Abstract
Background
With GAVI support, Vietnam introduced Haemophilus influenzae type b (Hib) vaccine in 2010 without evidence on cost-effectiveness. We aimed to analyze the cost-effectiveness of Hib vaccine from societal and governmental perspectives.
Method
We constructed a decision-tree cohort model to estimate the costs and effectiveness of Hib vaccine versus no Hib vaccine for the 2011 birth cohort. The disease burden was estimated from local epidemiologic data and literature. Vaccine delivery costs were calculated from governmental reports and 2013 vaccine prices. A prospective cost-of-illness study was conducted to estimate treatment costs. The human capital approach was employed to estimate productivity loss. The incremental costs of Hib vaccine were divided by cases, deaths, and disability-adjusted life years (DALY) averted. We used the WHO recommended cost-effectiveness thresholds of an intervention being highly cost-effective if incremental costs per DALY were below GDP per capita.
Result
From the societal perspective, incremental costs per discounted case, death and DALY averted were US$ 6,252, US$ 26,476 and US$ 1,231, respectively; the break-even vaccine price was US$ 0.69/dose. From the governmental perspective, the results were US$ 6,954, US$ 29,449, and US$ 1,373, respectively; the break-even vaccine price was US$ 0.48/dose. Vietnam's GDP per capita was US$ 1,911 in 2013. In deterministic sensitivity analysis, morbidity and mortality parameters were among the most influential factors. In probabilistic sensitivity analysis, Hib vaccine had an 84% and 78% probability to be highly cost-effective from the societal and governmental perspectives, respectively.
Conclusion
Hib vaccine was highly cost-effective from both societal and governmental perspectives. However, with GAVI support ending in 2016, the government will face a six-fold increase in its vaccine budget at the 2013 vaccine price. The variability of vaccine market prices adds an element of uncertainty. Increased government commitment and improved resource allocation decision making will be necessary to retain Hib vaccine.
Introduction
Haemophilus influenza type b (Hib) is an infectious bacterium transmitted from person to person through close contact. Hib can cause meningitis, pneumonia and other less frequent diseases, primarily in children less than five years [1, 2]. In Vietnam, the incidence of Hib meningitis was estimated to be 18/100,000 (95% confidence interval, 15.1-21.6) in children less than five years [3].
Hib vaccine has been used in developed countries since the early 1990s [2]. The herd immunity effect of Hib vaccine has led to a higher vaccine effectiveness compared to efficacy and a near-elimination state in countries with high coverage [4, 5]. Thanks to global efforts in infectious disease prevention, the Global Alliance for Vaccine and Immunization (GAVI) was established and has provided new and underused vaccine support to low-income countries, including Hib vaccine, since 2000. Using GAVI funding, Vietnam introduced the combined diphtheria-tetanus-pertussis-hepatitis B-Hib (DTP-HepB-Hib or “pentavalent”) vaccine in 2010 [6].
According to the country application for GAVI, Vietnam co-financed $0.30 per dose in 2010 and increased the rate by 10% annually during 2011-2015 [6]. However, in 2012 Vietnam moved from the low-income country group to the intermediate group and was responsible for a $0.20/dose rate with a 15% annual increase. The 2013 UNICEF pentavalent vaccine procurement price (one-dose vial) for GAVI was six times more expensive than the combination price of DTP and HepB vaccines [7]. This is a significant financial burden for the Vietnamese government if it decides to cover the pentavalent vaccine in the Expanded Program for Immunization (EPI) after GAVI support ends in 2016. The objective of this study was to analyze the cost-effectiveness of the introduction of the Hib vaccine versus no Hib vaccine in Vietnam. Given that no such analysis was available before the decision of vaccine introduction was made, the study will provide a useful impact assessment to support future decision making.
Methods
Study design
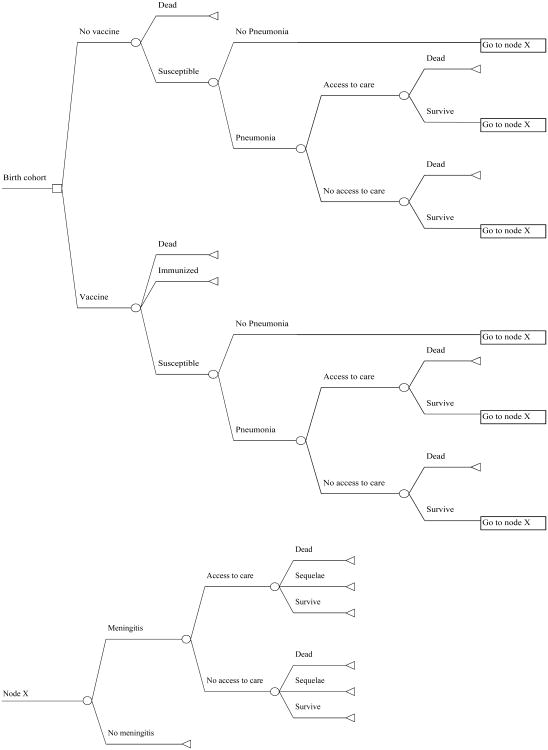
A decision-tree cohort model was developed to estimate the impact of Hib vaccine on disease burden and costs for the 2011 Vietnamese birth cohort (figure 1). Two vaccine schedules in comparison were the pentavalent vaccine administered at 2, 3, and 4 months after a HepB birth dose versus 3 doses of DTP and 3 doses of HepB vaccines. Microsoft Excel 2007 was used to develop the model. Model inputs were derived from local studies and the literature. Model outputs included numbers of Hib meningitis, meningitis sequelae, and Hib pneumonia cases, deaths, and disability-adjusted life years (DALY). Disease burdens from other Hib diseases, such as epiglottitis and cellulitis which are relatively rare, were excluded due to lack of data.
