Abstract
Aims
Angiogenesis is defined as the sprouting of capillaries from pre-existing vasculature. It is a complex process that includes endothelial proliferation, migration, and tube formation. Previous data have demonstrated a high expression level of manganese-superoxide dismutase (MnSOD) in endothelial cells and suggested an important role of MnSOD in several cardiovascular diseases. In addition, manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP) has been shown to mimic some of the effects of MnSOD in various tissues. However, its effect on the vasculature remains unknown.
Methods and Results
HUVECs were treated with MnTBAP. Migration, tube formation, and capillary sprouting assays were performed to evaluate the pro-angiogenic effect in vitro. Matrigel Plug assay was performed to assess capillary ingrowth in vivo. Compared to control, treatment with MnTBAP revealed increased cell migration, tube formation and capillary sprouting along with more capillary ingrowth in the matrigel plug assay. This effect was mediated through a mitofusin (Mfn)-1-dependent pathway. Expression of Tie-2, Ang-2 and VEGF mRNA was increased in muscle tissues after ligation in MnTBAP treated mice. However, revascularization in the hindlimb ischemia model was not statistically significant at day 10 in MnTBAP treated mice.
Conclusion
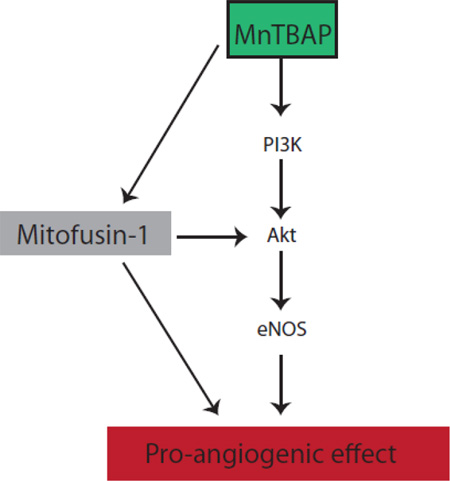
In summary, our data demonstrate a strong pro-angiogenic, but less pro-arteriogenic effect of MnTBAP in HUVECs mediated by Mfn-1.
Keywords: MnTBAP, endothelial cell, angiogenesis
Graphical abstract
1. INTRODUCTION
Angiogenesis is defined as the sprouting of capillaries from pre-existing vasculature. It is a complex process that includes endothelial proliferation, migration, and tube formation, all mediated by the crosstalk of several growth factors[3]. Although tremendous efforts have been made to understand the underlying molecular mechanisms in the past, there are still many aspects in the process of angiogenesis, which are not fully understood. One of the main initiator of angiogenesis is local hypoxia, which results from restricted blood flow downstream of the stenosis. In response to hypoxia, hypoxia-inducible factors (HIFs) are activated to regulate angiogenic genes such as vascular endothelial growth factor (VEGF)[13, 17]. Previous data have demonstrated increased expression of the manganese-superoxide dismutase (MnSOD) mRNA and protein in endothelial cells upon stimulation with VEGF. This effect was dependent on NADPH-oxidase-derived ROS, suggesting that VEGF signaling is sensitive to the redox state of the endothelium[1].
The MnSOD belongs to the superoxide dismutase (SOD) family and is the primary antioxidant defense against superoxide radicals within the mitochondria. It is highly expressed in the endothelium[1] and protects the cells against oxidative damage by converting superoxide to H2O2 and O2, and inhibiting free radical reactions[6]. The importance of MnSOD is underlined by the fact that MnSOD-null mice die between 3–13 days of age with cardiac, visceral and metabolic defects[14]. Interestingly, administration of the SOD mimetic manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP) rescued part of the systemic pathology in the MnSOD-null mice and dramatically prolonged their survival[18]. MnTBAP is a manganic porphyrin complex, which possesses similar effects as MnSOD in catalyzing the dismutation of superoxide radicals (O2−)[7]. Because the endothelium expresses high levels of MnSOD and MnSOD is also induced by VEGF[1, 25], we thought to investigate the potential impact of MnTBAP in regulating angiogenesis.
Our data indicate that MnTBAP activates pro-angiogenic signaling pathways through regulation of the mitochondrial fission protein mitofusin 1 and that this activation enhances angiogenic functions in endothelial cells.
2. MATERIALS AND METHODS
2.1 Animals
7–10 week-old C57/BL6J mice (Charles River Laboratories) were used for the study. Animals were maintained in the animal facility at the University Hospital Freiburg in a 22°C room with a 12-hour light/dark cycle and received drinking water ad libitum. Mice were fed regular chow. The Standing Committee on Animals at University Freiburg approved all protocols pertaining to experimentation with animals. All experiments have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
2.2 Cell cultures
All experiments were performed according to the principles outlined in the Declaration of Helsinki for the use of human tissue. Human umbilical vein endothelial cells (HUVECs) were freshly isolated from human umbilical veins of newborns and cultured as previously described[9].
2.3 Reactive oxygen species (ROS) production
ROS production was detected using 2´,7´-dichlorofluorescin diacetate (DCFDA) cellular ROS detection assay (Abcam). Briefly, cells were treated with MnTBAP (5µM and 50µM) and Tert-Butyl Hydrogen Peroxide (TBHP) as a positive control. Fluorescence was determined using a fluorescent microplate reader.
2.4 Tube formation assay
Culture plates were coated with Matrigel (BD Bioscience) according to the manufacturer’s instructions. HUVECs were pretreated with vehicle, VEGF or MnTBAP (5µM) in 1% FBS/EBM for 20 hours. A total 0.2×105 cells were cultured on Matrigel for 2h at 37°C. Cells were fixed with 4% PFA and pictures were taken from 4 random microscopic fields. For fluorescent staining, carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) was used.
