic pathways (Schmahmann and Pandya, 2006). Reduced structural integrity of this region in the ASD group may therefore affect long-range communication in the right hemisphere among a large number of cortical regions including frontal, parietal, temporal, and occipital areas. Furthermore, changes in white matter underlying the left central sulcus and in the posterior limb of the internal capsule could reflect loss of integrity of fibers belonging to corticospinal tracts crucial for somatosensory and tactile sensation (Schmahmann and Pandya, 2006). White matter abnormalities in the corticospinal tracts have also been observed in a previous study by Brito and colleagues (2009).
Our findings of age-related increases in FA and decreases in MD suggest continued white matter maturation during later childhood and adolescence in typically developing children, consistent with previous studies (Alexander et al., 2007; Ben Bashat et al., 2007; Lee et al., 2007). In contrast, ASD participants failed to show significant age-related changes for either FA or MD. This pattern of findings is mostly consistent with those from Alexander et al. (2007). However, these latter investigators were able to detect a correlation between MD and age in both their autism and control groups. This difference in findings may relate to a comparatively wider age range (7–33 years) in the ASD group studied by Alexander and colleagues.
Two other ASD studies have reported correlations between age and DTI measures. Pugliese et al. (2009) found overall similar age-related decreases in MD for several limbic pathways in a group with Asperger’s disorder compared with healthy controls, but failed to detect significant correlations between age and FA. Since this study included participants in a much wider age range than ours, from 9 to 54 years, age-dependent effects of adolescent development may have been compounded with aging effects in participants older than 40 years. Examining participants between the ages of 7 and 33 years, Lee et al. (2007) reported increase of FA with age in a TD control group, but not in participants with ASD, for superior temporal white matter; however, decreases in MD and radial diffusion with age were found for both groups. Our findings, together with those from previous studies, suggest that age-dependent white matter changes in school-age children and adolescents with ASD are similar in direction (increasing FA, decreasing MD and radial diffusion) compared to those in typical development, but diminished and affected by greater variability. Strong cognitive and neurofunctional variability has been previously reported (Bertoglio and Hendren, 2009; Müller et al., 2003) and may be attributed to the known heterogeneity of the disorder (Geschwind and Levitt, 2007; Happé et al., 2006).
In a recent TBSS study, Cheng et al. (2010) remarkably reported numerous tracts with greater FA in adolescents with ASD, compared to a TD group, whereas only few sites of decreased FA were identified. These results are not only largely inconsistent with our findings, but also with the existing DTI literature in older children, adolescents and adults with ASD, which has exclusively reported reduced FA (Alexander et al., 2007; Barnea-Goraly et al., 2004; Brito et al., 2009; Catani et al., 2008; Ke et al., 2009; Keller et al., 2007; Lee et al., 2007; Pardini et al., 2009; Thakkar et al., 2008). Partially divergent findings have, to our knowledge, been limited to younger children (Cheung et al., 2009; Kumar et al., 2009; Sundaram et al., 2008) and infants (Ben Bashat et al., 2007). Although our results strongly diverge from those reported by Cheng et al. (2010), we are therefore confident that they are representative of white matter compromise in school-age children and adolescents with ASD, given the broad consistency with previous studies.
While whole brain and ROI-based studies of white matter in ASD have been carried out in a number of studies (Alexander et al., 2007; Barnea-Goraly et al., 2004; Ben Bashat et al., 2007; Brito et al., 2009; Catani et al., 2008; Cheung et al., 2009; Ke et al., 2009; Keller et al., 2007; Lee et al., 2007; Pardini et al., 2009; Sundaram et al., 2008; Thakkar et al., 2008), only few have presented tract-based analyses (Cheng et al., 2010; Kumar et al., 2009; Pugliese et al., 2009). TBSS is a tract-based technique that optimizes spatial normalization and permits exploratory analyses without the need for a priori ROI selection, which is beneficial in ASD research, given that the pattern of tract-specific impairments in this disorder is not fully understood. Our findings suggest that white matter compromise in ASD is robust across a large number of fiber tracts, and can be identified by reduced FA as well as increased MD and radial diffusion for most of these. Our results therefore indicate that ASD is not a disorder characterized by dysfunction of one or a few functional networks, but by white matter abnormalities affecting many or almost all networks. In view of the high general functional level of our ASD cohort, with mean IQ above 100, such widespread white matter compromise is remarkable, possibly suggesting compensatory processes undetected by DTI measures.