Figure 1. The decision-tree model for the 2011 Vietnamese birth cohort.
The analysis was conducted from the societal and governmental perspectives. All costs were presented in 2013 US dollar (US$) (one US$ is equal to 21,125 Vietnamese Dong [8]) and adjusted for inflation [9]. Costs were discounted at 6.9% (the difference between the 2011 inflation rate and the interest rate for public lending banks [10]). Effectiveness was discounted at 5% [11]. The study was approved by the Institutional Review Board of the University of Texas in Houston, USA and the National Institute of Hygiene and Epidemiology, Vietnam.
Hib associated morbidity and mortality estimates
Table 1 presents estimates and ranges for the base-case and sensitivity analyses. Hib meningitis incidence was based on a retrospective study using the WHO Rapid Assessment Tool (RAT) [3]. We chose the RAT which reported a higher incidence estimate than that of a study conducted during 2000-2002 [12] as RAT estimates were more updated and representative. Data on children less than five years of age with a diagnosis of meningitis were collected from five hospitals in Hanoi and Ho Chi Minh City in 2004-2005 and entered into the RAT standardized worksheet for burden calculation. Hib meningitis sequelae rate and case-fatality rate (CFR) for treated cases were derived from population-based surveillance of bacterial meningitis in Hanoi [12]. Incidence of clinical pneumonia was estimated from hospital-based surveillance in Khanh Hoa during 2005- 2006 [13]. Because of the lack of hospital-based CFR data, we calculated CFR for treated pneumonia cases from the same RAT study [3].
Table 1. Model inputs.
| Parameters | Base-case value | One-way sensitivity analysis range | PSA distribution | Reference |
|---|---|---|---|---|
| Epidemiologic data | ||||
| 2011 live birth cohort | 1,444,753 | N/A | N/A | |
| Incidence of Hib meningitis (per 100,000 children per year) | 23.22 | 19.48-27.86 | gamma | [3] |
| Incidence of clinical pneumonia (per child per year) | 0.0293 | 0.0273-0.0566 | gamma | [13] |
| Proportion of clinical pneumonia caused by Hib | 0.05 | 0.01-0.09 | beta | [16] |
| Hib meningitis sequelae rate | 0.103 | 0.048-0.159 | beta | [12] |
| Hib meningitis case fatality rate-treated | 0.043 | 0.019-0.097 | beta | [12] |
| Hib meningitis case fatality rate-untreated | 1 | 0.5-1 | N/A | |
| Hib pneumonia case fatality rate-treated | 0.099 | 0.058-0.168 | beta | [3] |
| Hib pneumonia case fatality rate-untreated | 0.2 | 0.1-0.4 | N/A | |
| Vaccine effectiveness against Hib meningitis | 0.91 | 0.73-0.97 | beta | [18] |
| Vaccine effectiveness against clinical pneumonia | 0.05 | 0.01-0.09 | beta | [16] |
| Coverage rate-HepB birth dose | 0.485 | 0.348-0.621 | beta | [20] |
| Coverage rate-3 doses of HepB | 0.894 | 0.826-0.962 | beta | [20] |
| Coverage rate-3 doses of DTP | 0.913 | 0.86-0.966 | beta | [20] |
| Coverage rate-3 doses of DTP-HepB-Hib | 0.913 | 0.86-0.966 | beta | [20] |
| Hospitalization days per Hib menigitis case | 8.3 | 7.8-8.7 | normal | [22] |
| Hospitalization days per Hib pneumonia case | 9.5 | 4.5-14.5 | normal | [22] |
| Successful rate of access to health care | 0.71 | 0.64-0.78 | beta | [15] |
| Cost data* | ||||
| Acute Hib diseases | ||||
| Direct medical cost per pneumonia case | 189 | 176-202 | gamma | [22] |
| Direct medical cost per meningitis case | 316 | 129-502 | gamma | [22] |
| Direct non-medical cost per meningitis case | 164 | 44-284 | gamma | [22] |
| Direct non-medical cost per pneumonia case | 53 | 43-63 | gamma | [22] |
| Time cost of caregiver per meningitis case | 147 | 5-288 | gamma | [22] |
| Time cost of caregiver per pneumonia case | 63 | 47-79 | gamma | [22] |
| Sequelae meningitis | ||||
| Rehospitalization-direct medical cost | 316 | 129-502 | gamma | Assumption & [22] |
| Rehospitalization-direct non-medical cost | 164 | 44-284 | gamma | Assumption & [22] |
| Rehospitalization-time cost of caregiver | 147 | 5-288 | gamma | Assumption & [22] |
| Proportion of sequelae case need rehospitalization the same year of acute case | 0.213 | 0.096-0.33 | beta | [28] |
| Average number of outpatient visit per year (visit) | 0.99 | 0.06-2.55 | gamma | [28] |
| Cost of transportation per visit | 7.36 | 4.52-10.20 | gamma | [22] |
| Cost per outpatient visit | 3.16 | N/A | N/A | [27] |
| Cost of medication | 6.32 | N/A | N/A | Assumption |
| Unemployment rate (%) | 2.22 | N/A | N/A | [30] |
| Productivity loss per year | 549 | N/A | N/A | [29] |
Note: PSA, probabilistic sensitivity analysis; N/A, not available; Hib, Haemophilus influenzae type b; DTP, diphtheria-tetanus-pertussis; HepB, hepatitis B.