2.5 Matrigel plug assay
7–8 week-old female C57/BL6J mice were used. Growth factor-reduced Matrigel (BD Biosciences) was mixed with heparin (5 U/l) and matrigel containing the respective growth factors or vehicle was injected subcutaneously into the abdominal flanks of the mice. Mice were daily treated subcutaneously with either NaCl or MnTBAP (5 mg/kg body weight; Calbiochem 475870, USA). After 10 days, plugs were isolated, fixed in 4% paraformaldehyde (PFA) and sectioned for further staining. Blood vessel infiltration was analyzed in 10 random sections stained with Hematoxylin and Eosin. Images were taken with Zeiss Axioplan 2/ Axiovision Rel 4.8
2.6 In vitro scratch assay
In vitro scratch assay was performed as described[15]. Briefly, HUVECs were seeded into 6cm dishes and grown to monolayer. A scratch was scraped into the monolayer using a pipette tip. Cell debris were removed and cells were grown for 7 hours to allow migration. Images were taken and number of migrated cells per microscopic field were measured with Zeiss Axioplan 2/ Axiovision Rel 4,8.
2.7 BrdU proliferation assay
Proliferation assay was performed using colorimetric BrdU-incorporation ELISA (Roche, Penzberg, Germany). HUVECs were treated with MnTBAP for 7 hours. VEGF was used as a positive control. Absorbance was measured using an ELISA reader according to the manufacturer's instruction.
2.8 Hindlimb ischemia model
Unilateral femoral artery in 9–10 week-old male mice was ligated. Briefly, the left femoral artery was exposed, isolated from the femoral nerve and vein, and ligated distally to the origin of their arteria profunda femoris. MnTBAP was administered daily (5mg/kg BW) after surgery. Blood flow recovery was measured using laser doppler before and after surgery as indicated. Mice were numbered randomly for surgery and laser Doppler measurement. The investigator was blinded to the treatment groups. After 10 days, mice were euthanized and muscles were isolated.
2.9 Immunohistology
For immunohistochemistry, frozen OCT embedded sections of 6µm thickness were used. Tissue sections were washed in PBS for 20 minutes at room temperature and incubated for 1 hour at 38C° with the fluorescence dye as indicated. All photographs were taken with Zeiss Axioplan microscope and analyzed with Zeiss Axiovision. For quantitative evaluation of capillary density, number of isolectin-positive capillaries was counted in three random regions of at least eight evenly distributed tissue sections of the M. gastrocnemius at day 10 after hindlimb ligation. Analyses were performed by an investigator blinded to the treatment groups.
2.10 Western blot analysis
For western blotting, cells were lysed using RIPA buffer. Equal amounts of protein (30–50µg) were loaded and separated by 7.5% SDS-PAGE. Membranes were incubated overnight at 4°C with the appropriate primary antibody followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG (Dako). Blots were visualized by an ECL system (Pierce). Antibodies were purchased from Cell Signaling.
2.11 siRNA transfection
Mitofusin 1 siRNA was purchased from Life Technologies. Scrambled negative control Alexa Fluor was purchased from Qiagen, Hilden, Germany. For transfection, Lipofectamine RNAiMAx was used according to the manufacturer´s protocol (Life Technologies). Transfection efficiency was confirmed by Western blot.
2.12 RNA extraction and quantitative real-time PCR
Total RNA was extracted using Aurum RNA Mini Kit (Bio-Rad). Quantitative real-time PCR was performed using MyiQ lightcycler software (Bio-Rad). The following primers were used: VEGF (Forward: 5´-TCACCAAAGCCAGCACATAGGAGA-3´; Reverse: 5´- TTTCTCCGCTCTGAACAAGGCTCA-3´), Ang-2 (Forward: 5´-AGA AAG TTC TGG ACA TGG AGG GCA-3´; Reverse: 5´-TCT CCA TTA GGT CAT GCT GCT GCT-3´), Tie-2 (Forward: 5´- TGT TGA AAG CTT CCC AGG GAC TCA-3´; Reverse: 5´- AAA GGA GCA AGC TGA CTC CAC AGA-3´), TGFβ (Forward: 5´- GGA GCA ACA TGT GGA ACT CT; Reverse: 5´-CGT GAG CTG TGC AGG TGC T), COX1 (Forward: 5´-AGA AGG TCT CCC CTG GTG AA-3′; Reverse: 5′-GGG GGT AGT GCA TCA ACA CA-3´), LPL1 (Forward: 5´- AAG GCC TAC AGG TGC AGT TC -3´; Reverse: 5´-CCA GAT TGT TGC AGC GGT TC -3´), hRP (Forward: 5’-GCA CCA CGT CCA ATG ACA T-3’; Reverse: 5’-´GTG CGG CTG CTT CCA TAA-3’). hRP was used as an endogenous control and quantification was calculated using the ΔΔCT method.
2.13 Cell viability assay
Cells were transfected with siRNA as indicated. Cell viability was assessed in 96-well plates using Promega CellTiter-Fluor kit (Promega, Madison, USA).
2.14 Statistical analysis
All experiments were performed in triplicate. Animal numbers are stated with the different experimental results. Results are given as mean ± SEM. Statistical analysis was performed using GraphPad Prism 4.0, USA and comparisons were calculated by Student´s t-test. A p value <0.05 was considered significant.