Supplementary Material
Key points.
Previous DTI studies have shown white matter compromise in ASD; however, tract-specific evidence remains limited.
In this study, we applied an unbiased whole-brain quantitative estimation of DTI indices of the white matter tracts in children and adolescents with ASD.
Results indicate compromised white matter integrity in the ASD group in several major fiber tracts. In tracts for which significant group differences were detected, age was positively correlated with FA and negatively correlated with MD and radial diffusion in the TD, but not the ASD group.
Our findings reveal white matter compromise affecting numerous tracts in children and adolescents with ASD. Age-dependent effects further suggest that maturational changes are diminished in ASD from school-age childhood into young adulthood.
Acknowledgments
This study was supported by the National Institutes of Health, R01-DC006155 and R01-MH081023, with additional funding from NIDCD 1T32 DC007361-03 (author BK). Special thanks to the children and families who participated.
References
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Badaruddin DH, Andrews GL, Bolte S, Schilmoeller KJ, Schilmoeller G, Paul LK, et al. Social and behavioral problems of children with agenesis of the corpus callosum. Child Psychiatry Hum Dev. 2007;38:287–302. doi: 10.1007/s10578-007-0065-6. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Bertoglio K, Hendren RL. New developments in autism. Psychiatr Clin North Am. 2009;32:1–14. doi: 10.1016/j.psc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr, Rodrigues LS, Gasparetto EL, et al. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging. 2009;19:337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. Journal of Neuroscience. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, et al. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am J Neuroradiol. 2006;27:879–881. [PMC free article] [PubMed] [Google Scholar]
- Field AS, Alexander AL, Wu YC, Hasan KM, Witwer B, Badie B. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. Journal of Magnetic Resonance Imaging. 2004;20:555–562. doi: 10.1002/jmri.20169. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9:1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hirono N, Mori E, Ishii K, Ikejiri Y, Imamura T, Shimomura T, et al. Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64:552–554. doi: 10.1136/jnnp.64.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Adam JM. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2008:1–18. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, et al. Alterations in Frontal Lobe Tracts and Corpus Callosum in Young Children with Autism Spectrum Disorder. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 2001. [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA, Kleinhans N, Kemmotsu N, Pierce K, Courchesne E. Abnormal variability and distribution of functional maps in autism: an FMRI study of visuomotor learning. Am J Psychiatry. 2003;160:1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyden A, Carlsson M, Carlsson A, Gillberg C. Interhemispheric transfer in high-functioning children and adolescents with autism spectrum disorders: a controlled pilot study. Dev Med Child Neurol. 2004;46:448–454. doi: 10.1017/s001216220400074x. [DOI] [PubMed] [Google Scholar]
- Pardini M, Garaci FG, Bonzano L, Roccatagliata L, Palmieri MG, Pompili E, et al. White matter reduced streamline coherence in young men with autism and mental retardation. Eur J Neurol. 2009;16:1185–1190. doi: 10.1111/j.1468-1331.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell’Acqua F, Thiebaut de SM, Murphy C, et al. The anatomy of extended limbic pathways in Asperger syndrome: a preliminary diffusion tensor imaging tractography study. Neuroimage. 2009;47:427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. Int J Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord . Autism Diagnostic Interview - Revised. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press; 2006. [Google Scholar]
- Shinoura N, Suzuki Y, Tsukada M, Katsuki S, Yamada R, Tabei Y, et al. Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase. 2007;13:127–130. doi: 10.1080/13554790701399254. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18:633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, et al. Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry. 2006;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Werring DJ, Brassat D, Droogan AG, Clark CA, Symms MR, Barker GJ, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain. 2000a;123(Pt 8):1667–1676. doi: 10.1093/brain/123.8.1667. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. Journal of Neurology, Neurosurgery and Psychiatry. 2000b;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.