Costs are in 2013 US$ unless indicated otherwise.
Incidences and CFR were adjusted for limited access to care using the average reported proportion of under-five children with suspected pneumonia taken to an appropriate health provider in Vietnam according to UNICEF surveys during 1997-2011 [14, 15]. We assumed a 100% CFR for untreated meningitis and CFR of untreated pneumonia twice as that of a treated case. Due to the difficulty in estimating Hib pneumonia incidence, the number of clinical pneumonia cases was calculated, five percent of which were assumed attributable to Hib [13, 16, 17].
Vaccine coverage and effectiveness estimates
We used 91% for three-dose Hib vaccine effectiveness against meningitis from a meta-analysis of case-control studies [18]. Hib vaccine effectiveness against clinical pneumonia was estimated in two meta-analysis studies; one summarized efficacy for three doses [16] while the other used the efficacy after one dose [19]. To be consistent with the three-dose schedule, we used the three-dose effectiveness of 5% [16]. Based on government yearly reports, we averaged coverage rates for hepatitis B birth dose (48.5%), three doses of pentavalent (91.3%), hepatitis B (89.4%) and DTP vaccines (91.3%) [20].
Disability-adjusted life year estimates
DALYs were estimated as recommended in the 1996 Global Burden of Disease Study [21]. Age weighting was applied. Based on reported lengths of stay, durations of acute meningitis and pneumonia episodes were approximated at 9.5 and 8.3 days, respectively [22]. The duration of meningitis sequelae was assumed to be throughout patients' lives. The disability weight of 0.21 for a severe, acute episode of an infectious disease was used for both meningitis and pneumonia episodes [23]. We estimated the average disability weight for meningitis sequelae using weights of health states equivalent to different types of sequelae (developmental delay, hearing/vision loss, hydrocephalus, paralysis, seizure disorder, and vegetative state), adjusted for the percentage of sequelae types [12, 23]. The final weight was 0.343.
Cost estimates
Vaccine delivery costs
According to the country's application [6], no cold chain expansion or additional costs for vehicles and transportation were needed. Hence, only vaccines, injection supplies and other vaccine introduction costs were included. 2013 vaccine prices were used (US$ 2.59/pentavalent dose, US$ 0.141/DTP dose and US$ 0.29/hepatitis B dose). Vaccine wastage rates were set at 5%, 15% and 35% for the pentavalent vaccine, HepB vaccine and DTP vaccine, respectively [6]. Costs of injection supplies were the sum of costs of syringes, safety boxes and waste management, where costs of safety boxes and waste management were set at 20% of syringe costs [24]. Wastage rate of syringes was 10% and buffer stock rates for the new vaccine and syringes were 25% in the first year of introduction. Vaccine introduction costs were based on government reports, which detailed the costs of information-education-communication activities (conferences, advertisements, field trips) and training of immunization personnel [25, 26].
Disease treatment costs
These costs included direct medical costs from the governmental perspective, and both direct (medical and non-medical) and indirect costs from the societal perspective. We conducted a prospective cost-of-illness study in Bach Mai hospital to estimate mean direct medical costs (DMC), direct non-medical costs (DNMC), and caregiver's time costs among under-five pneumonia and meningitis patients [22]. 180 pneumonia patients and 15 meningitis patients were recruited. Cost categories of meningitis were between one to three times higher than those of pneumonia. Because there were no data on treatment costs of meningitis sequelae available, we based our estimate on several assumptions. Yearly, a disabled child was assumed to consume one outpatient visit at a cost of US$ 3.16/visit [27], drugs at a cost of twice that of outpatient costs, and transportation costs estimated from the cost-of-illness study [22]. Twenty-one percent of disabled children were assumed to experience one re-hospitalization in the following year at a cost equal to that of the acute episode [28].
To estimate indirect costs, we added productivity loss of household members to care for a sick child during the acute phase and a disabled child, and future lost productivity of the disabled child. Since it has been controversial to assign a dollar value to a life, we did not include productivity loss from death in the base-case, but only in a scenario analysis. The friction human capital approach was used to estimate productivity loss, using the minimum wage rate in 2011 [29] and adjusted for the 2011 national unemployment rate of 2.22% [30].
Patients with sequelae were assumed to have the same disability-adjusted life expectancy at birth as that of patients in the other Asian Islands Region [21]. Applying the United Nation model life table, life expectancy was 55 years for a disabled child less than one year and 61 years for those from 1-5 years [31]. It was assumed that one person cared for the disabled child full time from disease onset until death [28]. To estimate productivity loss from death and disability, we subtracted 18 (the average age of starting to work and earn income) from 58 (the average age of retirement) and multiplied by productivity loss per year. Finally, costs of adverse events were ignored because of well documented safety of the vaccine.
Cost-effectiveness analysis
Incremental cost-effectiveness ratios (ICERs) were presented as the incremental costs per Hib case, death, and DALY averted. As the Vietnamese Government has not determined an official policy for using cost-effectiveness evidence for health care decision making, we used the WHO-recommended thresholds [32]. We assumed that Hib vaccine was cost-effective if incremental costs per DALY averted were less than three times the 2013 Vietnamese gross domestic product (GDP) per capita of $1,911, and highly cost-effective if incremental costs per DALY averted were less than GDP per capita.