3. RESULTS
3.1 MnTBAP activates Akt and eNOS signaling
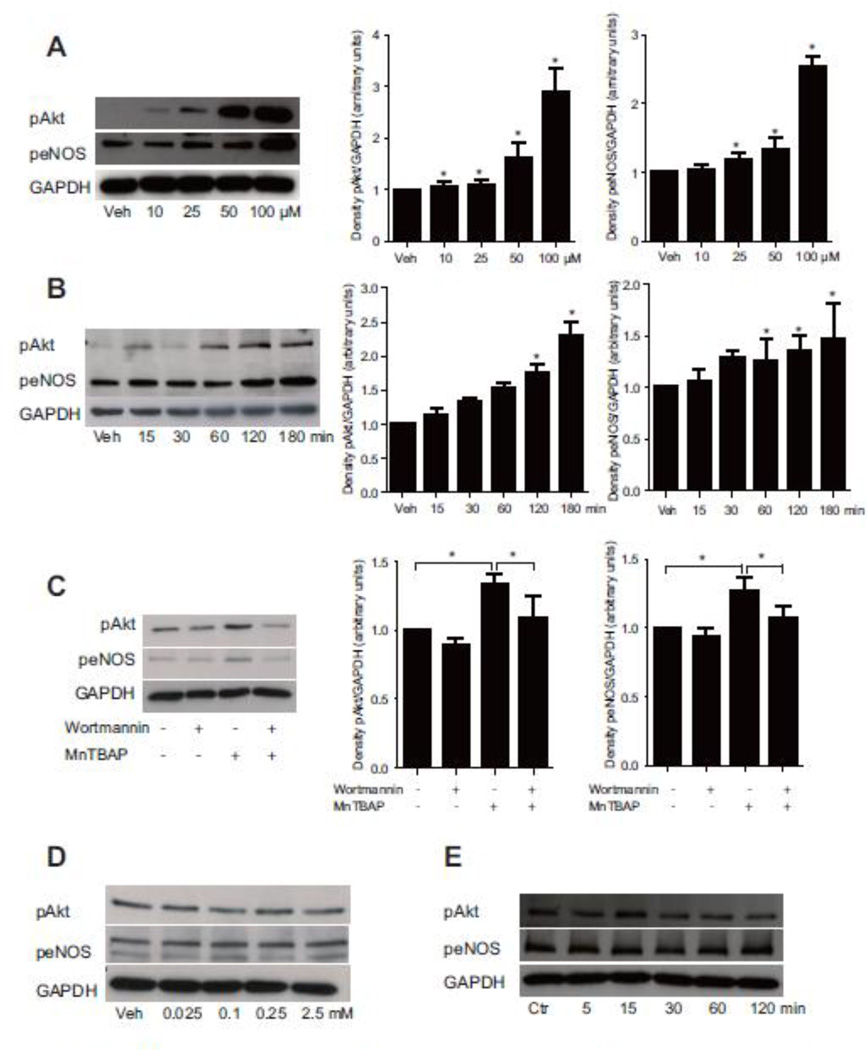
Akt, the serine/ threonine kinase, has been shown to play a pivotal role in angiogenesis. To investigate whether MnTBAP has pro-angiogenic effects in vitro, HUVECs were treated with MnTBAP in a dose- and time-dependent manner. Akt and eNOS were activated by increasing MnTBAP concentration (Fig 1A). This phosphorylation was time-dependent, and reached a maximum at 180 minutes (Fig 1B). Furthermore, pre-treatment with the phosphoinositol-3 kinase (PI3K)- inhibitor, wortmannin, inhibited MnTBAP-induced phosphorylation of Akt and eNOS by 1.52-fold and 1.4-fold, respectively (Fig 1C). Together, these data demonstrate that MnTBAP affects angiogenic signaling, and suggest that Akt and eNOS activation are PI3K-dependent.
Figure 1. MnTBAP modulates pro-angiogenic signaling pathways.
MnTBAP activates Akt and eNOS in a concentration- (A) and time-dependent manner (B). Pre-treatment with wortmannin inhibits MnTBAP induced phosphorylation of Akt and eNOS (C). Stimulation with increasing concentration of NAC does not result in activation of Akt or eNOS (D). Treatment of HUVECs with NAC for different time also shows no significant activation of Akt or eNOS (E). Data are presented as mean±SEM and are representative of at least 3 independent experiments. *P ≤ 0.05.
Because previous reports have suggested that MnTBAP has an anti-oxidative effect similar to N-acetylcysteine (NAC), we next investigated whether the pro-angiogenic effect of MnTBAP could be mediated through its effect on ROS. MnTBAP did not affect ROS production at the lower concentration of 5µM, but reduced ROS generation at 50µM. This finding suggests a concentration-dependent effect of MnTBAP on ROS generation under non-challenging conditions (Supplemental figure 1). In a next step, we evaluated whether NAC could also activate the pro-angiogenic signaling pathway in HUVECs. Interestingly, Akt and eNOS signaling remained unaffected by even high NAC doses or extended treatment periods (Fig 1D, E).
3.2 MnTBAP modulates endothelial migration, proliferation and tube formation
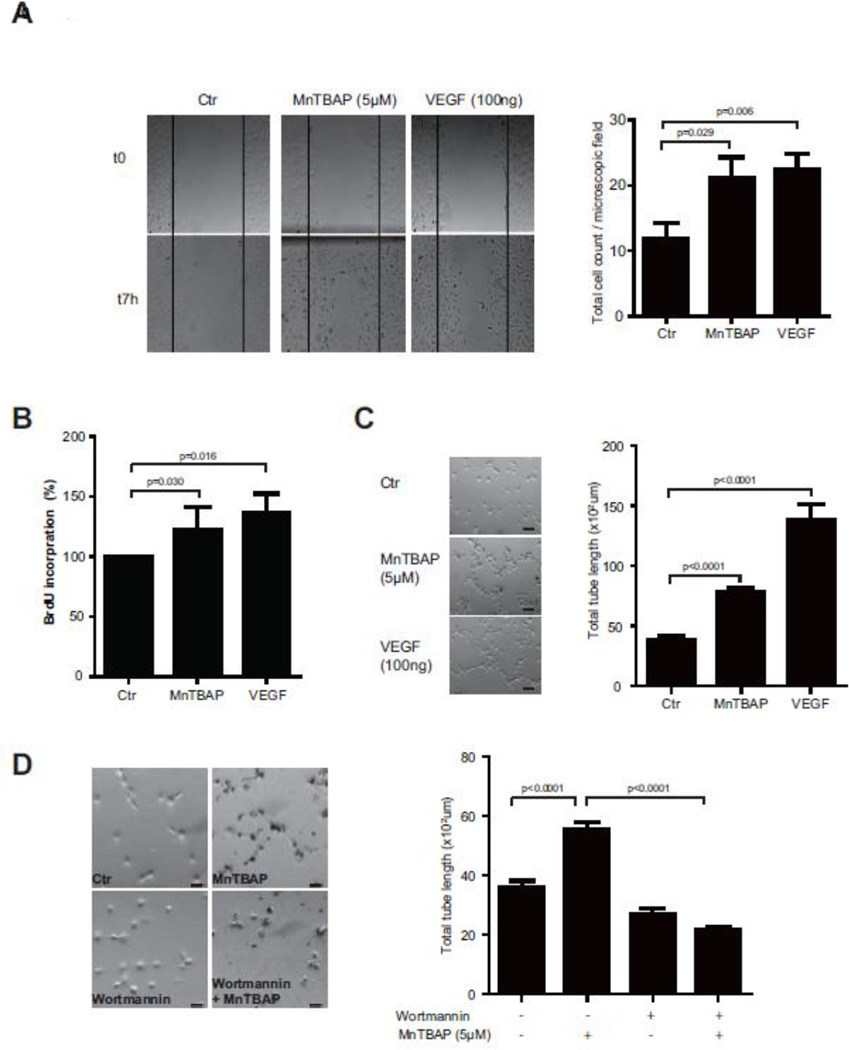
Having demonstrated that MnTBAP activates the pro-angiogenic signaling pathway in HUVECs, we next investigated its effect on endothelial cells using various functional assays. As demonstrated in the scratch assay, MnTBAP treatment enhanced cell migration toward the scratch area by 1.8-fold compared to control treatment (Fig 2A). This effect was similar to VEGF treated cells. MnTBAP treatment also increased cell proliferation (Fig 2B). Furthermore, in the tube formation assay, MnTBAP (5µM) treatment increased total tube length from 36.1×102 ± 208.7µm in control cells to 55.4×102 ± 243.4µm in stimulated cells (Fig 2C, Supplemental figure 3A). Treatment with the PI3K inhibitor wortmannin reduced total tube formation length to 21.8×102 ± 101.7µm (Fig 2D, Supplemental figure 3B).
Figure 2. MnTBAP promotes cell migration, proliferation and tube formation.
MnTBAP enhances cell migration towards the scratch area. Total number of migrated cells per microscopic field after 7h is shown in the bar graph (A). MnTBAP treatment increases proliferation of endothelial cells (B). Treatment with MnTBAP increases total tube length in the tube formation assay (C). This effect is dimished after pre-treatment with wortmannin (D). Data are presented as mean±SEM and are representative of at least 3 independent experiments. Scale bar: 100µm.
3.3 MnTBAP stimulates capillary sprouting and in vivo endothelial cell ingrowth
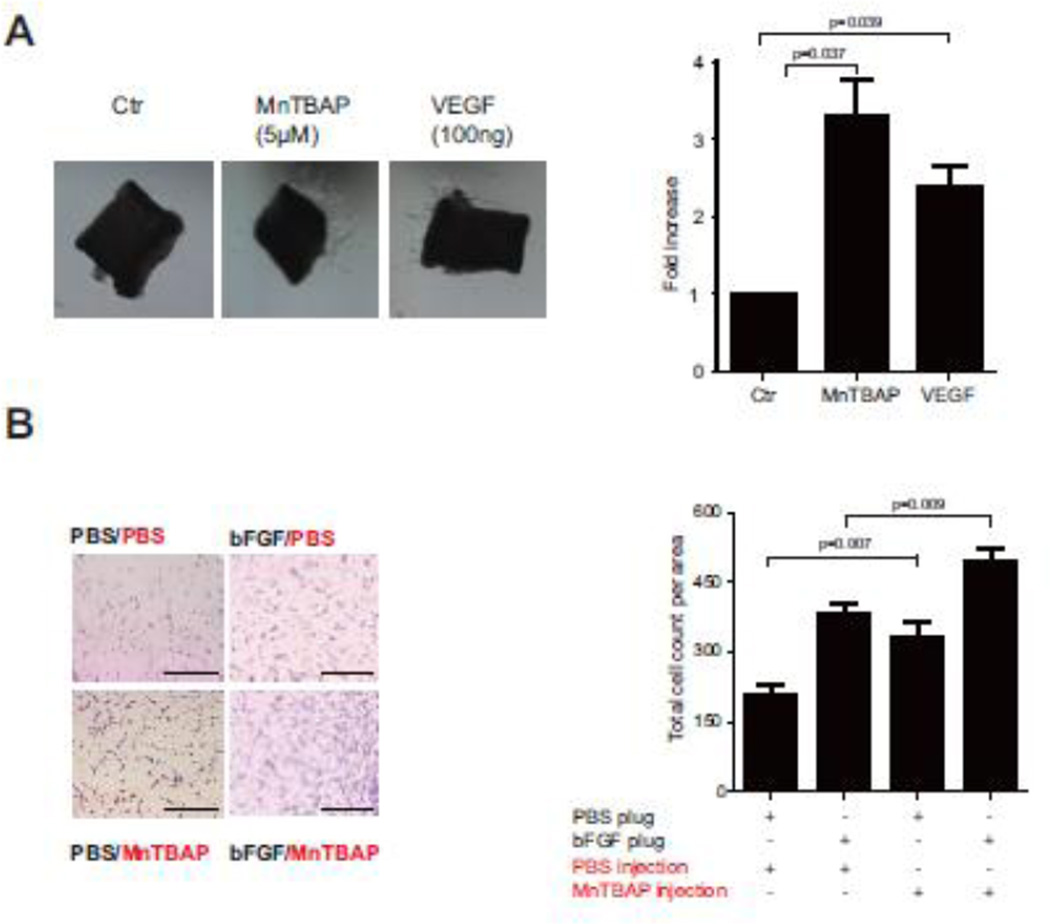
To further evaluate the pro-angiogenic effect of MnTBAP, capillary sprouting assay was performed using the mouse aortic ring assay. MnTBAP treatment increased total sprout length by 3.3-fold compared to control, which was similar to the effect of the positive control VEGF (Fig 3A). To further investigate the functional effect of MnTBAP in vivo, Matrigel plug assay was performed. Mice were injected subcutaneously with Matrigel and treated with MnTBAP or vehicle. bFGF and PBS served as positive and negative controls. Application of MnTBAP increased the amount of ingrowing endothelial cells from 210 ± 22 to 330 ±32 cells in comparison to unstimulated control plugs (Fig 3B). Furthermore, MnTBAP increased the ingrowing cells to 494 ± 27 cells/ area, when bFGF was added additionally to the plugs (Fig 3B). These data confirm the impact of MnTBAP in regulating endothelial cell ingrowth.