Sensitivity analysis
Deterministic sensitivity analysis was conducted for all model inputs with ranges specified in table 1. Tornado diagram was used to present parameters of which variations changed incremental costs per DALY averted ≥10%. We conducted univariate scenario analysis for 0% discount rate for cost and effectiveness, 5% discount rate for cost, a shorter life expectancy for disabled children (30 years), and the addition of productivity loss from death. We also examined different values for Hib meningitis incidence (34/100,000 children/year [16]) and clinical pneumonia incidence (0.35 episode/child-year [33]). Break-even vaccine prices were estimated. Probabilistic sensitivity analysis with 1,000 runs of Monte Carlo simulation was employed.
Results
Impacts of Hib vaccine
Table 2 presents the impact of Hib vaccine on disease burden and vaccine delivery costs. The model predicts that Hib vaccine prevented a discounted number of 1,579 cases, 373 deaths and 8,016 DALYs in the 2011 birth cohort. The vaccine also prevented 76 meningitis sequelae cases.
Table 2. The impact of Haemophilus influenzae type b vaccine on discounted disease burden and program costs.
| Hib vaccine | No Hib vaccine | Incremental value | |
|---|---|---|---|
| Health outcome | |||
| Case | 8,756 | 10,334 | 1,579 |
| Death | 1,022 | 1,395 | 373 |
| DALY | 20,535 | 28,551 | 8,016 |
| Costs (US$) | |||
| Vaccine delivery cost | |||
| Vaccine costs | 14,069,633.04 | 2,665,366 | |
| Program implementation cost | 3,107,207.00 | 2,851,423 | |
| Subtotal | 17,176,840.04 | 5,516,789 | 11,660,051 |
| Disease treatment cost | |||
| Societal perspective | 2,496,575 | 4,285,342 | 1,788,767 |
| Governmental perspective | 1,459,322 | 2,139,691 | 680,368 |
| Total cost | |||
| Societal perspective | 19,673,415 | 9,802,131 | 9,871,284 |
| Governmental perspective | 18,636,162 | 7,656,480 | 10,979,682 |
Note: Hib, Haemophilus influenzae type b; DALY, disability-adjusted life year.
The introduction of Hib vaccine has imposed an incremental vaccine delivery cost of $11.6 million. While the program actually saved resources on syringes, safety boxes and waste management, the high price of pentavalent vaccine caused a three-fold increase in vaccine delivery costs. Regarding treatment costs, after discounting the vaccine saved the society a total of $1.0 million for medical costs and $606 thousand for sequelae-related productivity loss. From the governmental perspective, the total discounted disease treatment costs saved were $703,000.
Cost-effectiveness analysis
Table 3 shows the cost-effectiveness results for the base-case. The base-case ICERs per discounted case, death and DALY prevented from the societal perspective were US$ 6,252, US$ 26,476 and US$ 1,231, respectively. The ICER per DALY averted was less than 2013 Vietnam's GDP per capita, indicating that Hib vaccine was highly cost-effective. From the governmental perspective, the base-case ICER per DALY prevented was US$ 1,370, which was also below the GDP per capita. The break-even vaccine price was US$ 0.69/dose and US$ 0.48/dose from the societal and governmental perspectives, respectively.
Table 3. Base-case and univariate scenario analysis (2013 US$).
| ICER/Case | ICER/Death | ICER/DALY | Break-even price | |
|---|---|---|---|---|
| Societal perspective | ||||
|
| ||||
| Base-case | 6,252 | 26,476 | 1,231 | 0.69 |
| Life expectancy of a sequelae case = 30 years | 6,338 | 26,840 | 1,271 | 0.67 |
| Discount rate=0% for cost and effectiveness | 2,235 | 8,240 | 385 | 1.81 |
| Discount rate=5% for cost | 5,963 | 25,253 | 1,175 | 0.78 |
| Meningitis incidence = 34/100,000 child-year | 4,282 | 17,322 | 803 | 0.84 |
| Clinical pneumonia incidence = 0.35/child-year | 1,465 | 10,119 | 490 | 0.91 |
| Productivity loss from death included | 5,688 | 24,084 | 1,120 | 0.86 |
|
| ||||
| Governmental perspective | ||||
|
| ||||
| Base-case | 6,954 | 29,449 | 1,370 | 0.48 |
| Life expectancy of a sequelae case = 30 years | 6,953 | 29,444 | 1,394 | 0.48 |
| Discount rate=0% for cost and effectiveness | 5,350 | 19,720 | 923 | 0.72 |
| Discount rate=5% for cost | 6,883 | 29,147 | 1,356 | 0.5 |
| Meningitis incidence=34/100000 child-year | 5,037 | 20,381 | 945 | 0.54 |
| Clinical pneumonia incidence=0.35/child-year | 1,720 | 11,883 | 576 | 0.61 |
Note: ICER, incremental cost-effectiveness ratio; DALY, disability-adjusted life year.