Figure 3. MnTBAP elicits angiogenic functions in endothelial cells.
Aortic rings isolated from mice treated with MnTBAP show increased total sprout length and higher sprout density, n=6 (C). Application of MnTBAP to mice with subcutaneous implanted Matrigel results in more ingrowing endothelial cells, n=8 (D). Data are presented as mean±SEM. Scale bar: 100µm.
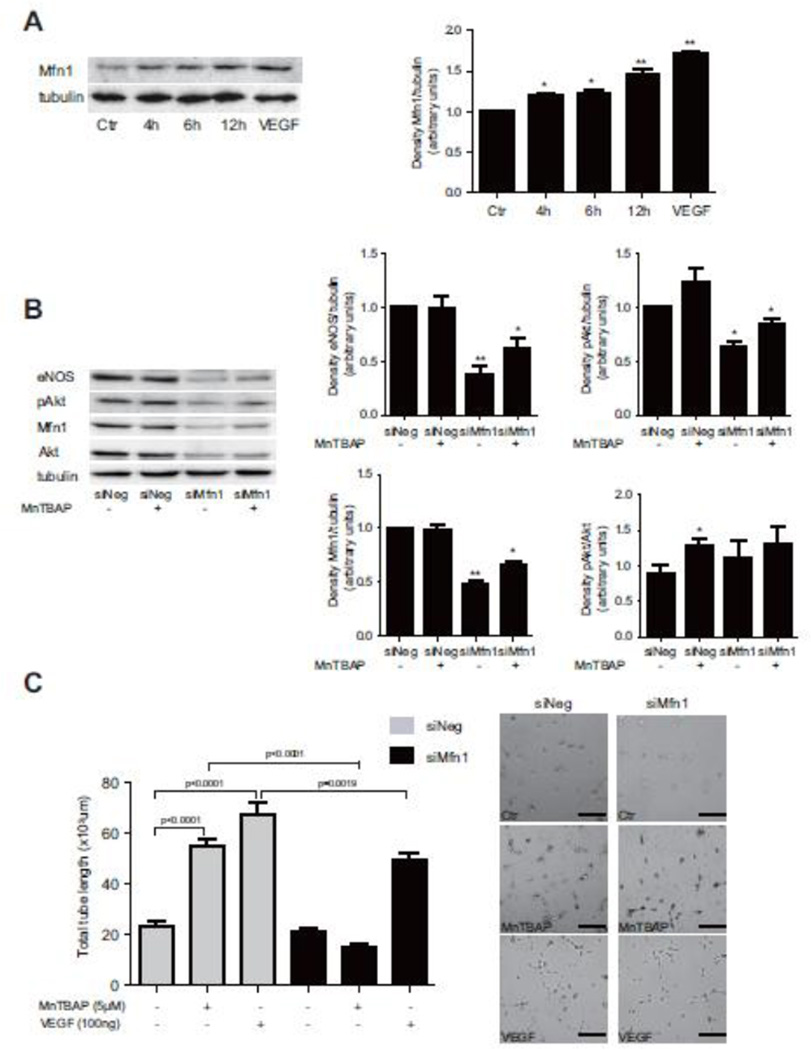
3.4 Mfn-1 expression in HUVECs is essential for MnTBAP-induced tube formation
Because previous data have suggested a potential role of the mitochondrial fission regulatory pathway in mediating pro-angiogenic effects in endothelial cells, we next investigated whether the mitochondrial fusion protein, mitofusin (Mfn)-1, is also modulated by MnTBAP. MnTBAP increased the expression level of the Mfn-1 protein in a time-dependent manner (Fig 4A). The mtDNA:nDNA ratio was not affected (Supplemental figure 5A). To address the potential role of Mfn-1 in mediating MnTBAP induced angiogenic functions, Mfn-1 expression was knocked-down with siRNA and tube formation assay was repeated. Knockdown efficiency of siMfn-1 was confirmed by western blot (Supplemental figure 4A). Two different siRNA mixtures were tested and both mixtures showed significant knock-down efficiency of the Mfn-1 protein. However, on the cellular level, mixture 2 appeared to be more toxic to the cells. We therefore decided to continue all further experiments with siMfn-1 mixture 1. Ablation of Mfn-1 with siMfn-1 mixture did not affect cell viability (Supplemental figure 4B). Mfn-1 knock-down significantly inhibited MnTBAP-mediated Akt and eNOS activation (Fig 4B). In line with this finding, endothelial networks were less extensive in HUVECs with Mfn-1 knockdown and endothelial tubes were shorter than control (Fig 4C, Supplemental figure 3C). This finding suggests that Mfn-1 is essential for MnTBAP-induced tube formation in endothelial cells.
Figure 4. Mfn-1 is expressed in HUVECs and mediates pro-angiogenic response.
Treatment with MnTBAP increases Mfn-1 expression in a time-dependent manner (A). MnTBAP-mediated activation of Akt and eNOS is inhibited after silencing of Mfn-1 (B). MnTBAP induced tube formation is prevented after silencing of Mfn-1 in comparison to control cells (C). Data are presented as mean±SEM and are representative for 3 independent experiments. *P≤0.05, **P≤ 0.005. Scale bar: 100µm.
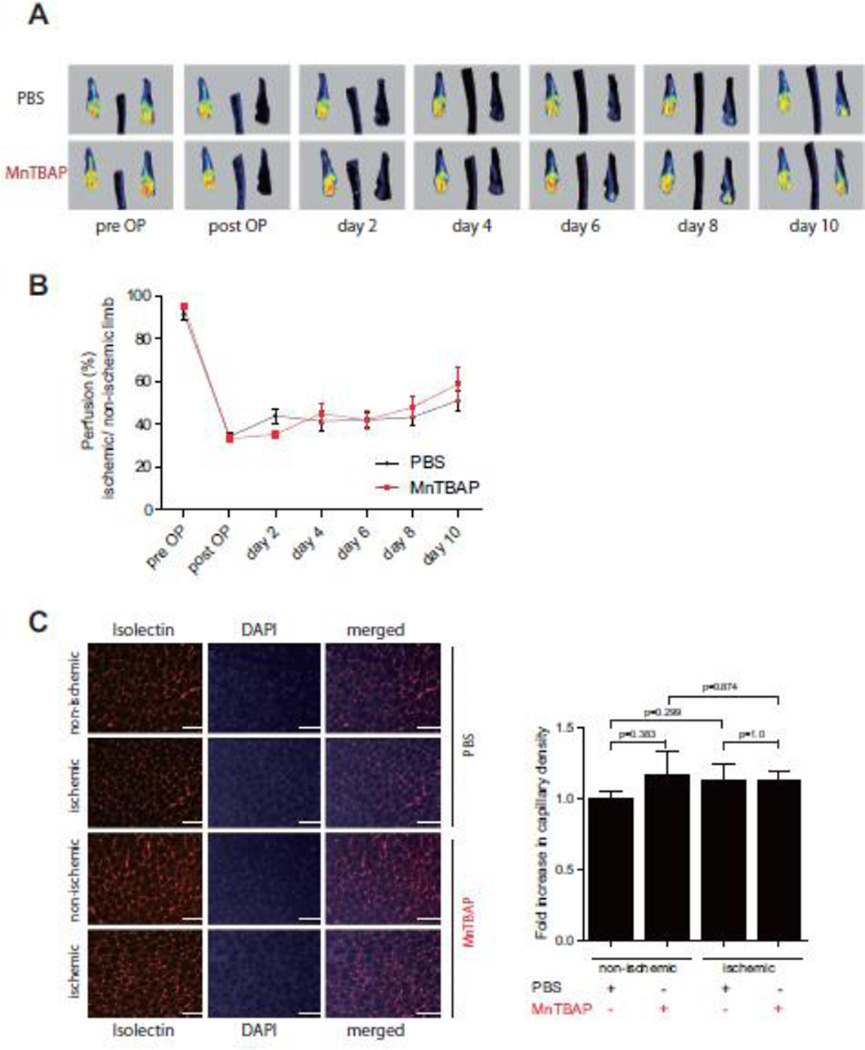
3.5 MnTBAP increases expression of pro-angiogenic factors under ischemic conditions, but does not promote revascularization in the hind-limb ischemia mouse model
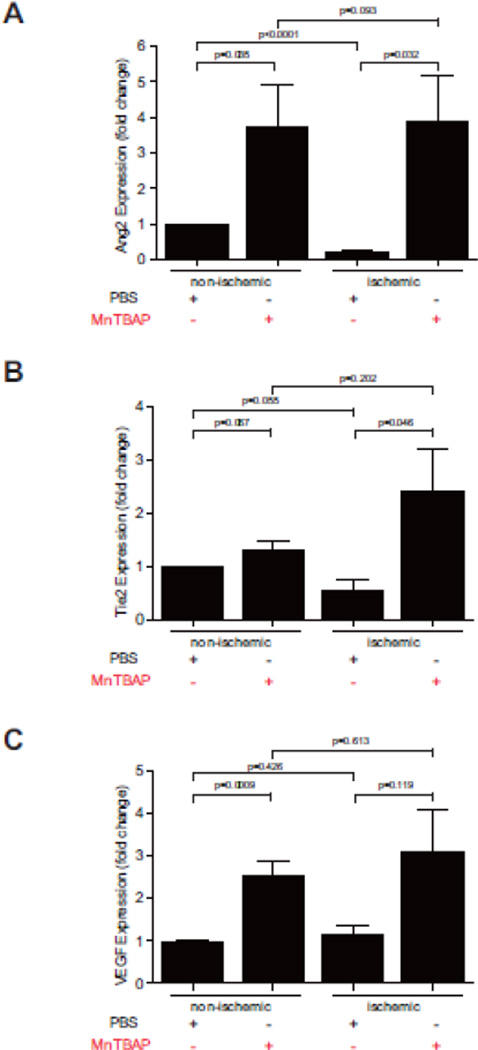
Having demonstrated a pro-angiogenic effect of MnTBAP, we next investigated the impact of MnTBAP in promoting revascularization under ischemic conditions using the hind-limb ischemia model. Mice were treated with MnTBAP (5mg/kg BW) daily after ligation. Laser Doppler perfusion imaging (LDPI) measurements over 10 days revealed a time-dependent increase of perfusion (Fig 5A, B). However, this change was not significant. Similarly, MnTBAP treatment did not significantly increase capillary density after ligation in comparison to control (Fig 5C). To further explore the effect of MnTBAP on pro-angiogenic mediators during the course of revascularization, real-time PCR was performed using muscle tissues 10 days after femoral artery ligation. Compared to the control group, MnTBAP treatment increased mRNA expression of Tie-2, Ang-2 and VEGF by 1.3, 3.8, and 2.6-fold in the non-ischemic group and by 4.3, 18, and 2.7-fold in the ischemic group, respectively (Fig 6, Supplemental figure 6). To further investigate the negative effect of MnTBAP on revascularization in the hindlimb ischemia model, expression of ICAM and TGFβ, which are mediators of arteriogenesis, were determined. ICAM upregulation was prevented by MnTBAP upon stimulation with TNF (Supplemental figure 5B) and TGFβ expression in the gastrocnemius muscles was also decreased in MnTBAP treated mice (Supplemental figure 5C).
Figure 5. Effect of MnTBAP on revascularization in the hindlimb ischemia model.
Representative images of laser Doppler perfusion Imaging (LDPI) during 10 days after femoral artery ligation (A, B). Immunhistochemistry of isolectin staining in M. gastrocnemius cryosections 10 days after arterial ligation (C). Data are represented as mean±SEM; n = 8 per group. Scale bar: 100µm.
Figure 6. MnTBAP induces expression of pro-angiogenic factors during revascularization.
Expression of Ang-2 (A), Tie-2 (B) and VEGF (C) mRNA in ischemic and non-ischemic tissue of M. gastrocnemius 10 days after femoral artery ligation. n = 6 per group; mean±SEM.
4. DISCUSSION