Sensitivity analysis
The scenario analyses (Table 3) showed that Hib vaccine remained highly cost-effective for any scenario and from any perspective. The most important variable was CFR of untreated meningitis of which variation caused incremental costs per DALY averted to increase above GDP per capita, but still below three times of the GDP (Figure 2). In probabilistic sensitivity analysis, Hib vaccine had a mean of US$ 1,279 per DALY averted (95% CI: 606-3,011) and 84% chance of being highly cost-effective from the societal perspective (Figure 3). From the governmental perspective, the Hib vaccine had a mean US$ 1,437 per DALY averted (95% CI: 725-2,840) and 78% chance of being highly cost-effective.
Figure 2.
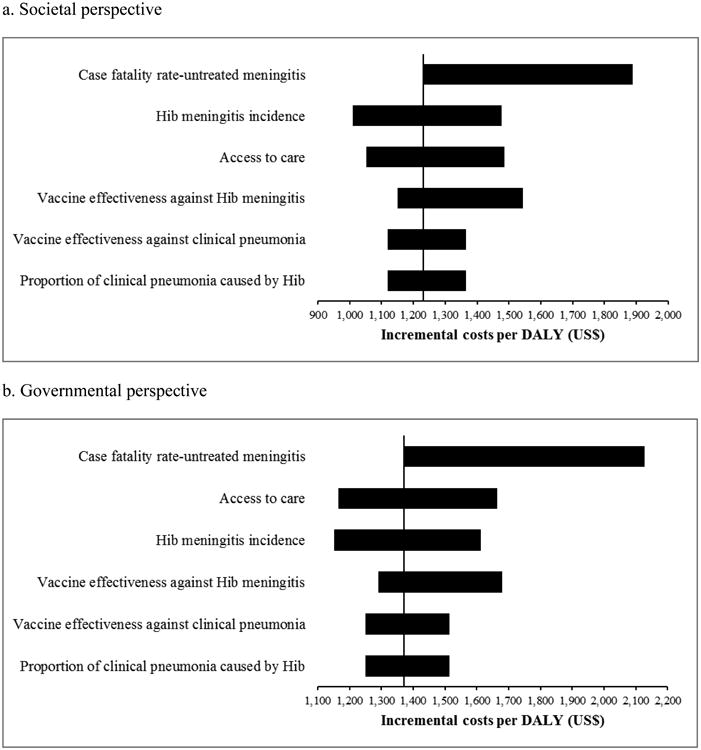
Tornado diagram of influential model inputs on incremental costs per disability-adjusted life year (DALY). Hib, Haemophilus influenzae type b.
Figure 3.
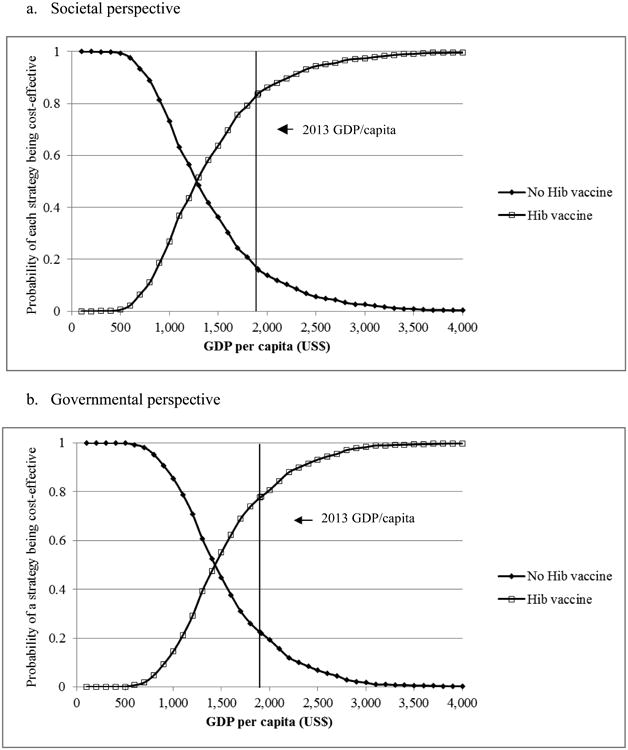
Cost-effectiveness acceptability curves of Hib vaccine versus no Hib vaccine. Hib, Haemophilus influenzae type b; GDP, Gross Domestic Product.
Discussion
Our study found that incremental costs per DALY averted of Hib vaccine compared to no vaccine was US$ 1,231 from the societal perspective and US$ 1,370 from the governmental perspective. According to the WHO thresholds, the introduction of Hib vaccine in Vietnam has been highly cost-effective. Both deterministic and probabilistic sensitivity analyses showed that our results were robust to variations in model inputs. In addition, Hib vaccine remained highly cost-effective in all scenario analyses. Our findings were consistent with other studies conducted in both lower-middle and upper-middle income countries, such as Uzbekistan, India, Indonesia, Kenya, Iran and Columbia [14, 34-39]. In high-income countries, Hib vaccine cost-effectiveness analyses were largely conducted during the 1990s when the vaccine was first licensed. The large majority of these studies concluded that the vaccine was cost-saving. This was partly due to higher meningitis incidence rates than what has been found in Vietnam, and in particular due to considerable meningitis sequelae treatment costs, which in many instances also included special schooling [40, 41]. Our study identified another highly cost-effective vaccine for Vietnam to consider, in addition to hepatitis B, rotavirus, and HPV vaccines [42-44].