The mitochondrial manganese SOD (MnSOD) belongs to the SOD superfamily. The importance of MnSOD in cell biology is evident in MnSOD knock-out mice. Depending on their genetic background, these mice die within weeks after birth and exhibit a variety of phenotypes including neurodegeneration and cardiac abnormalities[14]. Administration of the MnSOD mimetic MnTBAP rescued part of the phenotypes in these mice and prolonged their survival[18]. Interestingly, recent studies also demonstrated a potential therapeutic relevance of MnTBAP in preventing diabetes[11] and obesity[21]. In most cases, the beneficial effect of MnTBAP was linked to its anti-oxidative properties. Similarly, previous work showed that treatment with MnTBAP restored endothelial dysfunction caused by endothelial microparticles[19]. Pre-treatment with MnTBAP also decreased the cytotoxic effects of Sorafenib on endothelial cells[4].
Here, we demonstrate that MnTBAP enhances angiogenic functions in endothelial cells, which is at least partly independent from its anti-oxidative effect. Our findings suggest that MnTBAP activates the pro-angiogenic signaling via PI3K/Akt/eNOS, which are not seen under the anti-oxidant NAC. Stimulation with MnTBAP results in increased cell migration, tube formation and capillary sprouting. In parallel, MnTBAP treatment also promotes capillary ingrowth in vivo, suggesting a pro-angiogenic effect. Since MnSOD is primarily expressed in mitochondria, we next investigated whether MnTBAP also affects the mitochondrial regulatory pathway. Indeed, several studies have suggested that vascular endothelial cells rely upon mitochondria as signaling rather than energy-producing moieties[22]. In this context, the mitofusin proteins have been attributed to have an important role in regulating mitochondrial membrane fusion[24]. The Mfn proteins belong to the dynamin-related GTPase family[5]. There are two isoforms – Mfn-1 and Mfn-2, and both isoforms are expressed in endothelial cells. Recent data indicate that their expression can be increased by VEGF, suggesting an important role for Mfn proteins in regulating endothelial cell function and survival[16]. While previous data have demonstrated increased Mfn-1 and Mfn-2 expression in HUVECs upon VEGF stimulation[16], our data show that the expression level of Mfn-1 is also increased by MnTBAP. We were not able to detect the expression of Mfn-2 protein, despite the use of several antibodies from different companies. However, Lugus et al. showed that only Mfn-1 was important for activation of the Akt and eNOS pathway in HUVECs upon stimulation with VEGF, but not Mfn-2[16]. This finding suggests an isotype specific function of Mfn in endothelial cells. Our data demonstrated a loss of the pro-angiogenic effect of MnTBAP when Mfn-1 was depleted, which is in line with a previous work showing that ablation of Mfn-1 in HUVEC prevents VEGF-induced tube formation[16].