In general, a vaccine is recommended for the EPI when there is sufficient evidence on the burden of disease and the safety and efficacy of the vaccine [4]. While the safety and efficacy of Hib vaccine has been shown in various areas around the world [45], data on Hib disease burden is limited, especially in Asia. Imperfect laboratory techniques, high antibiotics use and inadequate specimen handling and transport have been suggested as possible explanations [4]. Although vaccine probe studies are able to provide better data on disease burden, only two studies have been conducted in Asia [46, 47]. In Vietnam, two epidemiologic studies estimated Hib disease burden before vaccine introduction. One study was a population-based surveillance and the other was a hospital-based surveillance [3, 12]. While both used appropriate case definitions and laboratory techniques, they failed to account for both missing cases and meningitis cases without lumbar puncture, so the reported incidence was likely underestimated. Inappropriate antibiotic use in Vietnam due to common self-medication practices and the ease of antibiotics purchase without prescription is another important issue that probably interferes with true estimates of Hib disease incidences [48]. Although Hib vaccine remained highly cost-effective in sensitivity analyses, the ICERs were especially sensitive to variations in determinants of disease burden, emphasizing the need to have a more precise disease burden estimate.
Our study has several limitations. First was the lack of local data for some model inputs, including CFRs for untreated Hib cases and costs of treatment and care for sequelae cases. However, variations in these parameters did not change our conclusion in sensitivity analyses. Secondly, the disease burden and costs might be underestimated because incidences and costs of other Hib diseases than meningitis and pneumonia were excluded. However, inclusion of other Hib diseases would make Hib vaccine more cost-effective, or even cost-saving. Thirdly, costs of vaccine adverse events were not accounted for, making the ICERs underestimated. Finally, the study did not account for herd immunity effects of Hib vaccine.
Although Hib vaccine introduction was found to be highly cost-effective in Vietnam, our study does not determine the affordability and sustainability of the program in the future. To better prepare recipient countries for self-financing, GAVI mandates a co-financing policy depending on the GNI per capita and GAVI eligibility threshold [49]. A country belongs to low-income country group if its GNI per capita is below the World Bank (WB) low-income threshold. A country is in intermediate group if its GNI per capita is above the WB low-income threshold but below GAVI threshold. A graduating country is the one with GNI per capita above GAVI threshold. The graduating country has to co-finance at a higher rate compared to the other two groups and the rate increases linearly to the full projected vaccine price after four years. Vietnam moved into the intermediate group in 2012. The 2013 Vietnam GNI was US$ 1,740 [50], which was above GAVI threshold; so GAVI has determined the country to be joining the graduating group in 2015. Therefore, the country has only one more year to apply for GAVI new vaccine support and must pay the full projected vaccine price after the next few years. Besides the issue of a six-fold increase in the vaccine budget (should the Hib vaccine remains at current 2013 GAVI-supported price), Vietnam is facing the uncertainty of market vaccine price variation after GAVI support ends. Although graduated countries can access the GAVI price for Crucell's pentavalent vaccine until 2020 [49], there is uncertainty regarding vaccine prices for different presentations (e.g. monovalent Hib vaccine) and from different manufactures after 2020. Therefore, the question of post-GAVI affordability and/or sustainability in Vietnam becomes of significance and requires a careful scrutiny into the government's EPI budget. Hopefully, adequate resources will be available to continue this highly cost-effective Hib vaccine program, which is contributing significantly to child health improvement and achievement of Millenium Development Goal 4 in Vietnam.
Acknowledgments
The study was funded by Small Grant program of the International Society for Infectious Diseases, Dissertation Research Award of the University of Texas School of Public Health, and the Fogarty International Center training grant # D43 TW007669
Role of the funding source: The sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflict of interest: None of the authors has any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Phuc Le, University of Texas School of Public Health.
Ulla K. Griffiths, London School of Hygiene and Tropical Medicine.
Dang Duc Anh, Vietnam National Institute of Hygiene and Epidemiology.
Luisa Franzini, University of Texas School of Public Health.
Wenyaw Chan, University of Texas School of Public Health.
J. Michael Swint, University of Texas School of Public Health.
References
- 1.Bennette JV, Platonov AE, Mary PE, Slack Mala P, Burton AH, Robertson SE. Haemophilus influenzae type b meningitis in the pre-vaccine era: a global review of incidence, age distributions, and case-fatality rate. World Health Organization; 2002. [Google Scholar]
- 2.Funkhouser A, Steinhoff MC, Ward J. Haemophilus influenzae disease and immunization in developing countries. Rev Infect Dis. 1991 May-Jun;13(Suppl 6):S542–54. doi: 10.1093/clinids/13.supplement_6.s542. [DOI] [PubMed] [Google Scholar]
- 3.Nyambat B, Dang DA, Nguyen HA, Mai TQ, Rani M, Slack MP, et al. Rapid assessment of Hib disease burden in Vietnam. BMC Public Health. 2011;11:260. doi: 10.1186/1471-2458-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO position paper on Haemophilus influenzae type b conjugate vaccines. (Replaces WHO position paper on Hib vaccines previously published in the Weekly Epidemiological Record. Wkly Epidemiol Rec. 2006 Nov 24;81(47):445–52. [PubMed] [Google Scholar]
- 5.Peltola H, Salo E, Saxen H. Incidence of Haemophilus influenzae type b meningitis during 18 years of vaccine use: observational study using routine hospital data. BMJ. 2005 Jan 1;330(7481):18–9. doi: 10.1136/bmj.38301.657014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Government of Socialist Republic of Viet Nam. GAVI Application form for country proposals for support to Immunization Services, Injection Safety and New and Under-Used vaccines. 2008 [Google Scholar]
- 7.Unicef. [Accessed July 20, 2013];Supplies and Procurement. Available at: http://www.unicef.org/supply/index_57476.html.
- 8.Vietcombank. Currency exchange rate. [Accessed June 1, 2013]; Available at: http://www.vietcombank.com.vn/exchangerates/
- 9.General Statistics Office. Consumer price index. [Accessed April 1, 2014]; Available at: http://www.gso.gov.vn/default.aspx?tabid=393&idmid=3&ItemID=13152.
- 10.Griffiths UK, Korczak VS, Ayalew D, Yigzaw A. Incremental system costs of introducing combined DTwP-hepatitis B-Hib vaccine into national immunization services in Ethiopia. Vaccine. 2009 Feb 25;27(9):1426–32. doi: 10.1016/j.vaccine.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Drummond M, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Third. Oxford University Press; 2005. [Google Scholar]
- 12.Anh DD, Kilgore PE, Kennedy WA, Nyambat B, Long HT, Jodar L, et al. Haemophilus influenzae type B meningitis among children in Hanoi, Vietnam: epidemiologic patterns and estimates of H. Influenzae type B disease burden. Am J Trop Med Hyg. 2006 Mar;74(3):509–15. [PubMed] [Google Scholar]
- 13.Anh DD, Kilgore PE, Slack MP, Nyambat B, Tho le H, Yoshida LM, et al. Surveillance of pneumococcal-associated disease among hospitalized children in Khanh Hoa Province, Vietnam. Clin Infect Dis. 2009 Mar 1;48(Suppl 2):S57–64. doi: 10.1086/596483. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths UK, Clark A, Shimanovich V, Glinskaya I, Tursunova D, Kim L, et al. Comparative economic evaluation of Haemophilus influenzae type b vaccination in Belarus and Uzbekistan. PLoS One. 6(6):e21472. doi: 10.1371/journal.pone.0021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unicef. [Accessed 6-1-2013];Proportion of children aged 0-59 months with suspected pneumonia taken to an appropriate health provider. Available at: http://www.childinfo.org/pneumonia_careseeking.php.
- 16.Watt JP, Wolfson LJ, O'Brien KL, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 17.Wolfson L, O'Brien KL, Watt JP, Henkle E, Deloria-Knoll M, et al. Methods to estimate the global burden of disease due to Haemophilus influenzae type b and Streptococcus pneumoniae in children less than 5 years of age. Lancet web annex. 2009:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 18.O'Loughlin RE, Edmond K, Mangtani P, Cohen AL, Shetty S, Hajjeh R, et al. Methodology and measurement of the effectiveness of Haemophilus influenzae type b vaccine: systematic review. Vaccine. 2010;28(38):6128–36. doi: 10.1016/j.vaccine.2010.06.107. [DOI] [PubMed] [Google Scholar]
- 19.Theodoratou E, Johnson S, Jhass A, Madhi SA, Clark A, Boschi-Pinto C, et al. The effect of Haemophilus influenzae type b and pneumococcal conjugate vaccines on childhood pneumonia incidence, severe morbidity and mortality. Int J Epidemiol. 2010;39(Suppl 1):i172–85. doi: 10.1093/ije/dyq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Government of Vietnam. [Accessed 5-18-2013];GAVI Alliance-Annual Progress Report 2011. 2012 Available at: http://www.gavialliance.org/country/vietnam/documents/#apr.
- 21.Murray CJ, Lopez AD. Global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Boston: Harvard University Press; 1996. [Google Scholar]
- 22.Le P, Griffiths UK, Anh DD, Franzini L, Chan W, Pham H, et al. The economic burden of pneumonia and meningitis among children less than five years old in Hanoi, Vietnam. Trop Med Int Health. 2014;19(11):1321–7. doi: 10.1111/tmi.12370. [DOI] [PubMed] [Google Scholar]
- 23.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estimating the potential cost-effectiveness of using Haemophilus influenzae type b vaccine-Field test version 1. World Health Organization; 2001. Vaccine Assessment and Monitoring Team, Department of Vaccines and Biologicals. [Google Scholar]
- 25.Ministry of Health National Institute of Hygiene and Epidemiology, Expanded Program on Immunization. Planning for the Expanded Program on Immunization in 2009. 2008 Dec; [Google Scholar]
- 26.Ministry of Health, National Institute of Hygiene and Epidemiology, Expanded Program on Immunization. Planning for the Expanded Program on Immunization in 2010. 2010 Mar; [Google Scholar]
- 27. [Accessed June 23, 2013];Estimates of Unit Costs for Patient Services for Viet Nam, WHO-CHOICE. Available at: http://www.who.int/choice/country/vnm/cost/en/index.html.
- 28.Griffiths UK, Dieye Y, Fleming J, Hajjeh R, Edmond K. Costs of meningitis sequelae in children in Dakar, Senegal. Pediatr Infect Dis J. Nov;31(11):e189–95. doi: 10.1097/INF.0b013e3182615297. [DOI] [PubMed] [Google Scholar]
- 29.The Government of Vietnam. No. 22/2011/ND-CP-Regulation on average salary. 4-4-2011
- 30.General Statistics Office. Unemployment rate. [Accessed 6-1-2013]; Available at: http://www.gso.gov.vn/default.aspx?tabid=387&idmid=3&ItemID=12834.