To further evaluate the impact of MnTBAP on revascularization under ischemic conditions, we performed the hindlimb ischemic model. MnTBAP treatment increased expression of the pro-angiogenic mediators Tie-2, Ang-2 and VEGF after ligation. Surprisingly, revascularization observed via LDPI was not significantly increased in MnTBAP treated mice. Different factors may explain this outcome. One factor could be an insufficient dosage of MnTBAP. Although most published in vivo studies also treated mice with MnTBAP at a dosage of 5mg/kg BW, some studies have used dosages up to 15mg/kg BW [4, 8, 10]. However, our data suggest, that higher concentration of MnTBAP may elicit toxic effects on the cellular level. We therefore selected the lower dose of MnTBAP. Another factor, which may have affected the outcome in our hindlimb ischemia model, could be that limb perfusion reflected by LDPI is mainly dependent on arteriogenesis, but less on angiogenesis. The lack of significant reperfusion we observed, may suggest that MnTBAP mainly increased angiogenesis. Arteriogenesis requires the interplay between different cells aside from endothelia cells, including inflammatory cells and smooth muscle cells (SMC). Adhesion molecules also play an important role in the course of arteriogenesis [23] and the intercellular adhesion molecule (ICAM)-1 has been shown to allow adhesion of monocytes and to mediate shear stress-induced arteriogenesis [12]. We now show, that MnTBAP inhibits ICAM-1 expression in vitro, which may negatively affect the interaction of monocytes with the vessel wall. Finally, previous works suggest that MnTBAP may have cell-specific effects. While our data demonstrate a proliferative effect of MnTBAP on endothelial cells, other studies have shown an anti-proliferative effect of MnTBAP in smooth muscle cells using a carotid endarterectomy model[10]. MnTBAP also reversed the hyperproliferative phenotype of SMC as seen in pulmonary artery hypertension[2]. Smooth muscle cell proliferation is partly regulated by TGFβ, which is also a potent chemoattractant for circulating monocytes[20, 26]. Interestingly, our data revealed an inhibitory effect on TGFβ expression in MnTBAP treated mice. This finding may further explain the less pronounced effect of MnTBAP on arteriogenesis, we observed in the ischemic mouse hindlimb model.
Taken together, our data support that MnTBAP elicits a pro-angiogenic effect in endothelial cells. This effect is mediated through an Mfn-1 dependent pathway, resulting in PI3K/ Akt/ eNOS signaling activation.
Supplementary Material
ACKNOWLEDGEMENT
We are indebted to Ute Wering and Franziska Pankratz for excellent technical assistance. This work is supported by grants from the National Institutes of Health (HL052233) to J.K. Liao, the Deutsche Forschungsgesellschaft (GE 2156/1-1, ZH 231/1-1) to C. Gensch and Q. Zhou and the German Heart Foundation (F/33/12) to Q. Zhou.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
REFERENCE
- 1.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. Faseb J. 2001;15:2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6:1102–1103. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 4.Coriat R, Nicco C, Chereau C, Mir O, Alexandre J, Ropert S, Weill B, Chaussade S, Goldwasser F, Batteux F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol Cancer Ther. 11:2284–2293. doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- 5.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 6.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 7.Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- 8.Garcia-Ruiz I, Solis-Munoz P, Fernandez-Moreira D, Grau M, Colina F, Munoz-Yague T, Solis-Herruzo JA. High-fat diet decreases activity of the oxidative phosphorylation complexes and causes nonalcoholic steatohepatitis in mice. Dis Model Mech. 7:1287–1296. doi: 10.1242/dmm.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–812. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 10.Hirschberg K, Radovits T, Korkmaz S, Loganathan S, Zollner S, Seidel B, Pali S, Barnucz E, Merkely B, Karck M, Szabo G. Combined superoxide dismutase mimetic and peroxynitrite scavenger protects against neointima formation after endarterectomy in association with decreased proliferation and nitro-oxidative stress. Eur J Vasc Endovasc Surg. 2010;40:168–175. doi: 10.1016/j.ejvs.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 12.Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 15.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 16.Lugus JJ, Ngoh GA, Bachschmid MM, Walsh K. Mitofusins are required for angiogenic function and modulate different signaling pathways in cultured endothelial cells. J Mol Cell Cardiol. 51:885–893. doi: 10.1016/j.yjmcc.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 18.Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- 19.Mezentsev A, Merks RM, O'Riordan E, Chen J, Mendelev N, Goligorsky MS, Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H1106–H1114. doi: 10.1152/ajpheart.00265.2005. [DOI] [PubMed] [Google Scholar]
- 20.Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 21.Pires KM, Ilkun O, Valente M, Boudina S. Treatment with a SOD Mimetic Reduces Visceral Adiposity, Adipocyte Death, and Adipose Tissue Inflammation in High Fat-Fed Mice. Obesity (Silver Spring) doi: 10.1002/oby.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 24.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, Tatsumi H, Satoh S, Senda T, Nakata T, Fujii J, Taniguchi N. Manganese-superoxide dismutase in endothelial cells: localization and mechanism of induction. Am J Physiol. 1993;265:H1173–H1178. doi: 10.1152/ajpheart.1993.265.4.H1173. [DOI] [PubMed] [Google Scholar]
- 26.van Royen N, Hoefer I, Buschmann I, Heil M, Kostin S, Deindl E, Vogel S, Korff T, Augustin H, Bode C, Piek JJ, Schaper W. Exogenous application of transforming growth factor beta 1 stimulates arteriogenesis in the peripheral circulation. Faseb J. 2002;16:432–434. doi: 10.1096/fj.01-0563fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.