- 31.United Nations. [Accessed 5-18-2013];Model Life Tables for Developing Countries. 1982 Available at: http://www.un.org/esa/population/publications/Model_Life_Tables/Model_Life_Tables.htm.
- 32. [Accessed June 1, 2013];Cost-effectiveness threshold –WHO CHOICE. Available at: http://www.who.int/choice/costs/CER_thresholds/en/
- 33.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86(5):408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akumu AO, English M, Scott JA, Griffiths UK. Economic evaluation of delivering Haemophilus influenzae type b vaccine in routine immunization services in Kenya. Bull World Health Organ. 2007;85(7):511–8. doi: 10.2471/BLT.06.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvis Guzmán N, De La Hoz Restrepo F, Vivas Consuelo D. The cost-effectiveness of Haemophilus influenzae type b vaccine for children under 2 years of age in Colombia. Rev Panam Salud Publica. 2006;20(4):248–55. doi: 10.1590/s1020-49892006000900005. [DOI] [PubMed] [Google Scholar]
- 36.Broughton EI. Economic evaluation of Haemophilus influenzae type B vaccination in Indonesia: a cost-effectiveness analysis. J Public Health (Oxf) 2007;29(4):441–8. doi: 10.1093/pubmed/fdm055. [DOI] [PubMed] [Google Scholar]
- 37.Gessner BD, Sedyaningsih ER, Griffiths UK, Sutanto A, Linehan M, Mercer D, et al. Vaccine-preventable Haemophilus influenza type B disease burden and cost-effectiveness of infant vaccination in Indonesia. Pediatr Infect Dis J. 2008;27(5):438–43. doi: 10.1097/INF.0b013e318165f1ba. [DOI] [PubMed] [Google Scholar]
- 38.Gupta M, Prinja S, Kumar R, Kaur M. Cost-effectiveness of Haemophilus influenzae type b (Hib) vaccine introduction in the universal immunization schedule in Haryana State, India. Health Policy Plan. 2013;28(1):51–61. doi: 10.1093/heapol/czs025. [DOI] [PubMed] [Google Scholar]
- 39.Moradi-Lakeh M, Shakerian S, Esteghamati A. Immunization against Haemophilus Influenzae type b in Iran; Cost-utility and Cost-benefit Analyses. Int J Prev Med. 2012;3(5):332–40. [PMC free article] [PubMed] [Google Scholar]
- 40.Garpenholt O, Silfverdal SA, Levin LA. Economic evaluation of general childhood vaccination against Haemophilus influenzae type b in Sweden. Scand J Infect Dis. 1998;30(1):5–10. doi: 10.1080/003655498750002222. [DOI] [PubMed] [Google Scholar]
- 41.Zhou F, Bisgard KM, Yusuf HR, Deuson RR, Bath SK, Murphy TV. Impact of universal Haemophilus influenzae type b vaccination starting at 2 months of age in the United States: an economic analysis. Pediatrics. 2002;110(4):653–61. doi: 10.1542/peds.110.4.653. [DOI] [PubMed] [Google Scholar]
- 42.Fischer TK, Anh DD, Antil L, Cat ND, Kilgore PE, Thiem VD, et al. Health care costs of diarrheal disease and estimates of the cost-effectiveness of rotavirus vaccination in Vietnam. J Infect Dis. 2005;192(10):1720–6. doi: 10.1086/497339. [DOI] [PubMed] [Google Scholar]
- 43.Goldie SJ, Diaz M, Kim SY, Levin CE, Van Minh H, Kim JJ. Mathematical models of cervical cancer prevention in the Asia Pacific region. Vaccine. 2008;26(Suppl 12):M17–29. doi: 10.1016/j.vaccine.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Tu HAT, de Vries R, Woerdenbag HJ, Li SC, Le HH, van Hulst M, et al. Cost-Effectiveness Analysis of Hepatitis B Immunization in Vietnam: Application of Cost-Effectiveness Affordability Curves in Health Care Decision Making. Value in Health Regional Issues. 2012;1(1):7–14. doi: 10.1016/j.vhri.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Danovaro-Holliday MC, Garcia S, de Quadros C, Tambini G, Andrus JK. Progress in vaccination against Haemophilus influenzae type b in the Americas. PLoS Med. 2008;5(4):e87. doi: 10.1371/journal.pmed.0050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baqui AH, El Arifeen S, Saha SK, Persson L, Zaman K, Gessner BD, et al. Effectiveness of Haemophilus influenzae type B conjugate vaccine on prevention of pneumonia and meningitis in Bangladeshi children: a case-control study. Pediatr Infect Dis J. 2007;26(7):565–71. doi: 10.1097/INF.0b013e31806166a0. [DOI] [PubMed] [Google Scholar]
- 47.Gessner BD, Sutanto A, Linehan M, Djelantik IG, Fletcher T, Gerudug IK, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365(9453):43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 48.Nga DTT, Chuc NT, Hoa NP, Hoa NQ, Nguyen NT, Loan HT, et al. Antibiotic sales in rural and urban pharmacies in northern Vietnam: an observational study. BMC Pharmacol Toxicol. 2014;15(1):6. doi: 10.1186/2050-6511-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.GAVI. [Accessed February 12, 2015];FAQs about co-financing. Available at: http://www.gavi.org/support/apply/faqs-about-co-financing/
- 50.World Bank. [Accessed January 6, 2015];GNI per capita, Atlas method (current US$) Available at: http://data.worldbank.org/indicator/NY.GNP.PCAP.CD.